Abstract
Human immunodeficiency virus type 1 (HIV-1) vectors transduce rhesus blood cells poorly due to a species-specific block by TRIM5α and APOBEC3G, which target HIV-1 capsid and viral infectivity factor (Vif), respectively. We sought to develop a lentiviral vector capable of transducing both human and rhesus blood cells by combining components of both HIV-1 and simian immunodeficiency virus (SIV), including SIV capsid (sCA) and SIV Vif. A chimeric HIV-1 vector including sCA (χHIV) was superior to the conventional SIV in transducing a human blood cell line and superior to the conventional HIV-1 vector in transducing a rhesus blood cell line. Among human CD34+ hematopoietic stem cells (HSCs), the χHIV and HIV-1 vectors showed similar transduction efficiencies; in rhesus CD34+ HSCs, the χHIV vector yielded superior transduction rates. In in vivo competitive repopulation experiments with two rhesus macaques, the χHIV vector demonstrated superior marking levels over the conventional HIV-1 vector in all blood lineages (first rhesus, 15 to 30% versus 1 to 5%; second rhesus, 7 to 15% versus 0.5 to 2%, respectively) 3 to 7 months postinfusion. In summary, we have developed an HIV-1-based lentiviral vector system that should allow comprehensive preclinical testing of HIV-1-based therapeutic vectors in the rhesus macaque model with eventual clinical application.
Hematopoietic stem cell (HSC) gene therapy is potentially curative for a variety of hereditary disorders, including congenital (and acquired) immunodeficiency syndromes, chronic granulomatous disease, the thalassemias, and sickle cell disease. In a landmark gene therapy trial that utilized a retroviral vector to correct HSCs derived from patients with X-linked severe combined immunodeficiency, proof of principle was established in the human setting, with several successes that soon followed (1, 11, 12, 24, 27, 28, 33). Subsequent adverse events associated with insertional mutagenesis have led to a reassessment of the risks associated with retroviral vector integration (6, 12), and a bias of murine leukemia virus (MLV)-based gammaretroviral vectors to integrate near transcription start sites (18, 24, 40) has prompted exploration of alternative vector types.
Lentiviral vectors based on human immunodeficiency virus type 1 (HIV-1) are ideal for the transfer of potentially therapeutic genes to HSCs, since lentiviral vectors can transduce nondivided cells, including hematopoietic stem cells, and can efficiently introduce complex payloads, such as the β-globin gene and key regulatory elements, an attribute not shared by MLV vectors (23). In addition, unlike MLV vectors, HIV-1 vectors display a tendency to integrate equally into active genes (24, 40). Moreover, current-generation HIV-1 vectors have been further modified to prevent activation of genes surrounding integration sites by self-inactivation (SIN) of the viral long terminal repeats (30). Finally, tissue-specific promoters/enhancers widely utilized in lentiviral vectors may further reduce the risk of integrating vectors (41).
Intrinsic immunity, in contrast to innate or adaptive immunity, is comprised of restriction factors that play an important role in species-specific resistance to viral infections and may thus limit the efficacy of viral vectors. Such restriction factors are responsible for the resistance of nonhuman primates to HIV-1 infection. These factors potentially restrict the permissivity of HIV-1-based lentiviral vectors in certain nonhuman primates, especially rhesus macaques, which provide an extensively utilized preclinical model for evaluating the safety and potential efficacy of viral vector strategies for gene therapy approaches to human disease. Among these restriction factors, tripartite motif-containing 5 isoform-α (TRIM5α) and apolipoprotein B mRNA-editing catalytic polypeptide 3G (APOBEC3G) have both been shown to restrict HIV-1 infection and in combination may potentially decrease the transduction efficiency of HIV-1-based viral vectors (31). TRIM5α binds to the HIV-1 capsid (hCA) to initiate E3 ubiquitin ligase-mediated degradation and prevent viral replication (37). In the absence of HIV-1 viral infectivity factor (HIV-1 Vif), APOBEC3G is incorporated into viral particles, promotes dC-to-dU mutations in minus-strand proviral cDNA in the newly infected cells, and suppresses productive infection (22, 34).
Previously, HIV-1-based vectors with mutations in the cyclophilin A (CypA) binding site were shown to escape baboon or rhesus TRIM5α binding (21, 29). But this modification was not sufficient to completely overcome the resistance of rhesus blood cells to HIV-1 vector transduction in vitro (29) or in our rhesus experiments (17). In contrast, HSCs of pigtailed macaques can be easily transduced with HIV-1 vectors (39), and new data suggest that alternative splice variants and chimeric transcripts with CypA and TRIM5α explain in part the lack of resistance in this model (26). Here we describe our development of an HIV-1-based vector system, substituting simian immunodeficiency virus (SIV) capsid (sCA) and SIV Vif in HIV-1 vectors, for efficient transduction of HSCs from both humans and rhesus macaques.
MATERIALS AND METHODS
Plasmid construction.
A series of vectors was constructed utilizing combination plasmids derived from both HIV-1 and SIV. Standard HIV-1- and SIV-based plasmids were kindly provided by Arthur Nienhuis (13). An HIV-1-based plasmid, pCRV1/stHIVGagpol, containing sCA instead of hCA, was kindly provided by Theodora Hatziioannou (16). The HIV-1 Gag/Pol-plus-sCA gene was cut out of pCRV1/stHIVGagpol and exchanged for the HIV-1 Gag/Pol gene in the pCAG-KGP1.1R plasmid to construct the second pCAG-stHIVGagpol plasmid (HIV1+sCA) (Fig. 1; see also the supplemental material) (15). Using the PCR technique, the sCA in a SIV-based Gag/Pol plasmid (pCAG-SIVgprre) was then substituted for hCA (pCAG-SIVgprre-hCA, SIV-hCA) (see Methods in the supplemental material) (13).
FIG. 1.
Plasmid construction. In the Gag/Pol plasmid, the sCA was substituted for the hCA (top). The SIV Vif expression cassette (large open rectangle), which includes the EF1α promoter (square) and SIV Vif, was inserted into the HIV1-Rev/Tat plasmid (bottom). EF1α-p, EF1α promoter; CA, capsid; CAG-p, CAG promoter.
The SIV-Vif expression cassette under the control of the EF1α promoter, without its first intron, was inserted backward into the upstream region of the CAG promoter in the HIV1 Rev/Tat plasmid (pCAG4-RTR2) (15) to construct a plasmid that expressed both HIV-1 Rev/Tat and SIV Vif (pCAG4-RTR2 sEF1α-sVif) (Fig. 1; see also Methods in the supplemental material). In the same way, we constructed plasmids which expressed SIV Rev/Tat and HIV-1 Vif (pCAG4-RTR-SIV sEF1α-hVif) or SIV-Vif (pCAG4-RTR-SIV sEF1α-sVif).
Chimeric lentiviral vector preparation.
SIN lentiviral vectors were prepared and titrated as described previously (14). Chimeric vectors refer to any vector containing elements of both HIV-1 and SIV (Fig. 2). Chimeric lentiviral vectors were prepared in 10-cm dishes by cotransfection of 293T cells in various combinations with 6 μg of Gag/Pol plasmids, 2 μg of Rev/Tat (and Vif) plasmids, 2 μg of vesicular stomatitis virus glycoprotein envelope expression plasmids (pCAGGS-VSVG), and 10 μg of vector plasmids that expressed the enhanced green fluorescent protein (EGFP) under the control of the MSCV long-terminal-repeat U3 promoter (HIV-1 based, pCL20c MpGFP; SIV-based, pCL20c SLFR MSCV-GFP). Conventional HIV-1 (HIV-1 Gag, Pol, Rev, and Tat) or conventional SIV (SIV Gag, Pol, Rev, and Tat) vectors were also prepared as described previously (13). All types of vectors were pseudotyped with the vesicular stomatitis virus glycoprotein envelope.
FIG. 2.
Chimeric lentiviral vector preparations. Various combinations of chimeric HIV-1 (A) or SIV (B) vector constructs including Gag/Pol, Rev/Tat, and Vif were prepared using transient transfection of 293T cells. Viral titers were assessed by EGFP expression in HeLa cells by flow cytometry. HIV1+sCA, hCA sequence was exchanged for that of SIV; SIV+hCA, sCA was exchanged for that of HIV-1. Error bars represent standard errors of means (n = 3); *, P < 0.01.
Titers of lentiviral vectors were evaluated using HeLa cells. An aliquot containing 5 × 104 HeLa cells was split into 12-well dishes containing 1 ml Dulbecco's modified Eagle medium (DMEM) and 10% fetal bovine serum (FBS). After 24-h culture, the cells were transduced with vectors in a total 1 ml medium containing 8 μg/ml polybrene (hexadimethrine bromide; Sigma-Aldrich, St. Louis, MO). Three or four days later, EGFP expression was detected by flow cytometry analysis using FACSCalibur (BD Biosciences, Franklin Lakes, NJ).
Transduction of cell lines and CD34+ cells.
Human (CEMx174 and CRL-1991; ATCC, Manassas, VA) and rhesus (LCL8664 and CRL-1805; ATCC) blood cell lines were transduced with various types of chimeric lentiviral vectors of HIV-1 and SIV. These vectors were added to 12-well dishes containing 1 × 105 cells per 1 ml of RPMI medium containing 10% FBS and 8 μg/ml polybrene.
Rhesus HSCs were isolated by performing positive immunoselection for CD34+ cells from peripheral blood stem cells that were harvested by leukapheresis after five consecutive days of subcutaneous injection of 10 μg/kg of body weight granulocyte colony-stimulating factor (G-CSF) and 200 μg/kg stem cell factor (SCF) (both kindly provided by Amgen Inc., Thousand Oaks, CA) as previously described (8, 9, 19). CD34+ cells (1 × 105) were aliquoted into fibronectin CH-296-coated (RetroNectin; TaKaRa, Otsu, Shiga, Japan) 12-well plates in 1 ml serum-free X-VIVO10 medium (Lonza, Allendale, NJ) containing SCF, FMS-like tyrosine kinase 3 ligand, and thrombopoietin (all 100 ng/ml; R&D Systems, Minneapolis, MN) in accordance with a slightly modified version of the previous method (13). After overnight prestimulation, the medium with the same concentration of cytokines was changed, and the lentiviral vectors were added to the cells. After 24 h of transduction, the medium was changed again with the same cytokines (no viral vector); after an additional 2 to 3 days, EGFP expression was evaluated by flow cytometry.
Erythroid and myeloid culture.
Human and rhesus CD34+ cells were differentiated into erythroid or myeloid cells after prestimulation and transduction. Erythroid cells were induced by DMEM containing 30% FBS, bovine serum albumin, β-mercaptoethanol, dexamethasone, holotransferrin, SCF, erythropoietin (EPO), and transforming growth factor beta (4). Medium changes were made every 4 to 6 days, and cultures were continued for a total of 2 weeks. Human and rhesus erythroid cells were detected by human glycophorin A-phycoerythrin (PE)-conjugated antibody (clone GA-R2; BD Biosciences) and rhesus monkey red blood cell antibody (clone T3G6; StemCell Technologies, Vancouver, British Columbia, Canada) with rat anti-mouse immunoglobulin G1-PE antibody (clone A85-1; BD Biosciences), respectively. In myeloid culture, DMEM containing G-CSF and granulocyte-macrophage colony-stimulating factor instead of transferrin, EPO, and transforming growth factor beta was used for 1 week. Both human and rhesus myeloid cells were detected by human CD33-PE antibody (clone AC104.3E3; Miltenyi Biotec, Bergisch Gladbach, Germany). EGFP expression and PE signals were evaluated by using a FACSCalibur system.
Methylcellulose CFU assay.
Human and rhesus CD34+ cells were transduced with lentiviral vectors, and then 1 × 104 cells were cultured in 1 ml methylcellulose media (MethoCult H4230; StemCell Technologies) containing 100 ng/ml SCF, 10 ng/ml interleukin 3, 10 ng/ml granulocyte-macrophage colony-stimulating factor, and 5 U/ml EPO for 9 to 12 days (38). Total and EGFP-positive erythroid and myeloid colonies were enumerated by microscopy under UV light (see Fig. 6A).
FIG. 6.
EGFP expression in erythroid and myeloid cells following ex vivo transduction of human and rhesus CD34+ cells. Hematopoietic human (MOI = 5) or rhesus (MOI = 2) cells were transduced. (A) EGFP expression was detectable among erythroid and myeloid colonies derived from χHIV vector-transduced rhesus CD34+ cells by microscopy under UV light. (B) The percentages of EGFP-positive colonies are shown among total erythroid and myeloid colonies in the CFU assay of transduced human or rhesus CD34+ cells. Erythroid and myeloid liquid cultures were derived from transduced human (C) or rhesus (D) CD34+ cells. Transduction rates were determined by the percentage of EGFP-positive cells by flow cytometry. Error bars, standard errors of the means (n = 3); *, P < 0.01. GPA, glycophorin A.
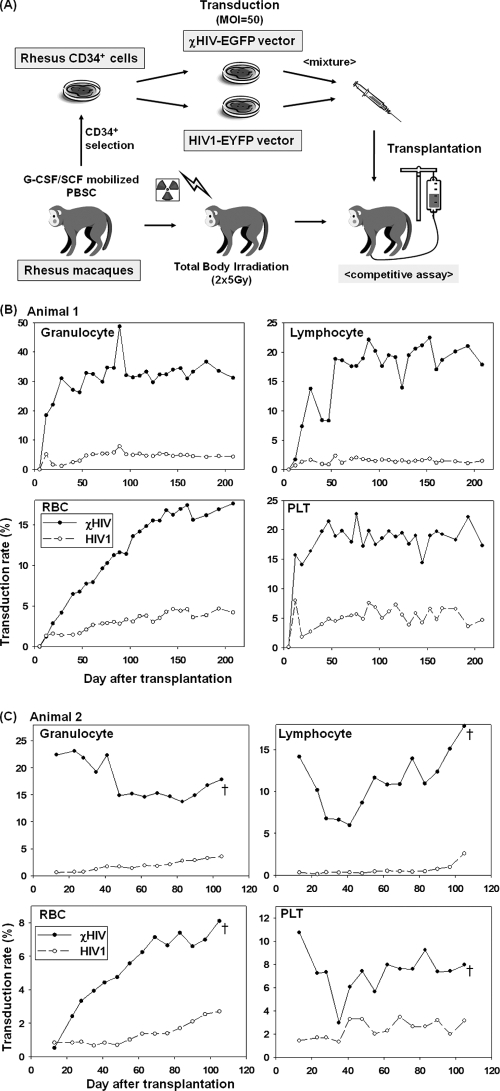
Competitive repopulation assay with the rhesus macaque.
G-CSF and SCF-mobilized peripheral blood stem cells were harvested from rhesus macaques, and CD34+ cells were immunoselected for transduction. Harvested rhesus CD34+ cells were cultured in X-VIVO10 medium (Lonza) containing SCF, FMS-like tyrosine kinase 3 ligand, and thrombopoietin on fibronectin CH-296-coated (TaKaRa) flasks overnight. Following prestimulation, half of the CD34+ cells were transduced with the chimeric vector found superior in the in vitro assays (χHIV-EGFP vector, no. 10, chimeric HIV1+sCA and no Vif) at an MOI of 50 in fresh X-VIVO10 medium containing the same cytokines for 24 h, and the other half were transduced with a conventional HIV-1 enhanced yellow fluorescent protein (EYFP) vector under the same conditions (see Fig. 7A). During ex vivo transduction, the rhesus macaques received a total 10 Gy total body irradiation split equally on two consecutive days. On day zero, two groups of transduced CD34+ cells were mixed and infused into the irradiated animals. After engraftment, peripheral blood EGFP and EYFP expression was evaluated periodically by flow cytometry.
FIG. 7.
In vivo transgene expression in the rhesus macaque competitive repopulation model. (A) Rhesus CD34+ cells were collected and split equally for transduction with either the χHIV vector (no. 10) expressing EGFP or the conventional HIV-1 vector (no. 1) expressing EYFP and infused simultaneously after lethal irradiation. The percentages of marked peripheral blood cells with respect to time after transplant are shown for animal 1 (B) and animal 2 (C). Animal 2 was euthanized at day 105 after transplantation due to progressive dyspnea, which was diagnosed as radiation pneumonitis at autopsy. PBSC, peripheral blood stem cells; PLT, platelets; †, euthanasia.
Statistical analysis.
Statistical analyses were performed using the JMP 7 software program (SAS Institute Inc., Cary, NC). Vector titers were compared using Dunnett's test (one-way analysis of variance [ANOVA]). Transduction rates after vector dose escalation studies of human and rhesus cell lines were compared using Tukey's honestly significant difference test (one-way ANOVA). Transduction rates for primary human and rhesus CD34+ cells were compared using the Student t test. A P value of <0.01 or 0.05 was deemed significant.
RESULTS
Chimeric lentiviral vectors can be produced with sufficient titers.
To evaluate whether chimeric lentiviral vectors could be prepared, we tested combinations of chimeric vectors encoding both HIV-1 and SIV gene products. The conventional HIV-1 vector had average titers of (9.2 ± 0.46) × 106 IU/ml. The addition of SIV Vif or the exchange for the sCA (no. 10 and 11) did not significantly change the titers of chimeric HIV-1 vectors ([8.4 ± 0.69] × 106 IU/ml [P = 0.73] and [8.1 ± 1.1] × 106 IU/ml [P = 0.73], respectively). In contrast, chimeric HIV-1 vectors with SIV Gag/Pol or SIV Rev/Tat had dramatically decreased titers ([0.18 to 4.5] × 105 IU/ml [P < 0.01]; Fig. 2A). The conventional SIV vector had average titers of [3.0 ± 0.11] × 106 IU/ml. Chimeric SIV vectors with hCA demonstrated very low titers ([5.5 ± 2.2] × 103 IU/ml; P < 0.01), while chimeric SIV vectors with both HIV-1 Gag/Pol and HIV-1 Rev/Tat showed similar titers ([3.8 ± 1.7] × 106 IU/ml [P = 0.88]; Fig. 2B).
Chimeric HIV-1 vectors efficiently transduce human and rhesus blood cell lines.
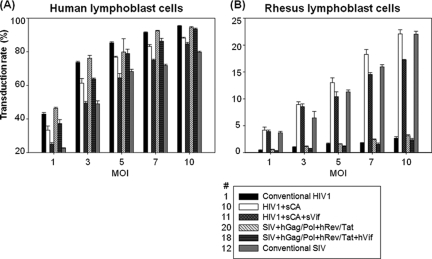
To evaluate the vectors with respect to viral capsid and cell type tropism, we exposed human and rhesus blood cell lines to three chimeric HIV-1 and three chimeric SIV lentiviral vectors (Fig. 3). The HIV1+sCA vector (no. 10) transduced both human and rhesus blood cell lines (80.8% ± 1.22% and 7.8% ± 0.47% at an MOI of 5, respectively), and the addition of SIV Vif (no. 11) did not substantially increase the transduction of a rhesus cell line (7.8% ± 0.66% at an MOI of 5) (Fig. 3A). On the other hand, the chimeric SIV+hCA vector (no. 22) transduced rhesus cell lines poorly (0.44% ± 0.05% at an MOI of 1; P < 0.01) (Fig. 3B). These data demonstrate that the transduction efficiency in human and rhesus blood cell lines depends upon the origin of the viral capsid and that the HIV1+sCA vectors were as efficient in transducing rhesus blood cell lines as the conventional SIV vector.
FIG. 3.
Transduction rate among human and rhesus blood cell lines. Human (CEMx174) and rhesus (LCL8664) blood cell lines were transduced with chimeric vectors made with various combinations of Gag/Pol and Vif. (A) HIV-1 vectors (no. 1, 4, 10, 11, and 12) MOI = 5. (B) SIV vectors (no. 1, 12, 15, 21, and 22); MOI = 1. Transduction rates were evaluated by determining the percentage of EGFP-positive cells by flow cytometry. Error bars, standard errors of means (n = 3); *, P < 0.01. hVif, HIV-1 VIF.
To confirm these initial findings, we performed dose escalation transductions on human and rhesus blood cell lines, using chimeric vectors with sufficient titer. The HIV1+sCA vectors allowed better transduction of a human blood cell line (P < 0.01 at all MOIs except 5) than the conventional SIV vector (Fig. 4A). The HIV1+sCA vectors also transduced a rhesus blood cell line as efficiently as the conventional SIV, while the conventional HIV-1 vector could not (P < 0.01 at all MOIs) (Fig. 4B). The addition of SIV Vif, however, modestly reduced the transduction rates for both the human (P < 0.05 at all MOIs except 5) and rhesus (P < 0.05 at an MOI of 5, 7, and 10) blood cell lines. The transduction patterns of the chimeric SIV vectors were similar to that of the HIV-1 vector. These data showed that among the HIV-1 vectors tested, vector no. 10, which contained sCA without Vif, was most optimal for human and rhesus blood cell line transduction. We hereinafter refer to this optimal vector system (HIV-1 vector with the sCA helper plasmid without Vif; no. 10) as the χHIV vector.
FIG. 4.
Transduction rate among human and rhesus lymphoblast cell lines following escalating doses of chimeric lentiviral vectors. Human (CEMx174) (A) or rhesus (LCL8664) (B) lymphoblast cell lines were transduced with chimeric HIV-1 vectors (no. 10 and 11) and SIV vectors (no. 18 and 20) and compared with conventional HIV-1 (no. 1) and SIV (no. 12) vectors. Transduction rates were determined by percentages of EGFP-positive cells by flow cytometry. Error bars, standard errors of means (n = 3). hVif, HIV-1 VIF.
χHIV vector transduces primary rhesus CD34+ cells better than the conventional HIV-1 vector.
To evaluate ex vivo transduction of our χHIV vector with human and rhesus HSCs, we mobilized human and rhesus peripheral blood CD34+ cells and transduced them with χHIV and conventional HIV-1 vectors at various MOIs (0.5 to 50). There were dose-dependent rates of transduction with both vectors in both target cell types, and transduction levels plateaued at an MOI of 10. In CD34+ cells from one healthy individual, χHIV and HIV-1 vectors showed equivalent transduction rates at lower MOIs (P < 0.01 at MOIs of 25 and 50) (Fig. 5A), while in rhesus CD34+ cells, the χHIV vector had superior transduction rates at all MOIs (P < 0.01 for all) (Fig. 5B). Interestingly, at MOI conditions greater than 5, the conventional HIV-1 vector could transduce rhesus CD34+ cells (42.7 to 47.1%), suggesting that rhesus TRIM5α can be saturated and can be partially overwhelmed by conventional hCA. These data demonstrate that the χHIV vector can efficiently transduce both human and rhesus primary CD34+ cells ex vivo.
FIG. 5.
Human and rhesus CD34+ cell transduction with χHIV vector. χHIV (no. 10) and conventional HIV-1 (no. 1) vectors were compared using CD34+ hematopoietic stem cells from one healthy individual (A) or one rhesus macaque (B) with MOIs ranging from 0.5 to 50. (C) CD34+ hematopoietic stem cells from one human volunteer and four rhesus monkeys were transduced at an MOI of 50. Transduction rates were determined by percentages of EGFP-positive cells by flow cytometry. Error bars, standard errors of the means (n = 3); *, P < 0.01.
To evaluate the potential individual variations regarding CD34+ cell transduction efficiency, we transduced CD34+ cells from four rhesus macaques with both vectors. While CD34+ cells from all four animals had varying levels of EGFP expression following transduction, the χHIV vector remained superior to the HIV-1 vector in transduction efficiency (P < 0.01) (Fig. 5C).
Next, we evaluated EGFP expression in differentiated erythroid and myeloid colonies in CFU assays (Fig. 6A). χHIV and conventional HIV-1 vectors produced equivalent EGFP-expressing colonies from human CD34+ cells (erythroid, 79% ± 0.79% versus 72% ± 0.64% [P < 0.01]; and myeloid, 34% ± 2.0% versus 37% ± 0.88% [P = 0.22], respectively). As expected, the χHIV vector produced a significantly higher percentage of EGFP-positive colonies from rhesus CD34+ cells than the conventional HIV-1 vector (erythroid, 77% ± 1.0% versus 15% ± 2.7% [P < 0.01]; and myeloid, 46% ± 1.8% versus 1.2% ± 0.59% [P < 0.01], respectively) (Fig. 6B). These CFU assay observations were then confirmed with liquid erythroid and myeloid cultures. Human erythroid and myeloid cells showed similar transgene expression (erythroid, 66% ± 4.0% versus 68% ± 2.3% [P = 0.61]; and myeloid, 36% ± 1.5% versus 37% ± 1.5% [P = 0.93], respectively) (Fig. 6C). Rhesus cells showed higher rates of transgene expression after χHIV transduction (erythroid, 51% ± 6.4% versus 11% ± 0.24% [P < 0.01]; myeloid, 27% ± 2.5% versus 6.1% ± 2.0% [P < 0.01], respectively) (Fig. 6D). These data confirmed that the χHIV vector could transduce rhesus HSCs in a manner superior to that of the conventional HIV-1 vector.
χHIV vector efficiently transduces hematopoietic stem cells in rhesus macaque competitive repopulation model.
We then went on to assess whether the χHIV vector could transduce rhesus HSCs capable of reconstituting in vivo hematopoiesis. We performed a competitive repopulation assay with two rhesus macaques in which half of the CD34+ cells were transduced with the χHIV vector at an MOI of 50 and the other half with the conventional HIV-1 vector under the same conditions. As expected, the χHIV vector showed better transduction rates in vitro than those of the HIV-1 vector (first rhesus, 61.1% ± 0.36% versus 28.0% ± 0.40%; and second rhesus, 48.6% ± 1.02% versus 30.7% ± 0.51%, respectively). Following transplantation, granulocyte recovery (>500 cells/μl) was observed on day 9 for the first animal and on day 12 for the second animal. After engraftment, complete blood counts were in the normal range and remained stable for both animals during follow up (see Fig. S1 in the supplemental material). For the first animal, in vivo gene marking levels from the χHIV vector slowly increased in all cell lineages (Fig. 7B). The percentage of cells expressing the transgene among all blood cells except red blood cells (RBCs) arrived at plateau levels (15 to 30%) 2 to 3 months after transplantation. Interestingly, the transgene expression levels in RBCs slowly increased over the 6 months posttransplantation. Marking levels from the conventional HIV-1 vector, however, remained at low levels (1 to 5%) in all lineages (Fig. 7B). In the second animal, marking levels from the χHIV vector among all blood cells except RBCs slowly decreased and finally arrived at plateau levels (7 to 15%) 2 months after transplantation (Fig. 7C). Transgene expression levels among RBCs were continuously increasing over the follow-up period. However, the conventional HIV-1 vector showed lower marking levels (0.5 to 2%) in all cell lineages. The vector copy number per transduced mononuclear cell in the peripheral blood was 2.5 for the χHIV vector and 1.2 for the HIV-1 vector in the first animal 7 months after transplantation. The second animal showed similar data (2.6 in the χHIV vector and 1.2 in the HIV-1 vector 3 months after transplantation). No dominant clone was detected by Southern blot analysis of peripheral blood cells in the first animal (see Fig. S2B in the supplemental material). Surface marker analysis of peripheral blood and bone marrow cells in the first animal demonstrated transduction rates with the χHIV vector that were superior to those with the HIV-1 vector in all cell lineages (see Fig. S3 in the supplemental material). These data demonstrate that the relative block to transduction of peripheral blood CD34+ cells by conventional HIV-1 vectors can be overcome by exchanging the entire sCA into the hCA.
The second animal had progressive dyspnea and bilateral diffuse ground-glass opacities on chest X-ray at day 97 despite being afebrile and having normal blood counts. Although 1 to 2 mg/kg prednisolone was instituted promptly, she was eventually euthanized at day 105 for progressive dyspnea. Postmortem examination showed the lungs contained alveoli and bronchioles filled with foamy macrophages and fibrin, alveolar walls variably thickened by maturing collagen, and variably hyperplastic type II pneumocytes. Bacterial culture of the lung was negative. These findings were consistent with radiation-induced pneumonitis with interstitial lung fibrosis.
DISCUSSION
Gene transfer to HSCs utilizing lentiviral vectors is a promising modality for the treatment of human diseases, and large-animal models remain important to inform the clinical effort. Among large-animal models, the rhesus macaque has proven well suited for preclinical safety and efficacy studies. Recently in a human trial of gene therapy for X-linked severe combined immunodeficiency using an MLV vector to transduce bone marrow-derived HSCs, four patients developed T-cell-type acute lymphoblastic leukemia caused by insertional mutagenesis (11, 12, 27), and additional events have heightened the need to better understand the underlying mechanism eliciting these events (28). New vector systems including SIN vectors have emerged as an alternative to MLV vectors. However, several groups have been unsuccessful in developing HIV-1 vectors for preclinical testing in rhesus models (29, 39), while others have achieved long-term marking of only 5% or less (2, 3). These events create a mandate to develop such relevant large-animal gene therapy models for preclinical testing of HIV-1 vectors.
TRIM5α and APOBEC3G are species-specific restriction factors that block retroviral infection (22, 31, 34, 36, 37). Rhesus TRIM5α can bind to the hCA, mediate HIV-1 RNA degradation, and block HIV-1 infection, while SIV (containing sCA) can escape rhesus TRIM5α-mediated viral degradation. These restriction factors have important implications in gene transfer experiments, and several strategies to modulate these factors have been employed to improve transduction by viral vectors. One such approach is to overwhelm all available TRIM5α by transducing at high MOIs. This strategy was demonstrated to be somewhat effective in our study (Fig. 5B), where the conventional HIV-1 vector at MOIs of 10 or higher were shown to transduce rhesus cells in vitro. This method, however, remains inferior to the χHIV vector and cannot promote efficient transduction of rhesus hematopoietic cells in vivo (Fig. 7B and C).
HIV-1 vectors that include mutations in the CypA-binding domain of the capsid sequence have been developed as another strategy to overcome the resistance to transduction in old world monkeys. These modified vectors have been reported to transduce both human and baboon CD34+ cells (21) but are not sufficient to allow escape from rhesus TRIM5α, and transduction rates among rhesus CD34+ cells remained suboptimal (17). By exchanging the entire hCA sequence with that of the sCA, we found that the χHIV vector efficiently transduces both human and rhesus CD34+ cells in vitro, suggesting that sCA escapes from rhesus TRIM5α binding. Our proof-of-concept results are further confirmed by a recent study which demonstrated that an experimental HIV-1 virus encoding sCA and SIV-Vif could replicate in rhesus peripheral blood cells through escape from rhesus TRIM5α and APOBEC3G, respectively (16). Our χHIV vector which includes sCA showed transduction efficiency for both human and rhesus blood cell lines superior to those of the alternative vectors.
As an additional strategy, we sought to incorporate Vif into our vector system in order to allow binding to APOBEC3G and enhance viral transduction. Our inclusion of SIV or HIV-1 Vif did not demonstrate the intended results and modestly decreased the viral titer during vector production. Although APOBEC3G plays no direct role in inhibiting transduction, several reasons explain why the addition of SIV Vif did not further improve transduction rates in rhesus blood cells. First, our lentiviral vectors do not contain the APOBEC3G protein in the virion particles because 293T cells do not express APOBEC3G in our vector production system (data not shown). Therefore, SIN lentiviral vectors that transduce only once theoretically do not require the SIV Vif gene to transduce rhesus HSCs. Second, when APOBEC3G is incorporated into HIV-1 virions during virus production, it can dramatically reduce viral infectivity in the absence of Vif (20). However, when there is a species mismatch between APOBEC3G and the genetically inserted Vif, the APOBEC3G-Vif complexes do not form, and APOBEC3G (produced by rhesus or human host cells) is allowed to suppress viral infection (5, 10, 16, 22, 35). Additionally, APOBEC3G has a Vif-independent pathway for inhibiting viral replication (7, 25). This prior observation is consistent with our escalating viral vector exposure experiments with primary CD34+ cells, where transduction rates plateaued well before 100%. Collectively, these observations suggest gene transfer to CD34+ cells is affected by restriction factors other than TRIM5α and APOBEC3G and thus warrants further exploration.
Though we succeeded in achieving high-level gene transfer to bulk CD34+ cells, clonogenic progenitors, and myeloid and erythroid cells differentiated in liquid culture in vitro, demonstration of transduction of the true HSCs requires transplantation experiments at a minimum. Recently it has been shown that HIV-1-based vectors efficiently transduce repopulating HSCs of the pigtailed macaque, in which TRIM5α undergoes alternative splicing (26, 39). While higher-level marking is feasible in this model, vector integration under the control of an alternatively spliced TRIM5α (26) may not be reliably predicted. In our rhesus competitive repopulation assay, preliminary gene marking levels in all blood lineages derived from the χHIV vector were significantly higher than that from the conventional HIV-1 vector and plateaued at levels of 15 to 30% 3 to 7 months after transplantation, suggesting that this high-level marking in peripheral blood cells originates from HSCs (32). Granulocytes and platelets, which have short half-lives, arrived at plateau levels of gene marking earlier (about 1 month), while lymphocytes and red blood cells, which have longer half-lives, reached a plateau in marking levels later (2 to 3 months). These early results thus suggest transduction of the true HSCs by our χHIV vector at potentially clinically relevant levels.
An important advantage of our vector system is that HIV-1 helper plasmids can be combined with the SIV vector plasmid to prepare vectors, while the HIV-1 vector plasmid could not be combined with SIV helper plasmids except by the exchanging of the capsid sequences only. Using our system, existing HIV-1-based therapeutic vector plasmids can therefore be utilized to produce χHIV vectors by simply replacing HIV-1 Gag/Pol plasmid with that of χHIV Gag/Pol (HIV1+sCA). Moreover, the χHIV system can produce high-titer vectors comparable to conventional HIV-1 vectors, with levels as high as 3 × 109 IU/ml following concentration by ultracentrifugation. Our vector system should thus allow testing of a variety of therapeutic vectors using conventional HIV-1 plasmids, already constructed in the large-animal rhesus model.
In summary, we developed an HIV-1 vector system that allows efficient transduction of both human and rhesus HSCs. We believe this vector system will prove invaluable for comprehensive preclinical safety and efficacy testing of a variety of potentially therapeutic vectors in the rhesus macaque model.
Supplementary Material
Acknowledgments
This work is supported by the intramural research program of the National Institute of Diabetes, Digestive, and Kidney Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health.
We thank Keyvan Keyvanfar and Martha Kirby for their assistance in flow cytometric analyses and the staff at 5 Research Court for their excellent care and handling of the rhesus macaques.
Footnotes
Published ahead of print on 22 July 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aiuti, A., S. Slavin, M. Aker, F. Ficara, S. Deola, A. Mortellaro, S. Morecki, G. Andolfi, A. Tabucchi, F. Carlucci, E. Marinello, F. Cattaneo, S. Vai, P. Servida, R. Miniero, M. G. Roncarolo, and C. Bordignon. 2002. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296:2410-2413. [DOI] [PubMed] [Google Scholar]
- 2.An, D. S., S. K. Kung, A. Bonifacino, R. P. Wersto, M. E. Metzger, B. A. Agricola, S. H. Mao, I. S. Chen, and R. E. Donahue. 2001. Lentivirus vector-mediated hematopoietic stem cell gene transfer of common gamma-chain cytokine receptor in rhesus macaques. J. Virol. 75:3547-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An, D. S., R. P. Wersto, B. A. Agricola, M. E. Metzger, S. Lu, R. G. Amado, I. S. Chen, and R. E. Donahue. 2000. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34(+) cells. J. Virol. 74:1286-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhanu, N. V., T. A. Trice, Y. T. Lee, and J. L. Miller. 2004. A signaling mechanism for growth-related expression of fetal hemoglobin. Blood 103:1929-1933. [DOI] [PubMed] [Google Scholar]
- 5.Bogerd, H. P., B. P. Doehle, H. L. Wiegand, and B. R. Cullen. 2004. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. USA 101:3770-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo, M., S. Hacein-Bey, G. de Saint Basile, F. Gross, E. Yvon, P. Nusbaum, F. Selz, C. Hue, S. Certain, J. L. Casanova, P. Bousso, F. L. Deist, and A. Fischer. 2000. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288:669-672. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, Y. L., V. B. Soros, J. F. Kreisberg, K. Stopak, W. Yonemoto, and W. C. Greene. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435:108-114. [DOI] [PubMed] [Google Scholar]
- 8.Donahue, R. E., M. R. Kirby, M. E. Metzger, B. A. Agricola, S. E. Sellers, and H. M. Cullis. 1996. Peripheral blood CD34+ cells differ from bone marrow CD34+ cells in Thy-1 expression and cell cycle status in nonhuman primates mobilized or not mobilized with granulocyte colony-stimulating factor and/or stem cell factor. Blood 87:1644-1653. [PubMed] [Google Scholar]
- 9.Donahue, R. E., K. Kuramoto, and C. E. Dunbar. November 2005. Large animal models for stem and progenitor cell analysis, chapter 22, unit 22A 1. Current protocols in immunology. Wiley Interscience, Hoboken, NJ. http://mrw.interscience.wiley.com/emrw/9780471142737/cp/cpim/article/im22a01/current/abstract. [DOI] [PubMed]
- 10.Goila-Gaur, R., and K. Strebel. 2008. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacein-Bey-Abina, S., A. Garrigue, G. P. Wang, J. Soulier, A. Lim, E. Morillon, E. Clappier, L. Caccavelli, E. Delabesse, K. Beldjord, V. Asnafi, E. MacIntyre, L. Dal Cortivo, I. Radford, N. Brousse, F. Sigaux, D. Moshous, J. Hauer, A. Borkhardt, B. H. Belohradsky, U. Wintergerst, M. C. Velez, L. Leiva, R. Sorensen, N. Wulffraat, S. Blanche, F. D. Bushman, A. Fischer, and M. Cavazzana-Calvo. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 118:3132-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacein-Bey-Abina, S., C. von Kalle, M. Schmidt, F. Le Deist, N. Wulffraat, E. McIntyre, I. Radford, J. L. Villeval, C. C. Fraser, M. Cavazzana-Calvo, and A. Fischer. 2003. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348:255-256. [DOI] [PubMed] [Google Scholar]
- 13.Hanawa, H., P. Hematti, K. Keyvanfar, M. E. Metzger, A. Krouse, R. E. Donahue, S. Kepes, J. Gray, C. E. Dunbar, D. A. Persons, and A. W. Nienhuis. 2004. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood 103:4062-4069. [DOI] [PubMed] [Google Scholar]
- 14.Hanawa, H., P. F. Kelly, A. C. Nathwani, D. A. Persons, J. A. Vandergriff, P. Hargrove, E. F. Vanin, and A. W. Nienhuis. 2002. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 5:242-251. [DOI] [PubMed] [Google Scholar]
- 15.Hanawa, H., D. A. Persons, and A. W. Nienhuis. 2005. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J. Virol. 79:8410-8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatziioannou, T., M. Princiotta, M. Piatak, Jr., F. Yuan, F. Zhang, J. D. Lifson, and P. D. Bieniasz. 2006. Generation of simian-tropic HIV-1 by restriction factor evasion. Science 314:95. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa, J., T. Ueda, L. Lisowski, M. M. Hsieh, K. Washington, O. Phang, M. Metzger, A. Krouse, R. E. Donahue, M. Sadelain, and J. F. Tisdale. 2009. Transient in vivo beta-globin production after lentiviral gene transfer to hematopoietic stem cells in the non-human primate. Hum. Gene Ther. 20:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hematti, P., B. K. Hong, C. Ferguson, R. Adler, H. Hanawa, S. Sellers, I. E. Holt, C. E. Eckfeldt, Y. Sharma, M. Schmidt, C. von Kalle, D. A. Persons, E. M. Billings, C. M. Verfaillie, A. W. Nienhuis, T. G. Wolfsberg, C. E. Dunbar, and B. Calmels. 2004. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hematti, P., S. Tuchman, A. Larochelle, M. E. Metzger, R. E. Donahue, and J. F. Tisdale. 2004. Comparison of retroviral transduction efficiency in CD34+ cells derived from bone marrow versus G-CSF-mobilized or G-CSF plus stem cell factor-mobilized peripheral blood in nonhuman primates. Stem Cells 22:1062-1069. [DOI] [PubMed] [Google Scholar]
- 20.Kao, S., H. Akari, M. A. Khan, M. Dettenhofer, X. F. Yu, and K. Strebel. 2003. Human immunodeficiency virus type 1 Vif is efficiently packaged into virions during productive but not chronic infection. J. Virol. 77:1131-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kootstra, N. A., C. Munk, N. Tonnu, N. R. Landau, and I. M. Verma. 2003. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. USA 100:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 23.May, C., S. Rivella, J. Callegari, G. Heller, K. M. Gaensler, L. Luzzatto, and M. Sadelain. 2000. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 406:82-86. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, R. S., B. F. Beitzel, A. R. Schroder, P. Shinn, H. Chen, C. C. Berry, J. R. Ecker, and F. D. Bushman. 2004. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2:E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman, E. N., R. K. Holmes, H. M. Craig, K. C. Klein, J. R. Lingappa, M. H. Malim, and A. M. Sheehy. 2005. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr. Biol. 15:166-170. [DOI] [PubMed] [Google Scholar]
- 26.Newman, R. M., L. Hall, A. Kirmaier, L. A. Pozzi, E. Pery, M. Farzan, S. P. O'Neil, and W. Johnson. 2008. Evolution of a TRIM5-CypA splice isoform in old world monkeys. PLoS Pathog 4:e1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nienhuis, A. W. 2008. Development of gene therapy for blood disorders. Blood 111:4431-4444. [DOI] [PubMed] [Google Scholar]
- 28.Ott, M. G., M. Schmidt, K. Schwarzwaelder, S. Stein, U. Siler, U. Koehl, H. Glimm, K. Kuhlcke, A. Schilz, H. Kunkel, S. Naundorf, A. Brinkmann, A. Deichmann, M. Fischer, C. Ball, I. Pilz, C. Dunbar, Y. Du, N. A. Jenkins, N. G. Copeland, U. Luthi, M. Hassan, A. J. Thrasher, D. Hoelzer, C. von Kalle, R. Seger, and M. Grez. 2006. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 12:401-409. [DOI] [PubMed] [Google Scholar]
- 29.Rits, M. A., K. A. van Dort, C. Munk, A. B. Meijer, and N. A. Kootstra. 2007. Efficient transduction of simian cells by HIV-1-based lentiviral vectors that contain mutations in the capsid protein. Mol. Ther. 15:930-937. [DOI] [PubMed] [Google Scholar]
- 30.Ryu, B. Y., M. V. Evans-Galea, J. T. Gray, D. M. Bodine, D. A. Persons, and A. W. Nienhuis. 2008. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood 111:1866-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuma, R., J. A. Noser, S. Ohmine, and Y. Ikeda. 2007. Inhibition of HIV-1 replication by simian restriction factors, TRIM5α and APOBEC3G. Gene Ther. 14:185-189. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, M., P. Zickler, G. Hoffman, S. Haas, M. Wissler, A. Muessig, J. F. Tisdale, R. G. Andrews, T. Wu, H.-P. Kiem, C. E. Dunbar, and C. von Kalle. 2002. Polyclonal long-term repopulating stem cell clones in a primate model. Blood 100:2737-2743. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzwaelder, K., S. J. Howe, M. Schmidt, M. H. Brugman, A. Deichmann, H. Glimm, S. Schmidt, C. Prinz, M. Wissler, D. J. King, F. Zhang, K. L. Parsley, K. C. Gilmour, J. Sinclair, J. Bayford, R. Peraj, K. Pike-Overzet, F. J. Staal, D. de Ridder, C. Kinnon, U. Abel, G. Wagemaker, H. B. Gaspar, A. J. Thrasher, and C. von Kalle. 2007. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J. Clin. Investig. 117:2241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehy, A. M., N. C. Gaddis, J. D. Choi, and M. H. Malim. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646-650. [DOI] [PubMed] [Google Scholar]
- 35.Simon, J. H., D. L. Miller, R. A. Fouchier, M. A. Soares, K. W. Peden, and M. H. Malim. 1998. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 17:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 37.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc. Natl. Acad. Sci. USA 103:5514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tisdale, J. F., Y. Hanazono, S. E. Sellers, B. A. Agricola, M. E. Metzger, R. E. Donahue, and C. E. Dunbar. 1998. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood 92:1131-1141. [PubMed] [Google Scholar]
- 39.Trobridge, G. D., B. C. Beard, C. Gooch, M. Wohlfahrt, P. Olsen, J. Fletcher, P. Malik, and H. P. Kiem. 2008. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood 111:5537-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, X., Y. Li, B. Crise, and S. M. Burgess. 2003. Transcription start regions in the human genome are favored targets for MLV integration. Science 300:1749-1751. [DOI] [PubMed] [Google Scholar]
- 41.Zychlinski, D., A. Schambach, U. Modlich, T. Maetzig, J. Meyer, E. Grassman, A. Mishra, and C. Baum. 2008. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 16:718-725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.