Abstract
Lentiviruses, including human immunodeficiency virus type 1 (HIV-1), typically encode envelope glycoproteins (Env) with long cytoplasmic tails (CTs). The strong conservation of CT length in primary isolates of HIV-1 suggests that this factor plays a key role in viral replication and persistence in infected patients. However, we report here the emergence and dominance of a primary HIV-1 variant carrying a natural 20-amino-acid truncation of the CT in vivo. We demonstrated that this truncation was deleterious for viral replication in cell culture. We then identified a compensatory amino acid substitution in the matrix protein that reversed the negative effects of CT truncation. The loss or rescue of infectivity depended on the level of Env incorporation into virus particles. Interestingly, we found that a virus mutant with defective Env incorporation was able to spread by cell-to-cell transfer. The effects on viral infectivity of compensation between the CT and the matrix protein have been suggested by in vitro studies based on T-cell laboratory-adapted virus mutants, but we provide here the first demonstration of the natural occurrence of similar mechanisms in an infected patient. Our findings provide insight into the potential of HIV-1 to evolve in vivo and its ability to overcome major structural alterations.
The envelope glycoprotein complex of the human immunodeficiency virus type 1 (HIV-1) is involved principally in virion attachment to target cell surfaces and in the entry process (15, 18, 27, 29, 52). Envelope glycoproteins (Env) are initially translated as a gp160 precursor glycoprotein, which is then processed during its trafficking through the secretory pathway, to yield a surface subunit gp120 noncovalently attached to a transmembrane subunit gp41. During HIV-1 assembly, Env proteins are incorporated at the surface of the viral particle as a trimeric structure consisting of three gp120/gp41 dimers (59, 62).
The gp41 consists of an ectodomain, a hydrophobic transmembrane anchor, and a cytoplasmic tail (CT). Lentiviruses, including HIV-1 and simian immunodeficiency virus (SIV), are unusual in having a transmembrane subunit with much longer CTs (∼150 amino acids) than most other retroviruses (20 to 50 amino acids) (27). Early studies with T-cell laboratory-adapted HIV-1 mutants showed that the gp41 CT region played an important role in regulating Env functions, the incorporation of Env into virus particles and, consequently, viral replication (16, 21, 35, 63). The integrity of the gp41 CT thus appears to be crucial for replication in primary T cells, macrophages, and in many transformed T-cell lines (1, 44). Viral variants with truncated gp41 are rarely isolated from infected patients. One study reported the isolation of a CD4-independent variant harboring a sharply truncated CT (64). However, this atypical isolate existed as a minority variant in the original quasispecies of the patient (54). SIV variants with truncated CTs obtained in cell culture in vitro have also been shown to revert rapidly (to full-length CT) when introduced into macaques (39). These observations indicate that the long CTs of lentiviruses, such as HIV-1 and SIV, have functions specific to viral replication and persistence in vivo.
Two groups of conserved sequence motifs have been identified in the gp41 CT that are likely to be involved in its functions. The first group, involved in regulating the intracellular trafficking of Env, includes a membrane-proximal tyrosine-based endocytic motif, Y712SPL, (9, 47); a diaromatic motif, Y802W803, implicated in the retrograde transport of Env to the trans-Golgi network (8), and a C-terminal dileucine motif recently identified as a second endocytic motif (7, 10, 60). We have also provided evidence for the existence of additional as-yet-unidentified signals in studies of primary HIV-1 (34). The second group of motifs consists of three structurally conserved amphipathic α-helical domains: lentivirus lytic peptides 1, 2, and 3 (LLP-1, LLP-2, and LLP-3) (11, 17, 33). LLP domains have been implicated in various functions, including Env fusogenicity and the incorporation of Env into HIV-1 particles (28, 32, 43, 45, 50, 61).
Several lines of evidence suggest that Env incorporation requires direct or indirect interactions between the matrix domain of the structural protein precursor Pr55Gag (matrix) and the gp41 CT during HIV-1 assembly. This possibility was first suggested by the observation that HIV-1 Env drives the basolateral budding of Gag in polarized cells (37, 48). A direct interaction between the matrix and a glutathione S-transferase fusion protein containing Env CT was subsequently observed in vitro (13). Synthetic peptides corresponding to various domains of the gp41 CT have also been shown to interact directly with Pr55Gag molecules (26). Furthermore, effects on viral infectivity of compensation between the CT and the matrix protein have been suggested by studies based on T-cell laboratory-adapted virus mutants (19, 40, 43). Finally, the cellular protein TIP47 was recently implicated in Env incorporation, based on its ability to bind both the matrix protein and the gp41 CT (38).
In a previous study describing the evolutionary dynamics of the glycan shield of HIV-1 Env, we identified a patient (patient 153) for whom the 15 env clones obtained during primary infection (early stage) encoded full-length Env, whereas the 15 env sequences from the HIV-1 present 6 years later (late stage) encoded truncated gp41 CTs (14). These late-stage sequences contained a deletion introducing an in-frame stop codon, resulting in a 20-amino-acid truncation of the Env. Note that, unlike a point mutation, this deletion cannot easily revert to the full-length form. Such a deletion affecting various known motifs of the gp41 CT would be expected to impair viral replication. However, the plasma viral load measured in patient 153 demonstrated that the virus had retained its ability to replicate.
In the present study, we explored the molecular mechanisms by which a primary HIV-1 maintained its capacity to replicate efficiently in this patient and demonstrated for the first time the occurrence of matrix and Env coevolution in vivo, providing insight into the ability of HIV-1 to overcome major structural alterations.
MATERIALS AND METHODS
Patient, samples, env, and matrix sequences.
Patient 153 is a man infected with an HIV-1 clade B virus selected from a cohort of patients identified at the time of symptomatic primary infection in the Department of Infectious Diseases of the Croix-Rousse Hospital, Lyon, France (5). The patient signed an informed consent form. Study protocols were approved by the Human Subjects Committee of the Hospices Civils de Lyon. The procedure used to clone full-length env genes from the DNA of peripheral blood mononuclear cells (PBMC) extracted from blood samples collected from patient 153 at the time of primary infection and six years later, has been described elsewhere (14). The corresponding env nucleotide sequences (15 early and 15 late clones) have been deposited in the GenBank database under accession numbers AY535489 through AY535518 (14). All of the late env clones contain a 23-bp deletion introducing an in-frame stop codon resulting in a 20-amino-acid truncation. This deletion does not affect the rev and tat gene sequences. One early env clone (E; accession number AY535491) and two late env clones (L1 and L2, accession numbers AY535509 and AY535516, respectively) were selected for further study.
The viral gag gene sequences encoding the matrix protein were also cloned from the DNA of PBMC collected from patient 153 at early and late stages. Amplification reactions were conducted by nested PCR, using the Platinum PCR SuperMix High Fidelity kit (Invitrogen). The outer primer pair used was M(+) (5′-GAAGCGCGCACGGCAAGAGG-3′) and M(−) (5′-GTTCCTGAAGGGTACTAGTAGTTCCTGC-3′). The inner primer pair AatII(+) (5′-CTAGCGGAGGCTAGACGTCGAGAGATGGGTGC-3′) and BsrGI153(−) (5′-GCCCTTGTAGGTTCTGTACAATAGGGTAATTTTGGCTG-3′) was designed to generate an AatII and a BsrGI restriction site at the 5′ and 3′ ends of the amplified sequence, respectively. The PCR products were inserted into pCR2.1 (Topo TA cloning kit; Invitrogen). Six early and ten late clones were sequenced. The nucleotide sequences were analyzed (Applied Biosystems) and the corresponding deduced amino-acid sequences were aligned by using CLUSTAL W (57), with manual corrections. One early gag clone (ME) and one late gag clone (ML) were selected for further study.
Six additional blood samples, collected from patient 153 between the early and late stages (n = 5) or 3 years after the late stage (n = 1), were used to analyze the evolution of viral env and gag sequences. Amplification reactions were conducted by nested PCR on PBMC DNA, using the Platinum PCR SuperMix High Fidelity kit (Invitrogen). The outer primer pair used to amplify the env gene sequences encoding the gp41 CT was CT(+) (5′-CACTGCTGTGCCTTGGAATG-3′) and CT(−) (5′-CTTGTAAGTCATTGGTCTTAAAGGTAC-3′). The inner primers used were NheI(+) (5′-ATAGTAGGAGGGCTAGCAGGTTTAAGAATAG-3′) and XbaI(−) (5′-TTATTGCAAAGCCCTTTCTAGACCCTGTCTCAC-3′). The gag sequences encoding the matrix protein were amplified with the primer pairs M(+)/M(−) and AatII(+)/BsrGI153(−) described above. All PCR products were inserted into pCR2.1 (Topo TA cloning kit; Invitrogen) and 10 clones of each were sequenced. The nucleotide sequences were analyzed (Applied Biosystems) and the corresponding deduced amino acid sequences of gp41 CTs or matrix proteins were aligned, using CLUSTAL W (57), with manual corrections.
In addition to the various sequence analyses conducted, HIV-1 RNA viral loads were determined on all blood samples from patient 153 available for the present study (Table 1). Treatment was initiated in March 2000, with a combination of Combivir (GlaxoSmithKline) and Viramune (Boehringer Ingelheim), and the patient was still on this treatment regimen when the last blood sample studied here was collected in February 2002.
TABLE 1.
Characteristics of patient 153 at the time of blood sample collection
| Date of blood sampleb | Viral load (log RNA copies/ml)c | gp41 CT truncationa | Matrix mutationa
|
||
|---|---|---|---|---|---|
| K25R | V34I | I45L | |||
| 05.03.1993 (early stage) | 4.96 | - (0/10) | - (0/10) | - (0/10) | - (0/10) |
| 10.18.1993 | 4.12 | - (0/10) | - (0/10) | - (0/10) | - (0/10) |
| 05.31.1994 | 2.87 | - (0/10) | - (0/10) | - (0/10) | - (0/10) |
| 01.12.1995 | 3.22 | + (10/10) | - (0/10) | + (3/10) | - (0/10) |
| 07.03.1997 | 3.86 | + (10/10) | - (0/10) | + (5/10) | - (0/10) |
| 06.02.1998 | 4.1 | + (10/10) | + (1/10) | + (6/10) | + (4/10) |
| 09.03.1999 (late stage) | 4.25 | + (10/10) | + (10/10) | + (10/10) | + (10/10) |
| 02.04.2002 | 3.15 | + (10/10) | + (10/10) | + (10/10) | + (10/10) |
The number of positive clones over the total number of clones tested is indicated in parentheses for each sample analyzed.
A treatment associating Combivir and Viramune was initiated in March 2000. Dates are given in the format “month.day.year.”
Plasma HIV RNA was quantified by using the Abbott RealTime HIV-1 assay.
Proviral constructs.
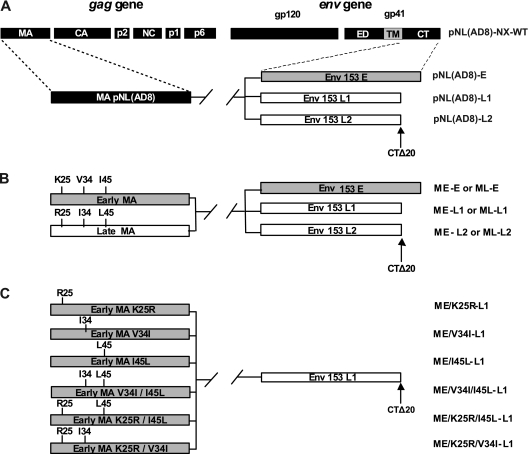
Replication-competent proviruses carrying env sequences encoding E, L1, or L2 gp41 CTs, alone or in combination with gag sequences encoding early or late matrix proteins, were constructed in a pNL(AD8) background. pNL(AD8) is an R5 derivative of pNL4-3 (20). We used pNL(AD8)-NX, which has been described elsewhere (34), as the starting material. This clone differed from pNL(AD8) by the insertion of NheI and XbaI restriction sites into the sequences corresponding to the membrane-spanning domain of gp41 and at the end of the env gene, respectively. The DNA sequences encoding the gp41 CTs of the E, L1, and L2 env genes were amplified by PCR with the Platinum PCR SuperMix High Fidelity kit (Invitrogen), using the primer pair NheI(+)/XbaI(−) described above. This primer pair was designed to generate NheI and XbaI restriction sites at the 5′ and 3′ ends of the amplified product, respectively. The PCR products were inserted into pCR2.1 (Topo TA cloning kit; Invitrogen), excised by digestion with NheI/XbaI and inserted into the corresponding sites of pNL(AD8)-NX, giving rise to pNL(AD8)-E, -L1, or -L2 (Fig. 1A). pNL(AD8)-Edel23 and pNL(AD8)-L1ins23 were obtained by overlap PCR, using appropriate primers. pNL(AD8)-Edel23 is a pNL(AD8)-E derivative containing the 23-bp deletion in the env sequence encoding the gp41 CT of the early clone E. Conversely, pNL(AD8)-L1ins23 was derived from pNL(AD8)-L1, in which the full-length env sequence of the late clone L1 has been restored.
FIG. 1.
Schematic representation of the proviral constructs used. The upper diagram shows the HIV-1 gag and env genes with the corresponding proteins. The parental viral proteins are indicated by boxes of different colors: black, NL(AD8); gray, early clones of HIV-1 from patient 153; white, late clones of HIV-1 from patient 153. The arrows indicate the stop codons shortening the gp41 CTs by 20 amino acids (clones L1 and L2). (A) Schematic representation of pNL(AD8)-NX proviral constructs harboring the sequences encoding the gp41 CTs derived from HIV-1 present at early (clone E) and late stages (clones L1 and L2) in patient 153. (B) Schematic representation of pNL(AD8)-NX proviral constructs carrying various combinations of sequences encoding the gp41 CTs (clones E, L1, and L2) and matrix proteins (early clone ME, late clone ML) derived from HIV-1 present at early or late stages in patient 153. (C) Schematic representation of pNL(AD8)-NX proviral constructs harboring the sequence encoding the truncated gp41 CT (clone L1) associated with the sequence encoding the early matrix protein (clone ME) with various combinations of the three amino acid substitutions (K25R, V34I, or I45L) found in all HIV-1 variants present at the late stage in patient 153.
Mutagenesis was then carried out in a pCR2.1 construct, which contained a 408-kb BssHII-SpeI fragment from pNL(AD8)-NX encompassing the sequence of the gag gene encoding the matrix protein. Sequences were modified with the QuikChange site-directed mutagenesis kit (Stratagene) and the following primers: AatII(+) described above, AatII(−) (5′-GCACCCATCTCTCGACGTCTAGCCTCCGCTAG-3′), BsrGI(+) (5′-CAGCCAAAATTACCCTATTGTACAGAACCTCCAGGGGC-3′), and BsrGI(−) (5′-GCCCCTGGAGGTTCTGTACAATAGGGTAATTTTGGCTG-3′). The resulting construct, pCR2.1(AD8)-M, contains AatII and BsrGI restriction sites at the 5′ and 3′ ends of the matrix coding sequence, respectively. The DNA sequences encoding the matrix proteins ME and ML, previously inserted into pCR2.1, were excised by digestion with AatII and BsrGI and inserted into the corresponding sites of pCR2.1(AD8)-M, giving rise to pCR2.1(AD8)-ME and -ML. Finally, the BssHII-SpeI fragment was excised from pCR2.1(AD8)-ME or -ML and inserted into the corresponding sites of pNL(AD8)-E, -L1, or -L2, as summarized in Fig. 1B.
Six additional pNL(AD8) constructs encoding early matrix mutants associated with the L1 gp41 CT were designed to test the relevance of the various amino acid substitutions distinguishing between early and late matrix proteins (Fig. 1C). Mutagenesis was carried out on the DNA sequence encoding the ME matrix protein inserted into the pCR2.1 vector. Sequences were modified with the QuikChange site-directed mutagenesis kit (Stratagene), using appropriate primers. After mutagenesis, the DNA sequences encoding the matrix mutants were excised by digestion with AatII and BsrGI before insertion into the corresponding sites of ME-L1, as described above. All of the proviral constructs generated were verified by DNA sequencing.
NLΔenv and NL F522Y proviruses were used as controls in some experiments (6, 46). NL F522Y encodes a nonfusogenic gp120/gp41 complex.
Cell culture.
293T and HeLa cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum and antibiotics (100 IU of penicillin and 100 μg of streptomycin/ml). MT4.R5 T cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics (4). TZM.bl cells were maintained in DMEM supplemented with 10% fetal calf serum, gentamicin (50 μg/ml), and 25 mM HEPES (51, 58). PBMC were obtained from the buffy coats of HIV-seronegative donors by Ficoll-Hypaque density gradient centrifugation. Monocytes were purified from PBMC by adhesion to the plastic of culture plates, as previously described (49). Monocyte-derived macrophages (MDMs) were obtained by allowing harvested monocytes to differentiate into macrophages for 7 days in 12-well plates (8 × 105 cells/well) containing RPMI 1640 medium supplemented with 10% human AB serum and antibiotics. Nonadherent PBMC were treated with phytohemagglutinin (5 μg/ml) in RPMI 1640 medium supplemented with interleukin-2 (10 ng/ml; Roche), 20% fetal calf serum, and antibiotics for 3 days. They were then washed free of phytohemagglutinin and maintained in RPMI 1640 medium supplemented with interleukin-2, 20% fetal calf serum, and antibiotics. Purified CD4+ T lymphocytes were negatively selected with biotin-antibody cocktail microbeads (CD4+ T-cell isolation kit II; Miltenyi Biotec, Germany).
Production of viral stocks.
Viruses for the analysis of replication kinetics in T cells and MDMs were obtained by transfecting 293T cells with proviral DNA constructs, as previously described (34). All virus stocks were sampled for detection of the p24 capsid protein by an Innotest HIV antigen enzyme-linked immunosorbent assay (ELISA) kit (Ingen) before freezing at −80°C.
Viruses for Env incorporation assays were generated by transfecting 293T cells and HeLa cells or infecting MT4.R5 cells, PBMC and MDMs. The procedure used to transfect HeLa cells with proviral DNA constructs has been described elsewhere (34). Viral supernatants were harvested 48 h after transfection, centrifuged at low speed, filtered, and used immediately for virus purification. For MT4.R5 and PBMC infections, the cells were exposed to the viruses produced in 293T cells (100 or 200 ng of p24 virus equivalent/3 × 106 cells/ml, respectively) for 2 h at 37°C, washed, and seeded at a concentration of 0.3 × 106 cells/ml in RPMI 1640 medium supplemented either with 10% fetal calf serum and antibiotics (MT4.R5 cells) or with interleukin-2, 20% fetal calf serum, and antibiotics (PBMC). Cells producing the viruses encoded by the ME-L1 construct, NLΔenv or NL F522Y were obtained after infection by vesicular stomatitis virus G protein (VSV-G)-pseudotyped viruses to compensate for the loss of infectivity. Pseudotyped viruses were generated by cotransfecting 293T cells with the proviral DNA constructs and a VSV-G expression plasmid (41). After infection, the MT4.R5 cells and PBMC were incubated for 3 days at 37°C and then washed, resuspended in an equal volume of medium, and incubated at 37°C for a further 3 days. Viral supernatants were harvested, centrifuged at low speed, filtered, and used immediately for virus purification. For MDM infection, the cells were also exposed to the virus produced in 293T cells (500 ng of p24 virus equivalent/1 ml/8 × 105 cells per well in 12-well plates) for 2 h at 37°C and washed extensively. Then, 2 ml of RPMI 1640 medium supplemented with 10% human AB serum and antibiotics was added per well, and the cells were incubated again at 37°C. Viral supernatants were harvested every 3 days over a 2-week period, centrifuged at low speed, filtered, and immediately used for virus purification. All virus stocks were sampled for quantification of the p24 capsid protein before virus purification.
Lysates of 293T cells were prepared at the time of virus collection for evaluation of the expression and processing of the viral envelope. Pelleted cells were washed with phosphate-buffered saline (PBS), repelleted by centrifugation, and resuspended in a 1% Triton in PBS supplemented with protease inhibitors. A sample was removed for p24 analysis, and cell lysates were then frozen at −80°C for subsequent Western blot analysis, as described below.
Viral replication kinetics.
The concentration of the viral stocks produced as described above from 293T cells was normalized on the basis of p24 capsid protein concentration. MT4.R5 cells were infected with 5 ng of p24 virus equivalent/106 cells/ml. Samples of culture supernatants were taken every 2 days for p24 capsid protein determination. After removal of the sample, cell suspensions were split 1:4 and returned to the incubator at 37°C. Phytohemagglutinin-stimulated PBMC were infected with 20 ng of p24 virus equivalent/106 cells/ml. The culture medium was sampled every 2 days for p24 capsid protein determination and was then completely replaced with fresh medium. MDMs were infected with 50 ng of p24 virus equivalent/8 × 105 cells/ml. The culture medium was sampled every 2 days for p24 capsid protein determination and was then completely replaced with fresh medium.
Determination of viral infectivity.
The infectivity of the virus stocks generated for Env incorporation assays was determined on TZM-bl cells in 96-well plates. TZM-bl cells express high levels of CD4 and CCR5 and contain β-galactosidase and luciferase reporter genes under the control of an HIV long terminal repeat (51). Eight 10-fold serial dilutions of each virus stock were prepared in complete medium (DMEM supplemented with 10% fetal calf serum, gentamicin [50 μg/ml], and 25 mM HEPES) containing 15 μg of DEAE-dextran/ml directly in the plates. TZM-bl cells (2 × 104/well) were then added. After 48 h, target cell infection was determined by staining with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Briefly, cells were washed twice with PBS and fixed with 80% acetone for 10 min at −20°C. A 50-μl portion of an X-Gal solution (4 mM potassium ferricyanide, 4 mM potassium ferrocyanide, 0.4 mg of X-Gal, and 2 mM MgCl2 in PBS) was added to each well. After 2 h at 37°C, the reaction was stopped by replacing the X-Gal solution with PBS, and the blue cells were counted, using a light microscope, for the last dilution giving more than 10 infected cells. The infectivity was determined as the number of infected cells per nanogram of p24 in the inoculum.
Env incorporation assays.
Viruses produced, as described previously, from 293T cells, HeLa cells, MT4.R5 cells, PBMC, and MDMs were used to assess Env incorporation into virions. Medium containing the viruses was overlaid on a 20% sucrose cushion in a Beckman SW28 tube, and particles were pelleted by centrifugation for 90 min at 50,000 × g and 4°C. Viral pellets were resuspended in a small volume of TNE buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 0.5 mM EDTA) supplemented with 1% Triton X-100 and protease inhibitors. An aliquot was removed for p24 capsid protein determination by ELISA, and resuspended pellets were frozen at −80°C for quantitative gp120 ELISA and Western blot analysis.
The quantitative gp120 ELISA was performed in Immulon-2 plates (Dynex) as previously described (34). Human monoclonal antibody (MAb) 2G12 was used for the detection of gp120 captured on the solid phase. Dilutions of purified gp120IIIB (Advanced Bioscience Laboratories) were used to construct a standard curve. Similar ELISA tests were also carried out with a pool of HIV-1-positive human sera for the detection of gp120.
Env incorporation was also assessed by Western blotting for viruses produced in HeLa cells, essentially as previously described (34). The membrane was initially probed for gp120 and p24, with specific goat polyclonal antibodies (AbD Serotec). After blot development, the antibodies were removed from the blot by incubation with Restore Western blot stripping buffer (Pierce), and the Western blotting procedure was then repeated with the 2F5 human MAb (Polymun Scientific) directed against gp41.
Subcellular distribution of Env and matrix proteins.
HeLa cells were transfected with DNA proviral construct and processed for immunofluorescence as previously described (34) with a mouse MAb specific for the matrix (p17) obtained from the National Institute for Biological Standards and Control (NIBSC) centralized facility for AIDS reagents (ARP342, from R.B. Ferns and R. S. Tedder) and an anti-Env human MAb 2G12 (Polymun Scientific). The transfection conditions used yielded ca. 5% positive cells with moderate Env staining. Images of representative cells were acquired with an Olympus FluoView 500 confocal microscope equipped with Argon (488-nm) and HeNe (546-nm) lasers, a 60× PlanApo oil-immersion objective lens, and Fluoview 4.3 software. The colocalization of Env and matrix proteins was quantified with Imariscoloc (Imaris, Bitplane), as previously described (34). Pearson channel correlation coefficients (R) in a studied volume (“1” indicating perfect colocalization and “0” indicating no correlation) were calculated for 10 cells for each sample and are expressed as means ± the standard deviation.
Analysis of cell-to-cell HIV transfer by flow cytometry.
Donor cells (primary CD4+ T lymphocytes or MT4.R5 cells) were infected with VSV-G-pseudotyped virions carrying various combinations of matrix and gp41 CT sequences. We used 10 and 100 ng of p24 virus equivalent per ml to infect 106 MT4.R5 cells, and 10, 100, or 500 ng of p24 virus equivalent per ml for 106 primary lymphocytes. At 36 h after infection, donor and target cells were mixed (1:1) in 96-well plates at a final concentration of 106 cells/ml, in a final volume of 200 μl. Before coculture, target cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 2.5 μM; Molecular Probes) for 10 min at 37°C to distinguish them from donor cells. After the times indicated, cells were stained for intracellular Gag expression (see below) and analyzed by flow cytometry, as previously described (56). HIV Gag protein was assayed after permeabilization and intracellular staining with anti-Gagp24 phycoerythrin MAb (KC57; Coulter). An isotype-matched MAb was used as negative control. Flow cytometry data were acquired with a FACSCalibur instrument (Becton Dickinson) and CellQuest software and were analyzed with FlowJo software (TreeStar).
RESULTS
The gp41 CT truncation of late-stage viruses from patient 153 markedly impairs virus replication.
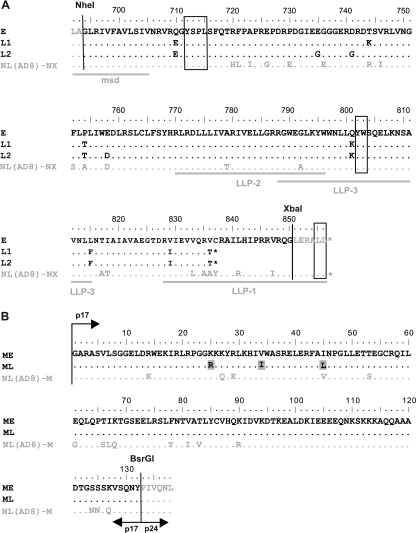
We first investigated the effects on virus replication of the truncation observed in the gp41 CT encoded by late HIV-1 env clones from patient 153. We transferred the gp41 CT coding sequences into an R5 molecular clone pNL(AD8) derivative [pNL(AD8)-NX-WT], as described in Materials and Methods (Fig. 1A). The gp41 CT coding sequences retained for the present study were derived from one early env clone (clone E) and two late env clones (clones L1 and L2) (Fig. 2A). The dominant sequence during the early stage of infection was that found in clone E (6 of 15 clones). The remaining early env clones encoded gp41 CTs differing by only one or two amino acid substitutions in different positions. The gp41 CT encoded by all 15 late env clones had a 20-amino-acid truncation, due to a 23-bp deletion introducing a stop codon. In addition, the sequences of the truncated gp41 CT encoded by the 15 late env clones differed from that encoded by the early clone E by five- to nine-amino-acid substitutions, most of these substitutions being common to several clones. The late gp41 CT coding sequences selected were derived from two representative late env clones (clone L1 and L2).
FIG. 2.
Amino acid sequences of gp41 CTs and matrix proteins from patient 153. (A) Sequence alignments for gp41 CTs are shown for an early viral variant with a full-length sequence (clone E), for two late viral variants harboring a 20-amino-acid truncation (clones L1 and L2), and for the NL(AD8)-NX-WT virus. The Env sequences upstream from the NheI restriction site and downstream from the XbaI restriction site are derived from NL(AD8). The highlighted domains include the C-terminal region of the membrane-spanning domain (msd) and the amphipathic alpha-helical domains LLP-1, LLP-2, and LLP-3. Trafficking motifs (Y712SPL, Y802W803, and L855L856) are boxed. Amino acid identity (.), stop codons (*), and substitutions are indicated. (B) Sequence alignments for matrix proteins are shown for an early viral variant (clone ME) and for a late viral variant (clone ML). The three amino acid substitutions specific to late matrix proteins are highlighted by gray shading. The matrix sequences upstream from the AatII restriction site (localized in the 5′ noncoding region of the viral genome) and downstream from the BsrGI restriction site (localized at the junction between the matrix protein p17 and the capsid protein p24) are derived from NL(AD8). Amino acid identity (.) and substitutions are indicated. Amino acid numbers correspond to the HXB2 sequence.
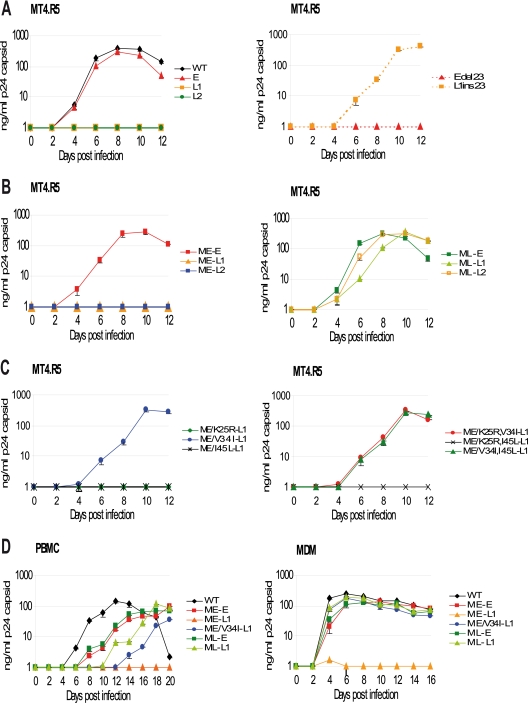
Viral stocks of NL(AD8) carrying early and late gp41 CT sequences were produced in 293T cells by transient transfection. No significant difference in p24 (capsid protein) production was found between clones, demonstrating the absence of unanticipated defects in the RNA sequence or Rev protein function. Western blot analysis on 293T cell lysates showed that the various glycoproteins were synthesized in a manner indistinguishable from that of the wild type (data not shown). We analyzed replication kinetics with the MT4.R5 T-cell line (Fig. 3A). Cells were infected with the viruses produced in 293T cells (5 ng of p24 virus equivalent/106 cells/ml), and p24 protein accumulation in the culture supernatants was quantified to compare replication kinetics between viruses. All of the data shown in Fig. 3A, B, and C were obtained in the same experiment, which simultaneously included all of the pNL(AD8)-NX constructs sequentially engineered during the present study, to facilitate comparative analyses. The replication kinetics of the virus carrying the early gp41 CT (E) closely resembled that of wild-type NL(AD8)-NX (Fig. 3A). In contrast, no virus replication was detected for the two late viruses encoding shortened gp41 CT (L1 and L2). As a negative control, we used the NLΔenv clone (12), which lacks Env and produced no detectable p24 in any of the cell types used here (data not shown). Thus, the NL(AD8)-NX virus harboring a truncated gp41 CT encoded by the late env clones from patient 153 failed to establish productive infection in MT4.R5 cells, whereas the NL(AD8)-NX virus carrying a full-length gp41 CT encoded by early env clones replicated at levels similar to those for the wild-type virus.
FIG. 3.
Replication kinetics of NL(AD8)-NX viruses carrying gp41 CTs and matrix proteins derived from the HIV-1 present at early or late stages in patient 153. Virus stocks, obtained by transfecting 293T cells with the indicated molecular clones, were normalized as a function of p24 capsid protein concentration and used to infect MT4.R5 cells (A, B, and C), PBMC (D, left), or MDMs (D, right), as described in Materials and Methods. Variations in p24 concentrations were monitored in the culture supernatant over time. Each experiment was performed at least twice in duplicate, with similar results obtained in terms of both the hierarchy and the extent of replication. Errors bars indicate the standard deviation for duplicate infections.
We generated the proviral constructs pNL(AD8)-Edel23, containing the 23-bp deletion in the sequence encoding the early clone, and pNL(AD8)-L1ins23, harboring a restored CT sequence in the context of the late clone L1, to exclude the possibility that changes other than gp41 CT truncation were responsible for the dramatic loss of replication capacity. NL(AD8)-L1ins23 and NL(AD8)-E had similar replication kinetics, whereas NL(AD8)-Edel23 and NL(AD8)-L1 displayed markedly impaired replication, demonstrating that the effects on viral replication observed for the NL(AD8)-NX virus carrying gp41 CT encoded by late env clones were due to the gp41 CT truncation (Fig. 3A).
Late-stage matrix protein restores the replication capacity of viruses harboring truncated gp41 CTs.
The plasma viral load measured in patient 153 at the late stage of infection (4.25 log viral RNA copies/ml) demonstrates the replication competence of the primary HIV-1 present in vivo, despite the presence of the gp41 CT truncation (Table 1). We hypothesized that the truncations observed in the gp41 CTs encoded by late env clones from patient 153 might be complemented by compensatory mutations in the matrix. We conducted a comparative analysis of matrix sequences obtained from patient 153 at early and late stages of infection. Six early and ten late gag clones were analyzed. Four of the six early gag clones encoded matrix proteins with identical amino acid sequences. The other two early clones differed by a single amino acid substitution. The 10 late gag clones encoded matrix proteins differing from those encoded by the early clones by three to six amino acid substitutions. Three of these substitutions (K25R, V34I, and I45L) were common to all late matrix sequences. One of the four identical early gag clones (ME) was selected for further analyses, together with one late gag clone (ML) encoding a matrix protein harboring only the three amino acid substitutions specific to late-stage viruses (Fig. 2B).
The selected early (ME) and late (ML) matrix sequences were cloned into the proviral constructs containing early and late env sequences (Fig. 1B). The viruses produced by the transfection of 293-T cells were used to infect MT4.R5 cells (Fig. 3B), as described above. Of the clones encoding the early matrix sequence, only the clone carrying the early env sequence (ME-E) replicated efficiently. Clones with the truncated gp41 CT (ME-L1 and ME-L2) did not produce detectable amounts of p24 in the supernatant. In contrast, expression of the late matrix sequence (ML) allowed the replication of clones carrying either early (ML-E) or late (ML-L1 and ML-L2) env sequences. Thus, the truncation observed in the gp41 CT encoded by the late env clones strongly decreased levels of virus replication in the presence of the early matrix sequence, whereas expression of a contemporary late matrix allele restored the ability of the virus to replicate.
The single V34I change in the matrix restores the replication capacity of viruses encoding truncated gp41.
The results described above demonstrate that the late matrix protein ML compensates for the viral replication defect induced by truncation of the gp41 CT L1 and L2. We evaluated the compensatory potential of the three amino acid substitutions between the early and late matrix proteins, using site-directed mutagenesis to introduce all of the possible combinations of the K25R, V34I, and I45L substitutions into the construct encoding the early matrix sequence coupled to a truncated late Env sequence (ME-L1) (Fig. 1C). Mutant virus phenotypes for viral spread in MT4.R5 cells were characterized as previously described (Fig. 3C). The ME-L1 viruses carrying the V34I substitution, alone or in combination with the K25R or I45L substitutions, replicated with kinetics similar to that of the ML-L1 virus (Fig. 3B and C). In contrast, viruses carrying the K25R and the I45L substitutions, alone or in combination, in matrix proteins with a valine residue in position 34, did not replicate during this experiment (Fig. 3C). Thus, the replication defect resulting from the truncation of the gp41 CT L1 and L2 may be reversed by a single amino acid substitution at position 34 in the late matrix protein.
As cell type may influence the replication capacity of viruses carrying mutated gp41 CT sequences, we characterized the replication kinetics of viruses with relevant combinations of matrix and envelope sequences, using PBMC and macrophages. Viral stocks were produced in 293T cells transiently transfected with the various proviral DNA constructs. An inoculum adapted to each cell type (20 ng of p24 virus equivalent/106 cells/ml for PBMC, 50 ng of p24 virus equivalent/8 × 105 cells/ml for macrophages), based on permissiveness to NL(AD8)-NX viruses, was used. We compared the replication kinetics between viruses by quantifying p24 protein accumulation in the culture supernatants. In PBMC, the reference clone NL(AD8)-NX replicated most rapidly (Fig. 3D). The two viruses carrying an early envelope sequence and different matrix alleles (ME-E and ML-E) replicated to similar extents. For viruses carrying a truncated CT, clear differences were observed as a function of matrix sequences: the clone carrying the early matrix sequence failed to replicate (ME-L1), the late matrix sequence allowed efficient virus replication (ML-L1), and the V34I mutation alone partially compensated for the loss of infectivity (ME/V34I-L1).
In macrophages, all of the NL(AD8)-NX viruses replicated with similar kinetics, with the exception of the ME-L1 virus, which displayed strong impairment of replication capacity, with p24 production peaking at 1.65 ng/ml 4 days after infection and then falling below 1 ng/ml for the rest of the experiment (Fig. 3D). Thus, the replication kinetics in relevant HIV target cells confirmed the impairment of virus replication due to gp41 truncation and its restoration by the late matrix protein. Residue 34 of the matrix protein was also confirmed to be a key element in compatibility with truncated gp41 CT.
The rescue of virus infectivity by matrix mutations is cell type independent.
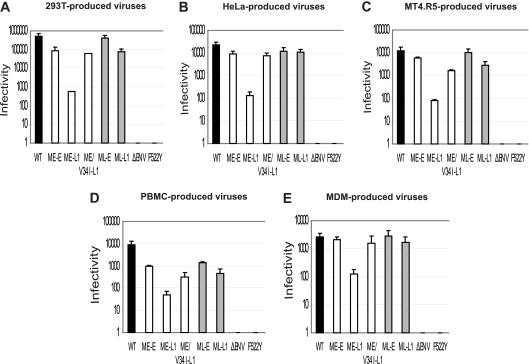
Several studies have reported differences in the intracellular transport and assembly process between cell types (22, 23, 30, 53). There may therefore be different requirements for compatibility between the viral matrix and envelope sequences. We thus analyzed the infectivity of virions produced in different cell types and carrying relevant combinations of matrix coding sequences and env genes from the early and late viruses found in patient 153. We used a single-round reporter assay based on CD4+ CCR5+ β-galactosidase indicator (TZM-bl) cells. This single-cycle assay tests for completion of the steps in the virus life cycle up to and including Tat expression and is therefore a useful tool for probing blocks of the early viral life cycle. The NL F522Y virus, which carries a nonfusogenic Env, and the NLΔenv virus were used as negative controls (6, 46). Viruses were produced by transiently transfecting 293T cells and HeLa cells with the various proviral DNA constructs or by infecting MT4.R5 cells, PBMC, or macrophages with the corresponding viruses, as described in Materials and Methods. To ensure efficient infection with defective ME-L1, NLΔenv, and NL F522Y viruses, we used VSV-G pseudotyping. Endpoint dilutions of each viral supernatant were used to infect TMZ-bl cells. After 48 h, we assessed target cell infection levels by staining with X-Gal. The infectivity was quantified as the number of infected cells per ng of p24 in the inoculum.
The infectious titer per ng of p24 was highest for viruses produced in 293-T cells, followed by viruses produced in HeLa and MT4.R5 cells (Fig. 4). Viruses carrying patient-derived sequences produced in PBMC and macrophages had lower levels of infectivity. The hierarchy of viral infectivities was conserved between cell types, suggesting similar requirements for matrix and envelope compatibility. In all cell types, virions carrying a truncated gp41 CT and the early matrix sequence (ME-L1) were markedly less infectious (1 to 2 logs less infectious) than viruses carrying full-length Env (ME-E and ML-E) and viruses in which the truncated CT was complemented by the late matrix (ML-L1) or an early matrix sequence carrying the V34I substitution (ME/V34I-L1). The replication defect observed for ME-L1 virus may therefore be attributed to a defect in the early steps of the virus replication cycle. The measurable single-cycle infectivity for the ME-L1 virus, however, suggests that the lack of detectable virus replication in multiple-cycle experiments is due to a cumulative effect of each infection cycle rather than to a complete loss of infectivity.
FIG. 4.
Singe-round infectivity of NL(AD8)-NX viruses carrying gp41 CTs and matrix proteins derived from HIV-1 present at the early or late stages in patient 153. Virus stocks of the indicated molecular clones, obtained by transfecting 293T (A) and HeLa cells (B) or by infecting MT4.R5 cells (C), PBMC (D), or MDMs (E), were used to infect TZM-bl cells, as described in Materials and Methods. Eight 10-fold serial dilutions of each virus stock were tested in 96-well plates. After 48 h, infection of the target cells was assessed by staining with X-Gal. The infectivity of each virus is expressed as the number of infected cells per ng of p24. The data are expressed as means ± the standard deviations.
The gp41 CT truncation decreases Env incorporation into virus particles, and this effect is reversed by matrix mutations.
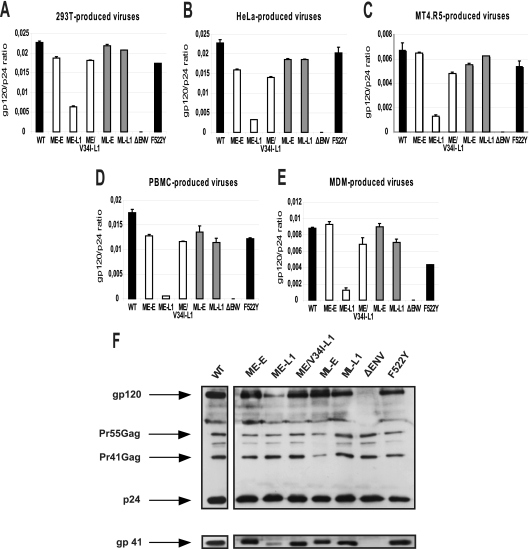
Studies with T-cell laboratory-adapted virus mutants have shown that the decrease in infectivity associated with certain substitutions, deletions, or truncations in the gp41 CT is accompanied by defective Env incorporation into virions (28, 32, 43, 45, 50). Our results for the single-cycle assay suggest that a lower efficiency of Env incorporation may be responsible for the impaired replication of the truncated CT variant, when not compensated for by the appropriate matrix mutations. We thus evaluated the particle protein composition of purified viruses encoding different combinations of matrix and envelope sequences. Virions were produced in 293T cells, HeLa cells, MT4.R5 cells, PBMC, and macrophages, as described above. Virions were pelleted from viral supernatants by centrifugation through a sucrose cushion. Virion-associated gp120 levels were quantified by a sensitive Env ELISA as described in Materials and Methods. Various antibodies (human MAb 2G12 and a pool of human sera from HIV-1-infected patients) for detecting the gp120 captured on the solid phase were tested. The data obtained with the human MAb 2G12 are reported here, since the background was lowest for this antibody. However, similar results were obtained with the pool of human sera, showing that the differences in gp120 incorporation between the various viruses did not depend on variations in the antigenic properties of Env. The highest Env incorporation levels, measured as the gp120/p24 ratio, were obtained for viruses produced by transfected 293-T cells (Fig. 5). Slightly lower levels of Env incorporation were observed for viruses produced by HeLa cells and PBMC. The gp120/p24 ratio was markedly lower for viruses produced in MT4.R5 cells and macrophages. Despite cell type-related differences, a comparison of virus clones clearly showed Env incorporation to be reduced only when the truncated CT gp41 was expressed together with the early matrix allele. This confirms that the replication defect of the ME-L1 virus is largely due to the impairment of Env incorporation. The matrix protein from the late virus population (ML-L1) and the single V34I mutation in the early matrix sequence (ME/V34I-L1) rescued the incorporation defect.
FIG. 5.
Env incorporation of NL(AD8)-NX viruses carrying gp41 CTs and matrix proteins derived from HIV-1 present at the early or late stages in patient 153. Virus stocks of the indicated molecular clones were obtained by transfecting 293T (A) and HeLa cells (B) or by infecting MT4.R5 cells (C), PBMC (D), or MDMs (E), as described in Materials and Methods. Env incorporation into virions was assessed, using viruses purified by centrifugation through a 20% sucrose cushion. Viral pellets were lysed in TNE buffer containing 1% Triton X-100 and gp120 was quantified by ELISA. The results shown were obtained from a representative experiment performed in duplicate. Errors bars indicate standard deviations. (F) Western blot analysis of Env incorporation into virions produced by HeLa cells. The upper panel shows the blot probed with anti-gp120 and anti-p24 antibodies. The positions of the Env glycoprotein gp120, the Gag precursor Pr55, the Gag processing intermediate Pr41 and the processed p24 capsid protein are indicated. The blot was stripped and reprobed with an anti-gp41 antibody to detect the Env glycoprotein gp41 (lower panel; see Materials and Methods).
For HeLa cells, the amounts of gp120, gp41, and p24 found in the various virus lysates were also assessed by Western blotting (Fig. 5F). The results were consistent with those obtained by ELISA, demonstrating that the observed difference in gp120 incorporation in ELISA reflected Env incorporation rather than excess shedding.
These results demonstrate that the defect in infectivity and replication kinetics of the ME-L1 virus resulted largely from the impaired incorporation of Env into virions. In addition, mutations selected in the late virus population in patient 153, including the V34I substitution in particular, compensated for the loss of Env incorporation in viruses harboring truncated gp41 CT in the various cell types.
The abnormal subcellular distribution of CT-truncated Env is reversed by the coexpression of matrix proteins carrying compensatory mutations.
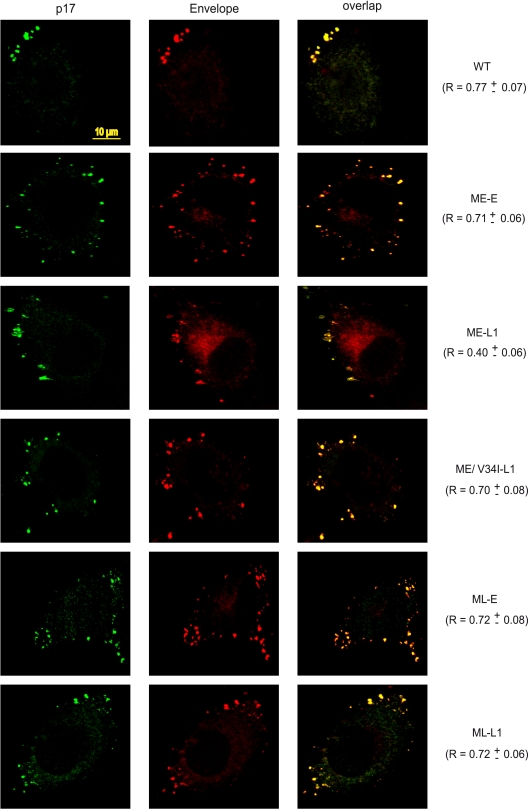
HeLa cells are a convenient model for analyzing the subcellular distribution of viral proteins. Confocal microscopy has shown that most of the assembling HIV-1 particles are present at the plasma membrane in these cells, giving a punctate pattern coinciding with Env staining (24, 34). We therefore investigated possible differences in the intracellular distribution of Env carrying full-length or truncated gp41 CTs produced together with the early, late, or ME/V34I matrix proteins by transiently transfecting HeLa cells. The steady-state intracellular distribution of Env was compared to that of the matrix protein, by labeling with an anti-p17 antibody that specifically recognizes the mature form of the protein. Cells were analyzed 40 h after transfection and treated with cycloheximide to eliminate newly synthesized Env from the early secretory pathway. The ImarisColoc module was used to assess the colocalization of Env and matrix protein, as described in Materials and Methods. Pearson channel correlation coefficient (R) for the studied volume (“1” indicating perfect colocalization, “0” indicating no correlation) was calculated for 10 cells from each sample and is expressed as the mean ± the standard deviation.
The NL(AD8)-NX-WT virus displayed punctate staining at the cell surface, with the highest degree of colocalization between Env and the p17 matrix protein (R = 0.77 ± 0.07) (Fig. 6). Similar levels of Env/p17 colocalization at the cell surface were observed for all other viruses, with the notable exception of the ME-L1 virus. For this virus, the colocalization of staining for Env and p17 was much weaker (R = 0.40 ± 0.06), whereas the intensity of diffuse cytosolic Env staining was markedly higher. Thus, the distribution of Env at the cell surface during viral assembly was strongly affected by truncation of the gp41 CT, which is consistent with the defect in Env incorporation observed for the ME-L1 virus. Expression of the late matrix protein gene normalized the distribution of the CT-truncated Env. The V34I substitution in the matrix protein was sufficient to restore a high degree of colocalization between Env and the p17 matrix protein at the cell surface for the virus harboring the truncated gp41 CT. These observations are consistent with the compensatory effect of matrix mutations on viral infectivity, replication kinetics, and Env incorporation.
FIG. 6.
Analysis of Gag and Env intracellular distributions by confocal microscopy. HeLa cells transfected with the indicated molecular clones were fixed and processed for immunofluorescence analysis with 2G12 anti-Env MAb and a mouse MAb specific for the matrix p17 protein. Before fixation, all of the cells were treated with 50 μg of cycloheximide/ml for 3 h. Protein distribution was assessed by confocal microscopy and the Pearson channel correlation coefficient (R) (“1” indicating perfect colocalization, “0” indicating no correlation) was calculated for each sample, as described in Materials and Methods. Scale bar, 10 μm.
gp41 CT truncation preceded the emergence of the V34I substitution in the matrix protein.
Our data show that mutations in the matrix sequence found in the late sample from patient 153 rescued an intrinsic replication defect associated with gp41 CT truncation. We investigated the order of appearance of these mutations in vivo by analyzing the matrix and gp41 CT sequences from five additional blood samples collected from patient 153 between the early and late stages of infection (Table 1). For each time point, 10 matrix and 10 gp41 CT sequence clones were analyzed (Table 1). The sequences encoding the truncated gp41 CT and the V34I substitution in the matrix protein were simultaneously detected in the proviral DNA isolated from PBMC collected 20 months after primary infection. At this time point, all 10 env sequences analyzed encoded the truncated gp41 CT, whereas only 3 of 10 gag sequence clones encoded a matrix protein harboring the V34I substitution, suggesting that gp41 CT truncation preceded the emergence of matrix mutations. The proportion of gag clones encoding a matrix protein with the V34I substitution gradually increased to 10 of 10 by the late stage. Two other substitutions (K25R and I45L) specific to the late matrix protein appeared after the V34I substitution, and their proportions increased over time. These results strongly suggest that gp41 CT truncation preceded the emergence of matrix changes, with V34I being the earliest and most prevalent mutation. These data are consistent with our data on the major role played by the V34I substitution in overcoming the deleterious effects of gp41 CT truncation. Note that the gp41 CT truncation and the three substitutions specific to the late matrix protein were still present in the 10 matrix and 10 gp41 CT sequence clones analyzed 29 months after the late stage (Table 1).
gp41 CT-truncated HIV-1 may spread by direct cell-to-cell transfer in the absence of matrix compensation.
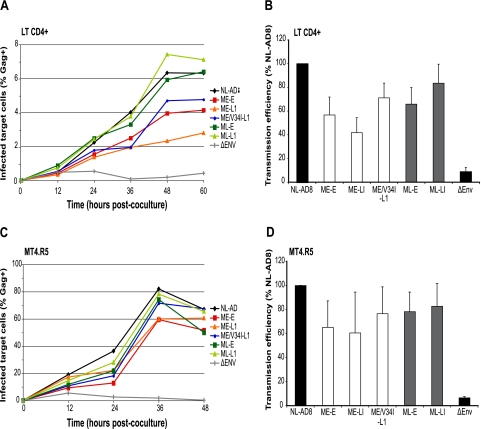
The analysis of sequential samples from patient 153 suggested that the gp41 truncation preceded the emergence of matrix mutations. We analyzed cell-to-cell spread as a possible mechanism supporting the replication of gp41-truncated viruses and facilitating the emergence of compensatory mutations. Cell-to-cell transfer is a more potent and rapid mechanism of viral propagation than infection by cell-free virus (55). The requirements for these two infection routes may differ. We used a previously described flow cytometry-based cell-to-cell virus transfer assay (56). Primary CD4+ T lymphocytes were infected with VSV-G-pseudotyped HIV-1 particles (100 ng of p24 virus equivalent/106 cells/ml), carrying different combinations of matrix and Env. At 36 h after infection, the cells were cocultured with autologous CFSE-labeled target T lymphocytes. CFSE labeling allows targets to be distinguished from donors and, thus, analysis of the emergence of newly infected cells in culture. The percentage of Gag+ cells among CFSE-labeled lymphocytes was determined at various times up to 60 h postcoculture to measure early productive virus-transfer events (Fig. 7A). Under the experimental conditions used here, infection with cell-free virus has a negligible impact on the emergence of Gag+ target cells (56). The percentage of Gag+ target cells in culture increased with time in experiments in which donor cells were infected by the reference clone NL(AD8)-NX-WT or by viruses carrying combinations of matrix and Env from patient 153, including that encoding a truncated gp41 not compensated by matrix mutations (ME-L1) (Fig. 7A). Thus, the ME-L1 virus can be transferred from infected cells to target cells in culture, although slightly less efficiently. A functional Env is required for cell-to-cell transfer of virus (56). Accordingly, when donor cells were infected by a VSV-G-pseudotyped virus defective for HIV-1 Env (NLΔenv) and cocultured with target cells, only a very small percentage of target cells became Gag+ (Fig. 7A). For this control virus, the proportion of Gag+ cells decreased over time, suggesting that the signal represents the endocytosis and degradation of viral particles by primary T lymphocytes. The transmission efficiency was calculated by comparing the area under the curve for each virus to that of the reference NL(AD8)-NX-WT clone in three independent cell-to-cell transfer experiments using different multiplicities of infection (Fig. 7B). Viruses encoding early or late Env displayed similar transfer efficiencies, suggesting that the size of the CT does not affect HIV-1 transfer. However, a trend toward slightly more efficient transfer was observed for viruses carrying the late matrix sequence, but this difference was not statistically significant.
FIG. 7.
Cell-to-cell HIV transfer. Cell-to-cell viral transfer was measured by a flow cytometry-based assay. Primary CD4+ T lymphocytes (A and B) or MT4R5 cells (C and D) were infected with VSV-G-pseudotyped HIV-1 particles carrying different combinations of matrix and Env. The cells were then cocultured with CFSE-labeled target cells, such that targets could be distinguished from donors. The appearance of Gag+ target cells was followed over time (A and C). The efficiency of transmission (B and D) was measured by calculating the area under the curve in three independent experiments, with values for the NL(AD8) clone being defined as 100%. Means and standard deviations are shown.
Similar results were obtained with MT4R5 cells (Fig. 7C and D). Gag+ cells appeared more rapidly among targets in this cell line than in primary lymphocytes and higher percentages of the target cells were productively infected. Also, in MT4.R5 cells, the ME-L1 virus displayed replication kinetics and a transfer efficiency similar to those for the other viruses. Overall, these findings demonstrate that cells producing the gp41-truncated virus, in the absence of matrix compensation, may productively infect target cells by cell-to-cell virus transfer.
DISCUSSION
The important role of the HIV-1 gp41 CT in regulating Env functions, incorporating Env into virus particles and, consequently, in viral replication has clearly been demonstrated in vitro with T-cell laboratory-adapted virus mutants (16, 19, 28, 43, 44, 50, 63). The strong conservation of CT length in primary isolates of HIV-1 and SIV also suggests that this factor plays a key role in viral replication and persistence in vivo (39, 54, 64). However, we describe here the emergence and dominance in vivo of a primary HIV-1 variant carrying a natural truncation of the gp41 CT. Env sequence analysis on longitudinal samples from patient 153 showed that the dominant virus population 6 years after primary infection harbored a C-terminal 20-amino-acid truncation, whereas the virus population present at the early stage of infection had a full-length Env sequence. This deletion may affect Env trafficking, incorporation, and function. The aims of the present study were thus to assess the functional consequences of the gp41 truncation and to identify the mechanisms allowing this virus to replicate efficiently in an infected patient despite this major Env alteration. Compensatory mutations emerged in the matrix protein, restoring Env incorporation and virus replication. To our knowledge, this is the first description of in vivo rescue of the impairment of viral replication due to a gp41 CT truncation.
The observed deletion in the gp41 encoded by late env clones from patient 153 encompassed both the C-terminal dileucine trafficking motif and much of the LLP-1 domain. We investigated its effects on virus replication by placing the gp41 CT coding sequences of early and late HIV-1 env clones from patient 153 in the context of the molecular clone pNL(AD8). NL(AD8)-NX viruses carrying the gp41 CT encoded by late env clones failed to establish productive infection in MT4.R5 cells. Analyses of purified virions demonstrated that the CT deletion strongly affected the efficiency of Env incorporation into virus particles and provided an explanation for the impairment of replication. These findings are consistent with previous studies showing that decreases in infectivity due to certain substitutions, deletions, or truncations in the gp41 CT, including those specifically affecting the LLP-1 domain, resulted from a defect in Env incorporation into virions (28, 32, 43, 50).
However, despite this deleterious truncation of the gp41 CT, the virus remained detectable in the plasma of patient 153 throughout the 9 years of follow-up, suggesting that functional complementation of gp41 CT truncation, probably involving the matrix protein, might have helped to maintain viral replication capacity. This hypothesis is based principally on the observation of compensation between the CT and matrix protein on viral infectivity of T-cell laboratory-adapted virus mutants (19, 40, 43). A comparative analysis of matrix sequences obtained from patient 153 at early and late stages of infection confirmed the occurrence of three substitutions (K25R, V34I, and I45L) in all late matrix sequences. Consistent with our hypothesis, the expression of a late matrix allele rescued replication of the CT-truncated virus. The restoration of virus replication by matrix mutations demonstrates that the CT-truncated Env is competent for all Env functions other than incorporation into virus particles. Furthermore, viruses carrying the late matrix protein and the early full-length gp41 CT were clearly replication competent. Thus, the substitutions observed in the late matrix protein are functionally compatible with a full-length Env. These data demonstrate the suitability of our model, derived from the NL(AD8) molecular clone, for use in the studies of the effects of compensation between the CT and matrix protein described here. However, it may be necessary to take into account more extensively the primary genetic context in which CT truncation arose, in analyses of other CT-truncated Env properties, such as their antigenicity.
We investigated the respective roles of the three amino acid substitutions differentiating early from late matrix proteins, by carrying out further studies with proviral constructs encoding early matrix sequences and all possible combinations of the K25R, V34I, and I45L substitutions coupled to the truncated late env sequence. Analyses of replication kinetics in MT4.R5 cells showed that the replication defect resulting from truncation of the gp41 CT was reversed by the single Val/Ile substitution at position 34 in the matrix protein. The V34I substitution in the matrix protein has been shown to restore the replication defect resulting from a small deletion in the LLP2/LLP3 domains of a T-cell laboratory-adapted virus mutant (43). However, we provide here the first demonstration of such mechanisms occurring in viruses circulating in a patient. Furthermore, these results represent the first description of a compensatory effect of amino acid substitutions in the matrix protein on the impairment of replication resulting from a truncation of the gp41 CT removing much of the LLP-1 domain. Restoration of the replication capacities of viruses harboring truncated gp41 CTs by the late matrix protein or V34I substitution was confirmed in PBMC and macrophages, demonstrating the key role of matrix residue 34 in compatibility with truncated CT gp41 in relevant HIV-1 target cells. The late matrix sequence and the V34I mutation alone restored the replication of viruses harboring the gp41 truncation in macrophages, whereas, in PBMC, the V34I substitution alone compensated only partially for the loss of infectivity. This difference suggests that the two additional substitutions (K25R and I45L) may help to optimize viral replication fitness in this cellular context.
The efficiency of Env incorporation into virus particles (expressed as the ratio of gp120 to p24) differed between cell types, but markedly low levels of CT-truncated Env incorporation and the rescue of Env incorporation by matrix mutations were observed in all cell types tested. Thus, the requirement for matrix and envelope compatibility with respect to the 20-amino acid CT truncation was similar in different cellular contexts, consistent with the infectivity data obtained for the single-round reporter assay. The single V34I substitution in the matrix protein, in particular, played a major role in compensating for Env truncation. The defective incorporation of CT-truncated Env and its rescue by matrix mutation were corroborated by confocal microscopy analyses conducted on HeLa cells. Only a partial redistribution of Env at the cell surface was observed for the virus harboring truncated gp41 CT and the early matrix sequence, whereas the V34I substitution in the matrix protein appeared to be sufficient to restore typical punctate pattern of p17 matrix protein staining coinciding with Env staining (24, 34). The exact nature of the mechanism of compensation between gp41 CT and matrix protein remains unclear. Matrix protein oligomerization creates holes into which the cytoplasmic tail of the gp41 envelope protein is thought to be inserted (2, 25, 43). The location of the V34I residue seems compatible with direct or indirect interaction with the LLP-1 domain (25). However, several aspects of this model remain undetermined, including the relevance of matrix protein-driven oligomerization in the context of the uncleaved Gag precursor and whether this process occurs in cells producing virus. Structural data for the intact CT of gp41 are also currently lacking.
Sequential analyses of samples collected from patient 153 between the early and late stages of infection indicated that gp41 CT truncation occurred before the emergence of matrix changes. The rapid selection of variants harboring a truncation in the gp41 CT contrasted with the slower emergence of variants carrying compensatory matrix substitutions over a period of several years, with V34I being the first and most prevalent matrix substitution (Table 1). The progressive emergence of the compensatory V34I matrix mutation was accompanied by an increase in viral load, which reached 4.25 log RNA copies/ml after 6 years (corresponding to the late stage) and 4.9 log 6 months later, when antiretroviral treatment was initiated. The changes in viral load observed in patient 153 suggest that strong selective pressure restrained virus replication 1 year after primary infection, leading to the selection of a variant harboring the truncated Env sequence. The progressive emergence of compensatory matrix mutations subsequently increased virus replication in vivo, conferring full pathogenic potential on the virus.
The radical switch observed in the env quasispecies is reminiscent of the purifying selection exerted by CTL responses on HIV-1 (36, 42). Two T-cell epitopes compatible with the HLA haplotype of patient 153 (A2 and B7) were identified (http://www.hiv.lanl.gov/content/immunology/maps/ctl/gp160.html) in the 20-amino-acid sequence deleted by CT truncation. A strong T-cell response specific for one of these epitopes inducing the selection of variants with a truncated gp41 CT is therefore a plausible hypothesis. Moreover, such T-cell responses against the B7 epitope were previously found in more than 20% of patients expressing this allele during primary infection (3). Unfortunately, we were unable to explore this possibility, because no cell samples from patient 153 were available. We cannot rule out the alternative possibility that CT truncation may be involved in antigenic variation of the ectodomain of Env and viral escape from neutralizing antibodies. Indeed, a potentially critical effect of even minor variations of the sequence of the CT on the antigenicity of the ectodomain of Env has recently been reported (31). To our knowledge, no treatment likely to affect HIV-1 replication was given to the patient before March 2000.
Our data also provide a potential mechanism for CT-truncated virus replication, allowing the emergence of compensatory matrix mutations. We have shown that, in the absence of compensatory matrix mutations, the HIV-1 variant harboring a truncated Env CT can spread by cell-to-cell transfer, a potent means of virus replication (55). This finding also suggests that the molecular requirements for direct virus transfer are less stringent than those underlying cell-free virus infection. Mutations affecting Gag or Env that strongly reduce Env incorporation into virus particles result in the number of Env spikes on the virus surface being insufficient for fusion pore formation and enlargement. In the context of cell-cell contacts, provided that the viral Env is competent for receptor binding and membrane fusion, several factors may facilitate the translocation of virus material. Even in the absence of syncytia, the large areas involved in cell-cell contact and the density of viral proteins and cellular receptors at these contact sites are likely to favor virus transfer. Furthermore, viral protein trafficking may be differently regulated in the presence of cellular contacts. Sustained virus replication creates suitable conditions for the emergence of compensatory mutations, which, in the case reported here, restored the infectivity of cell-free virions.
In conclusion, these data show that HIV-1 can undergo gp41 CT truncation removing a large part of the LLP-1 domain in vivo. In the patient studied here, the virus overcame this major structural change through a compensatory mechanism involving a single amino acid change in the matrix. Our data also reveal that it is possible for a virus with highly impaired Env incorporation to replicate through cell-to-cell transmission until the recovery of its full replicative and pathogenic capacity. These findings provide new insight into HIV-1 assembly and evolution potential in vivo.
Acknowledgments
We thank Eric O. Freed (National Cancer Institute at Frederick, Frederick, MD) for providing the pNL(AD8) proviral construct and Ali Amara (Unité de Pathogénie Virale, Institut Pasteur, Paris, France) for the MT4.R5 cell line. The TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; TZM-bl was obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc. We thank Marie Lambelé, Alain Moreau, Pierre-Yves Sizaret, and Sylvie Brunet for technical assistance. We are grateful to Arnaud Moris, Béatrice Labrosse, and Philippe Roingeard for helpful discussions on this work. Our confocal data were generated with the help of the RIO/IBiSA Microscopy Facility of François Rabelais University.
This work was supported by grants from SIDACTION (Paris, France) and from the French National Agency for Research on AIDS and Viral Hepatitis (ANRS). E.B. was supported by fellowships from the French Ministry of Research and SIDACTION. D.V. was supported by a fellowship from the ANRS.
Footnotes
Published ahead of print on 22 July 2009.
REFERENCES
- 1.Akari, H., T. Fukumori, and A. Adachi. 2000. Cell-dependent requirement of human immunodeficiency virus type 1 gp41 cytoplasmic tail for Env incorporation into virions. J. Virol. 74:4891-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfadhli, A., R. L. Barklis, and E. Barklis. 2009. HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology 387:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altfeld, M., E. T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M. N. Johnston, N. Burgett, M. E. Swartz, A. Yang, G. Alter, X. G. Yu, A. Meier, J. K. Rockstroh, T. M. Allen, H. Jessen, E. S. Rosenberg, M. Carrington, and B. D. Walker. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med. 3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, A., A. Vidy, G. Boulla, K. Mollier, J. Garcia-Perez, J. Alcami, C. Blanpain, M. Parmentier, J. L. Virelizier, P. Charneau, and F. Arenzana-Seisdedos. 2003. G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates. J. Virol. 77:2550-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ataman-Onal, Y., C. Coiffier, A. Giraud, A. Babic-Erceg, F. Biron, and B. Verrier. 1999. Comparison of complete env gene sequences from individuals with symptomatic primary HIV type 1 infection. AIDS Res. Hum. Retrovir. 15:1035-1039. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron, L., N. Sullivan, and J. Sodroski. 1992. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J. Virol. 66:2389-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot, G., K. Janvier, S. Le Panse, R. Benarous, and C. Berlioz-Torrent. 2003. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J. Virol. 77:6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 10.Byland, R., P. J. Vance, J. A. Hoxie, and M. Marsh. 2007. A conserved dileucine motif mediates clathrin and AP-2-dependent endocytosis of the HIV-1 envelope protein. Mol. Biol. Cell 18:414-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, S. S., S. F. Lee, and C. T. Wang. 2001. Cellular membrane-binding ability of the C-terminal cytoplasmic domain of human immunodeficiency virus type 1 envelope transmembrane protein gp41. J. Virol. 75:9925-9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavel, F., M. D. Hoggan, R. L. Willey, K. Strebel, M. A. Martin, and R. Repaske. 1989. Genetic recombination of human immunodeficiency virus. J. Virol. 63:1455-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 14.Dacheux, L., A. Moreau, Y. Ataman-Onal, F. Biron, B. Verrier, and F. Barin. 2004. Evolutionary dynamics of the glycan shield of the human immunodeficiency virus envelope during natural infection and implications for exposure of the 2G12 epitope. J. Virol. 78:12625-12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major coreceptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 16.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg, D., and M. Wesson. 1990. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein 41. Biopolymers 29:171-177. [DOI] [PubMed] [Google Scholar]
- 18.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 19.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabuzda, D. H., A. Lever, E. Terwilliger, and J. Sodroski. 1992. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 66:3306-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gousset, K., S. D. Ablan, L. V. Coren, A. Ono, F. Soheilian, K. Nagashima, D. E. Ott, and E. O. Freed. 2008. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 4:e1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grigorov, B., F. Arcanger, P. Roingeard, J. L. Darlix, and D. Muriaux. 2006. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J. Mol. Biol. 359:848-862. [DOI] [PubMed] [Google Scholar]
- 24.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill, C. P., D. Worthylake, D. P. Bancroft, A. M. Christensen, and W. I. Sundquist. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc. Natl. Acad. Sci. USA 93:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hourioux, C., D. Brand, P. Y. Sizaret, F. Lemiale, S. Lebigot, F. Barin, and P. Roingeard. 2000. Identification of the glycoprotein 41(TM) cytoplasmic tail domains of human immunodeficiency virus type 1 that interact with Pr55Gag particles. AIDS Res. Hum. Retrovir. 16:1141-1147. [DOI] [PubMed] [Google Scholar]
- 27.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 28.Jiang, J., and C. Aiken. 2007. Maturation-dependent human immunodeficiency virus type 1 particle fusion requires a carboxyl-terminal region of the gp41 cytoplasmic tail. J. Virol. 81:9999-10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and coreceptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 30.Jouve, M., N. Sol-Foulon, S. Watson, O. Schwartz, and P. Benaroch. 2007. HIV-1 buds and accumulates in “nonacidic” endosomes of macrophages. Cell Host Microbe 2:85-95. [DOI] [PubMed] [Google Scholar]
- 31.Kalia, V., S. Sarkar, P. Gupta, and R. C. Montelaro. 2005. Antibody neutralization escape mediated by point mutations in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41. J. Virol. 79:2097-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalia, V., S. Sarkar, P. Gupta, and R. C. Montelaro. 2003. Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J. Virol. 77:3634-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliger, Y., and Y. Shai. 1997. A leucine zipper-like sequence from the cytoplasmic tail of the HIV-1 envelope glycoprotein binds and perturbs lipid bilayers. Biochemistry 36:5157-5169. [DOI] [PubMed] [Google Scholar]
- 34.Lambele, M., B. Labrosse, E. Roch, A. Moreau, B. Verrier, F. Barin, P. Roingeard, F. Mammano, and D. Brand. 2007. Impact of natural polymorphism within the gp41 cytoplasmic tail of human immunodeficiency virus type 1 on the intracellular distribution of envelope glycoproteins and viral assembly. J. Virol. 81:125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, S. J., W. Hu, A. G. Fisher, D. J. Looney, V. F. Kao, H. Mitsuya, L. Ratner, and F. Wong-Staal. 1989. Role of the carboxy-terminal portion of the HIV-1 transmembrane protein in viral transmission and cytopathogenicity. AIDS Res. Hum. Retrovir. 5:441-449. [DOI] [PubMed] [Google Scholar]
- 36.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282-289. [DOI] [PubMed] [Google Scholar]
- 37.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Verges, S., G. Camus, G. Blot, R. Beauvoir, R. Benarous, and C. Berlioz-Torrent. 2006. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. USA 103:14947-14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luciw, P. A., K. E. Shaw, B. L. Shacklett, and M. L. Marthas. 1998. Importance of the intracytoplasmic domain of the simian immunodeficiency virus (SIV) envelope glycoprotein for pathogenesis. Virology 252:9-16. [DOI] [PubMed] [Google Scholar]
- 40.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Gottlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Picado, J., J. G. Prado, E. E. Fry, K. Pfafferott, A. Leslie, S. Chetty, C. Thobakgale, I. Honeyborne, H. Crawford, P. Matthews, T. Pillay, C. Rousseau, J. I. Mullins, C. Brander, B. D. Walker, D. I. Stuart, P. Kiepiela, and P. Goulder. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman, J. T., T. J. Sturgeon, P. Gupta, and R. C. Montelaro. 2007. Differential functional phenotypes of two primary HIV-1 strains resulting from homologous point mutations in the LLP domains of the envelope gp41 intracytoplasmic domain. Virology 367:102-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 48.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Bercoff, D., A. David, H. Sudry, F. Barre-Sinoussi, and G. Pancino. 2003. Fcgamma receptor-mediated suppression of human immunodeficiency virus type 1 replication in primary human macrophages. J. Virol. 77:4081-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piller, S. C., J. W. Dubay, C. A. Derdeyn, and E. Hunter. 2000. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J. Virol. 74:11717-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabakaran, P., A. S. Dimitrov, T. R. Fouts, and D. S. Dimitrov. 2007. Structure and function of the HIV envelope glycoprotein as entry mediator, vaccine immunogen, and target for inhibitors. Adv. Pharmacol. 55:33-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudner, L., S. Nydegger, L. V. Coren, K. Nagashima, M. Thali, and D. E. Ott. 2005. Dynamic fluorescent imaging of human immunodeficiency virus type 1 gag in live cells by biarsenical labeling. J. Virol. 79:4055-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha, K., H. Yan, J. A. Nelson, and B. Zerhouni-Layachi. 2005. Infection of human and non-human cells by a highly fusogenic primary CD4-independent HIV-1 isolate with a truncated envelope cytoplasmic tail. Virology 337:30-44. [DOI] [PubMed] [Google Scholar]
- 55.Sattentau, Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815-826. [DOI] [PubMed] [Google Scholar]
- 56.Sourisseau, M., N. Sol-Foulon, F. Porrot, F. Blanchet, and O. Schwartz. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J. Virol. 81:1000-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 60.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Honing, R. Benarous, and M. Thali. 2001. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adaptor. J. Virol. 75:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyss, S., A. S. Dimitrov, F. Baribaud, T. G. Edwards, R. Blumenthal, and J. A. Hoxie. 2005. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J. Virol. 79:12231-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, X., S. Kurteva, S. Lee, and J. Sodroski. 2005. Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J. Virol. 79:3500-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, X., X. Yuan, M. F. McLane, T. H. Lee, and M. Essex. 1993. Mutations in the cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein impair the incorporation of Env proteins into mature virions. J. Virol. 67:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zerhouni, B., J. A. Nelson, and K. Saha. 2004. Isolation of CD4-independent primary human immunodeficiency virus type 1 isolates that are syncytium inducing and acutely cytopathic for CD8+ lymphocytes. J. Virol. 78:1243-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]