Abstract
To determine the role of amino acid sequences of the hemagglutinin-neuraminidase (HN) cytoplasmic tail in Newcastle disease virus (NDV) replication and pathogenicity, we generated recombinant NDVs with a deletion or point mutation in the N-terminal cytoplasmic tail. The first 2-amino-acid deletion in the cytoplasmic tail did not affect the biological characteristics of NDV. However, a 4-amino-acid deletion and the substitution of alanine for serine at position 6 affected cell fusion, pathogenicity, and colocalization of the HN and M proteins of NDV, indicating that these residues of the HN cytoplasmic tail are critical for its specific incorporation into virions.
Newcastle disease virus (NDV) causes a highly contagious respiratory and neurologic disease in chickens, leading to severe economic losses in the poultry industry worldwide (1). NDV is a member of the family Paramyxoviridae and has a nonsegmented, negative-sense RNA genome consisting of six genes (3′-NP-P-M-F-HN-L-5′) (7). Infection of host cells by NDV is accomplished through the interaction of two surface glycoproteins, the fusion (F) and hemagglutinin-neuraminidase (HN) proteins. The F protein directs the membrane fusion between the viral and cellular membranes, while the HN protein mediates attachment to sialic acid, has neuraminidase activity, and plays a role in fusion promotion (4).
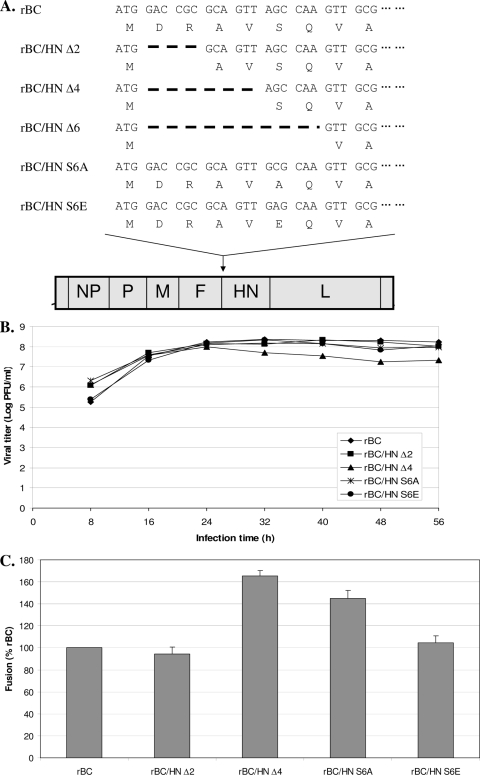
The HN protein of NDV is a type II transmembrane glycoprotein and possesses three spatially distinct domains: the ectodomain, transmembrane domain, and cytoplasmic tail. The globular ectodomain contains the sites for receptor binding and neuraminidase activity, and the transmembrane domain anchors to viral envelopes (8). The cytoplasmic tail domain contains 26 highly conserved amino acids whose functions are not well-known. In a plasmid-based expression system, truncation (23 amino acids) of the cytoplasmic tail caused improper orientation of the HN protein in the membrane insertion (13). In other paramyxoviruses, cytoplasmic tails of the HN proteins are known to play crucial roles in virus budding and assembly (10, 12). Our unsuccessful attempt to recover a recombinant NDV (rNDV) with complete deletion of the HN cytoplasmic tail also suggested that the cytoplasmic tail is required for assembly and budding of NDV. Therefore, in this study, we determined the role of amino acid sequences of the cytoplasmic tail in the NDV replication cycle. Since essential regions of the HN cytoplasmic tail for virus replication are unknown, we consecutively deleted the first 6 nucleotides (nt), 12 nt, or 18 nt of the HN cytoplasmic tail in a full-length antigenomic cDNA of NDV intermediate virulent (mesogenic) strain Beaudette C (BC) (6), thus maintaining the “rule of six” for the NDV genome (Fig. 1A). rNDVs were recovered using our standard protocol (6). We recovered rNDVs containing 2-amino-acid deletion and 4-amino-acid deletion of the HN cytoplasmic tail (rBC/HNΔ2 and rBC/HNΔ4, respectively), indicating that only these 4 amino acids are dispensable in generating infectious virions. Since rNDV containing 6-amino-acid deletion of the HN cytoplasmic tail could not be recovered, we wanted to know the role of amino acids at positions 5 and 6 in NDV replications. The serine residue at position 6 is a potential phosphorylation site. Therefore, to determine whether phosphorylation at this site is crucial for recovery of NDV, we additionally generated rNDVs with substitution of alanine and glutamic acid for serine (rBC/HNS6A and rBC/HNS6E, respectively) to confirm its crucial role in the recovery of rNDV.
FIG. 1.
Constructs of recombinant NDVs containing a deletion or point mutation in the N-terminal cytoplasmic tail of the HN protein and replication and fusion index of recovered viruses in infected cells. (A) Consecutively, 6 nt, 12 nt, or 18 nt of mRNA of the HN cytoplasmic tail in a full-length antigenomic cDNA of NDV was deleted. Deletions in the HN cytoplasmic tails are indicated by the large boldface dashes. In addition, serine at position 6 was substituted with alanine and glutamic acid was substituted by changing guanine to cytidine and adenosine, respectively. (B) In vitro replication of the mutant viruses was determined in virus-infected DF-1 cells at an MOI of 0.01. The viral titers were determined by plaque assay. (C) The fusion index was determined in virus-infected Vero cells at an MOI of 0.1. Cells were stained with hematoxylin-eosin, and the fusion index was calculated as a mean number of nuclei per cell. The assay was performed three times.
In vitro replication of recovered viruses was determined by plaque assay in virus-infected DF-1 cells at a multiplicity of infection (MOI) of 0.01 (5). All mutant viruses and the parental virus, rBC, grew to similar titers, indicating that alteration of the HN cytoplasmic tails did not affect their in vitro replication (Fig. 1B). Although the rBC/HNΔ4 mutant had grown well up to 24 h postinfection, a reduction of the viral titer was detected thereafter with rapid and extensive induction of syncytia. Therefore, we determined fusion promotion activity of the mutant viruses by quantitating syncytia in virus-infected Vero cells at an MOI of 0.1 at 30 h postinfection (8) and confirmed increased fusion promotion activity of rBC/HNΔ4 followed by rBC/HNS6A compared to that of rBC (Fig. 1C). Similarly, enhanced fusion activity was observed in other cytoplasmic tail-truncated paramyxoviruses, such as simian virus 5 and measles virus (2, 9). It has been postulated that interaction of matrix (M) protein with the cytoplasmic tails of the glycoproteins involves in a fusion-refractory conformation at the early stage of viral maturation (2). Therefore, these altered HN cytoplasmic tails could assist NDV in gaining its cell fusion competence by modulating this fusion-refractory conformation.
In general, the levels of the HN protein contents on the surfaces of virus-infected cells and in the virus particles were more affected by point mutation of serine than by truncation of the cytoplasmic tail. We analyzed surface expression of the HN protein on virus-infected DF-1 cells at an MOI of 0.1. At 24 h postinfection, the cells were labeled with a monoclonal antibody against the NDV HN protein followed by anti-Alexa Fluor 488 conjugate, fixed with 4% paraformaldehyde, and analyzed by a fluorescence-activated cell sorter (AriaII; BD Bioscience) with Flowjo program (Tree Star, Inc.) (Fig. 2A). The percentages of cells expressing the HN proteins were 89 (rBC), 78 (rBC/HNΔ2), 71 (rBC/HNΔ4), 64 (rBC/HNS6A), and 53 (rBC/HNS6E). To analyze incorporation of the HN proteins into the viral particles, the parental and mutant viruses harvested from allantoic fluid samples were purified through a 30% sucrose cushion. The viral proteins were separated on an 8% sodium dodecyl sulfate-polyacrylamide gel (Fig. 2B). We first examined whether the mutant viruses incorporated the same levels of other viral proteins. This assay was performed by determining the ratios of the P protein to M protein. We found that similar levels of the P and M proteins were present among the different mutant viruses (Fig. 2B). We then measured the levels of the HN proteins incorporated into the virus particles by determining the ratios of the HN protein to M protein (Fig. 2C). The pattern of incorporation of the HN proteins into the virus particles was similar with their cell surface expression. The HN protein contents of rBC/HNΔ2 and rBC/HNΔ4 were not significantly different from that of the parental virus (P > 0.05), indicating that truncation of the cytoplasmic tail did not impair its incorporation into the viral particles. In contrast, substitution of glutamic acid for serine decreased incorporation of the HN protein into the viral particles, indicating that serine plays an important role in both cell surface expression of the HN protein and its incorporation into the viral particles.
FIG. 2.
Effect of alteration of the HN cytoplasmic tail on incorporation of the HN proteins into viral particles and their surface expression in DF-1 cells. (A) Surface expression of the NDV HN protein in DF-1 cells was analyzed by a fluorescence-activated cell sorter. At 24 h postinfection, DF-1 cells infected with each virus were stained with monoclonal antibody against the HN protein followed by anti-Alexa Fluor 488 conjugate. (B) Ultracentrifuge-purified viruses from infected allantoic fluid samples were separated by electrophoresis, and the gel was then stained with Coomassie brilliant blue. (C) Ratios of HN protein to M-protein levels from the parental virus and the HN cytoplasmic tail mutant viruses were quantified.
We further determined the effect of cytoplasmic tail alteration on the pathogenicity of NDV in embryonated eggs and chicks (Table 1). The mean death time (MDT) was determined as the mean time (h) for the minimum lethal dose of virus to kill all the embryos after inoculation of 9-day-old specific-pathogen-free (SPF) embryonated chicken eggs with virus (1). The criteria for classifying the virulence of NDV strains are as follows: virulent strains take <60 h to kill embryos, intermediate virulent strains take 60 to 90 h to kill embryos, and avirulent strains take >90 h to kill embryos. Two mutant viruses (rBC/HNΔ2 and rBC/HNS6E) showed similar values of MDT compared to rBC (59 h). In contrast, the MDTs of rBC/HNΔ4 and rBC/HNS6A were 50 h and 51 h, respectively. Increased pathogenicity of these two mutants was also confirmed by an intracerebral pathogenicity index (ICPI) test in 1-day-old SPF chicks (1). The scale of the ICPI value in evaluating the virulence of NDV strains is from 0.00 (avirulent strains) to 2.00 (highly virulent NDV strains). The rBC/HNΔ4 virus had the highest ICPI value (1.61), followed by rBC/HNS6A (ICPI value of 1.58), among the parental and mutant viruses, probably due to their enhanced fusion promotion activity. In contrast, rBC/HNS6E had the lowest ICPI value (1.41), which would be associated with decreased HN protein contents detected in the viral particles and virus-infected cells. In our previous study, decreased HN protein contents in virus particles due to complete deletion of 5′ untranslated regions of the HN gene also resulted in attenuation of the virus in chickens (14). Consistently, rBC/HNΔ2 showed biological characteristics and pathogenicity similar to those of the parental virus, suggesting that aspartic acid and arginine are indispensable for the HN cytoplasmic tail of NDV.
TABLE 1.
Pathogenicity of the HN cytoplasmic tail mutant viruses in embryonated eggs and chicks
| Virus | MDT (h)a | ICPIb |
|---|---|---|
| rBC | 58 | 1.49 |
| rBC/HNΔ2 | 59 | 1.51 |
| rBC/HNΔ4 | 50 | 1.61 |
| rBC/HNS6A | 51 | 1.58 |
| rBC/HNS6E | 62 | 1.41 |
The mean time (in hours) for the minimum lethal dose of virus to kill all the inoculated embryos. NDV strains were classified by the following criteria: virulent strains take <60 h to kill embryos, intermediate virulent strains take 60 to 90 h to kill embryos, and avirulent strains take >90 h to kill embryos.
Pathogenicity of NDV in 1-day-old SPF chicks was evaluated by the ICPI value: virulent strains had ICPI values of 1.5 to 2.0, intermediate virulent strains had ICPI values of 1.0 to 1.5, and avirulent strains had ICPI values of 0.0 to 0.5.
The M protein plays a major role in virus assembly through its interaction with envelope glycoproteins and with the membranes of infected cells (11). To gain insight into the function of the amino acid sequences of the HN cytoplasmic tail in virus assembly, colocalization of the HN and M proteins was determined by confocal microscopy (LSM 510; Zeiss). Detection of the M and HN proteins was facilitated by coexpressing M protein and each altered HN protein using the pCAGGS expression system in 293T cells. In particular, the open reading frame of the M gene had been fused with an influenza virus hemagglutinin epitope tag (7 amino acid residues) followed by a stop codon and cloned into pCAGGS. After 24 h of transfection, the cells were fixed, permeabilized, stained with a monoclonal antibody against the NDV HN protein followed by anti-Alexa Fluor 488 and anti-HA Alexa Fluor 594 conjugates, and analyzed by confocal microscopy. The M and wild-type HN proteins were distributed in the nucleus and cytoplasm and in the cytoplasm, respectively, leading to their colocalization in the cytoplasm of infected cells (Fig. 3A). In contrast, cytoplasmic tail-altered HN proteins (4-amino-acid deletion and substitution of alanine for serine) were dominantly found on the cell surface with their colocalization with the M protein, indicating reduction of specificity in membrane insertion of these HN proteins (Fig. 3B and C). Furthermore, no colocalization of the 6-amino-acid deletion of cytoplasmic tail-altered HN protein with the M protein was detected (Fig. 3D), suggesting that this alteration had affected incorporation of the HN protein into virus particles and consequently virus recovery. Other paramyxoviruses (e.g., simian virus 5 and human respiratory syncytial virus) also showed a loss of intracellular interaction between the M protein and glycoproteins containing cytoplasmic tail-truncated domains (3, 12).
FIG. 3.
Localization of the NDV HN and M proteins in 293T cells. The M protein fused with an influenza virus hemagglutinin epitope tag and each HN variant containing altered cytoplasmic tails were expressed using the pCAGGS expression system in 293T cells. The cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, stained with a monoclonal antibody against the NDV HN protein followed by anti-Alexa Fluor 488 (green; HN) and anti-HA Alexa Fluor 594 (red; M) conjugates and analyzed by laser-scanning microscopy. (A) Wild-type HN, (B) HN Δ4, (C) HN S6A, and (D) HN Δ6.
In summary, we demonstrate that the cytoplasmic tail of HN plays a crucial role in the NDV life cycle. Our data suggest that the first 2 amino acids of the cytoplasmic tail are not absolutely required for NDV replication, but amino acids at positions 4 through 6 are critical for specific insertion of the HN protein into virion particles. Furthermore, our results indicate that the cytoplasmic tail of HN protein modulates the fusion activity of NDV. It will also be necessary to determine whether alteration of the HN cytoplasmic tail can affect interaction of the HN protein with the F protein.
Acknowledgments
We thank Daniel Rockemann, Yunsheng Wang, and our laboratory members for excellent technical assistance and Ireen Dryburgh-Barry for proofreading the manuscript.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Alexander, D. J. 1989. Newcastle disease, p. 114-120. In H. G. Purchase, L. H. Arp, C. H. Domermuth, and J. E. Pearson (ed.), A laboratory manual for the isolation and identification of avian pathogens, 3rd ed. American Association of Avian Pathologists, Dubuque, IA.
- 2.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghildyal, R., D. Li, I. Peroulis, B. Shields, P. G. Bardin, M. N. Teng, P. L. Collins, J. Meanger, and J. Mills. 2005. Interaction between the respiratory syncytial virus G glycoprotein cytoplasmic domain and the matrix protein. J. Gen. Virol. 86:1879-1884. [DOI] [PubMed] [Google Scholar]
- 4.Huang, Z., A. Panda, S. Elankumaran, D. Govindarajan, D. Rockemann, and S. K. Samal. 2004. The hemagglutinin neuraminidase protein of Newcastle disease virus determines tropism and virulence. J. Virol. 78:4176-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, Z., S. Elankumaran, A. S. Yunus, and S. K. Samal. 2004. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 78:10054-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamurthy, S., Z. Huang, and S. K. Samal. 2000. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology 278:168-182. [DOI] [PubMed] [Google Scholar]
- 7.Lamb, R. A., and G. D. Parks. 2007. Paramyxoviridae: the viruses and their replication, p. 1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 8.Panda, A., S. Elankumaran, S. Krishnamurthy, Z. Huang, and S. K. Samal. 2004. Loss of N-linked glycosylation from the hemagglutinin-neuraminidase protein alters virulence of Newcastle disease virus. J. Virol. 78:4965-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt, A. P., B. He, and R. A. Lamb. 1999. Involvement of the cytoplasmic domain of the hemagglutinin-neuraminidase protein in assembly of the paramyxovirus simian virus 5. J. Virol. 73:8703-8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takimoto, T., T. Bousse, E. C. Coronel, R. A. Scroggs, and A. Portner. 1998. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. J. Virol. 72:9747-9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng, M. N., S. S. Whitehead, and P. L. Collins. 2001. Contribution of the respiratory syncytical virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283-296. [DOI] [PubMed] [Google Scholar]
- 12.Waning, D. L., A. P. Schmitt, G. P. Leser, and R. A. Lamb. 2002. Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5. J. Virol. 76:9284-9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson, C., R. Gilmore, and T. Morrison. 1990. Aberrant membrane insertion of a cytoplasmic tail deletion mutant of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus. Mol. Cell. Biol. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan, Y., S. N. Rout, S. H. Kim, and S. K. Samal. 2009. Role of untranslated regions of hemagglutinin-neuraminidase gene in replication and pathogenicity of Newcastle disease virus. J. Virol. 83:5943-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]