Abstract
We previously reported that CD4C/human immunodeficiency virus (HIV)Nef transgenic (Tg) mice, expressing Nef in CD4+ T cells and cells of the macrophage/dendritic cell (DC) lineage, develop a severe AIDS-like disease, characterized by depletion of CD4+ T cells, as well as lung, heart, and kidney diseases. In order to determine the contribution of distinct populations of hematopoietic cells to the development of this AIDS-like disease, five additional Tg strains expressing Nef through restricted cell-specific regulatory elements were generated. These Tg strains express Nef in CD4+ T cells, DCs, and macrophages (CD4E/HIVNef); in CD4+ T cells and DCs (mCD4/HIVNef and CD4F/HIVNef); in macrophages and DCs (CD68/HIVNef); or mainly in DCs (CD11c/HIVNef). None of these Tg strains developed significant lung and kidney diseases, suggesting the existence of as-yet-unidentified Nef-expressing cell subset(s) that are responsible for inducing organ disease in CD4C/HIVNef Tg mice. Mice from all five strains developed persistent oral carriage of Candida albicans, suggesting an impaired immune function. Only strains expressing Nef in CD4+ T cells showed CD4+ T-cell depletion, activation, and apoptosis. These results demonstrate that expression of Nef in CD4+ T cells is the primary determinant of their depletion. Therefore, the pattern of Nef expression in specific cell population(s) largely determines the nature of the resulting pathological changes.
The major cell targets and reservoirs for human immunodeficiency virus type 1 (HIV-1)/simian immunodeficiency virus (SIV) infection in vivo are CD4+ T lymphocytes and antigen-presenting cells (macrophages and dendritic cells [DC]) (21, 24, 51). The cell specificity of these viruses is largely dependent on the expression of CD4 and of its coreceptors, CCR5 and CXCR-4, at the cell surface (29, 66). Infection of these immune cells leads to the severe disease, AIDS, showing widespread manifestations, including progressive immunodeficiency, immune activation, CD4+ T-cell depletion, wasting, dementia, nephropathy, heart and lung diseases, and susceptibility to opportunistic pathogens, such as Candida albicans (1, 27, 31, 37, 41, 82, 93, 109). It is reasonable to assume that the various pathological changes in AIDS result from the expression of one or many HIV-1/SIV proteins in these immune target cells. However, assigning the contribution of each infected cell subset to each phenotype has been remarkably difficult, despite evidence that AIDS T-cell phenotypes can present very differently depending on the strains of infecting HIV-1 or SIV or on the cells targeted by the virus (4, 39, 49, 52, 72). For example, the T-cell-tropic X4 HIV strains have long been associated with late events and severe CD4+ T-cell depletion (22, 85, 96). However, there are a number of target cell subsets expressing CD4 and CXCR-4, and identifying which one is responsible for this enhanced virulence has not been achieved in vivo. Similarly, the replication of SIV in specific regions of the thymus (cortical versus medullary areas), has been associated with very different outcomes but, unfortunately, the critical target cells of the viruses were not identified either in these studies (60, 80). The task is even more complex, because HIV-1 or SIV can infect several cell subsets within a single cell population. In the thymus, double (CD4− CD8−)-negative (DN) or triple (CD3− CD4− CD8−)-negative (TN) T cells, as well as double-positive (CD4+ CD8+) (DP) T cells, are infectible by HIV-1 in vitro (9, 28, 74, 84, 98, 99, 110) and in SCID-hu mice (2, 5, 91, 94). In peripheral organs, gut memory CCR5+ CD4+ T cells are primarily infected with R5 SIV, SHIV, or HIV, while circulating CD4+ T cells can be infected by X4 viruses (13, 42, 49, 69, 70, 100, 101, 104). Moreover, some detrimental effects on CD4+ T cells have been postulated to originate from HIV-1/SIV gene expression in bystander cells, such as macrophages or DC, suggesting that other infected target cells may contribute to the loss of CD4+ T cells (6, 7, 32, 36, 64, 90).
Similarly, the infected cell population(s) required and sufficient to induce the organ diseases associated with HIV-1/SIV expression (brain, heart, and kidney) have not yet all been identified. For lung or kidney disease, HIV-specific cytotoxic CD8+ T cells (1, 75) or infected podocytes (50, 95), respectively, have been implicated. Activated macrophages have been postulated to play an important role in heart disease (108) and in AIDS dementia (35), although other target cells could be infected by macrophage-tropic viruses and may contribute significantly to the decrease of central nervous system functions (11, 86, 97), as previously pointed out (25).
Therefore, because of the widespread nature of HIV-1 infection and the difficulty in extrapolating tropism of HIV-1/SIV in vitro to their cell targeting in vivo (8, 10, 71), alternative approaches are needed to establish the contribution of individual infected cell populations to the multiorgan phenotypes observed in AIDS. To this end, we developed a transgenic (Tg) mouse model of AIDS using a nonreplicating HIV-1 genome expressed through the regulatory sequences of the human CD4 gene (CD4C), in the same murine cells as those targeted by HIV-1 in humans, namely, in immature and mature CD4+ T cells, as well as in cells of the macrophage/DC lineages (47, 48, 77; unpublished data). These CD4C/HIV Tg mice develop a multitude of pathologies closely mimicking those of AIDS patients. These include a gradual destruction of the immune system, characterized among other things by thymic and lymphoid organ atrophy, depletion of mature and immature CD4+ T lymphocytes, activation of CD4+ and CD8+ T cells, susceptibility to mucosal candidiasis, HIV-associated nephropathy, and pulmonary and cardiac complications (26, 43, 44, 57, 76, 77, 79, 106). We demonstrated that Nef is the major determinant of the HIV-1 pathogenicity in CD4C/HIV Tg mice (44). The similarities of the AIDS-like phenotypes of these Tg mice to those in human AIDS strongly suggest that such a Tg mouse approach can be used to investigate the contribution of distinct HIV-1-expressing cell populations to their development.
In the present study, we constructed and characterized five additional mouse Tg strains expressing Nef, through distinct regulatory elements, in cell populations more restricted than in CD4C/HIV Tg mice. The aim of this effort was to assess whether, and to what extent, the targeting of Nef in distinct immune cell populations affects disease development and progression.
MATERIALS AND METHODS
Cloning and characterization of the mCD4, CD68, and CD11c regulatory sequences.
The cloning of the CD4E and CD4F regulatory elements has previously been described (47). For the isolation of the mCD4 promoter, we screened a mouse genomic library with a murine CD4 cDNA as a probe to isolate and clone ∼50 kbp of the genomic mouse CD4 gene. This includes ∼26 kbp of DNA upstream from 5′ end of the coding region, containing the previously characterized promoter, silencer, and enhancer elements of the mCD4 gene (83, 88). The mCD4 promoter was constructed by fusing the 5′ 11.5-kbp SalI-SacI fragment of the mouse CD4 genomic sequences (containing exon 1, intron 1, part of exon 2 up to the SacI site) to the 1.9-kbp mouse CD4 enhancer, as we have done previously (48).
The human CD68 promoter was isolated from a bacterial artificial chromosome clone (RP11-1867), essentially as described previously (38). The 2.9-kbp BstXI fragment upstream of the ATG initiation codon of the CD68 gene was first subcloned to destroy the ATG and ligated upstream of the 112-bp sequences containing the first intron of the CD68 gene generated by PCR 5′ primer 1212 (5′-TGATCTAGAGTCCCCTGGGCTTTTGGCAG-3′) and 3′ primer 1211 (5′-CCGGAATTCTGCTGGGGCTACTGGCAG-3′), as described previously (38).
The mouse CD11c promoter was prepared essentially as described previously (19). It was first isolated from a phage clone from a 129-mouse genomic library by hybridization with two genomic fragments used as probes, respectively, derived from regions between exons 2 and 3 and exons 2 and 6. These genomic probes were PCR amplified from mouse genomic DNA by using the primers 839 (GCTGTCTCCAAGTTGCTCAGA) and 840 (GAACTCAGCACCGTCCATGTG) and oligonucleotide 841 (GATGAACTCAGTAGAAAGCAGAG). A 4.8-kbp SacI-BspHI fragment containing the 5′ upstream region of the CD11c gene was excised from the identified phage clone and subcloned, and the ATG in exon 2 was mutated. The identity of this clone was confirmed by DNA sequencing.
Generation of Tg mice.
The mCD4/HIVNef, CD4E/HIVNef, CD4F/HIVNef, CD68/HIVNef, and CD11c/HIVNef transgenes were constructed by ligating the five mCD4, CD4E, CD4F, CD68, and CD11c regulatory sequences, respectively, upstream to the 8.8-kbp BssHII-SacI MutG fragment of the HIV-1 pNL4-3 clone, followed by the simian virus 40 (SV40) polyadenylation signal fragment, as described previously (43, 44).
In this HIVMutG (now designated HIVNef) fragment, all of the open reading frames of the HIV-1 genes, except for that of Nef, were interrupted. Therefore, the Tg sequences are able to express only the Nef protein. These transgene DNAs were excised from the pBR322 vector with AatII, purified, and microinjected into fertilized 1-day-old (C57BL/6 × C3H)F2 embryos to generate Tg mice, as previously described (43, 44). Tg lines were established from each construct by breeding as heterozygote for the transgene with normal C3H/HeNHsd mice obtained from Harlan (Madison, WI).
Transgene expression.
The levels of transgene RNA expression were determined by Northern blot analysis on different tissues of Tg lines using 32P-labled HIV-1 probes as previously described (44). The 32P-labeled(44, 46) bands were detected with the PhosphorImager screen and scanned with the StormImaging unit (Amersham Biosciences). Semiquantitation of the RNA bands was estimated with the ImageQuant software (Amersham Bioscience). The ratio of HIV-specific 8.8-kb signal relative to the 18S ribosome-specific signal was obtained. In addition, transgene RNA expression was estimated by real-time quantitative reverse transcription-PCR (RT-PCR), as described previously (78, 106). Tg Nef protein expression was assessed by Western blot analysis of lymphoid organs or purified cells from mice of different founder (F) lines, using rabbit anti-Nef antibody, as described previously (78, 103). Proteins were visualized by incubating the membranes with secondary anti-rabbit antibodies coupled to Alexa 680 fluorochrome, followed by scanning with Odyssey infrared imaging system (Licor). For semiquantitation of the protein bands, the membranes were stripped and reincubated with anti-actin antibodies. The actin-specific bands were visualized on the imaging system described above for the Nef-specific bands. The ratio of the Nef-specific signal relative to that of actin was obtained.
Histological analysis.
Histological assessment on lymphoid and nonlymphoid organs was carried out essentially as previously described (43, 44). Semiquantitative assessment of the histological phenotypes was done as previously described (45).
Flow cytometry and apoptosis analysis.
LSR-1 and FACSCalibur flow cytometers (BD Dickinson, San Jose, CA) were used for flow cytometry. Cell suspensions from lymphoid organs (thymuses, spleen, and lymph nodes [LN]) were prepared as described previously (44, 106). Cell surface staining for depletion and activation of T cells was performed with various antibodies, as described previously (106). Labeling of myeloid cells was performed with fluorescein isothiocyanate (FITC)- or allophycocyanin (APC)-CD11c, and phycoerythrin (PE)- or PECy7-CD11b (BD Pharmingen) and PE-F4/80 (Cedarlane Labs) antibodies. CellQuest software (BD Dickinson) was used for analysis.
Purification of lymphoid and myeloid cells.
CD4+ T cells, CD8+ T cells and B cells were purified by cell sorting, by positive selection using the MoFlo cell sorter (MoFlo, Colorado), as previously described (106). Peritoneal macrophages were harvested by injecting 10 ml of fresh medium (Iscove medium supplemented with 10% fetal bovine serum) into the peritoneal cavity of the animals, followed by plating on Petri dishes (Falcon) overnight. The attached macrophages were washed three times with phosphate-buffered saline (PBS). The purity of these cells (>90%) was confirmed by using anti-Mac1 antibodies.
FL cell transplantation.
Young (8- to 12-week-old) C3H/HeN (H-2k) (CD45.2+) non-Tg mice were lethally irradiated (950 rads) and injected via the tail vein with 4 × 106 fetal liver (FL) cells from Tg (CD45.1+ and/or CD45.2+) 14.5-day-old embryos. Chimeras were analyzed 3 to 12 months after transplantation.
Killing of C. albicans by peritoneal macrophages.
Elicited peritoneal macrophages were harvested 3 days after mice were given an intraperitoneal injection of 1 ml of sterile 0.04% sodium thioglycolate (Sigma-Aldrich). The cells were washed with PBS, treated with lysing buffer to remove erythrocytes, washed again with PBS, and resuspended in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 20 mM HEPES buffer, 2 mM l-glutamine, and 100 U of penicillin, streptomycin, and gentamicin/ml. To assay killing, C. albicans LAM-1 was grown in Sabouraud dextrose broth (BD Diagnostics, Sparks, MD) at 30°C for 18 h with rotary agitation. The cells were collected by centrifugation and washed twice with PBS. Then, 2.5 × 105 macrophages were incubated with FITC-labeled, live C. albicans blastoconidia in 100 μl of supplemented RPMI medium at a 1:1 effector/target cell ratio at 37°C. A negative control assay was performed at 4°C in the presence of cytochalasin B (5 μg/ml; Sigma-Aldrich). After incubation, macrophages were lysed with 2.5% sodium deoxycholate, and killed C. albicans cells were labeled with ethidium bromide (0.1 μg/ml) and quantitated by flow cytometry.
Inoculation of C. albicans.
Oral inoculation with C. albicans LAM-1 strain, assessments for signs of morbidity, quantification of C. albicans in the oral cavities of individual mice, and determination of the burdens of C. albicans in the gastrointestinal tracts and internal organs were conducted as described previously (26).
Statistical analysis.
Statistical analyses (analysis of variance, Sigmastat, and the Student t test) were performed as previously described (26, 78, 106).
RESULTS
Construction of Tg mice.
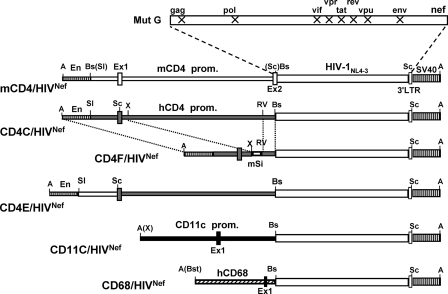
For the present study, five additional Tg strains expressing HIV-1 Nef through restricted cell-specific regulatory elements (mCD4, CD4E, CD4F, CD68, and CD11c) were generated (Fig. 1).
FIG. 1.
Structure of the transgenes. The mCD4/HIVNef, CD4E/HIVNef, CD4F/HIVNef, CD68/HIVNef, and CD11c/HIVNef DNAs were constructed as described in Materials and Methods. Symbols: En, mouse CD4 enhancer; hCD4 prom, human CD4 promoter; mSi, mouse silencer; mCD4 prom, mouse CD4 promoter; hCD68 prom, human CD68 promoter; CD11c, mouse CD11c promoter; SV40, polyadenylation sequences from SV40; Ex1 and Ex2, Exons 1 and 2. X represents interruption of the ORF of the indicated HIV-1 genes. Restriction sites: A, AatII; Bs, BssHII; Bst, BstXI; RV, EcoRV; Sc, SacI, Sl, SalI; X, XbaI. Thin bars: regulatory sequences from human CD4 (░⃞), mouse CD4 (□), mouse CD11c (▪), or human CD68 (▒) gene. Thick bars: HIV genome; stippled boxes, SV40 sequences; boxes, exons.
The mCD4 sequences, upstream of the mouse CD4 gene coding region, were previously shown to specifically direct the expression of genes mainly in thymic and peripheral CD4+ T cells, but not in cells of the monocyte/macrophage lineage (23, 83, 88). The CD4E and CD4F regulatory elements were previously shown to promote high expression of surrogate genes in CD4+ T cells and in lymphoid and myeloid DC (CD4E) or in CD4+ T cells and lymphoid DC (CD4F), but not in most macrophages (47). The CD68 sequences represent the promoter of the human CD68 gene. These sequences were previously used to express surrogate genes in myeloid cells, including macrophages (34, 38, 62). Finally, the CD11c sequences were from the mouse CD11c gene and were previously reported to promote cell-specific expression of various genes in DC (14-16). These cell-specific regulatory sequences were ligated to HIVNef, as was done previously (44) (Fig. 1).
For each DNA construct, at least two independent Tg founder (F) lines expressing the transgene were generated and used in the present study: mCD4/HIVNef (F69457, F85838, F85840, and F86708), CD4E/HIVNef (F104990 and F104991), CD4F/HIVNef (F78558, F78559, and F95136), CD68/HIVNef (F115705, F115706, F115707, F115708, and F115709), and CD11c/HIVNef (F98501, F98502, F98503, F98504, F98506, and F98507).
Transgene expression.
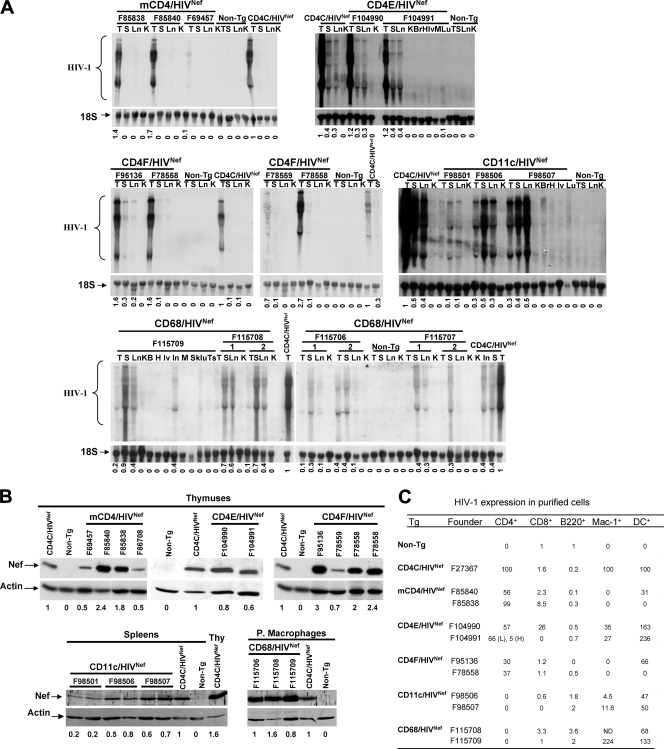
The levels of HIV transgene expression were first measured by both Northern (Fig. 2A) and Western (Fig. 2B) blotting of, respectively RNA and proteins from lymphoid and nonlymphoid organs of mice from different founder lines and compared to the levels of expression in the CD4C/HIVNef Tg mice (F27367) used in our earlier study (44). The levels of transgene expression varied among different founders, as expected, most likely as a result of variations of the transgene integration sites, with the following order: (i) in the thymus, for mCD4/HIVNef, F85840 > F85838 > (CD4C/HIVNef) F27367 > F69457 = F86708; for CD4E/HIVNef, F104991 = F104990 ≤ F27367 (CD4C/HIVNef); and for CD4F/HIVNef, F95136 ≥ F78558 > F27367 (CD4C/HIVNef) > F78559; (ii) in the peritoneal macrophages, for CD68/HIVNef, F115708 > F115706 = F27367 (CD4C/HIVNef) > F115709 = F115707; and (iii) in the spleen, for CD11c/HIVNef, F98507 = F27367 (CD4C/HIVNef) = F98506 > F98501. For CD68/HIVNef Tg mice, Nef expression was easily detectable in the LN, in the spleen, and in peritoneal macrophages, whereas CD11c-driven HIVNef expression was best detectable in the spleens and LN of Tg mice. These results showed that the levels of transgene expression were comparable to or higher than those of CD4C/HIVNef Tg mice in at least one line of each Tg strain.
FIG. 2.
Expression of HIV in Tg mice. (A) Northern blot analysis of HIV-1 RNA from various tissues of Tg mice of the indicated founder (F) lines and from non-Tg littermates. RNAs from CD4C/HIVNef Tg mice (F27367) serve as a positive control. Total RNAs were hybridized with 32P-labeled HIV-1-specific probes. The blots were striped and rehybridized with a 32P-labeled DNA oligonucleotide probe homologous to 18S rRNA. Also shown below each blot are the semiquantitative values for the levels of expression relative to CD4C/HIV Tg tissues (value of 1), measured as described in Materials and Methods. Symbols: T, thymus; S, spleen; Ln, lymph nodes; K, kidney; Br, brain; Lv, liver; Lu, lungs; H, heart; Sk, skin, Ts, testis; M, muscle. (B) Western blot analysis of Nef in protein extracts from different tissues. For mCD4/HIVNef, CD4E/HIVNef and CD4FNef Tg mice, thymuses from 1-month-old mice and from their non-Tg littermates were used. A thymus from CD4C/HIVNef Tg mouse was used as a positive control. For CD68/HIVNef and CD11c/HIVNef Tg mice, proteins were detected in peritoneal macrophages and spleen, respectively. The membranes were probed with rabbit anti-Nef antibodies and then stripped and blotted with anti-actin antibody. Proteins were visualized by incubating the membranes with secondary anti-rabbit antibodies coupled to Alexa 680 fluorochrome, followed by scanning with an Odyssey infrared imaging system (Licor). The actin-specific bands were visualized on the same imaging system as described above for the Nef-specific bands. The ratio of the Nef-specific signal relative to that of actin was obtained. The numbers below each lane represent the semiquantitative values for the levels of expression relative to CD4C/HIVNef Tg cells (value of 1), measured as described in Materials and Methods. (C) Quantitative RT-PCR analysis. The indicated cell populations were purified by cell sorting or by plating (for peritoneal macrophages) from the LN, spleens, and peritoneal cavities of the different founder strains, as described in Materials and Methods. Staining was performed with PE-coupled anti-mouse B220 (B cells), with APC-coupled anti-mouse CD4 (CD4+ T cells), with FITC-coupled anti-mouse CD8 (CD8+ T cells) or with FITC-coupled anti-mouse CD11c + PE-coupled anti-mouse CD11b (CD11c+CD11bHigh myeloid DC). Cells were sorted by gating on the PE-, APC-, FITC-, or PE+FITC-positive population. Total RNA was extracted from these cells and quantitative real-time RT-PCR was carried out to detect the HIV-1 transgene RNA, as described in Materials and Methods. The relative levels of expression are shown, with arbitrary values of 100 or 1. All values for the CD4+ cells, macrophages, or DC of each founder line of each strain are expressed relative to 100 of CD4C/HIVNef Tg CD4+ T cells, macrophages, or DC, respectively. All values for CD8+ T cells and B220+ B cells of each founder line of each strain are expressed relative to the value 1 of non-Tg CD8+ T cells or B220+ cells, respectively. Therefore, for each cell subset, a comparison was made between the different strains. In F104991 CD4E/HIVNef Tg mice, CD4+ T cells expressing high (H) or low (L) CD4 were analyzed. ND, not done.
To further document and monitor the identity of the cells expressing HIV-1 Nef, different cell populations were purified from the LN and spleen (CD4+ and CD8+ T cells, B cells, and DCs) and from the peritoneum (macrophages) of each Tg line and processed for quantitative real-time RT-PCR (Fig. 2C). Expression of the transgene was observed mainly in CD4+ T cells of mCD4/HIVNef Tg mice; in CD4+ T cells and DC (one founder) of CD4F/HIVNef Tg mice; in CD4+ T cells, DC, and to a lower extent in macrophages and CD8+ T cells (one founder) of CD4E/HIVNef Tg mice, thus confirming our previous data (47) on the specificity of these latter two promoters. For CD68/HIVNef Tg mice, high transgene expression was documented in macrophages (confirming data obtained by Western blot analysis [Fig. 2B]) and DC, but not at significant levels in lymphoid cells. Finally, the expression of HIV-1 in CD11c/HIVNef Tg mice was mainly detected in DC, as expected.
Development of very mild AIDS-like nonlymphoid organ diseases in CD4F/HIVNef Tg mice and their absence in mCD4/HIVNef and CD4E/HIVNef Tg mice.
In addition to severe immunodeficiency, the CD4C/HIVNef Tg mice also develop severe kidney, lung, and cardiac diseases and have a shorter life span (44, 55). To determine whether similar diseases affect these new Tg mice, groups of 15 to 20 Tg mice from each founder of mCD4/HIVNef, CD4F/HIVNef, and CD4E/HIVNef Tg strains and their non-Tg littermates were generated and observed for up to 12 months for the development of signs of illness (hypoactivity and weakness, ruffled hair, respiratory problems, weight loss [wasting], and diarrhea). During this observation period, these Tg mice survived as long as the non-Tg mice. At necropsy, they did not show any gross pathology, except that the peripheral LN were smaller (see below). Microscopic assessment of nonlymphoid tissues made in Tg mice at different ages (up to 10 months old) revealed no disease in the kidney and lungs of all founders of CD4E/HIVNef, CD4F/HIVNef, and mCD4/HIVNef Tg mice. In fact, these nonlymphoid organs seemed undistinguishable from those of their non-Tg littermates. In contrast, older (>12-month-old) CD4F/HIVNef Tg mice showed identical, but much milder Tg-specific pathologies in the nonlymphoid organs similar to those observed previously in CD4C/HIVNef Tg lines (43, 44).
The complete absence of nonlymphoid organ diseases in mCD4/HIVNef and CD4E/HIVNef Tg mice indicates that high expression of Nef in only CD4+ T cells or in some DC subsets and macrophages (47) is not sufficient to induce all of the AIDS-like phenotypes in mice. These data strongly suggest that Nef must be expressed in other cell subset(s) to initiate the development of the multiple organ lesions observed in CD4C/HIVNef Tg animals.
The number of CD4+ T cells is decreased in Tg mice expressing Nef through the mCD4, CD4E, or CD4F regulatory elements.
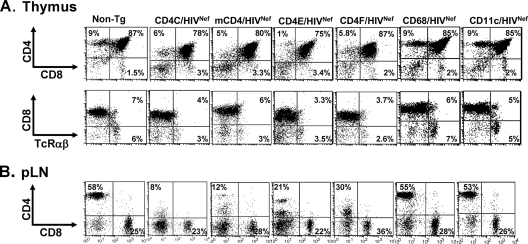
Compared to that of their age-matched non-Tg littermates, thymuses of mCD4/HIVNef, CD4E/HIVNef, or CD4F/HIVNef Tg mice displayed a lower cellularity (Table 1) than did the thymuses of CD4C/HIVNef Tg mice (44). Fluorescence-activated cell sorting (FACS) analysis confirmed that the absolute number of DP CD4+ CD8+ and SP CD4+ thymocytes of young (<3-month-old) Tg animals from most founder lines were decreased relative to that of their non-Tg littermates (Fig. 3A and Table 1). In addition, the CD4 cell surface expression was downregulated on both SP and DP thymocytes (Fig. 3A and Table 1). FACS analysis of cells from the pLN (Fig. 3B and Table 2) or spleens (data not shown) of mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg mice revealed a severe CD4+ T-cell depletion similar to that in CD4C/HIVNef Tg mice, which resulted in an alteration of the CD4/CD8 cell ratio (Fig. 3B and Table 2). Furthermore, the CD4 surface protein on the pLN CD4+ T cells was severely downregulated (Fig. 3B and Table 2).
TABLE 1.
Thymic cell surface marker analysis in HIVNef Tg micea
| Mouse lineb | Mean no. of cells (10−6) ± SD in different subpopulations
|
CD4/CD8 | Mean fluorescence (%) ± SDc
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | CD4+ CD8+ | CD4+ CD8− | CD4− CD8+ | Mean TcR count ± SD | CD4 | CD8 | SpCD4 | ||
| Non-Tg | 120 ± 37 | 99 ± 31 | 10.5 ± 3.4 | 2.3 ± 0.6 | 13.8 ± 5.1 | 4.0 ± 0.8 | 100 | 100 | 100 |
| CD4C/HIVNef | 65 ± 18* | 55 ± 15* | 2.9 ± 1.1* | 2.6 ± 0.6* | 5.4 ± 1.6* | 1.1 ± 0.2* | 37 ± 2.8* | 53 ± 9.4* | 20 ± 4.5* |
| mCD4/HIVNef | |||||||||
| F85838 | 56 ± 26* | 44 ± 21* | 4.6 ± 2.4* | 1.7 ± 0.8* | 5.6 ± 3* | 2.6 ± 0.9 | 46 ± 10* | 52 ± 4* | 25 ± 9* |
| F85840 | 92 ± 49 | 74 ± 42 | 5.4 ± 2* | 3.4 ± 1.1 | 1.6 ± 0.5* | 7.0 ± 3* | 45 ± 7* | 51 ± 7* | 37 ± 13* |
| CD4F/HIVNef | |||||||||
| F78558 | 84 ± 36 | 68 ± 27 | 4.7 ± 2.3* | 4.4 ± 2.0 | 5.6 ± 2.6* | 1.0 ± 0.1* | 36 ± 3.1* | 57 ± 4.5* | 32 ± 10* |
| F95136 | 45 ± 23* | 39 ± 20* | 1.7 ± 0.9* | 1.6 ± 1* | 3.0 ± 1.7* | 1.1 ± 0.1* | 10 ± 0.6* | 64 ± 1.3* | 31 ± 2* |
| CD4E/HIVNef | |||||||||
| F104990 | 30 ± 6.2* | 25 ± 5.0* | 1.2 ± 0.2* | 1.1 ± 0.2* | 2.2 ± 0.4* | 1.1 ± 0.1* | 38 ± 2* | 55 ± 0.7* | 53 ± 13* |
| F104991 | 43 ± 9.6* | 31 ± 7.8* | 4.2 ± 1.4* | 2 ± 0.3 | 4.3 ± 0.9* | 2.2 ± 0.5* | 47 ± 21* | 70 ± 8* | 60 ± 6.1* |
| CD68/HIVNef | |||||||||
| F115706 | 114.9 ± 21.7 | 95.0 ± 17.8 | 9.3 ± 1.2 | 2.7 ± 0.5 | 17.0 ± 2.9 | 4.9 ± 1.6 | 93.7 ± 10.5 | 94.4 ± 9.4 | 100 |
| F115708 | 86.5 ± 9.5 | 70.9 ± 13 | 7.8 ± 1.5 | 3.2 ± 1.3 | 13.6 ± 5.8 | 2.6 ± 0.6 | 78 ± 12 | 82 ± 3 | 100 |
| F115709 | 82.5 ± 30.2 | 68.4 ± 25.5 | 6.7 ± 2.3 | 2.6 ± 1.1 | 12.6 ± 5.3 | 2.6 ± 0.6 | 84.1 ± 11.8 | 99.9 ± 18.4 | 100 |
| CD11c/HIVNef | |||||||||
| F98501 | 88.3 ± 23.0 | 75.3 ± 21.4 | 6.1 ± 0.9 | 1.7 ± 0.4 | 11.2 ± 2.4 | 3.0 ± 0.3 | 99.9 ± 5.9 | 96.7 ± 5.8 | 99.9 ± 18.4 |
| F98506 | 91.4 ± 15 | 73 ± 13 | 12.4 ± 3.1 | 2.0 ± 0.4 | 14.4 ± 3.4 | 5.9 ± 0.9 | 96 ± 4 | 92 ± 2.7 | 100 |
| F98507 | 107 ± 27.1 | 84.6 ± 19.2 | 12 ± 3.8 | 3.5 ± 2 | 14 ± 4.8 | 4.0 ± 1.2 | 100 | 100 | 100 |
*, P < 0.05 according to the Student t test, comparing Tg with non-Tg groups. TcR, T-cell receptor.
FACS analysis was performed on four to nine mice (1.5 to 3 months old) for each Tg line, including the positive control CD4C/HIVNef Tg mice, except for CD11c/HIVNef and CD68/HIVNef mice (both founders), which were analyzed at 2 to 4 months of age. The non-Tg control values were obtained by pooling the results of all non-Tg littermates from different lines.
The mean fluorescence values for CD4 and CD8 in DP CD4+ CD8+ and CD4 in SP T cells were obtained by calculating the ratio of CD4 or CD8 staining in Tg thymuses relative to those of non-Tg thymuses (100%). Mean values were then calculated with the values for each line.
FIG. 3.
FACS analysis of thymic and peripheral T lymphocytes from Tg mice. Thymus (A) and peripheral lymph node (pLN) (B) cells from a representative Tg mouse from each founder line expressing higher HIV Nef levels (mCD4/HIVNef [F85840], CD4E/HIVNef [F104991], CD4F/HIVNef [F95136], CD68/HIVNef [F115709], and CD11c/HIVNef [F98507]), as well as from a CD4C/HIVNef Tg mouse and a non-Tg littermate, were analyzed the same day by flow cytometry for the expression of CD4, CD8, and TcRαβ. The percentage of T cells found in each quadrant is indicated. These data are representative of at least three independent experiments with four to nine (2- to 3-month-old) mice in each line.
TABLE 2.
Peripheral LN cell surface marker analysis in HIVNef Tg micea
| Mouse lineb | Mean no. of cells (10−6) ± SD in different subpopulations
|
Mean CD4/CD8 ratio ± SD | Mean fluorescence (%) ± SDc
|
||||
|---|---|---|---|---|---|---|---|
| Total | CD4+ | CD8+ | TcR | CD4 | CD8 | ||
| Non-Tg | 17.4 ± 5.2 | 9.0 ± 2.5 | 3.8 ± 1.9 | 13.0 ± 4.8* | 2.5 ± 0.5* | 100 | 100 |
| CD4C/HIVNef | 5.8 ± 3.9* | 0.9 ± 0.6* | 1.4 ± 1* | 2.1 ± 1.5* | 0.6 ± 0.1* | 15 ± 9* | 80 ± 20 |
| mCD4/HIVNef | |||||||
| F85838 | 3.9 ± 1.8* | 0.6 ± 0.3* | 1.0 ± 0.5* | 1.6 ± 0.8* | 0.5 ± 0.1* | 30 ± 5* | 95 ± 5 |
| F85840 | 3.3 ± 0.7* | 0.3 ± 0.1* | 0.9 ± 0.4* | 1.4 ± 0.5* | 0.5 ± 0.2* | 21 ± 13* | 92 ± 2 |
| CD4F/HIVNef | |||||||
| F78558 | 3.8 ± 1.4* | 1.1 ± 0.4* | 1.2 ± 0.4* | 2.3 ± 0.9* | 0.9 ± 0.1* | 16 ± 3* | 90 ± 10 |
| F95136 | 6.0 ± 3.5* | 0.8 ± 0.7* | 1.7 ± 0.1* | 2.7 ± 1.8* | 0.4 ± 0.2* | 21 ± 9* | 97 ± 7 |
| CD4E/HIVNef | |||||||
| F104990 | 3.6 ± 0.9* | 0.7 ± 0.2* | 0.9 ± 0.2* | 1.6 ± 0.4* | 0.8 ± 0.1* | 26 ± 8* | 75 ± 11* |
| F104991 | 6.8 ± 0.3* | 1.1 ± 0.4* | 1.2 ± 0.1* | 2.1 ± 0.3* | 0.9 ± 0.4* | 28 ± 20* | 70 ± 2* |
| CD68/HIVNef | |||||||
| F115708 | 11.1 ± 1.1* | 5.7 ± 0.3* | 2.2 ± 0.2 | 8 ± 0.5* | 2.5 ± 0.2 | 93 | 91 |
| F115709 | 12.8 ± 5 | 6.1 ± 2.1 | 3.1 ± 1.4 | 6.9 ± 5 | 2 ± 0.1 | 100 | 90 |
| CD11c | |||||||
| F98506 | 15 ± 6.2 | 8.4 ± 4.3 | 3.2 ± 1.4 | 11.6 ± 5.6 | 2.5 ± 0.4 | 100 | 100 |
| F98507 | 10.8 ± 1.7* | 5.9 ± 1.0* | 2.1 ± 0.6 | 8.1 ± 1.6* | 2.8 ± 0.3 | 100 | 100 |
*, P < 0.05 according to the Student's t test, comparing Tg with non-Tg growth.
FACS analysis was performed on four to nine mice (1.5 to 3 months old) for each Tg line, except for CD4F/HIVNef (F95136) and CD68/HIVNef mice (both founders), which were analyzed at 2 to 4 months of age. The non-Tg control values were obtained by pooling the results of all non-Tg littermates from different lines.
The mean fluorescence values for CD4 and CD8 T cells were obtained by calculating the ratio of Tg CD4 or CD8 relative to that of non-Tg lymph nodes (100%). Mean values were then calculated with the values for each line.
These results show that when Nef is expressed under the regulation of the mCD4, CD4E, or CD4F sequences, thymic and peripheral CD4+ T cells are severely depleted to a degree comparable to that observed in CD4C/HIVNef Tg mice, confirming previous studies in Tg mice expressing Nef with different T-cell-specific promoters (12, 65, 89).
Peripheral mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg CD4+ T cells exhibit an activated/memory-like phenotype.
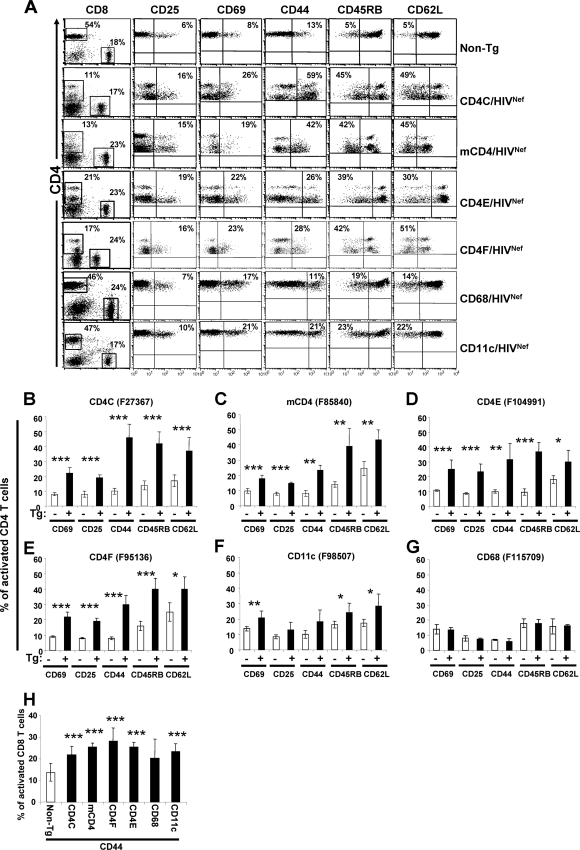
We next examined whether peripheral CD4+ T cells from mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg mice exhibit an activated/memory-like phenotype as in CD4C/HIVNef Tg mice (106) or in other Tg mice showing T-cell-restricted Nef expression (58, 65, 89). FACS analysis showed that a larger proportion of Tg CD4+ T cells expressed CD69 and CD25 cell surface markers and were CD44high, CD45RBlow, and CD62Llow cells (Fig. 4A to E). Similarly, Tg CD8+ T cells showed signs of activation in these three Tg lines, i.e., a higher percentage of CD8+ CD44+ cells, but not of the other activation markers mentioned above, relative to non-Tg controls (Fig. 4H).
FIG. 4.
Immunophenotype of activation markers on pLN CD4+ and CD8+ T cells from Tg mice. (A) Three-color FACS analysis (CD4-APC, CD8-FITC, and CD25-, CD44-, CD45RB-, CD62L-, or CD69-PE) of CD4+ T cells was performed on pLN from a representative Tg mouse from each strain expressing HIV at high levels, as well as from control CD4C/HIVNef Tg mouse and a non-Tg littermate, as described in the legend to Fig. 3. Isotype control antibody for each activation marker was used as a negative control. The number in the upper quadrant represents the percentage of cells in this quadrant. (B to G) Quantification of the percentages (mean ± the standard error of the mean [SEM]) of CD4+ T cells expressing CD25, CD69 and CD44 cell surface markers, or being negative for CD45RB and CD62L, as analyzed and shown in panel A. The data were pooled from five to seven non-Tg mice and six to eight Tg mice (3 to 7 months old) from each Tg mouse founder line as described for Fig. 3A. Statistical analyses were performed by using the Student t test by comparing the Tg CD4+ T cells with non-Tg CD4+ T cells expressing the indicated cell surface markers. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (H) Histograms representing the percentages (mean ± the SEM) of CD8+ T cells expressing CD44 in different Tg strains. These were obtained and analyzed as described in panels B to G.
These data indicate that this activated phenotype can segregate from other Nef-induced organ diseases (e.g., kidney, lung) and is not sufficient for their development.
Enhanced apoptosis/death of peripheral CD4+ T cells from mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg mice.
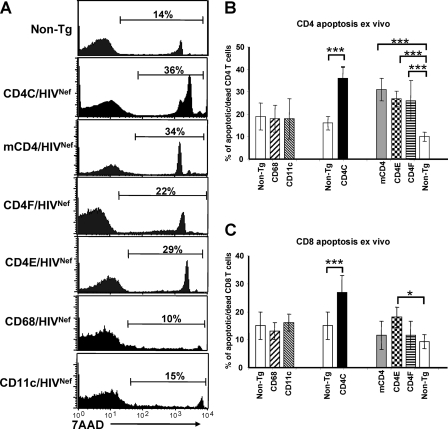
We also measured indices of apoptosis in cells of peripheral LN from mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg mice by using 7-aminoactinomycin D (7AAD) staining. As shown by FACS analysis, the percentage of 7AAD-positive (apoptotic/dead cells) CD4+ T cells was larger in all of these Tg mice than in their non-Tg littermates (Fig. 5A and B), and comparable to that in CD4C/HIVNef Tg mice (79). Such enhanced apoptosis was not observed in Tg CD8+ T cells from the mCD4/HIVNef and CD4F/HIVNef Tg mice and was modest in those of CD4E/HIVNef Tg mice (Fig. 5C). Together, these results suggest that restricted Nef expression in CD4+ T cells is sufficient to induce their death by apoptosis.
FIG. 5.
Assessment of cell death of pLN CD4+ and CD8+ T cells from Tg mice. (A) FACS profiles of apoptotic and/or dead CD4+ T cells. The cells isolated from pLN of a Tg mouse from each strain, as described in the legend to Fig. 3, were stained with anti-CD4 and 7AAD and were analyzed by FACS, by gating on CD4+ T cells. The percentage of apoptotic and/or dead CD4+ T cells from these representative mice is shown, corresponding to the high and intermediate 7AAD-positive cells. (B and C) Quantitation of apoptotic and/or dead CD4+ (B) or CD8+ (C) T cells from pLN. The data were analyzed by FACS as described in panel A and were pooled from results on 5 to 12 Tg (dark bar) and their 11 non-Tg littermate (white bar) 2- to 3-month-old mice from each line, as indicated. Bars in the histogram represent the percentage (mean ± the SEM) of 7AAD-positive CD4+ (B) or CD8+ (C) T cells. Statistical analysis was performed by using the Student t test. *, P < 0.05; ***, P < 0.001.
CD4+ T cells are only slightly depleted in CD68/HIVNef and CD11c/HIVNef Tg mice.
It has been shown that expression of HIV-1 genes in macrophages or DC has detrimental effects on CD4+ T cells in vitro (6, 7, 36, 64, 90). To examine the consequences of myeloid cell-restricted Nef expression on T cells in vivo, we generated CD68/HIVNef and the CD11c/HIVNef Tg mice (Fig. 1), expressing HIV-1 Nef at undetectable levels in CD4+ T cells, but at high levels in macrophages and DC or in DC only, respectively (Fig. 2B and C). Mice from each of the CD68/HIVNef and CD11c/HIVNef Tg strains remained healthy and fertile and survived as long as non-Tg mice during the whole observation period, for up to 12 months. Macroscopic and microscopic assessment performed blindly on young (<6-month-old) and older (7- to 12-month-old) Tg mice from founder lines expressing the highest levels of Nef (F115708 and F115709 for CD68/HIVNef and F98506 and F98507 for CD11c/HIVNef) revealed no disease in the kidneys of these Tg mice (see Table S1 in the supplemental material). However, mild lung disease (LIP) and pulmonary hypertension were occasionally observed in one CD68/HIVNef and in both CD11c/HIVNef founder Tg mice (see Table S1 in the supplemental material). In contrast to what is usually observed in CD4C/HIVNef Tg mice, the numbers of thymocytes in CD68/HIVNef and CD11c/HIVNef Tg mice were in the normal range and comparable to those of age-matched non-Tg littermates (Table 1). However, a modest decrease of pLN cellularity was observed in older (3- to 6-month-old) CD11c/HIVNef and CD68/HIVNef Tg mice (Table 2). In addition, splenomegaly was a frequent manifestation in older CD11C/HIVNef Tg mice but rarely observed in CD68/HIVNef Tg mice (data not shown) and may reflect an accumulation of CD11b+ cells (see below).
To determine whether cooperation between Nef-expressing cells would lead to the development of more extensive pathological changes, we generated CD68HIVNef × mCD4/HIVNef, CD68/HIVNef × CD4E/HIV Nef, and CD68/HIVNef × CD4F/HIVNef double Tg lines. Analysis of histological changes in different organs and of the distribution of the major lymphoid populations (by FACS analysis) of these double Tg mice revealed no additional phenotypic changes compared to those observed in single Tg mice from each strain (data not shown).
FACS analysis of thymic cell populations (DN CD4− CD8− cells, DP CD4+ CD8+ cells, and SP CD4+ CD8− or CD8+ CD4− T cells) of CD68/HIVNef and CD11c/HIVNef Tg mice revealed that they were comparable in percentage and absolute cell numbers to those of their non-Tg littermates (Fig. 3A and Table 1). However, the percentage and absolute cell numbers of CD4+ T cells in pLN of one line of CD11c/HIVNef (F98507) and of CD68/HIVNef (F115708) Tg mice was modestly decreased (Fig. 3B and Table 2 and data not shown), although such mild depletion was not apparent in mesenteric LN of several founders (data not shown). In addition, staining for the activation markers CD25, CD69, CD44, CD45RB, and CD62L showed that a slightly higher percentage of CD4+ T cells expressed CD69 and were CD45RBlow and CD62Llow in both young and older CD11c/HIVNef Tg mice relative to their control littermates (Fig. 4F). Such enhanced activation was rarely observed in CD68/HIVNef Tg mice (Fig. 4G). Apoptosis and/or death of pLN CD4+ and CD8+ T cells was not significantly different in CD68/HIVNef and CD11c/HIVNef Tg mice relative to that in their control littermates (Fig. 5B and C). In addition, FACS analysis revealed an accumulation of CD11b+ cells in the spleen of CD11c/HIVNef Tg mice from the two founder lines studied compared to non-Tg mice but not in other lymphoid organs examined (LN and thymus) (data not shown). This cell population was further studied with markers for macrophages and DC, since this latter cell subset has previously been shown to display abnormalities in CD4C/HIV Tg mice (76). The percentage of myeloid CD11bhi CD11c+ DC was significantly increased in the spleens and pLN of CD11c/HIVNef and CD68/HIVNef Tg mice compared to those of non-Tg mice (Table 3), and these Tg DC showed signs of immaturity, exhibiting lower cell surface expression of major histocompatibility complex class II (data not shown). This immature myeloid DC phenotype is similar to that previously reported in CD4C/HIV Tg mice (76). The number of CD11b+ F4/80+ macrophages was increased in the spleens of CD11c/HIV Tg mice relative to those in control non-Tg mice (20 ± 5 versus 5 ± 2.4 × 106; P < 0.001), but this increase was milder in CD68/HIVNef Tg mice (9 ± 4 versus 6 ± 1).
TABLE 3.
FACS analysis of myeloid DC from CD11c/HIVNef and CD68/HIVNef Tg mice
| Mouse linea | Mean values obtained for CD11c+ cells ± SDb
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
LN
|
|||||
| Total no.c | % CD11bhid | % CD11bint/low | Total no. | % CD11bhi | % CD11bint/low | |
| Non-Tg | 1.8 ± 1.2 | 35 ± 4 | 52 ± 4 | 0.1 ± 0.1 | 25 ± 7 | 55 ± 9 |
| CD11c/HIVNef | 2.5 ± 0.8 | 41 ± 5†d | 46 ± 5† | 0.1 ± 0.06 | 39 ± 11* | 46 ± 6* |
| Non-Tg | 1.7 ± 0.4 | 35 ± 1.8 | 51 ± 1 | 0.5 ± 0.1 | 34 ± 4 | 54 ± 4.4 |
| CD68/HIVNef | 1.4 ± 0.3 | 45 ± 2.8‡ | 45 ± 1.7* | 0.4 ± 0.1 | 49 ± 3.9† | 42 ± 2.2† |
Tg CD11c/HIVNef (F98507) and CD68 (F115708) mice (3 to 6 months old) with their respective littermates. Number of animals: For spleen, 12 CD11c/HIVNef and 5 CD68/HIVNef Tg mice with, respectively, nine and five non-Tg littermates; for LN, eight CD11c/HIVNef and four CD68/HIVNef Tg mice with, respectively, five and four non-Tg littermates.
*, P ≤ 0.05; †, P < 0.01; ‡, P < 0.001.
That is, the absolute cell number (106).
Gated on total CD11c+ DC.
Together, these experiments show that Nef expression in macrophages or DC affects their maturation but is not sufficient for inducing disease in nonlymphoid organs and does not contribute significantly to CD4+ T-cell depletion.
Nonlymphoid organ diseases cannot be transferred by transplantation of fetal liver cells.
We next investigated whether organ diseases of Tg mice could be transferred by transplantation of Tg hematopoietic cells. For this, Tg and control non-Tg BM or FL cells were transplanted into lethally irradiated normal mice. Recipient mice were sacrificed 3 to 12 months posttransplantation. Transplantation was successful in all mice, as determined by reconstitution of the CD4+ and CD8+ T cells clearly derived from the donor BM cells (P. Chrobak et al., unpublished data). None of the 13 Tg chimeric mice generated developed Tg-specific organ diseases, as evaluated histologically (data not shown), indicating that the putative Nef-expressing cells responsible for inducing organ diseases are not transplantable hematopoietic cells.
Chronic oral carriage of C. albicans in Tg mice.
In order to determine whether expression of Nef in specific subsets of hematopoietic cells is required for inducing mucosal candidiasis, already documented in CD4C/HIVMutA Tg mice (26), CD4C/HIVNef, mCD4/HIVNef, CD4E/HIVNef, CD4F/HIVNef, CD68/HIVNef, and CD11c/HIVNef Tg mice and their non-Tg littermates were inoculated orally with C. albicans LAM-1.
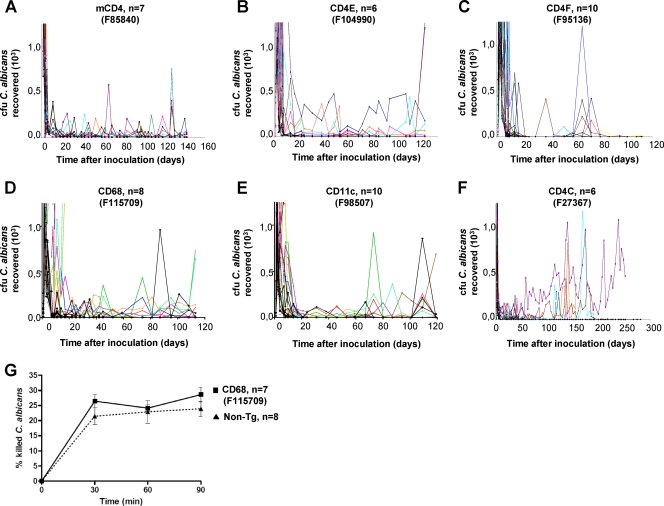
Longitudinal assessments of oral burdens of C. albicans showed that primary infection in non-Tg mice was self-limited, with uniform clearance of C. albicans from the oral cavities within 9 days after inoculation. In contrast, oral burdens were significantly elevated (P < 0.05) in Tg mice from all strains tested for at least 4 months (Fig. 6A to E), although to a lesser extent than in CD4C/HIVNef Tg mice (Fig. 6F). In addition, high burdens of C. albicans were found in the stomachs and small and large intestines of Tg mice from all strains but were only rarely found in the non-Tg animals (Table 4). Dissemination to internal organs (brain, lungs, liver, or kidneys) was limited in most Tg mice from all strains, a finding consistent with previous observations in CD4C/HIVMutA Tg mice (26).
FIG. 6.
Oral C. albicans burdens in Tg mice. (A to F) Tg mice (2 to 3 months old) from the indicated strains and positive control CD4C/HIVNef (F27367), as well as their non-Tg littermates, were inoculated intraorally with C. albicans, and the burdens were assessed longitudinally by sampling the oral cavities. Color lines represent individual Tg mice; black lines represent non-Tg littermates. The number (n) of mice inoculated in each group is indicated in each panel. (G) Phagocytosis and killing of C. albicans blastoconidia by peritoneal macrophages of non-Tg or CD68/HIVNef (F115709) Tg mice.
TABLE 4.
Viable CFU in organs of HIVNef Tg mice orally infected with C. albicans
| Variable | Mouse typea
|
||||||
|---|---|---|---|---|---|---|---|
| CD4C/HIVNef | CD4E/HIVNef | CD4F/HIVNef | mCD4/HIVNef | hCD68/HIVNef | CD11c/HIVNef | Control non-Tg | |
| No. of animals inoculated | 6 | 6 | 10 | 7 | 8 | 10 | 42 |
| Age at inoculation (days) | 76 | 78 | 36-76 | 130-133 | 66-89 | 76-82 | 36-130 |
| Mean age at assessment in days (range) | 293 (194-357)b | 189c | 175 (149-189)d | 275 (273-276)e | 189 (178-201)f | 193 (192-198)g | 195 (148-293)h |
| Organs culture positive for C. albicans | |||||||
| Brain | |||||||
| No. of mice | 2 | 1 | 0 | 0 | 2 | 0 | 0 |
| Mean C. albicans count (CFU/g) | 1.8 × 103 | 6.9 × 103 | NA | NA | 1.8 × 102 | NA | NA |
| Range (CFU/g) | 1.5 × 103-2.1 × 103 | NA | NA | NA | 3.1 × 101-3.2 × 102 | NA | NA |
| Lungs | |||||||
| No. of mice | 1 | 1 | 1 | 0 | 2 | 0 | 0 |
| Mean C. albicans count (CFU/g) | 4.5 × 103 | 1.8 × 103 | 8.3 × 100 | NA | 1.1 × 102 | NA | NA |
| Range (CFU/g) | NA | NA | NA | NA | 7.0 × 101-1.5 × 102 | NA | NA |
| Liver | |||||||
| No. of mice | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean C. albicans count (CFU/g) | 4.5 × 102 | NA | NA | NA | NA | NA | NA |
| Range (CFU/g) | 2.7 × 102-6.3 × 102 | NA | NA | NA | NA | NA | NA |
| Kidneys | |||||||
| No. of mice | 2 | 1 | 0 | 0 | 1 | 0 | 0 |
| Mean C. albicans count (CFU/g) | 2.8 × 102 | 6.9 × 102 | NA | NA | 5.0 × 101 | NA | NA |
| Range (CFU/g) | 1.1 × 102-4.4 × 102 | NA | NA | NA | NA | NA | NA |
| Stomachi | |||||||
| No. of mice | 5 | 4 | 5 | 7 | 7 | 6 | 9 |
| Mean C. albicans count (CFU/g) | 1.0 × 105 | 2.3 × 105 | 1.7 × 105 | 2.2 × 105 | 7.2 × 104 | 1.1 × 105 | 1.6 × 105 |
| Range (CFU/g) | 7.9 × 102-4.0 × 105 | 4.4 × 104-5.3 × 105 | 4.1 × 104-4.7 × 105 | 2.5 × 104-4.9 × 105 | 9.1 × 101-5.8 × 105 | 2.0 × 102-3.4 × 105 | 1.3 × 104-3.8 × 105 |
| Small intestinei | |||||||
| No. of mice | 5 | 6 | 5 | 7 | 6 | 7 | 9 |
| Mean C. albicans count (CFU/g) | 2.0 × 103 | 2.0 × 104 | 2.6 × 103 | 3.3 × 103 | 5.0 × 103 | 8.1 × 102 | 1.6 × 103 |
| Range (CFU/g) | 5.9 × 101-6.5 × 103 | 1.2 × 104-3.8 × 104 | 2.9 × 102-7.9 × 103 | 6.5 × 101-1.1 × 104 | 3.2 × 101-8.1 × 103 | 3.3 × 101-1.7 × 103 | 6.6 × 101-9.2 × 103 |
| Large intestinei | |||||||
| No. of mice | 5 | 4 | 5 | 7 | 6 | 8 | 9 |
| Mean C. albicans count (CFU/g) | 3.4 × 104 | 8.6 × 104 | 1.0 × 104 | 8.3 × 103 | 2.3 × 104 | 9.4 × 103 | 6.1 × 103 |
| Range (CFU/g) | 8.5 × 102-1.2 × 105 | 2.8 × 104-1.9 × 105 | 3.2 × 103-2.4 × 104 | 1.2 × 103-2.3 × 104 | 5.3 × 102-9.6 × 104 | 4.0 × 101-4.3 × 104 | 7.1 × 102-1.3 × 104 |
Mice studied included Tg and control non-Tg offspring derived from founder mouse F27367 (CD4C/HIVNef), F104990 (CD4E/HIVNef), F95136 (CD4F/HIVNef), F85840 (mCD4/HIVNef, high expresser) and F69457 (mCD4/HIVNef, low expresser), F115709 (hCD68/HIVNef), and F98507 (CD11c/HIVNef). NA, not applicable.
Four mice were nonsurvivors (assessments, days 194 to 338); two surviving mice were euthanized (assessment, day 357).
All six mice survived and were euthanized at 189 days of age.
All 10 mice survived and were euthanized at 149 to 189 days of age.
All seven mice survived and were euthanized at 273 to 276 days of age.
All eight mice survived and were euthanized at 178 to 201 days of age.
All 10 mice survived and where euthanized at 192 to 198 days of age.
Control non-Tg littermates were euthanized on the same day as the Tg mice.
Note that clearance of C. albicans from stomach and intestine of some non-Tg mice occurs more slowly than from the oral cavity, but without causing mucosal candidiasis as in Tg mice.
Chronic oral carriage of C. albicans in CD11c/HIVNef and CD68/HIVNef Tg mice suggested that Nef-expressing myeloid DC and/or macrophages may exhibit altered functions. We measured the capacity of elicited peritoneal macrophages from CD68/HIVNef Tg mice (F115709) to kill C. albicans. This latter line was chosen because it shows high transgene expression in both macrophages and DC. Unexpectedly, CD68/HIVNef Tg peritoneal macrophages were found to kill C. albicans to levels comparable to those of control non-Tg mice (Fig. 6G), suggesting that the susceptibility to candidiasis of these Tg mice does not result from a defective effector function of macrophages against C. albicans.
Together, these results indicate that restricted HIV Nef expression in DC combined or not with expression in macrophages and CD4+ T cells can lead to the development of persistent oral carriage of C. albicans in Tg mice.
DISCUSSION
Our previous studies have shown that CD4C/HIVNef Tg mice which express HIV-1 Nef in immature and mature CD4+ T cells and in cells of the myeloid/DC lineage develop severe AIDS-like pathologies (44, 57, 76, 77, 79, 106). In the present study, we used five distinct regulatory elements to drive expression of HIV-1 Nef in more restricted immune cell populations of Tg mice. We found that distinct phenotypes were associated with Nef expression in specific immune cell populations. We could also rule out the contribution of some Nef-expressing cell populations to the development of a number of phenotypes.
Restricted expression of Nef in CD4+ T cells, in macrophages and in DC is not sufficient for the development of nonlymphoid organ diseases.
We previously reported that nonlymphoid organ diseases (kidney, heart, and lung) developed in CD4−/− CD4C/HIV Tg mice in which the Nef-expressing CD4+ T cells were deleted (106). This strongly suggested that Nef expression in CD4+ T cells was dispensable for their development. A similar conclusion could be inferred from other studies of Tg mice whose Nef expression, driven by the CD3δ, CD2, or TcRβ promoter, was presumably restricted to T cells, although this was not documented (12, 65, 89). In the present study, we extended and confirmed these data by showing that the restricted Nef expression in CD4+ T cells of mCD4/HIVNef Tg mice did not induce lung and kidney diseases. Surprisingly, we also found that expression of Nef in CD4+ T cells plus DC (mCD4/HIVNef and CD4F/HIVNef), in CD4+ T cells plus macrophages plus DC [(mCD4/HIVNef × CD68/HIVNef) double Tg], in macrophages plus DC (CD68/HIVNef) and in DC (CD11c/HIVNef) was not sufficient to induce these latter organ diseases. These results are rather intriguing because these targeted cell populations represent the majority of the immune cells expressing the HIV transgene in the CD4C/HIV Tg mice (43, 47, 48, 77). We have ruled out that the lower pathogenicity for organ diseases in these five new Tg strains could result from lower levels of Nef expression than in CD4C/HIVNef Tg mice, by directly measuring HIV expression in each of the purified targeted cell populations (Fig. 2C). Therefore, our data strongly suggest that one or many, as-yet-unidentified, cell subset(s) expressing Nef under the regulation of the CD4C elements, but unable to support expression by the mCD4, CD4E, CD4F, CD68, or CD11c promoter and not originating from transplantable hematopoietic cell progenitors, is critical for the development of nonlymphoid organ diseases. This unknown population(s) could represent subsets of nontransplantable myeloid cells or DC not targeted by the CD68 or CD11c promoter, respectively. Alternatively, it could represent a cell population(s) different from the CD4+ T cells, myeloid cells, and DC and yet still expressing CD4 in human but not in mice (mimicked in Tg mouse by expression with the human CD4C or the mouse mCD4 regulatory elements, respectively). Endothelial cells and kidney glomerular epithelial cells (podocytes) could possibly represent such populations, although these populations are not known to express CD4. Some authors have reported infection of vascular cells (30, 92, 105) by HIV or SIV, and renal epithelial cells have been shown to be infected by HIV in patients with HIV-1-associated nephropathy (18, 20, 67). Moreover, expression of HIV Nef or other HIV gene products in kidney cells of Tg mice has been reported to induce kidney disease (17, 53, 54, 56, 111). It would be most important to identify the cell population(s) which appears to be so critical for inducing nonlymphoid organ diseases by Nef. Since the human CD4C regulatory elements seem to be active in this murine cell population(s), the corresponding human cell population(s) is likely to express the CD4 receptor. If such a population expresses an appropriate coreceptor, it may also be infectible by HIV-1 through its CD4/coreceptor and may represent a significant novel reservoir for the virus.
Restricted expression of Nef in several distinct cell subsets is sufficient to induce chronic oral carriage of C. albicans in Tg mice.
We found that the five newly generated HIV Tg strains studied here exhibit susceptibility to oral candidiasis comparable to that of CD4C/HIVNef Tg mice. Such results were expected in mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef Tg mice showing depletion and functional anomalies of CD4+ T cells, since our previous work has demonstrated that loss of CD4+ T cells and/or altered CD4+ T-cell phenotype and function determine, at least in part, the susceptibility to chronic oral carriage of C. albicans in CD4C/HIVMutA Tg mice (63). The susceptibility to candidiasis of CD68/HIVNef and CD11c/HIVNefTg mice also concurs with our previous observations, in which expression of the transgene in either or both CD4+ T cells and DCs abrogated the proliferative response of CD4+ T cells in C. albicans-pulsed DC/CD4+ T-cell cocultures in vitro (63). Therefore, successful control of C. albicans in the oral mucosa of the normal host likely requires the participation and interaction of both of these cell populations.
Nef-expressing DCs in CD68/HIVNef and CD11c/HIVNef Tg mice appear to be defective at generating a fully competent T-cell-mediated adaptive immune response to C. albicans, either as a result of intrinsic defects or because their CD4+ T cells are functionally impaired. Whatever the mechanism, the present data clearly show that mucosal candidiasis segregates independently of CD4+ T-cell loss and of organ diseases in these Tg mouse strains, suggesting a pathogenesis for candidiasis distinct from each of these two latter phenotypes, which themselves appear to segregate independently (45, 78, 103).
Expression of Nef in myeloid cells is not sufficient to induce depletion of CD4+ T cells.
Only a modest decrease of CD4+ T cells could be documented in pLN (but not in mesenteric LN) of older, but not of younger, CD11c/HIVNef or CD68/HIVNef Tg mice. In addition, the numbers of thymic CD4+ T-cell subsets in these Tg mice were comparable to those of their non-Tg littermates. These results indicate that high expression of Nef in either or both DC and macrophages has little influence on the production and/or maintenance of these lymphoid T-cell populations. This result rules out a model by which CD4+ T cells would be depleted in CD4C/HIVNef Tg mice as a consequence of their contact with these Nef-expressing myeloid cells or with factor(s) secreted from them. These in vivo results appear to contrast with previous studies showing that HIV-1-infected human DC or macrophages can mediate CD4+ T-cell death in vitro (6, 36, 64, 90).
In humans, a major contribution of the presence of this pool of HIV-1-infected myeloid cells may be primarily to facilitate CD4+ T-cell infection, a process well documented (3, 87, 107) but which cannot be scored in the Tg biological system used here.
Expression of Nef in CD4+ T cells is necessary and sufficient to induce their depletion.
If expression of Nef in macrophages and DC is not sufficient to induce CD4+ T-cell depletion, our results strongly suggest that its expression in CD4+ T cells is necessary and sufficient for their loss. First, we found that in each strain expressing Nef in CD4+ T cells (mCD4/HIVNef, CD4E/HIVNef, and CD4F/HIVNef), the peripheral CD4+ T cells were depleted to a level comparable to that observed in CD4C/HIVNef Tg mice. Second, we could establish that the diseases in nonlymphoid organs (and the toxic circulating factors produced by them) do not constitute an important contributor to the CD4+ T-cell depletion because significant depletion of DP and SP CD4+ thymocytes and peripheral CD4+ T cells still occurs in the absence of organ lesions in these Tg mice. Third, the absence of significant CD4+ T-cell depletion in CD68/HIVNef and CD11c/HIVNef Tg mice has ruled out Nef-expressing macrophages and DC as likely cell subsets involved in major loss of CD4+ T cells. Fourth, it would be very unlikely that the putative Nef-expressing cell population(s) that we postulated to be responsible for the nonlymphoid organ diseases (see above) would be involved in CD4+ T-cell depletion. Indeed, analysis of several Nef mutants and alleles expressed in Tg mice has shown that thymic/peripheral CD4+ T-cell depletion and organ disease are two phenotypes segregating independently (45, 78, 103). Thus, expression of Nef in CD4+ T cells appears to be required and may be sufficient for their depletion, although the contribution of all possible CD4+ cell populations (such as the rare accessory CD4+ CD3− cells [61]) has not been studied. These results confirm and extend previous work on other Tg mice expressing Nef through T-cell-restricted promoters (12, 65, 89), although Nef was expressed in both CD4+ and CD8+ T cells in all of these Tg mice. Interestingly, we found that the extent of thymocyte depletion in mice from one mCD4/HIVNef (F85840) and one CD4F/HIVNef (F78558) founder line was relatively modest despite the higher levels of Nef expression in thymocytes of these mice relative to those of control CD4C/HIVNef Tg mice. This may suggest additional complexity in the mechanisms of thymocyte versus peripheral CD4+ T-cell depletion induced by Nef, a phenomenon previously observed in our work on Nef mutants or alleles (45, 78, 103) and requiring further studies.
Concluding remarks.
The use of distinct cell-specific promoters to express Nef in Tg mice has thus revealed the critical role of unique cell populations in the induction of specific phenotypes. These studies strongly suggest that the nonlymphoid organ diseases (lung and kidney) and immune disease (CD4+ T-cell loss) are induced by Nef expression in distinct cell populations. These studies have also revealed the minimal role of Nef-expressing macrophages and DC in inducing these phenotypes and have suggested the existence of another, as-yet-unidentified, Nef-expressing cell subset responsible for inducing the organ diseases and possibly representing another reservoir of Nef-expressing cells. In humans, much of the cell specificity of HIV-1 is provided by its receptor/coreceptor expressed on the target cells. However, since the phenotypes observed in CD4C/HIVNef Tg mice are very similar to those arising in AIDS patients, and since HIV-1 Nef interacts with effectors very well conserved (PAK2, hck, etc.) between mice and humans, the consequences of Nef expression in distinct human cell populations are likely to be very similar to those described in these Tg mice. If indeed this mouse model of AIDS is relevant, it would predict that infection of human immature thymocytes (CD4+ CD8+ [DP]) and possibly their progenitors (CD3− CD4+, CD4− CD8− [DN], or CD3− CD4− CD8− [TN]), known to be infectible with HIV-1 in vitro (9, 28, 74, 84, 98, 99, 110) and in SCID-hu mice (2, 5, 91, 94), is the major explanation for the severe changes observed in thymus (smaller volume, depletion of CD4+ T cells, loss of architecture) of HIV-1-infected subjects (40, 59, 73, 81, 102; reviewed in references 33 and 68). It would also predict the existence of another reservoir of Nef-expressing cells (not yet identified) that are critical for the development of nonlymphoid organ phenotypes in some patients.
Supplementary Material
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research, HIV/AIDS Research Program, to P.J., Z.H., and L.D.R. and from the NIH (HL-083470) to P.J. P.J. is the recipient of a Canada Research Chair.
We thank Benoît Laganière, Ginette Massé, Pascale Jovert, Valérie Côté, Karina Lamarre, Stéphanie Lemay, Marie-Eve Higgins, Caroline Larocque, Jonathan Kergoat, Eve-Lyne Thivierge, Jean-René Sylvestre, Francine Aumont, Daniel Rivas, and Isabelle Corbin for their excellent technical assistance. We are grateful to Michel Robillard and Qinzhang Zhu from the transgenic core facility for generating Tg mice. We thank Eric Massicotte and Martine Dupuis from the Cell Biology core for cell sorting; Miguel Chagnon, Michel Lamoureux, and Yves Lepage for statistical analysis; Denis G. Kay for early pathological assessment; and Paul Brochu and Johanne Poudrier for early work on the CD68 promoter and on the CD11c/HIV Tg mice, respectively.
Footnotes
Published ahead of print on 15 July 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Agostini, C., and G. Semenzato. 1996. Immunologic effects of HIV in the lung. Clin. Chest Med. 17:633-645. [DOI] [PubMed] [Google Scholar]
- 2.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 3.Ancuta, P., K. J. Kunstman, P. Autissier, T. Zaman, D. Stone, S. M. Wolinsky, and D. Gabuzda. 2006. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344:267-276. [DOI] [PubMed] [Google Scholar]
- 4.Auewarakul, P., K. Sangsiriwut, S. Suwanagool, and C. Wasi. 2001. Target cell populations of human immunodeficiency virus type 1 in peripheral blood lymphocytes with different chemokine receptors at various stages of disease progression. J. Virol. 75:6384-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autran, B., P. Guiet, M. Raphael, M. Grandadam, H. Agut, D. Candotti, P. Grenot, F. Puech, P. Debre, and J. Y. Cesbron. 1996. Thymocyte and thymic microenvironment alterations during a systemic HIV infection in a severe combined immunodeficient mouse model. AIDS 10:717-727. [DOI] [PubMed] [Google Scholar]
- 6.Badley, A. D., D. Dockrell, M. Simpson, R. Schut, D. H. Lynch, P. Leibson, and C. V. Paya. 1997. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J. Exp. Med. 185:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badley, A. D., J. A. McElhinny, P. J. Leibson, D. H. Lynch, M. R. Alderson, and C. V. Paya. 1996. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J. Virol. 70:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banapour, B., M. L. Marthas, R. J. Munn, and P. A. Luciw. 1991. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). Virology 183:12-19. [DOI] [PubMed] [Google Scholar]
- 9.Bonyhadi, M. L., L. Su, J. Auten, J. M. McCune, and H. Kaneshima. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1073-1080. [DOI] [PubMed] [Google Scholar]
- 10.Borda, J. T., X. Alvarez, I. Kondova, P. Aye, M. A. Simon, R. C. Desrosiers, and A. A. Lackner. 2004. Cell tropism of simian immunodeficiency virus in culture is not predictive of in vivo tropism or pathogenesis. Am. J. Pathol. 165:2111-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 12.Brady, H. J., D. J. Pennington, C. G. Miles, and E. A. Dzierzak. 1993. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. EMBO J. 12:4923-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brocker, T., A. Gulbranson-Judge, S. Flynn, M. Riedinger, C. Raykundalia, and P. Lane. 1999. CD4 T-cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 29:1610-1616. [DOI] [PubMed] [Google Scholar]
- 15.Brocker, T., M. Riedinger, and K. Karjalainen. 1997. Driving gene expression specifically in dendritic cells. Adv. Exp. Med. Biol. 417:55-57. [DOI] [PubMed] [Google Scholar]
- 16.Brocker, T., M. Riedinger, and K. Karjalainen. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruggeman, L. A., S. Dikman, C. Meng, S. E. Quaggin, T. M. Coffman, and P. E. Klotman. 1997. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J. Clin. Investig. 100:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruggeman, L. A., M. D. Ross, N. Tanji, A. Cara, S. Dikman, R. E. Gordon, G. C. Burns, V. D. D'Agati, J. A. Winston, M. E. Klotman, and P. E. Klotman. 2000. Renal epithelium is a previously unrecognized site of HIV-1 infection. J. Am. Soc. Nephrol. 11:2079-2087. [DOI] [PubMed] [Google Scholar]
- 19.Chiodoni, C., P. Paglia, A. Stoppacciaro, M. Rodolfo, M. Parenza, and M. P. Colombo. 1999. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J. Exp. Med. 190:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen, A. H., N. C. Sun, P. Shapshak, and D. T. Imagawa. 1989. Demonstration of human immunodeficiency virus in renal epithelium in HIV-associated nephropathy. Modern Pathol. 2:125-128. [PubMed] [Google Scholar]
- 21.Collman, R. G., C. F. Perno, S. M. Crowe, M. Stevenson, and L. J. Montaner. 2003. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: new answers yield new questions. J. Leukoc. Biol. 74:631-634. [DOI] [PubMed] [Google Scholar]
- 22.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crocker, P. R., W. A. Jefferies, S. J. Clark, L. P. Chung, and S. Gordon. 1987. Species heterogeneity in macrophage expression of the CD4 antigen. J. Exp. Med. 166:613-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe, S. M., and R. S. Kornbluth. 1994. Overview of HIV interactions with macrophages and dendritic cells: the other infection in AIDS. J. Leukoc. Biol. 56:215-217. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani, H., B. A. Puffer, R. W. Doms, and V. M. Hirsch. 2003. Unique pattern of convergent envelope evolution in simian immunodeficiency virus-infected rapid progressor macaques: association with CD4-independent usage of CCR5. J. Virol. 77:6405-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Repentigny, L., F. Aumont, J.-S. Ripeau, M. Fiorillo, D. G. Kay, Z. Hanna, and P. Jolicoeur. 2002. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J. Infect. Dis. 185:1103-1114. [DOI] [PubMed] [Google Scholar]
- 27.de Repentigny, L., D. Lewandowski, and P. Jolicoeur. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 17:729-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Rossi, A., M. L. Calabro, M. Panozzo, D. Bernardi, B. Caruso, G. Tridente, and L. Chieco-Bianchi. 1990. In vitro studies of HIV-1 infection in thymic lymphocytes: a putative role of the thymus in AIDS pathogenesis. AIDS Res. Hum. Retrovir. 6:287-298. [DOI] [PubMed] [Google Scholar]
- 29.Dimitrov, D. S. 2000. Cell biology of virus entry. Cell 101:697-702. [DOI] [PubMed] [Google Scholar]
- 30.Eugenin, E. A., S. Morgello, M. E. Klotman, A. Mosoian, P. A. Lento, J. W. Berman, and A. D. Schecter. 2008. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. Am. J. Pathol. 172:1100-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 32.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 33.Gaulton, G. N., J. V. Scobie, and M. Rosenzweig. 1997. HIV-1 and the thymus. AIDS 11:403-414. [DOI] [PubMed] [Google Scholar]
- 34.Gavin, A. L., B. Duong, P. Skog, D. Ait-Azzouzene, D. R. Greaves, M. L. Scott, and D. Nemazee. 2005. DeltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J. Immunol. 175:319-328. [DOI] [PubMed] [Google Scholar]
- 35.Gendelman, H. E., S. A. Lipton, M. Tardieu, M. I. Bukrinsky, and H. S. Nottet. 1994. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 56:389-398. [DOI] [PubMed] [Google Scholar]
- 36.Godard, C. M., H. Serriès, C. Fulachier, and J. C. Chermann. 1997. Apoptosis of CD4+ T cells induced after contact with HIV1-infected or non-infected macrophages. Institute Pasteur/Elsevier 148:383-396. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Scarano, F., and J. Martin-Garcia. 2005. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 5:69-81. [DOI] [PubMed] [Google Scholar]
- 38.Gough, P. J., S. Gordon, and D. R. Greaves. 2001. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology 103:351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grody, W. W., S. Fligiel, and F. Naeim. 1985. Thymus involution in the acquired immunodeficiency syndrome. Am. J. Clin. Pathol. 84:85-95. [DOI] [PubMed] [Google Scholar]
- 41.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 42.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna, Z., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and P. Jolicoeur. 1998. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol. 72:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna, Z., D. G. Kay, N. Rebai, A. Guimond, S. Jothy, and P. Jolicoeur. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163-175. [DOI] [PubMed] [Google Scholar]
- 45.Hanna, Z., E. Priceputu, C. Hu, P. Vincent, and P. Jolicoeur. 2006. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology 346:40-52. [DOI] [PubMed] [Google Scholar]
- 46.Hanna, Z., E. Priceputu, D. G. Kay, J. Poudrier, P. Chrobak, and P. Jolicoeur. 2004. In vivo mutational analysis of the N-terminal region of HIV-1 Nef reveals critical motifs for the development of an AIDS-like disease in CD4C/HIV transgenic mice. Virology 327:273-286. [DOI] [PubMed] [Google Scholar]
- 47.Hanna, Z., N. Rebai, J. Poudrier, and P. Jolicoeur. 2001. Distinct regulatory elements are required for faithful expression of human CD4 in T cells, macrophages and dendritic cells of transgenic mice. Blood 98:2275-2278. [DOI] [PubMed] [Google Scholar]
- 48.Hanna, Z., C. Simard, and P. Jolicoeur. 1994. Specific expression of the human CD4 gene in mature CD4+ CD8− and immature CD4+ CD8+ T cells, and in macrophages of transgenic mice. Mol. Cell. Biol. 14:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harouse, J. M., A. Gettie, R. C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 284:816-819. [DOI] [PubMed] [Google Scholar]
- 50.He, J. C., M. Husain, M. Sunamoto, V. D. D'Agati, M. E. Klotman, R. Iyengar, and P. E. Klotman. 2004. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J. Clin. Investig. 114:643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirsch, V. M., P. M. Zack, A. P. Vogel, and P. R. Johnson. 1991. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J. Infect. Dis. 163:976-988. [DOI] [PubMed] [Google Scholar]
- 52.Hu, Q. X., A. P. Barry, Z. X. Wang, S. M. Connolly, S. C. Peiper, and M. L. Greenberg. 2000. Evolution of the human immunodeficiency virus type 1 envelope during infection reveals molecular corollaries of specificity for coreceptor utilization and AIDS pathogenesis. J. Virol. 74:11858-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Husain, M., V. D. D'Agati, J. C. He, M. E. Klotman, and P. E. Klotman. 2005. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS 19:1975-1980. [DOI] [PubMed] [Google Scholar]
- 54.Husain, M., G. L. Gusella, M. E. Klotman, I. H. Gelman, M. D. Ross, E. J. Schwartz, A. Cara, and P. E. Klotman. 2002. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J. Am. Soc. Nephrol. 13:1806-1815. [DOI] [PubMed] [Google Scholar]
- 55.Jolicoeur, P., D. G. Kay, M. Cool, S. Jothy, N. Rebai, and Z. Hanna. 1999. A novel mouse model of HIV-1 disease. Leukemia 13:S78-S80. [DOI] [PubMed] [Google Scholar]
- 56.Kajiyama, W., J. B. Kopp, N. J. Marinos, P. E. Klotman, and P. Dickie. 2000. Glomerulosclerosis and viral gene expression in HIV-transgenic mice: role of nef. Kidney Int. 58:1148-1159. [DOI] [PubMed] [Google Scholar]
- 57.Kay, D. G., P. Yue, Z. Hanna, S. Jothy, E. Tremblay, and P. Jolicoeur. 2002. Cardiac disease in transgenic mice expressing human immunodeficiency virus-1 Nef in cells of the immune system. Am. J. Pathol. 161:321-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koenen, P. G., F. M. Hofhuis, M. A. Oosterwegel, and K. Tesselaar. 2007. T cell activation and proliferation characteristic for HIV-Nef transgenic mice is lymphopenia induced. J. Immunol. 178:5762-5768. [DOI] [PubMed] [Google Scholar]
- 59.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 60.Lackner, A., P. Vogel, R. A. Ramos, J. D. Kluge, and M. Marthas. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145:428-439. [PMC free article] [PubMed] [Google Scholar]
- 61.Lane, P. J., F. M. Gaspal, and M. Y. Kim. 2005. Two sides of a cellular coin: CD4+ CD3− cells regulate memory responses and lymph node organization. Nat. Rev. Immunol. 5:655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang, R., R. L. Rutschman, D. R. Greaves, and P. J. Murray. 2002. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 168:3402-3411. [DOI] [PubMed] [Google Scholar]
- 63.Lewandowski, D., M. Marquis, F. Aumont, A. C. Lussier-Morin, M. Raymond, S. Senechal, Z. Hanna, P. Jolicoeur, and L. de Repentigny. 2006. Altered CD4+ T-cell phenotype and function determine the susceptibility to mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J. Immunol. 177:479-491. [DOI] [PubMed] [Google Scholar]
- 64.Lichtner, M., C. Maranon, P. O. Vidalain, O. Azocar, D. Hanau, P. Lebon, M. Burgard, C. Rouzioux, V. Vullo, H. Yagita, C. Rabourdin-Combe, C. Servet, and A. Hosmalin. 2004. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res. Hum. Retrovir. 20:175-182. [DOI] [PubMed] [Google Scholar]
- 65.Lindemann, D., R. Wilhelm, P. Renard, A. Althage, R. Zinkernagel, and J. Mous. 1994. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J. Exp. Med. 179:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Littman, D. R. 1998. Chemokine receptors: keys to AIDS pathogenesis? Cell 93:677-680. [DOI] [PubMed] [Google Scholar]
- 67.Marras, D., L. A. Bruggeman, F. Gao, N. Tanji, M. M. Mansukhani, A. Cara, M. D. Ross, G. L. Gusella, G. Benson, V. D. D'Agati, B. H. Hahn, M. E. Klotman, and P. E. Klotman. 2002. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat. Med. 8:522-526. [DOI] [PubMed] [Google Scholar]
- 68.McCune, J. M. 1997. Thymic function in HIV-1 disease. Semin. Immunol. 9:397-404. [DOI] [PubMed] [Google Scholar]
- 69.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehandru, S., M. A. Poles, K. Tenner-Racz, V. Manuelli, P. Jean-Pierre, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81:599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller, C. J., M. Marthas, J. Greenier, D. Lu, P. J. Dailey, and Y. Lu. 1998. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J. Virol. 72:3248-3258. (Erratum, 72:6277.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T-cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 101:12324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papiernik, M., Y. Brossard, N. Mulliez, J. Roume, C. Brechot, F. Barin, A. Goudeau, J. F. Bach, C. Griscelli, and R. Henrion. 1992. Thymic abnormalities in fetuses aborted from human immunodeficiency virus type 1 seropositive women. Pediatrics 89:297-301. [PubMed] [Google Scholar]
- 74.Pedroza-Martins, L., K. B. Gurney, B. E. Torbett, and C. H. Uittenbogaart. 1998. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J. Virol. 72:9441-9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plata, F., B. Autran, L. P. Martins, S. Wain-Hobson, M. Raphael, C. D. M. Mayaud, J. M. Guillon, and P. Debre. 1987. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature 328:348-351. [DOI] [PubMed] [Google Scholar]
- 76.Poudrier, J., X. Weng, D. G. Kay, Z. Hanna, and P. Jolicoeur. 2003. The AIDS-like disease of CD4C/HIV Tg mice is associated with accumulation of immature CD11bhi dendritic cells. J. Virol. 77:11733-11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poudrier, J., X. Weng, D. G. Kay, G. Paré, E. L. Calvo, Z. Hanna, M. H. Kosco-Vilbois, and P. Jolicoeur. 2001. The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-g and IL-6. Immunity 15:173-185. [DOI] [PubMed] [Google Scholar]
- 78.Priceputu, E., Z. Hanna, C. Hu, M.-C. Simard, P. Vincent, S. Wildum, M. Schindler, F. Kirchhoff, and P. Jolicoeur. 2007. Primary human immunodeficiency virus type 1 nef alleles show major differences in pathogenicity in transgenic mice. J. Virol. 81:4677-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Priceputu, E., I. Rodrigue, P. Chrobak, J. Poudrier, T. W. Mak, Z. Hanna, Hu.C., D. G. Kay, and P. Jolicoeur. 2005. The Nef-mediated AIDS-like disease of CD4C/HIV transgenic mice is associated with increased Fas/FasL expression on T cells and T cell death, but is not prevented in Fas, FasL, TNFR-1, and ICE-deficient nor in Bcl2-expressing transgenic mice. J. Virol. 79:6377-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reyes, R. A., D. R. Canfield, U. Esser, L. A. Adamson, C. R. Brown, C. Cheng-Mayer, M. B. Gardner, J. M. Harouse, and P. A. Luciw. 2004. Induction of simian AIDS in infant rhesus macaques infected with CCR5- or CXCR4-utilizing simian-human immunodeficiency viruses is associated with distinct lesions of the thymus. J. Virol. 78:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenzweig, M., D. P. Clark, and G. N. Gaulton. 1993. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS 7:1601-1605. [DOI] [PubMed] [Google Scholar]
- 82.Ross, M. J., and P. E. Klotman. 2004. HIV-associated nephropathy. AIDS 18:1089-1099. [DOI] [PubMed] [Google Scholar]
- 83.Sawada, S., J. D. Scarborough, N. Killeen, and D. R. Littman. 1994. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell 77:917-929. [DOI] [PubMed] [Google Scholar]
- 84.Schnittman, S. M., S. M. Denning, J. J. Greenhouse, J. S. Justement, M. K. J. Baseler, B. F. Haynes, and A. S. Fauci. 1990. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCRαβ+ and TCRγδ+ to human immunodeficiency virus infection: a mechanism for CD4+ (T4) lymphocyte depletion. Proc. Natl. Acad. Sci. USA 87:7727-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schramm, B., M. L. Penn, R. F. Speck, S. Y. Chan, E. De Clercq, D. Schols, R. I. Connor, and M. A. Goldsmith. 2000. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J. Virol. 74:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz, L., L. Civitello, A. Dunn-Pirio, S. Ryschkewitsch, E. Berry, W. Cavert, N. Kinzel, D. M. Lawrence, R. Hazra, and E. O. Major. 2007. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J. Neurovirol. 13:274-283. [DOI] [PubMed] [Google Scholar]
- 87.Sewell, A. K., and D. A. Price. 2001. Dendritic cells and transmission of HIV-1. Trends Immunol. 22:173-175. [DOI] [PubMed] [Google Scholar]
- 88.Siu, G., A. L. Wurster, D. Duncan, T. M. Soliman, and S. M. Hedrick. 1994. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO J. 13:3570-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T-cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sperber, K., P. Beuria, N. Singha, I. Gelman, P. Cortes, H. Chen, and T. Kraus. 2003. Induction of apoptosis by HIV-1-infected monocytic cells. J. Immunol. 170:1566-1578. [DOI] [PubMed] [Google Scholar]
- 91.Stanley, S. K., J. M. McCune, H. Kaneshima, J. S. Justement, M. Sullivan, E. B. M. Boone, J. Adelsberger, M. Bonyhadi, and J. Orenstein. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steffan, A. M., M. E. Lafon, J. L. Gendrault, C. Schweitzer, C. Royer, D. Jaeck, J. P. Arnaud, M. P. Schmitt, A. M. Aubertin, and A. Kirn. 1992. Primary cultures of endothelial cells from the human liver sinusoid are permissive for human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 89:1582-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9:853-860. [DOI] [PubMed] [Google Scholar]
- 94.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 95.Sunamoto, M., M. Husain, J. C. He, E. J. Schwartz, and P. E. Klotman. 2003. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 64:1695-1701. [DOI] [PubMed] [Google Scholar]
- 96.Tersmette, M., R. E. de Goede, B. J. Al, I. N. Winkel, R. A. Gruters, H. T. H. H. Cuypers, and F. Miedema. 1988. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J. Virol. 62:2026-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trillo-Pazos, G., A. Diamanturos, L. Rislove, T. Menza, W. Chao, P. Belem, S. Sadiq, S. Morgello, L. Sharer, and D. J. Volsky. 2003. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 13:144-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uittenbogaart, C. H., D. J. Anisman, B. D. Jamieson, S. Kitchen, I. Schmid, J. A. Zack, and E. F. Hays. 1996. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS 10:F9-F16. [DOI] [PubMed] [Google Scholar]
- 99.Valentin, H., M. T. Nugeyre, F. Vuillier, L. Boumsell, M. B. S. F. Schmid, and R. A. Pereira. 1994. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J. Virol. 68:3041-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vigano, A., S. Vella, N. Principi, D. Bricalli, N. Sala, A. Salvaggio, M. Saresella, A. Vanzulli, and M. Clerici. 1999. Thymus volume correlates with the progression of vertical HIV infection. AIDS 13:F29-F34. [DOI] [PubMed] [Google Scholar]
- 103.Vincent, P., E. Priceputu, D. Kay, K. Saksela, P. Jolicoeur, and Z. Hanna. 2006. Activation of p21-activated kinase 2 and its association with Nef are conserved in murine cells but are not sufficient to induce an AIDS-like disease in CD4C/HIV transgenic mice. J. Biol. Chem. 281:6940-6954. [DOI] [PubMed] [Google Scholar]
- 104.Wang, X., T. Rasmussen, B. Pahar, B. Poonia, X. Alvarez, A. A. Lackner, and R. S. Veazey. 2007. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 109:1174-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ward, J. M., T. J. O'Leary, G. B. Baskin, R. Benveniste, C. A. Harris, P. L. Nara, and R. H. Rhodes. 1987. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am. J. Pathol. 127:199-205. [PMC free article] [PubMed] [Google Scholar]
- 106.Weng, X., E. Priceputu, P. Chrobak, J. Poudrier, D. G. Kay, Z. Hanna, T. W. Mak, and P. Jolicoeur. 2004. CD4 T cells from CD4C/HIV nef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro, but are dispensable for the development of a severe AIDS-like organ disease. J. Virol. 78:5244-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu, L., and V. N. Kewalramani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yearley, J. H., C. Pearson, R. P. Shannon, and K. G. Mansfield. 2007. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res. Hum. Retrovir. 23:515-524. [DOI] [PubMed] [Google Scholar]
- 109.Yunis, N. A., and V. E. Stone. 1998. Cardiac manifestations of HIV/AIDS: a review of disease spectrum and clinical management. J. Acquir. Immune Defic. Syndr. 18:145-154. [DOI] [PubMed] [Google Scholar]
- 110.Zaitseva, M. B., S. Lee, R. L. Rabin, H. L. Tiffany, J. M. Farber, K. W. Peden, P. M. Murphy, and H. Golding. 1998. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J. Immunol. 161:3103-3113. [PubMed] [Google Scholar]
- 111.Zhong, J., Y. Zuo, J. Ma, A. B. Fogo, P. Jolicoeur, I. Ichikawa, and T. Matsusaka. 2005. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 68:1048-1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.