Abstract
The amino (N) terminus of the human papillomavirus (HPV) minor capsid protein L2 can induce low-titer, cross-neutralizing antibodies. The aim of this study was to improve immunogenicity of L2 peptides by surface display on highly ordered, self-assembled virus-like particles (VLP) of major capsid protein L1, and to more completely characterize neutralization epitopes of L2. Overlapping peptides comprising amino acids (aa) 2 to 22 (hereafter, chimera or peptide 2-22), 13 to 107, 18 to 31, 17 to 36, 35 to 75, 75 to 112, 115 to 154, 149 to 175, and 172 to 200 of HPV type 16 (HPV16) L2 were genetically engineered into the DE surface loop of bovine papillomavirus type 1 L1 VLP. Except for chimeras 35-75 and 13-107, recombinant fusion proteins assembled into VLP. Vaccination of rabbits with Freund's adjuvanted native VLP induced higher L2-specific antibody titers than vaccination with corresponding sodium dodecyl sulfate-denatured proteins. Immune sera to epitopes within residues 13 to 154 neutralized HPV16 in pseudovirion neutralization assays, whereas chimera 17-36 induced additional cross-neutralization to divergent high-risk HPV18, -31, -45, -52, and -58; low-risk HPV11; and beta-type HPV5 (titers of 50 to 10,000). Aluminum hydroxide-monophosphoryl lipid A (Alum-MPL)-adjuvanted VLP induced similar patterns of neutralization in both rabbits and mice, albeit with 100-fold-lower titers than Freund's adjuvant. Importantly, Alum-MPL-adjuvanted immunization with chimeric HPV16L1-HPV16L2 (peptide 17-36) VLP induced neutralization or cross-neutralization of HPV16, -18, -31, -45, -52, and -58; HPV6 and -11; and HPV5 (titers of 50 to 100,000). Immunization with HPV16 L1-HPV16 L2 (chimera 17-36) VLP in adjuvant applicable for human use induces broad-spectrum neutralizing antibodies against HPV types evolutionarily divergent to HPV16 and thus may protect against infection with mucosal high-risk, low-risk, and beta HPV types and associated disease.
The more than 100 types of human papillomaviruses (HPV) identified to date (14) are the etiological agents of skin and mucosal papillomas or warts. Persistent infection with high-risk mucosal types, most often HPV type 16 (HPV16) and HPV18, causes cervical cancer, which constitutes the second leading fatal cancer in women worldwide, causing 274,000 deaths per year. Substantial morbidity results from other noncervical HPV-related conditions, such as anogenital warts or anal cancer (23).
The development of current prophylactic papillomavirus vaccines was launched by observations that recombinantly expressed major capsid protein L1 self-assembles into virus-like particles (VLP). These empty viral capsids are composed of 360 L1 molecules and resemble native virions in both structure and immunogenicity, yet are nononcogenic and noninfectious. Moreover, VLP cannot replicate because the cells in which VLP are made contain only L1 and no other papillomavirus genes. Subunit VLP vaccines induce high-titer and type-restricted antibody responses to conformational L1 epitopes (12, 26, 39, 44). When applied to women prior to infection, available vaccines targeting the most prevalent high-risk types, HPV16 and HPV18, have demonstrated up to 100% efficacy against persistent infection and associated disease caused by the included types and thus are potentially able to prevent ∼70% of cervical high-grade dysplasias and probably cancers (22, 46). Therefore, use of currently licensed L1 vaccines necessitates continuation of cytological cervical screening of women. The prevention of 96% of cervical cancer would require immunity to seven high-risk HPV types (HPV16, -18, -31, -33, -45, -52, and -58) (32) and the development of more highly multivalent (and presumably costly) L1 VLP vaccines.
The search for alternative broader-spectrum immunogens drew attention to the minor capsid protein L2, which is immunogenically subdominant in the context of coexpressed L1-L2 capsids (38). Immunization of animals with the amino (N)-terminal peptide of L2 demonstrated its ability to elicit low-titer neutralizing antibodies that protect against challenge with cognate papillomavirus types in vivo (16, 19), cross-neutralize heterologous types in vitro (25, 33, 38), and confer cross-protection in vivo (17).
This study addresses two major issues that may further the development of L2-based broader-spectrum vaccines. First, the N terminus of L2 is more closely examined for potential neutralization epitopes, by incorporating peptides into papillomavirus VLP as peptide-presenting platforms (7, 21, 42). Moreover, we take advantage of the immunogenic characteristics of virion surfaces, such as the dense repetitive surface array of VLP, to induce strong and enduring immune responses to displayed L2 epitopes.
MATERIALS AND METHODS
Baculovirus expression of chimeric L1-L2 proteins.
L2 peptides of HPV16 were genetically engineered into the DE surface loops of bovine papillomavirus type 1 (BPV1) L1 (by insertion between amino acids [aa] 133 and 134) or HPV16 L1 (between aa 136 and 137).
Reverse-orientated (back-to-back) synthetic oligonucleotides (1; primer pairs available upon request) encoding HPV16 L2 aa 18 to 31 were used for insertion into the BPV1 L1 by an inverse-touchdown PCR, using the baculovirus transfer vector BPV1 L1-pEVmod (26). To avoid nonspecific products, the annealing temperature was decreased by 1.5°C over seven cycles, followed by an additional 30 cycles at the final touchdown temperature. Subsequently, the inserted L2 sequences were joined by blunt-end ligation. Silent mutations were introduced into the C-terminal L1 open reading frame [poly(A) tract] (2) to avoid mutations of the amplimer. The mutated L1-L2 sequence was recloned into vector pEVmod, and inserted L2-epitopes were further elongated by reapplication of PCR (3).
The following series of peptides spanning the N terminus of HPV16 L2 were incorporated into BPV1 L1 pEVmod employing a newly established SstII (CCGCGG) restriction enzyme site inserted between aa 133 and 134 by inverse-touchdown PCR using a primer pair (4). Double-stranded oligonucleotides (with flanking SstII sites) encoding HPV16 L2 peptides 2-22 (5) and 35-75 (6) were generated by synthetic oligonucleotide annealing or by generating PCR-amplimers encoding HPV16 L2 75-112 (7), 115-154 (8), 149-175 (9), 172-200 (10), and 13-107 (11), respectively, and cloned into the SstII site of BPV L1 (aa 133 and 134). SstII (CCGCGG) codes for proline (P)-arginine (R), added to both ends of L2 in the translated fusion proteins.
A plasmid encoding HPV16 L1 (derived from genomic clone 114K [27]) plus HPV16 L2 aa 17 to 36 (hereafter referred to as “16L1-16L2 17-36”) was generated by subcloning of codon-optimized synthetic oligonucleotides (Geneart, Germany) into the BglII to KpnI sites of baculovirus transfer vector pSynwtVI− (27). Recombinant expression vectors were verified by bidirectional sequencing (VBC-Biotech, Vienna, Austria).
Recombinant baculoviruses were generated by cotransfection of Sf-9 insect cells with transfer vectors and linearized baculovirus DNA (BaculoGold; BD Biosciences). Expression and purification of VLP were performed as described previously (26). In brief, chimeric proteins were expressed by infection of insect cells with amplified baculovirus stocks for 3 days. Subsequently, harvested cells were lysed by sonication in phosphate-buffered saline (PBS), 0.8 M NaCl, 2 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride. Following addition of 0.5% Brij58, proteins were incubated overnight at 4°C. High-molecular-mass structures were purified by ultracentrifugation on sucrose-PBS cushions (35% [wt/vol]) and CsCl-PBS (29% [wt/wt]) density gradients for at least 24 h each.
Disassembly of VLP into pentameric capsomers was achieved under reducing conditions by extensive dialyzing into 10 mM Tris HCl (pH 7.9), 10 mM dithiothreitol, 7.5% 2-mercaptoethanol (2-ME) (adapted from reference 31). Immunizations were performed with pentamers redialyzed into equivalent buffer without 2-ME. For immunizations with denatured antigen, VLP were dialyzed against PBS (0.5 M NaCl, 1 mM CaCl2, 0.02% Tween 80) and boiled in 4% sodium dodecyl sulfate (SDS).
Western blotting.
Sf-9 cells were infected for 3 days, harvested, denatured in lysis buffer (2% 2-ME), and analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting. Expression of L1 proteins was verified by monoclonal antibody (MAb) AU1 recognizing the linear BPV L1 epitope DTYRYI (BAbCO) or Camvir-1 raised against HPV16 L1 (BD Pharmingen). To verify antigenicity of L2 peptides, samples were probed with MAb RG-1 directed to HPV16 L2 aa 17 to 36 (18) or polyclonal rabbit sera raised against His-tagged HPV16 L2 aa 1 to 88 or His-tagged HPV16 L2 aa 11 to 200 (33).
TEM.
For transmission electron microscopy (TEM), purified particles were loaded on glow-discharged carbon-coated copper grids, fixed on 2.5% glutaraldehyde, negatively stained with 1% uranyl acetate, and visualized with a JEOL 1010 electron microscope at 80 kV and ×30,000 magnification.
Immunization.
Proteins were extensively dialyzed against PBS containing 0.5 M NaCl, 1 mM CaCl2, and 0.02% Tween 80. Two New Zealand White (NZW) rabbits were immunized with each 50 μg native or SDS-denatured particles in complete Freund's adjuvant (CFA), followed by three boosts 4, 6, and 8 weeks later in incomplete Freund's adjuvant (IFA) (Charles River, Kisslegg, Germany). Alternatively, immunogens were administered in a 10:1 mixture of aluminum hydroxide gel (A8222; Sigma-Aldrich) and monophosphoryl lipid A (S6322; Sigma-Aldrich) (referred to as “Alum-MPL”) prepared according to the manufacturer's protocols. BALB/c mice were given 10 μg of antigen and boosted 4, 8, and 10 weeks after the first injection. Sera were collected 14 days after the last boost and stored at −20°C.
ELISA.
Specific antibody titers in antisera raised against BPV1 L1- HPV16 L2 (further referred to as “BL1-16L2”) peptides 2-22, 75-112, 115-154, 149-175, and 172-200 were determined by enzyme-linked immunosorbent assay (ELISA), using His-tagged HPV16 L2 1-88 or HPV16 L2 11-200 polypeptides to coat the microtiter plates.
These antigens were generated using the pET28A vector (33) transformed into Escherichia coli Rosetta DE3 (Novagen), induced by isopropyl-β-d-thiogalactopyranoside (IPTG), and affinity purified on Ni-nitrilotriacetic acid (NTA) columns (Qiagen). Eluted proteins were pooled, verified by Western blotting, quantified via the BCA (bicinchoninic acid) protein assay kit (Pierce), and stored at −20°C.
The ELISA was performed as described previously (18). Maxisorb plates (Nunc) were coated overnight with 0.1 μg/well L2 peptide in carbonate buffer (pH 9.6) and blocked with 1% bovine serum albumin (BSA)-PBS. Ten-fold serial dilutions of antisera were incubated in triplicate wells in BSA-PBS-0.05% Tween 20. Following three washes with PBS-0.05% Tween 20, peroxidase-conjugated antibody in BSA-PBS-0.05%Tween 20 (1:5,000) was added and this mixture was incubated for 1 h at room temperature. Finally, plates were washed and developed by adding the substrate ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Boehringer Mannheim]. The optical density at 405 nm (OD405) was determined using an ELISA reader (Dynatech).
ELISA using synthetic biotinylated HPV16 L2 18-31 peptide.
Antisera raised against BL1-16L2 18-31, BL1-16L2 17-36, and 16L1-16L2 17-36 were examined by ELISA using the biotinylated peptide HPV16 L2 18-31 (LYKTCKQAGTCPPD; JPT Peptide Technologies, Berlin, Germany) as the antigen (42). One microgram of peptide/well was added to Nunc streptavidin plates overnight (as specified by the Nunc streptavidin general coating protocol). Plates were washed with PBS, blocked overnight with 0.5% nonfat dry milk-powder-PBS at 4°C, and incubated with serially diluted antisera for 1 h at room temperature. Following three washes with PBS, a 1:10,000 dilution of conjugate was added, plates were washed and then developed with ABTS, and OD405 was determined.
Pseudovirion neutralization assay.
Pseudovirions were produced in 293TT cells and purified on Optiprep gradients (Sigma) as described by Buck et al. (http://home.ccr.cancer.gov/lco/protocols.asp) with minor modifications. The following plasmids for expression of L1 and L2 capsid proteins or secreted alkaline phosphatase (SEAP) were used. The following packaging plasmids were provided by J. Schiller, NIH: HPV5, p5sheLL; HPV6, p6sheLL; HPV16, p16L1h and p16L2h; HPV18, peL1fB and peL2bhb; HPV45, p45shell; and cottontail rabbit papillomavirus (CRPV), pCRPVL1 and pCRPVL2. (Plasmid maps and references may be found at http://home.ccr.cancer.gov/Lco/plasmids.asp.) The following plasmids were provided by Kanda, Tokyo: HPV31, p31L1h and p31L2h (28); HPV52, p52L1h and p52L2h (unpublished); and HPV58, p58L1h and p58L2h (28). The HPV11 L1 puF3 (no. 1170), HPV11 L2 puF3 (no. 1244), and HPV11 L1/L2 pIRES (no. 1245) packaging plasmids (unpublished) were provided by M. Müller, Heidelberg, Germany. The target plasmid pYSEAP was provided by J. Schiller, NIH.
Expression vectors for packaging capsid proteins were cotransfected with reporter plasmid pYSEAP, and capsid yield was detected colorimetrically. Neutralization assays were performed according to an adapted protocol (http://home.ccr.cancer.gov/lco/neutralizationassay.htm). Pseudovirions were preincubated with 1:2 serial dilutions of sera starting at 1:100 in duplicate wells on ice for 1 h. Following infection with pseudovirion solutions, 293TT cells were incubated for 72 h at 37°C and SEAP activity was determined in clarified cell supernatants colorimetrically at 405 nm (1). Neutralization titers were reported as the reciprocals of the highest dilution causing 50% reduction of SEAP activity in each assay, compared to preimmune sera of the same dilution. When reduction of SEAP was close to 50% at a 1:100 dilution, sera were reevaluated at 1:50.
RESULTS
Previous studies reported that immunization with peptide 1-88 of HPV16 L2 induced low-titer humoral immune responses to homologous HPV16 and cross-neutralization of heterologous types in vitro (33) and that vaccination with peptide 11-200 of HPV16 L2 confers cross-protection in vivo against challenge by CRPV and rabbit oral papillomavirus (ROPV) (17). In order to enhance antibody titers generated by immunization, L2 peptides were incorporated into a surface displayed site of L1, presumably resulting in a 360-fold array of L2 on the capsid surface.
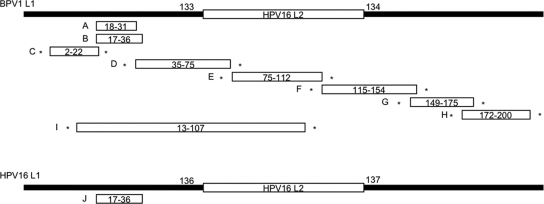
Previously, peptides up to 9 aa in length have been successfully expressed by the DE loop on the BPV1 VLP surface without compromising the ability to assemble into immunogenic VLP (21, 42). Therefore, the DE loop of L1 was chosen for insertion to display the L2 peptide on the surface of the assembled chimeric BPV1 VLP (Fig. 1). The use of BPV1 capsids as carrier avoids induction of neutralizing anti-HPV L1 antisera that might obscure detection of low-titer anti-HPV16 L2 (cross)-neutralizing antibodies. Coding sequences for nine partially overlapping HPV16 L2 peptides 18-31 (protein A), 17-36 (protein B, corresponding to the epitope of MAb RG-1) (18), 2-22 (protein C), 35-75 (protein D), 75-112 (protein E), 115-154 (protein F), 149-175 (protein G), 172-200 (protein H), and 13-107 (protein I) were inserted between codons 133 and 134 of BPV1 L1. An expression vector for an additional chimeric L1-L2 fusion protein with insertion of HPV16 L2 17-36 into HPV16 L1 (16L1-16L2 17-36) (protein J) was also generated. As HPV16 is the most important high-risk type, causing 50% of cervical cancers worldwide, we reasoned that the use of HPV16 L1 VLP as a carrier for HPV16 L2 would enable induction of a combined high-titer anti-HPV16 L1 and a broadly cross-neutralizing anti-L2 immune response. Here, insertion into the DE loop of HPV16 L1 between codons 136 and 137 was chosen by sequence alignment.
FIG. 1.
Summary of chimeric L1-L2 fusion proteins A to J. HPV16 L2 peptides with the indicated amino acid residues were inserted into the DE loop of BPV1 L1 protein (between residues 133 and 134) or HPV16 L1 protein (between residues 136 and 137). Solid lines indicate L1 proteins, and open bars indicate L2 peptides. Asterisks indicate that 2 aa (Pro and Arg) were added N and C terminal to the respective peptide resulting from restriction enzyme sites used for cloning. The schemes are not drawn to scale.
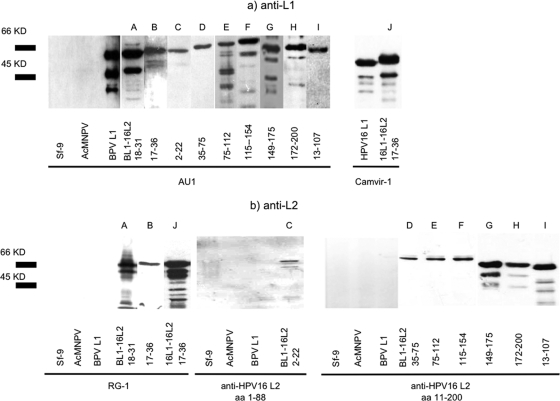
Recombinant baculoviruses were generated and used for infection of Sf-9 insect cells. Three days later, cells were lysed and analyzed by SDS-polyacrylamide gel electrophoresis. Western blotting of recombinant proteins with MAb AU1 (anti-BPV L1) and Camvir-1 (anti-HPV16 L1) showed migration within a range of 45 to 60 kDa (Fig. 2a, proteins A to J). As expected, migration of most L1-L2 fusion proteins was slightly slower than that of wild-type L1 proteins.
FIG. 2.
(a) Analysis of chimeric fusion proteins (by Western blotting of Sf-9 cell lysates infected by recombinant baculoviruses. MAb AU1 or Camvir-1 detected BPV1 L1 or HPV16 L1 of chimeric VLP as major bands within a range of 45 to 60 kDa. Reactivity to lower-molecular-mass bands is likely due to proteolytic degradation products. Both MAbs were nonreactive with lysates of uninfected Sf-9 cells or wild-type baculovirus (Autographa californica multiple nucleopolyhedrosis virus [AcMNPV])-infected insect cells. Wild-type BPV1 L1 or HPV16 L1 proteins were used as controls. (b) Antigenicity of incorporated L2 peptides was verified with MAb RG-1 (proteins A, B, and J) or polyclonal rabbit sera to HPV16 L2 aa 1-88 (protein C) and HPV16 L2 aa 11-200 (proteins D, E, F, G, H, and I), respectively. Proteins D, E, and F were used as gradient-purified fusion proteins.
Antigenicity of inserted HPV16 L2 peptides was verified by MAb RG-1 (anti-HPV16 L2 17-36) or polyclonal rabbit sera raised against HPV16 L2 1-88 or HPV16 L2 11-200 as appropriate (Fig. 2b). Several faster-migrating bands are probably caused by protein degradation.
Native L1 VLP trigger higher titer neutralizing antibodies than subunit pentamers, and the pentamers are dramatically more immunogenic than monomeric, denatured L1 protein (26, 45). Thus the assembly status of chimeric L1-L2 proteins was determined by TEM. In case of equivocal morphological formations, assembly into capsomers was further distinguished by ELISA performed with conformation-dependent MAb 5B6, whose binding is dependent on BPV1 L1 assembly into pentamers (43).
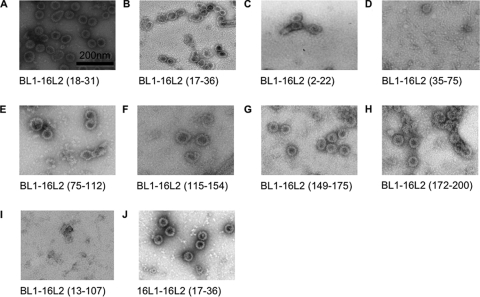
As shown in Fig. 3, TEM demonstrated assembly into full-size VLP (∼50 to 60 nm in diameter) of BL1-16L2 18-31 (protein A), 17-36 (protein B), 2-22 (protein C), 75-112 (protein E), 115-154 (protein F), 149-175 (protein G), 172-200 (protein H), and 16L1-16L2 17-36 (protein J). Chimeric proteins BL1-16L2 35-75 (protein D) and BL1-16L2 13-107 (protein I) existed in less-ordered conformations, suggesting the presence of L1 pentamers or protein aggregates. To further distinguish these possibilities, ELISAs using MAb 5B6 (37) and MAb AU1 were performed. Neither of the protein preparations that lacked VLP (proteins D and I) reacted with 5B6, suggesting that chimeric proteins shown for D and I were unable to assemble into pentamers. Moreover, results demonstrated enhanced binding of MAb AU1 with denatured BL1-16L2 13-107 (protein I), compared to native preparations, but not for BL1-16L2 35-75 (protein D), suggesting a partially conformation-dependent formation of the former (protein I) but not the latter (protein D) (data not shown). Consequently, we refrained from immunization with BL1-16L2 35-75.
FIG. 3.
TEM of purified particle preparations (magnification, ×30,000). Chimeric proteins BL1-16L2 18-31 (A), 17-36 (B), 2-22 (C), 75-112 (E), 115-154 (F), 149-175 (G), and 172-200 (H) and 16L1-16L2 17-36 (J) assemble into VLP, with a size of ∼50 to 60 nm. BL1-16L2 35-75 (D) and BL1-16L2 13-107 (I) do not unequivocally form capsomers or VLP.
Taken together, 8 out of 10 chimeric proteins were able to assemble into VLP, presenting up to 44 aa of 16L2 (BL1-16L2 115-154 [protein F] plus 4 aa encoded by SstII flanking restriction enzyme sites) within the DE surface loop of BPV1 L1, and 20 aa within HPV16 L1 (protein J), respectively (Fig. 1 and 3).
L2-specific serum antibodies.
Immunogenicity of chimeric L1-L2 VLP and humoral immune responses to displayed L2 peptides were determined by immunization of NZW rabbits. Each antigen was administered either as native particles or SDS-denatured antigen in order to determine the impact of particle structure on immunogenicity. Immunizations were performed using the potent adjuvant CFA or IFA. Antigens that induced broadly cross-neutralizing antibody responses were further administered using human-applicable Alum-MPL as the adjuvant. Moreover, inbred BALB/c mice were inoculated with antigen-Alum-MPL formulations in order to encompass an alternative mammalian system.
Two NZW rabbits were immunized in CFA/IFA with BL1-16L2 18-31 (protein A), 17-36 (protein B), 2-22 (protein C), 75-112 (protein E), 115-154 (protein F), 149-175 (protein G), and 172-200 (protein H), each as native or SDS-denatured preparations. Due to its incomplete assembly, BL1-16L2 13-107 (protein I) was inoculated as the native preparation only.
By ELISA, L2-specific immune responses were detected using synthetic peptide HPV16 L2 18-31 or bacterially expressed HPV16 L2 1-88 or 11-200 proteins, respectively, as antigens (Table 1). Apart from BL1-16L2 2-22 (protein C), all VLP preparations induced significant antibody responses (titers ranging from 2,500 to 312,500), while corresponding denatured proteins each elicited antibody levels that were typically five times lower (titers of 500 to 12,500). Preimmune sera were nonreactive in all cases.
TABLE 1.
Evaluation of rabbit antisera by HPV16 L2 peptide ELISAa
| Rabbit antiserum (CFA/IFA) | Titer with antiserum to BL1-16L2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 18-31 (protein A) | 17-36 (protein B) | 2-22 (protein C) | 75-112 (protein E) | 115-154 (protein F) | 149-175 (protein G) | 172-200 (protein H) | 13-107 (protein I) | |
| Native protein | ||||||||
| NZW #1 | 62,500 | 62,500 | 0 | 2,500 | 62,500 | 62,500 | 312,500 | 500 |
| NZW #2 | 62,500 | 62,500 | 0 | 2,500 | 62,500 | 62,500 | 312,500 | 500 |
| Denatured protein | ||||||||
| NZW #3 | 12,500 | 2,500 | 0 | 500 | 2,500 | 12,500 | 12,500 | x |
| NZW #4 | 12,500 | 100 | 0 | 500 | 2,500 | 2,500 | 12,500 | x |
Two NZW rabbits each were vaccinated with the indicated chimeric BL1-16L2 proteins, either as native VLP or SDS-disrupted preparations, using Freund's CFA or IFA as the adjuvant. The L2 ELISA was performed using HPV16 L2 1-88 (for sera raised against BL1-16L2 2-22), HPV16 L2 18-31 (BL1-16L2 18-31 and BL1-16L2 17-36), and HPV16 L2 11-200 (BL1-16L2 75-112, BL1-16L2 115-154, BL1-16L2 149-175, BL1-16L2 172-200, and BL1-16L2 13-107) as ELISA antigens. Sera were serially end-point diluted and tested in triplicates. SDS-disrupted protein BL1-16L2 13-107 was not used as an immunogen (x).
These results demonstrate improved immunogenicity of epitopes present on native VLP, compared to analogous denatured proteins. The complete absence of a detectable humoral response to L2 by BL1-16L2 2-22 (protein C) immunization suggests that the N-terminal 20 aa of HPV16 L2 do not represent a B-cell epitope in rabbits. Moreover, the inability of BL1-16L2 13-107 (protein I) to assemble into VLP may be responsible for inducing only a modest anti-L2 immune response (titers of 500) (Table 1).
In vitro neutralization.
Pseudovirion neutralization assays take advantage of papillomavirus-based gene transfer vectors (pseudovirions) to mimic papillomavirus infection and its inhibition in vitro (4). Infection of cell cultures with L1 and L2, encapsidating the reporter plasmid pYSEAP leads to expression of SEAP, which can be measured in the supernatant, whereas preincubation of pseudovirions with neutralizing antibodies prevents infection and decreases the amount of SEAP. It has been shown that neutralizing antibodies correlate with protection of animals from viral challenge in vivo (17, 18).
Neutralization assays were performed with pseudovirions of L2-homologous type HPV16 and L2-divergent high-risk HPV18 (Table 2). Sera unable to neutralize infection with either type were not further evaluated. All sera were tested in 10-fold serial dilutions from 1:100 to 1:1,000,000, evidence of lower antibody levels was reevaluated for serum dilutions of 1:50, whereas titers of <50 were considered insignificant.
TABLE 2.
Pseudovirion neutralization assays of rabbit sera raised against indicated native or denatured BL1-16L2 proteins using Freund's CFA or IFA as the adjuvanta
| Pseudovirions | Neutralizing antibody titer with antiserum to BL1-16L2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Native 18-31 (protein A) | Denatured 18-31 (protein A) | Native 17-36 (protein B) | Denatured 17-36 (protein B) | Native 75-112 (protein E) | Denatured 75-112 (protein E) | Native 115-154 (protein F) | Denatured 115-154 (protein F) | Native 13-107 (protein I) | |
| HPV16 | 0 | 100 | 1,000 | 100 | 100 | 50 | 1,000 | 100 | 100 |
| 100 | 100 | 10,000 | 100 | 0 | 0 | 100 | 50 | 0 | |
| HPV18 | 0 | 100 | 100-1,000 | 100 | 0 | 0 | 0 | 0 | 0 |
| 0 | 100 | 1,000 | 0 | 0 | 0 | 0 | 0 | 0 | |
| HPV31 | 0 | 0 | 0 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 0 | 0 | x | x | 0 | x | x | ||
| HPV45 | 0 | 100 | 100 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 0 | 100-1000 | x | x | 0 | x | x | ||
| HPV52 | 0 | 0 | 0 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 100 | 100 | x | x | 0 | x | x | ||
| HPV58 | 0 | 0 | 100 | 100 | 0 | x | 0 | 0 | 50 |
| 0 | 100 | 1,000 | 0 | x | x | 0 | x | x | |
| HPV6 | 0 | 0 | 0 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 0 | 0 | x | x | 0 | 0 | x | ||
| HPV11 | 0 | 0 | 0 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 0 | 50-100 | x | x | 0 | 0 | x | ||
| HPV5 | 0 | 1,000 | 100 | 100 | 0 | x | 0 | 0 | 0 |
| 100 | 100 | 10,000 | 0 | x | x | 0 | x | x | |
| CRPV | 0 | 0 | 0 | 0 | 0 | x | 0 | 0 | 0 |
| 0 | 50 | 0 | x | x | 0 | x | x | ||
| Native 2-22 (protein C) | Denatured 2-22 (protein C) | Pentameric 18-31 (protein A) | Native 149-175 (protein G) | Denatured 149-175 (protein G) | Native 172-200 (protein H) | Denatured 172-200 (protein H) | |||
| HPV16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| HPV18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
Titers of two animals each are shown. Sera that neutralized neither HPV16 nor HPV18 were not tested (x) for the remaining pseudovirion types. Assays were performed with serum dilutions ranging from 1:100 to 1:1,000,000. When a lower titer of neutralization was suspected, sera were reevaluated at dilution of 1:50.
As expected from negative ELISA results, BL1-16L2 2-22 (protein C) antiserum did not contain any detectable neutralizing antibodies. One of two rabbits immunized with BL1-16L2 18-31 (protein A) native VLP developed neutralizing antisera to HPV16 (titer of 100) and nonrelated beta HPV5 (100). On the contrary, SDS-denatured antigen induced neutralizing antibodies to those and four additional HPV types (titers of both animals given in parentheses), HPV16 (100 and 100), HPV18 (100 and 100), HPV45 (100 and 0), HPV52 (0 and 100), HPV58 (0 and 100), and HPV5 (1,000 and 100), as well as CRPV (0 and 50). Therefore, it was concluded that presentation of peptide HPV16 L2 18-31 in chimeric VLP was disadvantageous to induction of neutralizing antibodies. In addition, animals immunized with BL1-16L2 18-31 (protein A) disassembled into pentavalent capsomers were incapable of inducing neutralizing antibodies (not shown).
Immunization of rabbits with BL1-16L2 17-36 (protein B) VLP (comprising the RG-1 epitope) induced neutralizing antibodies against five high-risk HPV types, HPV16 (1,000 and 10,000), HPV18 (100 to 1,000 and 1,000), HPV45 (100 and 100 to 1,000), HPV52 (0 and 100), HPV58 (100 and 1,000); low-risk type HPV11 (0 and 50 to 100); and beta HPV type 5 (100 and 10,000). Immune sera to disrupted VLP caused less-distinct titers to HPV16 (100 and 100), HPV18 (100 and 0), HPV58 (100 and 0), and HPV5 (100 and 0), and neutralization was undetectable for HPV45, HPV52, and HPV11.
Vaccination with chimeric particles BL1-16L2 75-112 (protein E) and BL1-16L2 115-154 (protein F) neutralized HPV16 pseudovirions with titers of (100 and 0) (protein E) and (1,000 and 100) (protein F), respectively, but did not cross-neutralize any other pseudovirions tested. Corresponding denatured antigens elicited modest titers of (50 and 0) (protein E) and (100 and 50) (protein F), respectively.
Although both native and denatured BL1-16L2 149-175 (protein G) and BL1-16L2 172-200 (protein H) induced pronounced 16L2-specific immune responses by ELISA, antisera were nonneutralizing for HPV16 and HPV18. One of two animals inoculated with BL1-16L2 13-107 (protein I) evolved neutralizing antibodies against HPV16 (100) and HPV58 (50) (Table 2).
Therefore, neutralization epitopes could be mapped within N-terminal HPV16 L2 aa 17 to 148. However, induction of cross-neutralization to closely related genital high-risk (HPV52 and HPV58), as well as phylogenetically divergent high-risk types (HPV18 and HPV45), genital low-risk (HPV11), beta HPV (HPV5), and animal papillomavirus (CRPV) was restricted to previously reported HPV16 L2 residues aa 17 to 36 (the RG-1 epitope). The importance of flanking aa 17 and 32 to 36 is emphasized by insufficient neutralization of sera raised against construct 18-31. Moreover, presentation on VLP surfaces can improve immunogenicity of displayed epitopes as compared to linear fusion proteins. To determine whether chimeric VLP retained the capability of inducing neutralizing antibodies to conformation-dependent epitopes of carrier protein L1, BPV1 pseudovirion neutralization assays were performed (Table 3). Antisera induced by chimeric VLP (BL1-16L2 18-31 [protein A], 17-36 [protein B], 75-112 [protein E], 115-154 [protein F], and 149-175 [protein G]) neutralized BPV1 pseudovirions with titers ranging from 100,000 to 1,000,000, whereas two chimeras (BL1-16L2 2-22 [protein C] and 172-200 [protein H]) raised lower titers of neutralizing antibodies (1,000 to 10,000). Therefore, the insertion of four out of six peptides did not interfere with induction of high-titer neutralizing antibodies against L1, irrespective of the size of the incorporated peptides.
TABLE 3.
Neutralization of BPV1 pseudovirions by sera raised against native BL1-16L2 chimeric VLPa
| Pseudovirions | BPV1 neutralizing antibody titer with antiserum to BL1-16L2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Native 18-31 (protein A) | Native 17-36 (protein B) | Native 2-22 (protein C) | Native 75-112 (protein E) | Native 115-154 (protein F) | Native 149-175 (protein G) | Native 172-200 (protein H) | Native BL1/L2 | |
| BPV1 | 1,000,000 | 100,000 | 10,000 | 1,000,000 | 100,000 | 100,000 | 1,000 | 100,000 |
| 100,000 | 1,000,000 | 10,000 | 100,000 | 100,000 | 1,000,000 | 10,000 | ||
Assays were performed with serum dilutions from 1:100 to 1:1,000,000. The BPV L1/L2 VLP rabbit serum was raised against coexpressed wild-type BPV L1 plus L2 VLP.
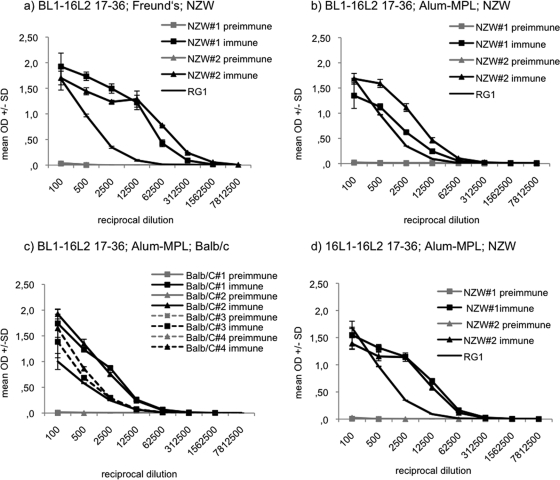
As Freund's adjuvant is not approved for human use, two additional rabbits were immunized with BL1-16L2 17-36 (protein B) using Alum-MPL as the adjuvant. A comparable formulation (ASO4) is used in the approved HPV L1 vaccine Cervarix. In addition, four BALB/c mice were vaccinated with the same antigen-adjuvant formulation to further our observations of cross-neutralization to a different mammalian system. Peptide ELISA detected L2-specific antibody responses with titers of 2,500 to 12,500 in both rabbits and mice (Fig. 4b and c). Chimeric VLP formulated on Freund's adjuvant (Fig. 4a) induced at least five times higher antibody titers than Alum-MPL.
FIG. 4.
BPV L1-HPV16 L2 (BL1-16L2) 17-36 immunizations of rabbits and mice, using Freund's or Alum-MPL adjuvant and HPV16 L1-HPV16 L2 (16L1-16L2) 17-36 immunizations of rabbits, using Alum-MPL adjuvant for evaluation by L2 peptide ELISA. ELISAs were performed in triplicates for fivefold serial serum dilutions from 100 to 7,812,500. ELISAs were performed using synthetic peptide HPV16 L2 18-31 as the antigen. Data from BL1-16L2 17-36 antisera indicate L2-specific antibody titers of 62,500 to 312,500 in NZW rabbits using Freund′s adjuvant (a), titers of 12,500 in NZW rabbits using Alum-MPL (b), and titers of 2,500 to 12,500 in BALB/c using Alum-MPL (c). Data from 16L1-16L2 17-36 antisera indicate L2-specific antibody titers of 12,500 in NZW rabbits using Alum-MPL (d). MAb RG-1 is directed against HPV16 L2 17-36 (18). Data are shown as mean OD ± standard deviations.
Both rabbits immunized with the Alum-MPL formulation elicited antisera capable of neutralizing high-risk HPV16 (100 and 100), HPV18 (100 and 100), HPV58 (100 and 100), and beta-type HPV5 (50 and 50) (Table 4). In addition, one of the rabbits' sera neutralized high-risk type HPV45 (100) and low-risk HPV6 (titers from 50 to 100) and HPV11 (100). Thus, with immunization schedules similar to those with Freund's adjuvant, VLP immunizations using Alum-MPL induced titers that were 1 or 2 orders of magnitude lower. Out of four mice immunized with BL1-16L2 17-36, one mouse developed neutralizing antibodies against HPV16 only (titer of 100), two mice elicited antibodies against HPV16 (1,000 and 50 to 100), and -18 (1,000 and 100), and one animal developed antibodies against HPV18 (100), -31 (100), -45 (100), -52 (100), and -58 (100) (Table 4).
TABLE 4.
Pseudovirion neutralization assays for antisera of two NZW rabbits and four BALB/c mice immunized with BL1-16L2 17-36 and two NZW rabbits immunized with 16L1-16L2 17-36, using Alum-MPL as the adjuvanta
| Pseudovirions | Neutralizing antibody titer with Alum-MPL
|
|||||||
|---|---|---|---|---|---|---|---|---|
| BL1-16L2 17-36
|
16L1-16L2 17-36
|
|||||||
| NZW rabbit
|
BALB/c mouse
|
|||||||
| 1 | 2 | 1 | 2 | 3 | 4 | NZW rabbit 1 | NZW rabbit 2 | |
| HPV16 | 100 | 100 | 0 | 1,000 | 100 | 50-100 | 100,000 | 100,000 |
| HPV18 | 100 | 100 | 100 | 1,000 | 0 | 100 | 1,000 | 1,000 |
| HPV31 | 0 | 0 | 100 | 0 | 0 | 0 | 10,000 | 1,000 |
| HPV45 | 100 | 0 | 100 | 0 | 0 | 0 | 1,000 | 100 |
| HPV52 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 50 |
| HPV58 | 100 | 100 | 100 | 0 | 0 | 0 | 1,000 | 1,000 |
| HPV6 | 50-100 | 0 | 0 | 0 | 0 | 0 | 100 | 50 |
| HPV11 | 100 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| HPV5 | 50 | 50 | 0 | 0 | 0 | 0 | 100 | 50 |
| CRPV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Assays were performed in duplicates for 10-fold serial serum dilutions of 1:100 to 1:1,000,000. When a lower titer of neutralization was suspected, sera were reevaluated at a dilution of 1:50.
To further develop HPV capsids as potential vaccine carrier, we incorporated the cross-protective epitope HPV16 L2 17-36 (RG-1) into HPV16 L1 (derived from the German HPV16 variant 114K [27]). Analogous to the insertion site for BPV1 L1 between aa 133 and 134, for HPV16 insertion between aa 136 and 137 was used. These amino acids are located within the DE surface loop, adjacent to a hypervariable immunodominant segment of L1. Assembly into VLP was observed by TEM (Fig. 3J). Two NZW rabbits were vaccinated with native VLP adjuvanted with Alum-MPL. As examined by peptide ELISA, immunization induced L2-specific antibodies in both animals with titers of 1:12,500 (Fig. 4d).
When analyzed by pseudovirion neutralization assays, both rabbit sera neutralized HPV16 with titers of 100,000, which largely reflects the antibody response to the HPV16 L1 VLP carrier. Moreover, robust neutralization of heterotypic high-risk types HPV18 (1,000 and 1,000), HPV31 (10,000 and 1,000), HPV45 (1,000 and 100), HPV52 (100 and 50), and HPV58 (1,000 and 1,000) was observed. In addition, neutralization of low-risk types HPV6 (100 and 50), HPV11 (100) by one of the rabbits' sera, and beta-type HPV5 (100 and 50) was detected (Table 4). As expected, rabbit antiserum #4835 (J. Schiller), raised against HPV16 L1 VLP, adjuvanted with Freund's neutralized HPV16 (1,000,000) and closely related HPV31 (1, 000) only (data not shown). These results eliminate the impact of carrier L1 on the broad range of cross-neutralization and indicate that the insertion of L2 peptides does not interfere with high-titer antibody responses to L1.
DISCUSSION
The impact of recently introduced HPV L1 VLP vaccines on the burden of HPV-associated anogenital neoplasias and their cost-effectiveness are the subject of ongoing studies. Current vaccines target HPV types that cause 90% of anogenital warts (HPV6 and HPV11) and/or 70% of cervical cancers (HPV16 and HPV18). Protection by L1 VLP vaccination is primarily type restricted with partial cross-protection against some phylogenetically related mucosal types (3, 47). Thus, cytological screening programs in vaccinated women cannot be abandoned, and the considerable costs for introduction of prophylactic immunization programs in the population must be borne in addition to screening costs. In addition, currently available vaccines are too costly at the moment for use in developing countries, where 80% of the global cervical cancer burden occurs.
Previous studies have demonstrated that vaccination with N-terminal or full-length L2 peptides or proteins can induce a low-titer, yet protective antibody response to a wide range of divergent papillomavirus types and species. The HPV16 L1-HPV16 L2 VLP vaccination strategy described herein triggers with a single construct both high-titer conformation-dependent neutralizing antibodies similar to monovalent HPV16 L1-VLP vaccination and significant levels of antibodies to a highly conserved region of L2 that cross-neutralize a broad spectrum of pathogenic HPV types.
Crystallization of small (T = 1) L1 HPV16 VLP has revealed the atomic structure of the viral capsid, in particular the hypervariable surface loops that contain the immunodominant and conformation-dependent epitopes that are recognized by neutralizing antibodies and determine the viral serotype (10). To augment immunogenicity of L2, peptides covering the N terminus of HPV16 L2 were inserted into corresponding sites of the DE loop of BPV1 L1 and HPV16 L1. The tolerated lengths of the inserted L2 peptide that still allowed for VLP assembly were 44 and 20 residues, respectively. The amino acid sequence of the insert appears to be an additional limitation for VLP assembly. It is noteworthy that sequence analysis strongly predicts the presence of a transmembrane region at aa 45 to 67 which may account for the failure of the 35-75 and 13-107 L2 constructs to assemble, and its absence from all of the constructs that do assemble. These observations extend previous studies and confirm that the immunogenic DE loop is a suitable site for antigen presentation in two different papillomavirus genera (21, 42).
Papillomavirus VLP-based vaccinations induce strong humoral (6, 11, 26, 27, 39) and cell-mediated immune responses to L1 and incorporated antigens (21, 30, 34, 41, 43, 49, 50). The antibody response to VLP is strongly dependent on the density of surface-presented epitopes (2, 8). Accordingly, rabbits immunized with native chimeric VLP demonstrate robust immune responses to L2 by ELISA, with, on average a fivefold-higher titer than vaccinations with SDS-disrupted proteins (Table 1). Assuming that this difference would likely be even greater in the absence of adjuvant, peptide display on L1 VLP surfaces appears to represent a useful strategy to overcome immune subdominance of L2 in its natural context (38).
A comprehensive examination of B-cell epitopes within the N terminus of HPV16 L2 was conducted using overlapping peptides. As chimeric BL1-16L2 69-81 and 108-120 have been published previously (42), adjacent peptides were designed without overlap. Neutralization of homologous HPV16 pseudovirions was achieved by immunizations with chimeric proteins comprising HPV16 L2 aa 17 to 148 (Table 3). These observations support previous findings that protective neutralizing antibody response to L2 of divergent PV types is mediated by N-terminal 150 aa of BPV4 L2 (5, 9), BPV1 L2 (33), CRPV-ROPV L2 (16), and HPV16 L2 (15, 24, 33).
Importantly, efficient cross-neutralization of a larger panel of mucosal HPV types was restricted to sera raised against incorporated HPV16 L2 residues aa 17 to 36, the previously described RG-1 epitope (18). Residues within this highly conserved region between different papillomavirus genera and types were previously determined to interact with a secondary cell surface receptor (48), while its critical involvement in virus infectivity is allocated to a later stage of infection (35).
The use of Alum-MPL as the adjuvant narrowed the gap between the immunization conditions of animal studies given herein and established L1 subunit vaccines. The formulation approximates the proprietary adjuvant ASO4 of Cervarix, whose adjuvant characteristics have been attributed to the activation of innate immunity by proinflammatory cytokine pathways as well as induction of memory B cells (20). Neutralizing antibody patterns between individual rabbits vaccinated with BL1-16L2 17-36 in Freund's adjuvant or Alum-MPL differ considerably, showing 1- to 2-log differences in titer or even negativity to some types, which might be due to antibodies below the detection level, rather than different epitope processing and presentation in individuals. In agreement with previous observations (15, 29), despite similar L2 ELISA titers, a large variation in neutralization levels was found in sera of inbred BALB/c mice vaccinated with BL1-16L2 17-36 plus Alum-MPL, indicating differential targeting of neutralizing and nonneutralizing L2 epitopes. It remains to be seen whether this is related to the use of Alum-MPL as compared to Freund's adjuvant. Overall, cross-neutralization was induced in two different mammals and using Alum-MPL as the adjuvant, which is applicable for human use.
Most importantly, the levels of neutralizing antibodies induced herein presumably are protective in vivo (16, 18, 19). Sensitivity of papillomaviruses to neutralization by low-titer antisera may in part be due to the unusually slow uptake kinetics into cells allowing prolonged access to neutralizing antibodies (13). In addition, a vaginal challenge model of mice points to the fact that the virus initially binds to the basement membrane of mechanically disrupted epithelium and eventually adsorbs to the epithelial cells, while they regain contact with the basement membrane (36).
Despite modification of the L1 VLP surface by L2 insertion, immunization with chimeric BL1-16L2 VLP induced high-titer anti-BPV1 L1 antibodies, irrespective of inserted peptide length. Similarly, 16L1-16L2 17-36 VLP induced high titers of HPV16 neutralizing antibodies, similar to HPV16 wild-type L1 VLP vaccination, indicating that the major immunogenic epitopes of L1 are retained. It is noteworthy that immunization of rabbits with native chimeric 16L1-16L2 17-36 VLP, adjuvanted with Alum-MPL, induced robust neutralization of high-risk HPV types 16, 18, 31, 45, 52, and 58, low-risk types 6 and 11, and beta HPV type 5, with neutralization titers ∼10- to 100-fold higher than those of sera raised against the comparable BL1-16L2 17-36 VLP under identical vaccination conditions (Table 4). These results indicate a favorable presentation of the RG-1 epitope within the amino acid composition of HPV16 L1 as the carrier. It is therefore tempting to speculate that cross-neutralization epitopes within the highly conserved region 17-36 of L2 of divergent HPV types can be displayed in an analogous manner.
The appraisal of potential second-generation HPV vaccines will be dependent on competitive costs, relevant to the establishment of vaccination programs in developing countries. Due to the possibility of large-scale production and stability, L2 (poly)peptides are promising immunogens to fulfill these requirements. Still, enhanced levels of neutralizing antibodies remain tied to the use of potent adjuvants (16, 40), carrying a risk to induce unwanted reactogenicity. Coadministration of Cervarix and L2 peptides does not diminish neutralizing antibody responses to either of the immunogens (R. Roden, unpublished observation) but does not circumvent expense and delivery restrictions. In further studies, it will be important to determine whether the slow kinetics of the declining immune response over time as observed with L1 VLP vaccines are similar to or different from those of chimeric L1-L2 VLP vaccines for both anti-L1 and anti-L2 neutralization titers.
Chimeric L1-L2 VLP are a promising strategy to develop broader-spectrum HPV vaccines. Their potential use is based on L1 VLP as the vaccine carrier, which are well tolerated and which, to date, can provide long-term protection of 8 years. As the obtained yield is similar to that of HPV16 wild-type L1 VLP (data not shown), the HPV16 L1-HPV16-L2 17-36 VLP vaccine described herein may provide broad cross-protection to heterotypic HPV types, without increased costs, in addition to high-level and type-restricted protection against homologous HPV16.
Acknowledgments
This research was supported by a grant to R.K. from the Austrian Science Foundation (P18990B13) and in part by grants to R.R. from the National Institutes of Health (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and CA118790).
R.K. is a coinventor on L1 patents licensed to GlaxoSmithKline (GSK) and Merck (MSD). R.R. is a paid consultant of Merck & Co, and Knobbe, Martens, Olson and Bear LLC. R.R. has received an unrestricted educational grant from GSK. R.R. is a coinventor on L2 patents licensed to PaxVax, Inc., Shantha Biotechnics, and Acambis, Inc., and on pseudovirion technology licensed to GSK and MSD. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Alphs, H. H., R. Gambhira, B. Karanam, J. N. Roberts, S. Jagu, J. T. Schiller, W. Zeng, D. C. Jackson, and R. B. Roden. 2008. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc. Natl. Acad. Sci. USA 105:5850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., and R. M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15:235-270. [DOI] [PubMed] [Google Scholar]
- 3.Brown, D. R., S. K. Kjaer, K. Sigurdsson, O. E. Iversen, M. Hernandez-Avila, C. M. Wheeler, G. Perez, L. A. Koutsky, E. H. Tay, P. Garcia, K. A. Ault, S. M. Garland, S. Leodolter, S. E. Olsson, G. W. Tang, D. G. Ferris, J. Paavonen, M. Steben, F. X. Bosch, J. Dillner, E. A. Joura, R. J. Kurman, S. Majewski, N. Munoz, E. R. Myers, L. L. Villa, F. J. Taddeo, C. Roberts, A. Tadesse, J. Bryan, L. C. Lupinacci, K. E. Giacoletti, H. L. Sings, M. James, T. M. Hesley, and E. Barr. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J. Infect. Dis. 199:926-935. [DOI] [PubMed] [Google Scholar]
- 4.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445-462. [DOI] [PubMed] [Google Scholar]
- 5.Campo, M. S., B. W. O'Neil, G. J. Grindlay, F. Curtis, G. Knowles, and L. Chandrachud. 1997. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology 234:261-266. [DOI] [PubMed] [Google Scholar]
- 6.Carter, J. J., M. B. Hagensee, S. K. Lee, B. McKnight, L. A. Koutsky, and D. A. Galloway. 1994. Use of HPV 1 capsids produced by recombinant vaccinia viruses in an ELISA to detect serum antibodies in people with foot warts. Virology 199:284-291. [DOI] [PubMed] [Google Scholar]
- 7.Chackerian, B. 2007. Virus-like particles: flexible platforms for vaccine development. Expert Rev. Vaccines 6:381-390. [DOI] [PubMed] [Google Scholar]
- 8.Chackerian, B., P. Lenz, D. R. Lowy, and J. T. Schiller. 2002. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol. 169:6120-6126. [DOI] [PubMed] [Google Scholar]
- 9.Chandrachud, L. M., G. J. Grindlay, G. M. McGarvie, B. W. O'Neil, E. R. Wagner, W. F. Jarrett, and M. S. Campo. 1995. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 211:204-208. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, N. D., R. Hopfl, S. L. DiAngelo, N. M. Cladel, S. D. Patrick, P. A. Welsh, L. R. Budgeon, C. A. Reed, and J. W. Kreider. 1994. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J. Gen. Virol. 75:2271-2276. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, N. D., and J. W. Kreider. 1990. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J. Virol. 64:3151-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culp, T. D., and N. D. Christensen. 2004. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology 319:152-161. [DOI] [PubMed] [Google Scholar]
- 14.de Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 324:17-27. [DOI] [PubMed] [Google Scholar]
- 15.Embers, M. E., L. R. Budgeon, T. D. Culp, C. A. Reed, M. D. Pickel, and N. D. Christensen. 2004. Differential antibody responses to a distinct region of human papillomavirus minor capsid proteins. Vaccine 22:670-680. [DOI] [PubMed] [Google Scholar]
- 16.Embers, M. E., L. R. Budgeon, M. Pickel, and N. D. Christensen. 2002. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J. Virol. 76:9798-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambhira, R., S. Jagu, B. Karanam, P. E. Gravitt, T. D. Culp, N. D. Christensen, and R. B. S. Roden. 2007. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 81:11585-11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambhira, R., B. Karanam, S. Jagu, J. N. Roberts, C. B. Buck, I. Bossis, H. Alphs, T. Culp, N. D. Christensen, and R. B. S. Roden. 2007. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J. Virol. 81:13927-13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaukroger, J. M., L. M. Chandrachud, B. W. O'Neil, G. J. Grindlay, G. Knowles, and M. S. Campo. 1996. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J. Gen. Virol. 77:1577-1583. [DOI] [PubMed] [Google Scholar]
- 20.Giannini, S. L., E. Hanon, P. Moris, M. Van Mechelen, S. Morel, F. Dessy, M. A. Fourneau, B. Colau, J. Suzich, G. Losonksy, M. T. Martin, G. Dubin, and M. A. Wettendorff. 2006. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 24:5937-5949. [DOI] [PubMed] [Google Scholar]
- 21.Handisurya, A., S. Gilch, D. Winter, S. Shafti-Keramat, D. Maurer, H. M. Schatzl, and R. Kirnbauer. 2007. Vaccination with prion peptide-displaying papillomavirus-like particles induces autoantibodies to normal prion protein that interfere with pathologic prion protein production in infected cells. FEBS J. 274:1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-1255. [DOI] [PubMed] [Google Scholar]
- 23.Hu, D., and S. Goldie. 2008. The economic burden of noncervical human papillomavirus disease in the United States. Am. J. Obstet. Gynecol. 198:500.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawana, K., K. Matsumoto, H. Yoshikawa, Y. Taketani, T. Kawana, K. Yoshiike, and T. Kanda. 1998. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology 245:353-359. [DOI] [PubMed] [Google Scholar]
- 25.Kawana, K., H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1999. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J. Virol. 73:6188-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Dürst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo, K., Y. Ishii, H. Ochi, T. Matsumoto, H. Yoshikawa, and T. Kanda. 2007. Neutralization of HPV16, 18, 31, and 58 pseudovirions with antisera induced by immunizing rabbits with synthetic peptides representing segments of the HPV16 minor capsid protein L2 surface region. Virology 358:266-272. [DOI] [PubMed] [Google Scholar]
- 29.Kondo, K., H. Ochi, T. Matsumoto, H. Yoshikawa, and T. Kanda. 2008. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J. Med. Virol. 80:841-846. [DOI] [PubMed] [Google Scholar]
- 30.Lenz, P., P. M. Day, Y. Y. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy, M. P., W. I. White, F. Palmer Hill, S. Koenig, and J. A. Suzich. 1998. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. J. Virol. 72:32-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz, N., F. X. Bosch, X. Castellsague, M. Diaz, S. de Sanjose, D. Hammouda, K. V. Shah, and C. J. Meijer. 2004. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer 111:278-285. [DOI] [PubMed] [Google Scholar]
- 33.Pastrana, D. V., R. Gambhira, C. B. Buck, Y. Y. Pang, C. D. Thompson, T. D. Culp, N. D. Christensen, D. R. Lowy, J. T. Schiller, and R. B. Roden. 2005. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology 337:365-372. [DOI] [PubMed] [Google Scholar]
- 34.Pinto, L. A., J. Edwards, P. E. Castle, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, W. Kopp, J. W. Adelsberger, M. W. Baseler, J. A. Berzofsky, and A. Hildesheim. 2003. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J. Infect. Dis. 188:327-338. [DOI] [PubMed] [Google Scholar]
- 35.Richards, R. M., D. R. Lowy, J. T. Schiller, and P. M. Day. 2006. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 103:1522-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, J. N., C. B. Buck, C. D. Thompson, R. Kines, M. Bernardo, P. L. Choyke, D. R. Lowy, and J. T. Schiller. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857-861. [DOI] [PubMed] [Google Scholar]
- 37.Roden, R. B. S., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roden, R. B. S., W. H. Yutzy, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 39.Rose, R. C., W. Bonnez, C. Da Rin, D. J. McCance, and R. C. Reichman. 1994. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J. Gen. Virol. 75:2445-2449. [DOI] [PubMed] [Google Scholar]
- 40.Rubio, I., A. Bolchi, N. Moretto, E. Canali, L. Gissmann, M. Tommasino, M. Muller, and S. Ottonello. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949-1956. [DOI] [PubMed] [Google Scholar]
- 41.Rudolf, M. P., S. C. Fausch, D. M. Da Silva, and W. M. Kast. 2001. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J. Immunol. 166:5917-5924. [DOI] [PubMed] [Google Scholar]
- 42.Slupetzky, K., R. Gambhira, T. D. Culp, S. Shafti-Keramat, C. Schellenbacher, N. D. Christensen, R. B. Roden, and R. Kirnbauer. 2007. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine 25:2001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slupetzky, K., S. Shafti-Keramat, P. Lenz, S. Brandt, A. Grassauer, M. Sara, and R. Kirnbauer. 2001. Chimeric papillomavirus-like particles expressing a foreign epitope on capsid surface loops. J. Gen. Virol. 82:2799-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzich, J. A., S. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thones, N., A. Herreiner, L. Schädlich, K. Piuko, and M. Müller. 2008. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J. Virol. 82:5472-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, K. A. Ault, A. R. Giuliano, C. M. Wheeler, L. A. Koutsky, C. Malm, M. Lehtinen, F. E. Skjeldestad, S. E. Olsson, M. Steinwall, D. R. Brown, R. J. Kurman, B. M. Ronnett, M. H. Stoler, A. Ferenczy, D. M. Harper, G. M. Tamms, J. Yu, L. Lupinacci, R. Railkar, F. J. Taddeo, K. U. Jansen, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271-278. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler, C. M., S. K. Kjaer, K. Sigurdsson, O. E. Iversen, M. Hernandez-Avila, G. Perez, D. R. Brown, L. A. Koutsky, E. H. Tay, P. Garcia, K. A. Ault, S. M. Garland, S. Leodolter, S. E. Olsson, G. W. Tang, D. G. Ferris, J. Paavonen, M. Steben, F. X. Bosch, J. Dillner, E. A. Joura, R. J. Kurman, S. Majewski, N. Munoz, E. R. Myers, L. L. Villa, F. J. Taddeo, C. Roberts, A. Tadesse, J. Bryan, L. C. Lupinacci, K. E. Giacoletti, M. James, S. Vuocolo, T. M. Hesley, and E. Barr. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J. Infect. Dis. 199:936-944. [DOI] [PubMed] [Google Scholar]
- 48.Yang, R., P. M. Day, W. H. Yutzy IV, K.-Y. Lin, C.-F. Hung, and R. B. S. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, R., F. M. Murillo, K. Y. Lin, W. H. Yutzy IV, S. Uematsu, K. Takeda, S. Akira, R. P. Viscidi, and R. B. Roden. 2004. Human papillomavirus type-16 virus-like particles activate complementary defense responses in key dendritic cell subpopulations. J. Immunol. 173:2624-2631. [DOI] [PubMed] [Google Scholar]
- 50.Zamora, E., A. Handisurya, S. Shafti-Keramat, D. Borchelt, G. Rudow, K. Conant, C. Cox, J. C. Troncoso, and R. Kirnbauer. 2006. Papillomavirus-like particles are an effective platform for amyloid-beta immunization in rabbits and transgenic mice. J. Immunol. 177:2662-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]