Abstract
Intramuscular inoculation of rhesus macaques with one or more doses of recombinant vesicular stomatitis virus (rVSV) expressing human immunodeficiency virus type 1 (HIV-1) Gag (rVSVgag) typically elicits peak cellular immune responses of 500 to 1,000 gamma interferon (IFN-γ) enzyme-linked immunospots (ELISPOTS)/106 peripheral blood lymphocytes (PBL). Here, we describe the generation of a novel recombinant mumps virus (rMuV) expressing HIV-1 Gag (rMuVgag) and measure the Gag-specific cellular immune responses detected in rhesus macaques following vaccination with a highly attenuated form of rVSV expressing HIV-1 Gag (rVSVN4CT1gag1) and rMuVgag in various prime-boost combinations. Notably, peak Gag-specific cellular immune responses of 3,000 to 3,500 ELISPOTS/106 PBL were detected in macaques that were primed with rMuVgag and boosted with rVSVN4CT1gag1. Lower peak cellular immune responses were detected in macaques that were primed with rVSVN4CT1gag1 and boosted with rMuVgag, although longer-term gag-specific responses appeared to remain higher in this group of macaques. These findings indicate that rMuVgag may significantly enhance Gag-specific cellular immune responses when administered with rVSVN4CT1gag1 in heterologous prime-boost regimens.
The ability to recover infectious virus from genomic cDNA has enabled the development of nonsegmented negative-strand RNA viruses as candidate vaccine vectors (8, 20); vesicular stomatitis virus (VSV), which predominantly infects insects and livestock in nature (29, 51, 52), is one of the most extensively studied in this group of RNA viruses. Recombinant forms of VSV (rVSVs) have been tested in preclinical studies as potential vaccine vectors to combat a wide range of human diseases including human immunodeficiency virus (HIV)/AIDS (16, 28, 30, 31, 40-43). In one of these studies, nonhuman primates (NHPs) vaccinated with rVSV vaccine vectors expressing simian immunodeficiency virus (SIV) Gag and HIV Env proteins were protected from disease following challenge with a pathogenic SIV/HIV-1 recombinant (SHIV) (46). Although these prototypic rVSV vaccine vectors elicited robust SHIV-specific immune responses in NHPs and demonstrated protective efficacy in the SHIV challenge model, they were found to be insufficiently attenuated for human trials when tested in a stringent NHP neurovirulence model (27). This finding was addressed by the development of a highly attenuated rVSV vector (rVSVN4CT1gag1). This vector was attenuated by combination of a specific N gene translocation and G gene truncation (9), with the N gene in position 4 (N4), the G gene expressing a G protein with a single amino acid in the cytoplasmic tail (CT1), and the HIV-1 Gag gene added in the first position of the genome (gag1). The rVSVN4CT1gag1 vector caused no obvious signs of neurological disease in young mice following intracranial inoculation with >107 PFU of virus (12) and produced only very minimal, predominantly inflammatory lesions following intrathalamic inoculation of NHPs with 107 PFU of virus (unpublished data). Although rVSVN4CT1gag1 demonstrated reduced in vitro replication efficiency and in vivo virulence, it was as immunogenic in mice (12) and NHPs (unpublished data) as the much more virulent prototypic rVSV vectors that provided protection from disease in the SHIV challenge model.
Mumps virus (MuV), the agent of mumps in humans, is a nonsegmented negative-strand RNA virus in the family Paramyxoviridae. The incidence of mumps has been greatly reduced in the developed world by the introduction of live attenuated MuV vaccine strains over the past 30 to 35 years. The most commonly used MuV vaccine in the United States and Western Europe is the Jeryl Lynn strain, which has demonstrated excellent efficacy and an outstanding safety record for the >100 million doses administered to the pediatric population. A system for the recovery of the Jeryl Lynn strain of MuV from genomic cDNA has been described previously (10). This methodology has enabled targeted alteration of the MuV genome to study virus-associated neurovirulence and neuroattenuation (33) as well as the possibility of developing MuV as a vaccine vector for other pathogens.
There is currently no proven method of inducing broadly neutralizing antibodies in HIV type 1 (HIV-1) vaccinees. It has been postulated, however, that robust vaccine-induced cellular immune responses directed against one or more HIV-1 proteins may be sufficient to prevent HIV-1-infected humans from developing AIDS in the absence of broadly neutralizing antibodies (34). Although this hypothesis has been called into question recently following the results of an HIV-1 phase II clinical trial (the STEP trial), there is still reason to believe that a robust cellular immune response against specific cytotoxic T-lymphocyte epitopes within highly conserved regions of the viral proteome could result in a significantly reduced viral load following HIV-1 infection (45). One rational approach for maximizing vaccine-induced HIV-1-specific peak cellular immune responses is the administration of completely heterologous vaccine vectors in prime-boost regimens (2, 15, 24, 40), unlike the serotype switch used previously in rVSV prime-boost vaccination regimens (12, 46, 47). In general, the magnitudes of the resulting cellular immune responses were higher than those detected for comparable prime-boost regimens with homologous vectors (14, 46) although any associated enhancement of protective efficacy in challenge models remains unclear (3, 48). Here, we describe the generation of a novel rMuV vector expressing HIV-1 Gag (rMuVgag) and the immune responses elicited in rhesus macaques when this vector was administered with a highly attenuated rVSVN4CT1gag1 vector in heterologous prime-boost regimens.
MATERIALS AND METHODS
Cells and viruses.
Vero and baby hamster kidney (BHK) cell lines were obtained from the American Type Culture Collection and propagated at 37οC in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), sodium pyruvate (20 mM), and gentamicin (50 μg/ml). Recombinant mumps virus (rMuV) was derived from the Jeryl Lynn strain of MuV vaccine (Merck and Co., Inc., Westpoint, PA). Recombinant VSVN4CT1gag1 was derived from the Indiana serotype of rVSV.
Construction and rescue of rMuVgag and rVSVN4CT1gag1 vectors.
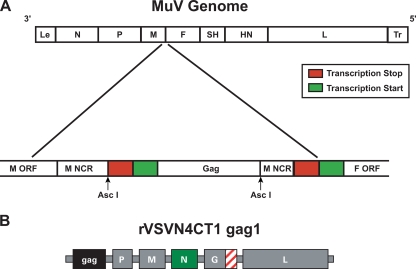
The construction and rescue of rMuV from genomic cDNA has previously been described in detail (10). For the purposes of this study, the plasmid containing rMuV genomic cDNA (pMuV) was modified to contain an AscI site in the 3′ noncoding region (NCR) of the M gene (pMuVAscI) to enable insertion of a transcription unit(s) (TU) between the MuV M and F genes for foreign gene expression. This was accomplished by generating two PCR products: one stretching from the unique BssHII site in the M gene into the M gene 3′ NCR and containing a primer-encoded AscI site at the 3′ end and the other stretching from the M gene 3′ NCR to the unique XhoI site in the L gene and containing a primer-encoded AscI site at the 5′ end. Both PCR products were gel purified, digested with AscI, and then ligated in vitro. The resulting DNA fragment was gel purified, trimmed with BssHII and XhoI, and cloned back into pMuV to generate pMuVAscI. The HIV-1 (strain Hxb2) p55 Gag gene was then PCR amplified from an existing cDNA template (17) with primers encoding AscI sites and MuV-specific transcription stop/start signals (19). The resulting PCR product was gel purified, trimmed with AscI, and cloned into pMuVAscI to generate pMuVgag (Fig. 1). A helper-virus-free method for the recovery of rMuV from genomic cDNA has been described recently (54) and was used for the rescue of rMuVgag. Briefly, nearly confluent Vero cell monolayers in T-150 flasks were trypsinized, rinsed, collected by centrifugation at 300 × g, and resuspended in Iscove's modified Dulbecco's medium supplemented with 200 μM 2-mercaptoethanol, 1% nonessential amino acids, 1% sodium pyruvate, and 1% dimethyl sulfoxide. Cells were then electroporated with a mixture of plasmids expressing T7 RNA polymerase under the control of the cytomegalovirus (CMV) promoter and with the MuV N, P, and L proteins and a positive-sense copy of the rMuVgag genome, all under the control of the T7 RNA polymerase promoter. Electroporated cells were collected by centrifugation at 300 × g for 5 min at room temperature (RT) and then transferred to a flask containing DMEM supplemented with 10% FBS, 200 μM 2-mercaptoethanol, 1% nonessential amino acids, and 1% sodium pyruvate. Cells were then incubated at 37°C in 5% CO2 for 3 h, followed by heat shock at 43°C in 5% CO2 for 3 h, and were then returned to 37°C in 5% CO2 for ∼18 h; the medium was then replaced, and incubation was continued for 5 to 7 days. The monolayers were scraped into the culture medium, agitated to break up cell clumps, and transferred onto 50% confluent Vero cell monolayers in T-150 flasks. The cell monolayers were observed daily for the development of MuV-induced cytopathic effect (CPE). Rescued rMuVgag was plaque picked and amplified on Vero cell monolayers and then tested for Gag expression by Western blotting, Gag-specific enzyme-linked immunosorbent assay (ELISA), and whole-infected-cell immunofluorescence. The integrity of the Gag gene open reading frame in rMuVgag was also verified by consensus nucleotide sequencing of reverse transcription-PCR-amplified cDNA spanning the Gag gene TU. Working stocks of rMuV and rMuVgag were prepared by adsorption of virus for 2 h at 37°C to newly confluent Vero cell monolayers at a multiplicity of infection (MOI) of 0.01 PFU/cell, followed by incubation at 37°C for 48 h in complete medium (DMEM containing 3.4 g/liter glucose and 2 mM glutamine, supplemented with 10% FBS and 1% sodium pyruvate). Infected-cell monolayers were then scraped into suspension and subjected to a single round of freeze-thaw in ethanol-dry ice, followed by incubation in a 37°C water bath. Cell debris was removed by centrifugation at 500 × g at 4°C for 10 min, and the supernatant was flash frozen in an ethanol-dry ice bath and stored at −80°C.
FIG. 1.
Diagrams showing genetic organization of rMuVgag (A) and rVSVN4CT1gag1 (B) genomes. Le and Tr are MuV leader and trailer sequences, respectively. An AscI site was generated in the rMuV M gene untranslated region by PCR mutagenesis. The HIV-1 p55 Gag gene with flanking M gene transcription stop (red box) and F gene transcription start (green box) signals was cloned into the AscI site. The rVSVN4CT1gag1 genome had the N gene translocated to position 4 and the HIV-1 p55 Gag gene at position 1 in the genome; the rVSV G gene encoded a truncated form of G protein (hashed box), containing a single amino acid in the cytoplasmic tail.
The construction, rescue, and in vitro and in vivo characterization of rVSVN4CT1gag1 have been described previously in detail (9, 12). Working stocks of rVSVN4CT1gag1 were prepared by adsorption of virus to confluent BHK cell monolayers at an MOI of 0.05 PFU/cell, followed by incubation at 32°C for 48 to 60 h. Cell debris was then removed from culture medium by centrifugation at 500 × g at 4°C for 10 min, and virus was purified by centrifugation (28,000 rpm at 4°C for 90 min in a Beckman SW-28 rotor) through a sucrose cushion (10% [wt/vol] in phosphate-buffered saline [PBS], pH 7.0) (9). Virus pellets were resuspended in PBS, pH 7.0, flash frozen in an ethanol-dry ice bath, and stored at −80°C. Virus titration was performed by plaque assay as previously described (9).
Titration of rMuV.
For titration of rMuV and rMuVgag, newly confluent Vero cell monolayers in six-well dishes were infected in duplicate with 10-fold dilutions of virus stock. Virus was adsorbed for 2 h at 37°C. The inoculum was then replaced with 3 ml of serum-free medium containing ∼0.8% (wt/vol; final concentration) molten agarose (SeaPlaque; Cambrex Bio Science Rockland Inc., Rockland, ME), and cells were incubated at 37°C for 4 to 5 days, after which the agarose overlay was removed, and cell monolayers were fixed with 10% (vol/vol) formaldehyde in PBS for 10 min at RT. The formaldehyde solution was then replaced with PBS, and virus plaques/syncytia were counted under low-power magnification on an inverted microscope.
Western blot analysis of HIV Gag expression.
Replicate confluent Vero cell monolayers in six-well plates were infected at an MOI of 5.0 PFU/cell. Virus inoculum (0.5 ml) was adsorbed for 15 min at RT, followed by 30 min at 37οC in 5% CO2 for rVSVN4CT1gag1 and 2 h at 37οC in 5% CO2 for rMuVgag. Additional growth medium was then added, and cells were incubated at 37οC in 5% CO2 for 24 to 48 h. At 24 and 48 h postinfection, cells were scraped into suspension and collected by centrifugation for 10 min at 3,000 × g. Supernatant was removed, and cell pellets were mixed with 0.5 ml of lysis buffer (0.05 M Tris-HCl, pH 7.5, 0.01 M NaCl, 1% Triton X-100). Cell lysates were then diluted 1:1 in Laemmli sample buffer (Bio-Rad) and heated at 90οC for 5 min to denature proteins. Samples were electrophoresed on 4 to 12% Bis-Tris polyacrylamide gel electrophoresis gels (NuPAGE) with a Precision-Plus protein standard (Bio-Rad), and proteins were then transferred to nitrocellulose membrane using the iBlot system (Invitrogen). The nitrocellulose membrane was then blocked in 5% milk in TTBS (0.02% Tween-20, 0.9% NaCl, 100 mM Tris-HCl, pH 7.5) overnight, followed by three 5-min washes in TTBS. The blot was incubated with an HIV-1 p24 Gag-specific monoclonal antibody (ImmunoDiagnostics, Inc.), diluted 1:2,000 in 5% milk in TTBS, for 1 h at RT, followed by three 5-min washes in TTBS. The blot was then incubated with secondary antibody, biotinylated goat anti-mouse immunoglobulin G (IgG; Vector Labs) diluted 1:2,000 in 5% milk in TTBS, for 1 h, followed by three 5-min washes in TTBS. Protein-antibody complexes were then visualized using Vectastain ABC and TMB (3,3′,5,5′-tetramethylbenzidine) substrate kits (Vector Labs).
Immunization of macaques.
A total of 15 captive-bred rhesus macaques (Macaca mulatta) of Indian origin were used in this study. Macaques were maintained in accordance with the Guide for the Care and Use of Laboratory Macaques (39). All macaques were seronegative for MuV, VSV, and HIV-1 Gag proteins prior to the start of the study. Two groups of five macaques each were inoculated subcutaneously (s.c.) at two dorsal sites with 1 × 107 PFU/animal of rMuVgag contained in 2 ml of PBS (1 ml/site). A third group was inoculated intramuscularly (i.m.) in each quadriceps with 5 × 107 PFU/animal of rVSVN4CT1gag1 contained in 2 ml of PBS (1 ml/site) (Table 1). Eight weeks later, macaques primed with rMuVgag were boosted with either a second s.c. dose of rMuVgag (1 × 107 PFU/animal; rMuVgag/rMuVgag) or an i.m. dose of rVSVN4CT1gag1 (5 × 107 PFU/animal; rMuVgag/rVSVN4CT1gag1), and macaques primed with rVSVN4CT1gag1 were boosted with an s.c. dose of rMuVgag (1 × 107 PFU/animal; rVSVN4CT1gag1/rMuVgag). Macaques receiving rMuVgag twice were then also boosted with rVSVN4CT1gag1 (5 × 107 PFU/animal; rMuVgag/rMuVgag/rVSVN4CT1gag1) 8 weeks after the second rMuVgag inoculation. Blood samples were collected from macaques at intervals pre- and postinoculation and were processed for measurement of rMuV, rVSV, and HIV-1 Gag-specific humoral and cellular immune responses.
TABLE 1.
Immunization schedule for rhesus macaques
| Group | Prime (wk 0)
|
Boost (wk 8)
|
Boost (wk 17)
|
No. of animals | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Route | Dose (PFU) | Vaccine | Route | Dose (PFU) | Vaccine | Route | Dose (PFU) | ||
| 1 | rMuVgag | s.c. | 1 × 107 | rMuVgag | s.c. | 1 × 107 | rVSVN4CT1gag1 | i.m. | 1 × 107 | 5 |
| 2 | rMuVgag | s.c. | 1 × 107 | rVSVN4CT1gag1 | i.m | 1 × 107 | 5 | |||
| 3 | rVSVN4CT1gag1 | i.m. | 1 × 107 | rMuVgag | s.c | 1 × 107 | 5 | |||
IFN-γ and IL-2 ELISPOT assays.
Ninety-six-well flat-bottom enzyme-linked immunospot (ELISPOT) assay plates (Millipore, Bedford, MA) were coated overnight with either a mouse anti-human gamma interferon (hIFN-γ) monoclonal antibody (clone 27; BD-Pharmingen, San Diego, CA) or with a goat anti-human interleukin-2 (IL-2) polyclonal antibody (R&D system, Minneapolis, MN) according to the manufacturer's recommendations. The plates were then washed three times with PBS and blocked for 2 h with PBS containing 5% FBS. Rhesus macaque whole blood (EDTA treated) was collected at intervals after immunization; peripheral blood lymphocytes (PBL) were isolated from whole blood by Accuspin tube (Sigma-Aldrich Co., St. Louis, MO) density gradient centrifugation and resuspended in complete R05 culture medium (RPMI 1640 medium supplemented with 5% FBS, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin sulfate, 1 mM sodium pyruvate, 1 mM HEPES, 100 mM nonessential amino acids) and shipped via overnight courier. Within 24 h of blood draw, the isolated macaque PBL were washed once with complete R05 culture medium and resuspended in complete R05 culture medium containing either 50 μg/ml phytohemagglutinin (Sigma), Gag peptide pools (15-mers overlapping by 11 amino acids; 2.5 μM final peptide concentration of each peptide) spanning HIVHXB2 Gag p55, or medium alone. Assays were performed in duplicate with 2 × 105 PBL/well (2 × 106 PBL/ml). Cells were incubated for 18 to 24 h at 37°C and then removed from the ELISPOT plate by first a rinse with deionized water and then six washes with PBS containing 0.25% Tween-20, followed by three additional rinses with PBS. Thereafter, ELISPOT plates were incubated with a biotinylated rabbit polyclonal anti-human IFN-γ (0.2 μg/well; Biosource, Camarillo, CA) diluted in PBS containing 1% bovine serum albumin for 2 h at RT; similarly, IL-2 ELISPOT plates were incubated at 4°C overnight with biotinylated goat anti-human IL-2 antibodies (R&D Systems, Minneapolis, MN) diluted in PBS containing 1% bovine serum albumin. ELISPOT plates were then washed six times with PBS containing 0.25% Tween-20 and incubated for 1 h at RT with streptavidin-horseradish peroxidase (HRP)-conjugated anti-cytokine antibody (BD-Biosciences, San Diego CA) diluted 1:250 (for IFN-γ) or 1:100 (for IL-2) in PBS containing 10% FBS and 0.005% Tween-20. Unbound conjugate was removed by rinsing plates six times with PBS containing 0.25% Tween-20. Chromogenic substrate (100 μl/well; 1-Step NBT/BCIP [nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate]; Pierce, Rockford, IL) was then added (100 μl/well) for 3 to 5 min (for IFN-γ) or 25 to 30 min (for IL-2) and rinsed away with water, after which the plates were air dried, and the resulting spots were counted using an Immunospot Reader (CTL Inc., Cleveland, OH). Peptide-specific IFN-γ or IL-2 ELISPOT responses were considered positive if the response (minus medium background) was more than threefold above the medium response and if ≥50 spot-forming colonies (SFC)/106 PBL were observed.
Intracellular cytokine staining.
Freshly isolated PBL were resuspended at ∼1 × 107 cells/ml in R05 culture medium and stimulated with 1 μM Gag peptide mix for 5 to 6 h at 37°C in the presence of brefeldin A (GolgiPlug, 1 μl/ml; BD Biosciences). Negative control tubes without peptide were also included. After stimulation, PBL were washed twice in flow cytometry wash buffer (PBS-2% FBS) and stained for 20 min in the dark at 4°C with surface marker-specific monoclonal antibodies: anti-CD3-Pacific Blue (clone SP34-2), anti-CD4-peridinin chlorophyll protein-Cy5.5 (clone L200), and anti-CD8-allophycocyanin-Cy7 (clone SK1), all obtained from BD Biosciences. An aqua fluorescent reactive dye (Invitrogen) was used for staining dead cells. PBL were washed and permeabilized according to the manufacturer's instructions (Cytofix/Cytoperm kit; BD Biosciences). After two washes with the supplied buffer, PBL were incubated on ice for 30 min with anti-IFN-γ-Alexa Fluor 700 (clone B27), anti-tumor necrosis factor alpha (TNF-α)-phycoerythrin-Cy7 (clone Mab11), and anti-IL-2-fluorescein isothiocyanate (FITC) (clone m21-17H12), all obtained from BD Biosciences. PBL were subsequently rinsed with the supplied buffer and fixed in BD stabilizing fixative buffer (BD Biosciences). Fixed PBL were stored at 4°C in the dark until analyzed by flow cytometry (within 24 h).
Flow cytometric analysis.
PBL were analyzed on an LSRII (BD Biosciences) instrument. A total of 5 × 105 to 5 × 106 events were collected per sample. Analysis was performed using FlowJo software (version 8.2; TreeStar, Inc.). An aqua stain (Aqua Bright) versus side scatter plot was used to exclude dead cells. A small lymphocyte gate was created using a forward scatter versus side scatter plot. Non-T cells were excluded by gating on CD3+ cells. CD8+ T cells were selected by gating on CD4− CD8+ T cells, and CD4+ T cells were selected by gating on CD4+ CD8− T cells. Functional analysis was performed by measuring the expression of each cytokine. Boolean combinations of single functional gates were created using FlowJo software to determine the frequency of each response based on all possible combinations of cytokine expression. Background responses detected in negative control wells were subtracted from those detected in peptide-stimulated samples for every specific functional combination. Frequencies were sent from the table editor of FlowJo for formatting in Pestle software (version 1.3; provided by M. Roederer, NIH, Bethesda, MD). All fluorochrome-labeled monoclonal antibodies were purchased from BD Pharmingen (San Diego, CA).
MuV ELISA.
Macaque sera were tested for the presence of MuV-specific IgG using an ELISA kit supplied by Diagnostic Automation, Inc. (Calabasas, CA). Briefly, serial twofold dilutions of serum samples were prepared in the provided serum diluent and then added to MuV antigen-coated 96-well plates. Plates were incubated for 30 min at RT and then rinsed five times to remove serum samples. HRP-conjugated rabbit anti-human IgG was then added to each well for 30 min at RT. The HRP conjugate was then rinsed off, and TMB substrate solution was added to allow color development, which was stopped by the addition of 1N H2SO4. Colorimetric analysis was performed at 450 nm using a Biotek plate reader (Biotek, Winooski, VT). The log10 values of serum dilutions were plotted against absorbance values, and the data were transformed into a linear regression using Origin, version 6.1, software. The geometric mean titers plus the standard error were determined for each group.
MuV neutralization assay.
The presence of MuV-neutralizing antibodies in macaque serum was detected by a standard virus neutralization assay. Briefly, 100 PFU of rMuV was incubated with duplicate twofold dilutions of serum for 1 h at 37°C. Each serum dilution was then mixed with 1.5 × 103 Vero cells in suspension and dispensed into separate wells of a 96-well dish. Cells were then incubated at 37°C in 5% CO2 for 4 days and monitored for viral CPE under the microscope. The neutralization titer was calculated as the reciprocal of the highest serum dilution that completely protected cell monolayers from viral CPE.
Statistical analysis.
Analysis of variance of IFN-γ ELISPOT data was performed on SAS, version 8.2, software using the type 1 GENMOD procedure with negative binomial distribution. A P value of less than 0.05 indicated that the test was statistically significant.
RESULTS
Rescue and in vitro characterization of rMuVgag and rVSVN4CT1gag1.
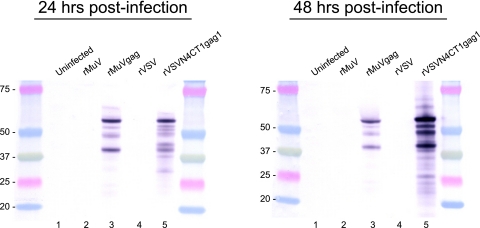
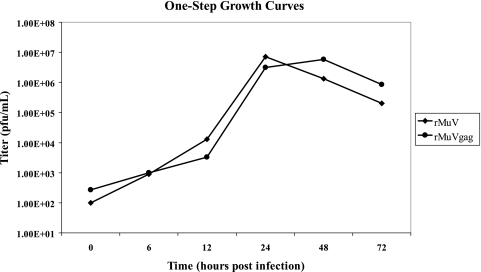
Previously, rMuV vectors were generated that expressed either β-galactosidase or luciferase from a TU inserted between the MuV M and F genes (unpublished data). These studies indicated that expression of the HIV-1 p55 Gag protein from a TU similarly positioned between the M and F genes was also feasible (Fig. 1). When we designed the expression cassette containing the Gag gene, the additional genetic sequence incorporated into the rMuV genome was limited to a multiple of six in order to preserve the essential stoichiometry of one N protein for every six nucleotides within the viral nucleocapsid structure, referred to as the “rule of six” (6). Failure to maintain this ratio of N protein to nucleotide prevents rescue of rMuV from cDNA (unpublished data). Following recovery of rMuVgag from genomic cDNA the gag open reading frame was verified by genome consensus sequencing. Robust expression of the encoded HIV-1 Gag p55 protein was detected by whole-infected-cell immunofluorescence (data not shown), by Western blot analysis of rMuVgag-infected Vero cells using a Gag-specific monoclonal antibody (Fig. 2), and by ELISA of infected-cell lysate (data not shown). In line with the data from Western blot analysis, the ELISA data indicated levels of Gag expression that were more than threefold greater in rVSVN4CT1gag-infected cells than in rMuVgag-infected cells when cell cytopathology was complete in each case at 30 to 35 h postinfection. A one-step growth study in Vero cells indicated that propagation of rMuVgag was slightly delayed relative to rMuV although a similar peak titer was achieved (Fig. 3) (also seen previously when reporter genes were added to the rMuV genome), indicating that insertion of the Gag gene between the M and F genes did not greatly reduce viral replication efficiency. By analogy with VSV (26), it was anticipated that expression of MuV genes (i.e., the F, SH, hemagglutinin-neuraminidase, and L genes) downstream of the Gag gene would be downregulated because they were displaced further away from the single 3′ transcription promoter, thereby affecting viral replication. However, downstream genes express proteins, which may not be needed in large quantities to sustain robust virus propagation due to their specific functional activities and more distal position from the 3′ transcription promoter. Therefore, it is likely that an incremental reduction in their expression levels did not significantly affect virus replication efficiency. As previously observed (12), the highly attenuated rVSVN4CT1gag1 vector also abundantly expressed Gag protein in infected Vero cells (Fig. 2).
FIG. 2.
Western blot analysis of HIV-1 Gag protein expressed in Vero cells by rMuVgag and rVSVN4CT1gag1. Replicate Vero cell monolayers in six-well plates were infected at an MOI of 5 PFU/cell with either rMuVgag or rVSVN4CT1gag1. Cell lysates were collected at 24 h and 48 h postinfection. The Gag protein was detected with an HIV-1 Gag-specific monoclonal antibody. Lanes 1, uninfected cells; lanes 2, rMuV-infected cells; lanes 3, rMuVgag-infected cells; lanes 4, rVSV-infected cells; lanes 5, rVSVN4CT1gag1-infected cells. Protein molecular mass markers (shown in kDa) flank each gel.
FIG. 3.
One-step growth curves for rMuV and rMuVgag. Replicate Vero cell monolayers in six-well plates were infected in duplicate with rMuV and rMuVgag at an MOI of 5 PFU/cell. Infected cell supernatants were collected at intervals postinfection and titrated by standard plaque assay. Each data point represents the average titer obtained from duplicate infections.
Gag-specific cellular immune responses.
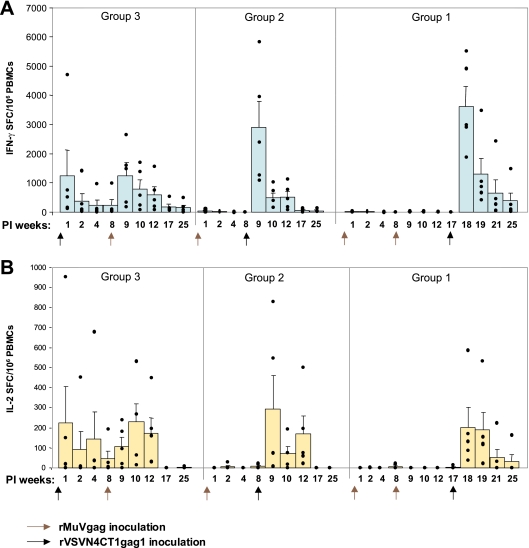
Gag-specific cellular immune responses elicited in macaques immunized with rVSVN4CT1gag1 and rMuVgag were assessed by IFN-γ and IL-2 ELISPOT assays. The vaccination regimens (Table 1) were designed to compare the magnitude, duration, and quality of immune responses elicited in macaques following alternative rMuVgag/rVSVN4CT1gag1 and rVSVN4CT1gag1/rMuVgag prime-boost strategies. Macaques primed with rVSVN4CT1gag1 had a robust T-cell response with one high outlier (average, ∼1,000 IFN-γ SFC/106 PBL) 1 week after dosing (Fig. 4A). At weeks 2 and 4 postprime, responses in these macaques had declined to an average of ∼400 and ∼300 IFN-γ SFC, respectively, rising back to an average of ∼1,000 IFN-γ SFC 1 week after boosting with rMuVgag. These responses then waned gradually to ∼150 IFN-γ SFC by week 17 postboost (week 25 postprime). The pattern of IL-2 ELISPOT responses (Fig. 4B) measured in these macaques was very similar to that obtained for IFN-γ ELISPOTS but at reduced magnitude. IL-2 ELISPOT responses peaked at ∼200 SFC/106 PBL at 1 week postprime with rVSVN4CT1gag1, declining to ∼50 SFC by week 8, and boosted back to ∼200 SFC 2 weeks postinoculation with rMuVgag. By week 17 postboost with rMuVgag, IL-2 ELISPOT responses were undetectable.
FIG. 4.
HIV-1 Gag-specific cellular immune responses in rhesus macaques. (A) HIV-1 Gag-specific IFN-γ ELISPOT responses at the indicated intervals after primary inoculation (PI). (B) HIV-1 Gag-specific IL-2 ELISPOT responses at the indicated intervals after primary inoculation. Data represent average ELISPOT responses with standard error limits (error bars) at weeks 1 to 25 during the study. Black dots represent individual animal responses. Arrows indicate the timing of each vector inoculation.
In contrast to the robust Gag-specific responses detected in macaques primed with rVSVN4CT1gag1, Gag-specific IFN-γ and IL-2 ELISPOT responses detected in macaques inoculated either once or twice with rMuVgag were virtually undetectable at week 1 postdosing. However, when these two groups of macaques were boosted with rVSVN4CT1gag1, very robust peak Gag-specific IFN-γ ELISPOT responses of 3,000 and 3,500 SFC/106 PBL were observed. A statistical treatment of these data showed that the difference in peak IFN-γ ELISPOT responses between groups of animals receiving the rVSVN4CT1gag1/rMuVgag1 regimen and the rMuVgag/ rMuVgag/rVSVN4CT1gag1 regimen was significant (P = 0.04). A comparison of the rVSVN4CT1gag1/rMuVgag and rMuV/rVSVN4CT1gag1 regimens did not quite achieve statistical significance (P = 0.09). Notably, in all 10 outbred macaques primed with rMuVgag, peak responses of >1,000 Gag-specific IFN-γ SFC were detected after boosting with rVSVN4CT1gag1. Although responses in these macaques waned relatively quickly, ∼200 IFN-γ SFC could still be detected in macaques primed twice with rMuVgag 8 weeks after boosting with rVSVN4CT1gag1.
The pattern of IL-2 ELISPOT responses in these macaques mirrored IFN-γ ELISPOT responses but at reduced magnitude (Fig. 4B). Also, differences in the magnitude of peak IL-2 ELISPOT responses detected for all three different vaccination regimens were not as remarkable as those seen for IFN-γ ELISPOT responses. Depletion of CD4+ and CD8+ T-cell populations from total IFN-γ-secreting PBL indicated a predominantly CD8+ T-cell Gag-specific response following either priming or boosting with rVSVN4CT1gag1 (data not shown). Similar depletion analyses for the rVSVN4CT1gag1/rMuVgag regimen indicated a more balanced CD4+/CD8+ response after boosting with rMuVgag.
Intracellular cytokine staining of PBL.
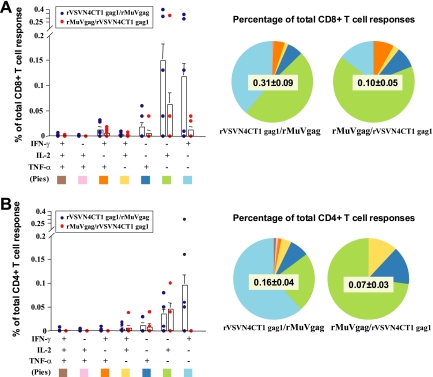
Cytokine production was measured in Gag-specific CD8+ and CD4+ T cells by intracellular cytokine staining (ICS) 25 weeks after primary inoculation (17 weeks postboost) to assess any qualitative and quantitative differences in longer-term responses between rVSVN4CT1gag1/rMuVgag and rMuVgag/rVSVN4CTgag1 vaccination regimens. For logistical reasons ICS was not performed for macaques primed twice with rMuVgag and boosted with rVSVN4CT1gag1. Overall, the percentage of total CD8+ and CD4+ T cells expressing intracellular cytokines was greater following the rVSVN4CT1gag1/rMuVgag vaccination regimen. Although the rMuVgag/rVSVN4CT1gag1 regimen elicited the highest peak IFN-γ responses 1 week after boosting, macaques receiving the rVSVN4CT1gag1/rMuVgag regimen had relatively more abundant IFN-γ- and IL-2-producing CD8+ T cells and IFN-γ-producing CD4+ T cells at week 25 postprimary inoculation. One factor that may have contributed to this outcome was a relatively higher CD4+ IL-2 response detected by ICS 1 week postboost in animals receiving the rVSVN4CT1gag1/rMuVgag regimen (data not shown). Within each vaccination regimen, the number of Gag-specific CD8+ T cells producing either IFN-γ or IL-2 or TNF-α alone was greater than those producing two or more cytokines (Fig. 5); this was also the case in CD4+ T cells for IL-2 and TNF-α production. Also, following the rVSVN4CT1gag1 boost, IFN-γ expression was detected in CD8+ T cells but not CD4+ T cells, which correlated with depletion study data performed 1 week after boosting (data not shown), while IL-2 and TNF-α were detected in both CD8+ and CD4+ T cells.
FIG. 5.
ICS in immunized animals at week 25 postinoculation. (A) Average percentage of CD8+ T cells expressing IFN-γ, IL-2, and TNF-α, with standard error (error bars). (B) Average percentage of CD4+ T cells expressing IFN-γ, IL-2, and TNF-α, with standard error (error bars).Dots represent individual animals. Proportions of cells expressing individual and multiple cytokines for each vaccination regimen are indicated by the size of colored regions of the pie charts, according to the key at the bottom of each panel.
Humoral immune responses.
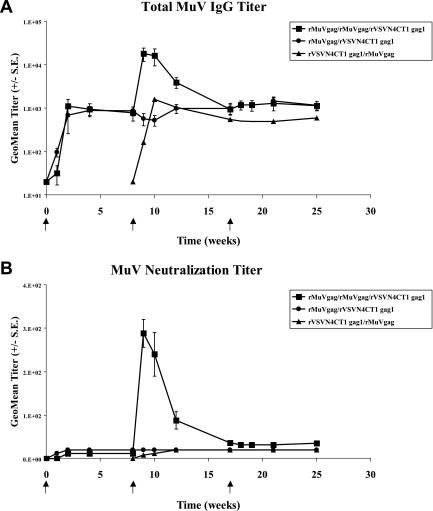
Only very low levels of Gag-specific IgG were detected following prime and boost inoculations in all vaccination regimens (data not shown). However, inoculation of macaques with a single dose of rMuVgag elicited a measurable MuV-specific IgG response, which was significantly boosted following a second rMuVgag inoculation (Fig. 6A). Relatively similar results were obtained in MuV neutralization assays, where neutralization titers were very low following a single dose of rMuVgag but increased significantly after the second rMuVgag inoculation (Fig. 6B). In contrast, and as previously observed, robust VSV neutralization titers were detected in macaques receiving a single dose of rVSVN4CT1gag1 (data not shown), reflecting the potent antigenic properties of the VSV G protein (42).
FIG. 6.
Detection of MuV-specific IgG and neutralizing antibody. Graphs show average MuV-specific IgG and MuV neutralization titers detected in animal sera at intervals following each vaccination regimen.
DISCUSSION
Protection against HIV-1/AIDS will likely require the elicitation of robust immune responses directed against critical epitopes within the HIV-1 proteome. In an attempt to reach this goal, a variety of vector systems and vaccination regimens have been tested in preclinical and clinical studies (2-5, 7, 18, 23, 24, 38, 44, 49). Here, we describe the introduction of rMuV as a potential HIV-1 vaccine vector and the immune responses detected in NHPs following heterologous prime-boost regimens with rMuVgag and a previously described (12), highly attenuated rVSVN4CT1gag1 vector. The rationale for testing rMuV as a potential HIV-1 vaccine vector was based on a number of criteria, including the ability of the Jeryl Lynn strain of MuV to infect and undergo limited dissemination throughout the human host (55), an excellent safety profile in young infants for the Jeryl Lynn strain of MuV vaccine used in this study, and the ability to modify the MuV genome to highly express one or more foreign proteins under the control of the single 3′ transcription promoter (unpublished data). Additionally, MuV is phylogenetically related to measles virus, which has also been modified to express HIV-1 proteins and has demonstrated potential as an HIV-1 vaccine vector (11, 35-37, 50). The potential of a prototypic rVSV vector as an HIV-1 vaccine has been well documented (42, 46). The rVSVN4CT1gag1 vector used here displayed a highly attenuated phenotype in mice and NHPs while retaining immunogenic properties that were similar to the much more virulent prototypic rVSV vectors (12) (unpublished data). Previous work showed that rVSV vectors could elicit robust Gag-specific cellular immune responses in rhesus macaques. The magnitude of peak cellular immune responses obtained in those studies was 500 to 1,000 IFN-γ SFC after primary immune responses were boosted with a different rVSV serotype (46). The immune responses obtained when NHPs were either primed with plasmid DNA expressing SIV Gag and then boosted with rVSV expressing SIV Gag (15) or primed with rVSV expressing SIV Gag and boosted with modified vaccinia virus Ankara expressing SIV Gag (40) were more robust than responses with prime-boost regimens comprising only rVSV vectors, with the caveat that differences in ELISPOT assays in different laboratories can affect the absolute ELISPOT values obtained. Here, we report that very robust peak HIV-1 Gag-specific cellular immune responses were elicited in rhesus macaques primed with rMuVgag and boosted with rVSVN4CT1gag1, further supporting the notion that stronger immune responses can be achieved in prime-boost vaccination regimens employing heterologous vectors. Importantly, Gag-specific cellular immune responses of >1,000 IFN-γ SFC/106 PBL were detected in all 10 outbred macaques primed with rMuVgag and boosted with rVSVN4CT1gag1, indicating efficient priming with a single dose of rMuVgag. This result contrasts with previous work, where most groups of macaques primed with rVSVgag and boosted with either a different serotype of rVSVgag or other heterologous vector contained some low-responder macaques (13) (unpublished observations). The data also indicate that the sequence of administration of vaccine vectors may be important in determining the magnitude and quality of the resulting immune response since priming macaques with rVSVN4CT1gag1 and boosting with rMuVgag did not elicit equivalent responses as the converse vaccination regimen. Such differences may reflect unique vector biology and the resulting specific interaction(s) of vector with the immune system. The reasons for the absence of measurable Gag-specific cellular immune responses in macaques primed either once or twice with rMuVgag are not clear but may reflect the unique interaction between MuV and humans and more restricted replication and dissemination of MuV in nonhuman hosts. It is therefore feasible that that rMuV may elicit much stronger immune responses to expressed foreign antigens in humans than indicated here in macaques.
No significant humoral immune response to HIV-1 Gag was detected in this study, possibly reflecting the largely intracellular location of Gag protein, which reduces availability for B-cell presentation, coupled with the known low level of Gag antigenicity and likely host range limitation of rMuV replication in macaques. Therefore, even though expressed Gag protein is myristylated and could hypothetically form virus-like particles (21, 22), a critical mass of Gag protein suitably displayed for antibody induction may not have been achieved. Similar arguments may hold true for the highly attenuated rVSVN4CT1gag1, which in mice appears to be restricted to a very small number of susceptible cells following i.m. inoculation (unpublished observations). A modest MuV-specific humoral immune response was detected in macaques after a single inoculation with rMuVgag. In humans this response is usually composed of antibodies to MuV F and hemagglutinin-neuraminidase surface glycoproteins and the abundant nucleocapsid (N) protein (32). Interestingly, in spite of a measurable humoral response to MuV after a single inoculation, little or no MuV-neutralizing antibody was detected, indicating the feasibility of administering a second dose of rMuVgag without risk of virus neutralization. After the second rMuVgag inoculation, peak levels of both MuV-specific IgG and neutralizing antibodies increased significantly, indicating that reinfection of macaques occurs in the presence of a low/modest humoral response.
One very important consideration affecting the suitability of rMuV vectors for use in adult humans is the effect of preexisting MuV-specific immune responses remaining from either childhood vaccination or natural infection. There is mounting evidence that immune responses to MuV wane significantly 10 to 15 years after one or two MuV immunizations (1, 25). The data obtained in this study indicate that following s.c. inoculation of macaques, rMuVgag can infect susceptible cells and express antigens in the presence of preexisting MuV-specific total IgG. To further address this question, a study is in progress to test the immunogenicity of an rMuVgag/rVSVN4CT1gag1 prime-boost regimen in rhesus macaques that are preimmunized with rMuV and have MuV-specific IgG and MuV neutralization titers that are similar to those found in young adult (18 to 30 years old) humans. Alternatively, rMuV vectors expressing heterologous antigens may in the future be considered for use in the pediatric population, eliminating concerns about preexisting immunity.
CD4+ and CD8+ T-cell ICS was performed to analyze both the magnitude and quality of longer-term Gag-specific responses in macaques. In contrast to the magnitude of peak cellular responses detected 1 to 2 weeks after boosting in each regimen, macaques that were primed with rVSVN4CT1gag1 and boosted with rMuVgag had a higher percentage of Gag-specific CD4+ and CD8+ T cells remaining at week 25 of the study than macaques primed with rMuVgag and boosted with rVSVN4CT1gag1. This finding could be due to differences in the number of IL-2-producing CD4+ cells present shortly after the boost; higher numbers of CD4+ IL-2-producing cells have been associated with improved memory T-cell responses (53). Also notable and in agreement with CD4+ and CD8+ T-cell depletion studies, the rVSVN4CT1gag1/rMuVgag regimen induced a more balanced CD4+/CD8+ T-cell response than the rMuVgag/rVSVN4CT1gag1 regimen. Again, these data hint at the importance of the specific virus vector-host interactions and the sequence of administration in determining the magnitude, quality, and durability of the resulting immune response.
Overall, the data obtained in this study demonstrate the potential of rMuV as a vaccine vector for HIV-1 when used in prime-boost regimens with rVSV vector(s). It is also possible that rMuV may be used either alone or in combination with rVSV and other vector systems to develop vaccines for other serious diseases.
Acknowledgments
We thank Chris Parks and Sue Witko for advice on virus rescue. We also thank Erik Johnson for traveling to immunization sites for final vaccine dose preparation.
The rVSVN4CT1gag1 component of this work was sponsored by an HIV-1 Vaccine Design and Development Team Contract from the National Institutes of Health and National Institute of Allergy and Infectious Diseases (HVDDT NO1-A1-25458).
Footnotes
Published ahead of print on 22 July 2009.
REFERENCES
- 1.Afzal, M. A., and P. D. Minor. 1999. Immune response and vaccine efficacy. Vaccine 17:1813. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belshe, R. B., C. Stevens, G. J. Gorse, S. Buchbinder, K. Weinhold, H. Sheppard, D. Stablein, S. Self, J. McNamara, S. Frey, J. Flores, J. L. Excler, M. Klein, R. E. Habib, A. M. Duliege, C. Harro, L. Corey, M. Keefer, M. Mulligan, P. Wright, C. Celum, F. Judson, K. Mayer, D. McKirnan, M. Marmor, and G. Woody. 2001. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J. Infect. Dis. 183:1343-1352. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, J. D., A. D. Cohen, S. Vogt, K. Schumann, B. Nath, L. Ahn, K. Lacy, M. L. Bagarazzi, T. J. Higgins, Y. Baine, R. B. Ciccarelli, R. S. Ginsberg, R. R. MacGregor, and D. B. Weiner. 2000. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 Env/Rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J. Infect. Dis. 181:476-483. [DOI] [PubMed] [Google Scholar]
- 6.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67:4822-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke, D. K., D. Cooper, M. A. Egan, R. M. Hendry, C. L. Parks, and S. A. Udem. 2006. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin. Immunopathol. 28:239-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke, D. K., F. Nasar, M. Lee, J. E. Johnson, K. Wright, P. Calderon, M. Guo, R. Natuk, D. Cooper, R. M. Hendry, and S. A. Udem. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, D. K., M. S. Sidhu, J. E. Johnson, and S. A. Udem. 2000. Rescue of mumps virus from cDNA. J. Virol. 74:4831-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combredet, C., V. Labrousse, L. Mollet, C. Lorin, F. Delebecque, B. Hurtrel, H. McClure, M. B. Feinberg, M. Brahic, and F. Tangy. 2003. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 77:11546-11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper, D., K. J. Wright, P. C. Calderon, M. Guo, F. Nasar, J. E. Johnson, J. W. Coleman, M. Lee, C. Kotash, I. Yurgelonis, R. J. Natuk, R. M. Hendry, S. A. Udem, and D. K. Clarke. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose, N. A., C. Ohlen, P. Polacino, C. C. Pierce, M. T. Hensel, L. Kuller, T. Mulvania, D. Anderson, P. D. Greenberg, S. L. Hu, and N. L. Haigwood. 2003. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J. Virol. 77:11563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan, M. A., S. Y. Chong, S. Megati, D. C. Montefiori, N. F. Rose, J. D. Boyer, M. K. Sidhu, J. Quiroz, M. Rosati, E. B. Schadeck, G. N. Pavlakis, D. B. Weiner, J. K. Rose, Z. R. Israel, S. A. Udem, and J. H. Eldridge. 2005. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 21:629-643. [DOI] [PubMed] [Google Scholar]
- 16.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megati, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 20:989-1004. [DOI] [PubMed] [Google Scholar]
- 17.Egan, M. A., S. Megati, V. Roopchand, D. Garcia-Hand, A. Luckay, S. Y. Chong, M. Rosati, S. Sackitey, D. B. Weiner, B. K. Felber, G. N. Pavlakis, Z. R. Israel, J. H. Eldridge, and M. K. Sidhu. 2006. Rational design of a plasmid DNA vaccine capable of eliciting cell-mediated immune responses to multiple HIV antigens in mice. Vaccine 24:4510-4523. [DOI] [PubMed] [Google Scholar]
- 18.Egan, M. A., W. A. Pavlat, J. Tartaglia, E. Paoletti, K. J. Weinhold, M. L. Clements, and R. F. Siliciano. 1995. Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-range-restricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. J. Infect. Dis. 171:1623-1627. [DOI] [PubMed] [Google Scholar]
- 19.Elango, N., T. M. Varsanyi, J. Kovamees, and E. Norrby. 1988. Molecular cloning and characterization of six genes, determination of gene order and intergenic sequences and leader sequence of mumps virus. J. Gen. Virol. 69:2893-2900. [DOI] [PubMed] [Google Scholar]
- 20.Finke, S., and K. K. Conzelmann. 2005. Recombinant rhabdoviruses: vectors for vaccine development and gene therapy. Curr. Top. Microbiol. Immunol. 292:165-200. [DOI] [PubMed] [Google Scholar]
- 21.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 22.Gottlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta, K., M. Hudgens, L. Corey, M. J. McElrath, K. Weinhold, D. C. Montefiori, G. J. Gorse, S. E. Frey, M. C. Keefer, T. G. Evans, R. Dolin, D. H. Schwartz, C. Harro, B. Graham, P. W. Spearman, M. Mulligan, and P. Goepfert. 2002. Safety and immunogenicity of a high-titered canarypox vaccine in combination with rgp120 in a diverse population of HIV-1-uninfected adults: AIDS Vaccine Evaluation Group protocol 022A. J. Acquir. Immune Defic. Syndr. 29:254-261. [DOI] [PubMed] [Google Scholar]
- 24.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hersh, B. S., P. E. Fine, W. K. Kent, S. L. Cochi, L. H. Kahn, E. R. Zell, P. L. Hays, and C. L. Wood. 1991. Mumps outbreak in a highly vaccinated population. J. Pediatr. 119:187-193. [DOI] [PubMed] [Google Scholar]
- 26.Iverson, L. E., and J. K. Rose. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477-484. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, J. E., F. Nasar, J. W. Coleman, R. E. Price, A. Javadian, K. Draper, M. Lee, P. A. Reilly, D. K. Clarke, R. M. Hendry, and S. A. Udem. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 360:36-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 29.Jonkers, A. H. 1967. The epizootiology of the vesicular stomatitis viruses: a reappraisal. Am. J. Epidemiol. 86:286-291. [DOI] [PubMed] [Google Scholar]
- 30.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipe, D. M., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.). 2007. Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 33.Lemon, K., B. K. Rima, S. McQuaid, I. V. Allen, and W. P. Duprex. 2007. The F gene of rodent brain-adapted mumps virus is a major determinant of neurovirulence. J. Virol. 81:8293-8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letvin, N. L. 2006. Progress and obstacles in the development of an AIDS vaccine. Nat. Rev. Immunol. 6:930-939. [DOI] [PubMed] [Google Scholar]
- 35.Lorin, C., C. Combredet, V. Labrousse, L. Mollet, P. Despres, and F. Tangy. 2005. A paediatric vaccination vector based on live attenuated measles vaccine. Therapie 60:227-233. [DOI] [PubMed] [Google Scholar]
- 36.Lorin, C., F. Delebecque, V. Labrousse, L. Da Silva, F. Lemonnier, M. Brahic, and F. Tangy. 2005. A recombinant live attenuated measles vaccine vector primes effective HLA-A0201-restricted cytotoxic T lymphocytes and broadly neutralizing antibodies against HIV-1 conserved epitopes. Vaccine 23:4463-4472. [DOI] [PubMed] [Google Scholar]
- 37.Lorin, C., L. Mollet, F. Delebecque, C. Combredet, B. Hurtrel, P. Charneau, M. Brahic, and F. Tangy. 2004. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 78:146-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 39.National Research Council. 1996. Guide for the Care and Use of Laboratory Macaques. National Academic Press, Washington, DC.
- 40.Ramsburg, E., N. F. Rose, P. A. Marx, M. Mefford, D. F. Nixon, W. J. Moretto, D. Montefiori, P. Earl, B. Moss, and J. K. Rose. 2004. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J. Virol. 78:3930-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reuter, J. D., B. E. Vivas-Gonzalez, D. Gomez, J. H. Wilson, J. L. Brandsma, H. L. Greenstone, J. K. Rose, and A. Roberts. 2002. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 76:8900-8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 45.Rolland, M., D. C. Nickle, and J. I. Mullins. 2007. HIV-1 group M conserved elements vaccine. PLoS Pathog. 3:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 47.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santra, S., Y. Sun, J. G. Parvani, V. Philippon, M. S. Wyand, K. Manson, A. Gomez-Yafal, G. Mazzara, D. Panicali, P. D. Markham, D. C. Montefiori, and N. L. Letvin. 2007. Heterologous prime/boost immunization of rhesus monkeys using diverse poxvirus vectors. J. Virol. 81:8563-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 50.Tangy, F., and H. Y. Naim. 2005. Live attenuated measles vaccine as a potential multivalent pediatric vaccination vector. Viral Immunol. 18:317-326. [DOI] [PubMed] [Google Scholar]
- 51.Tesh, R. B., B. N. Chaniotis, and K. M. Johnson. 1971. Vesicular stomatitis virus, Indiana serotype: multiplication in and transmission by experimentally infected phlebotomine sandflies (Lutzomyia trapidoi). Am. J. Epidemiol. 93:491-495. [DOI] [PubMed] [Google Scholar]
- 52.Tesh, R. B., P. H. Peralta, and K. M. Johnson. 1970. Ecologic studies of vesicular stomatitis virus. II. Results of experimental infection in Panamanian wild animals. Am. J. Epidemiol. 91:216-224. [DOI] [PubMed] [Google Scholar]
- 53.Williams, M. A., A. J. Tyznik, and M. J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441:890-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witko, S. E., C. S. Kotash, R. M. Nowak, J. E. Johnson, L. A. Boutilier, K. J. Melville, S. G. Heron, D. K. Clarke, A. S. Abramovitz, R. M. Hendry, M. S. Sidhu, S. A. Udem, and C. L. Parks. 2006. An efficient helper-virus-free method for rescue of recombinant paramyxoviruses and rhadoviruses from a cell line suitable for vaccine development. J. Virol. Methods 135:91-101. [DOI] [PubMed] [Google Scholar]
- 55.Yamanishi, K., M. Takahashi, S. Ueda, Y. Minekawa, and T. Ogino. 1973. Studies on live mumps virus vaccine. V. Development of a new mumps vaccine “AM 9” by plaque cloning. Biken J. 16:161-166. [PubMed] [Google Scholar]