Abstract
We examined chronic venous disorders (CVD) in persons who injected illicit drugs. The study design was cross-sectional, comparative stratified by age, gender, ethnicity, as well as by three types of drug use (noninjection; arm or upper body injection only; and legs with or without upper body injection). Subjects completed demographic, health, and substances abuse questionnaires and were evaluated using the clinical component of the Clinical-Etiology-Anatomy-Pathophysiology Classification. Seven hundred and thirteen participants were evaluated. Those who injected in the legs ± arms had significantly worse CVD. Thirty-nine percent of leg ± arm injectors vs. 4.2% or noninjectors or arm only injectors had moderate to severe CVD. Persons who injected in the legs ± arms were 9.14 times more likely to develop venous ulcers than those that injected in the arms and upper body only and 34.64 times more likely as those who never injected. CVD was associated with injecting in the groin, legs and feet as compared with other sites. The pattern of disorders associated with leg injection is consistent with the underlying pathology of chronic venous insufficiency.
Infrequently acknowledged as a drug use complication, chronic venous disease (CVD) is likely underappreciated in terms of its magnitude and impact on injection drug users. The initiation of injection use begins around 19.5 years of age;1 and, the typical user injects, often directly into the veins, up to four times per day.2 Injecting in the arms is most common and usually progresses to the veins in the lower extremities.3 In a cohort of injection drug users in methadone maintenance therapy, injecting in the groin, legs and feet accounted for 50% of the injecting years (8.7 mean years injecting in the legs/17.4 total mean years injecting).4 Veins are destroyed from the trauma of repeated injection, irritating qualities of the abused drugs and substances, and localized infection.5,6 Injecting may also lead to sclerosis and thrombosis of superficial and deep veins.3,5 McColl et al.7 reported the overall association of injecting drug via femoral vein puncture was a risk factor for deep vein thrombosis associated with 21.4% of all cases of deep vein thrombosis and even higher (52.4%) in women younger than 40 years of age. Even those who stop abusing drugs remain at risk for venous disease; damage that occurred during the active period of injecting persists and advances long after drug use ceases and venous disease may be advanced in mid-life. As a result of venous damage in the legs, the drug use population is important in the study of chronic venous disease.
The purposes of our study were two-fold: to examine CVD in a drug use population and to explore clustering of venous disorders. The specific aims were: (1) to quantify the distribution of the type and severity of venous disease in either leg; (2) to examine differences in CVD classification by site of injection/noninjection drug use; (3) identify the specific venous disorders that were most highly associated with site of injection/noninjection drug use controlling for age and comorbidities; and (4) using only participants who injected in the lower extremities, to examine the relationship between years of injecting and CVD severity.
METHODS
Participants (N = 713) were recruited from 12 methadone treatment clinics located in a large urban area between 2005 and 2007. The study was approved by the Institutional Review Board of the affiliated university. The study used a three-group, cross-sectional, comparative design. The groups were determined by site of injection/noninjection drug use: Group 1 consisted of noninjection drug users; Group 2 consisted of participants who injected drugs in their arms and/or upper body only. Group 3 consisted of participants with a history of injecting in their legs (leg ± arm). We enrolled participants into the three groups in a 1:1:2 distribution, respectively, based on our previous findings about leg injection.
To increase comparability of the groups, participants were stratified on age (25–39; 40–49; 50–65 years); gender (male, female); and ethnicity (African American; White). Participants selected to delay their methadone dose until after completion of the study so that they would be alert and respond to questions in an expedient manner.
All participants completed researcher read questionnaires. The Demographic Questionnaire obtained information about sex, race, education, employment, and age of each participant. The Health History Questionnaire asked the participant about medical diagnoses he/she was told (i.e., hypertension, myocardial infarction, heart disease, diabetes mellitus, and stroke). Participants were measured and weighed on a standard scale. The body mass index (BMI) was calculated from the person’s height and weight. The demographic and health history instruments were previously used.4 The test–retest reliability values for the Demographic and Health History Questionnaires were 0.99 and 0.86, respectively.8
The Drug History Questionnaire was used to obtain a detailed drug history from each participant; it is described elsewhere.9 For this report, the number of years of injecting in the hands, arms and above the waist and the years injecting in the groin, legs, and feet were used to describe the sample. The Drug History Questionnaire has a median κ value of 0.79.9
With the person seated and legs dependent, both legs were assessed using the clinical section of the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) Classification. 10 The clinical CEAP is a descriptive leg assessment which provides the following classification of CVD by disease severity: Class 0—no visible or palpable signs of venous disease; Class 1—telangiectasis or reticular veins; Class 2—varicose veins, distinguished from reticular veins by diameter of 3 mm or more; Class 3—edema; Class 4a—pigmentation or eczema; Class 4b—lipodermatosclerosis or atrophie blanche; Class 5—healed venous ulcers; Class 6—active venous ulcer.10 In the current study, the inter-rater reliability was 0.97 for the right leg and 0.94 for the left leg. We did not have Doppler studies to confirm anatomic distribution of reflux, but others have reported a high association between CEAP scores and venous reflux and ultrasound scans.11 Participants were compensated $40 for their time.
Statistical evaluation
The clinical CEAP was coded categorically and numerically to quantify the type and severity of venous disease in either leg. Chi-square tests of association were used to examine differences in classification by site of injection/noninjection drug use. Analysis of covariance was used to examine CVD severity in relation to the site of injection/noninjection drug use, age, gender, and ethnicity. The ten specific leg disorders making up the clinical CEAP were coded dichotomously for occurrence in either leg. Logistic regression was used to identify the specific disorders that were most highly associated with site of injection/noninjection drug use controlling for age and comorbidities. Categorical principle components analysis was used to examine the association among specific CEAP disorders and to identify the underlying dimensions. Finally, using only participants who injected in the legs, the relationship between years of injecting and CVD severity was examined r with polynomial and nonparametric regression.
RESULTS
Participants (N = 713) were 335 men (46.9%) and 378 women; they ranged in age from 25 to 65 years old, mean ± standard deviation (M ± SD) = 46.26 ± 9.06 years. Four hundred and forty (61.7%) were African American, 64.5% had a high school education or higher, and 30.0% were employed. Participants reported M ± SD of 2.91 ± 2.15 co-morbid health conditions. Their mean body mass index (BMI) was 28.05 (SD = 6.81). Those who injected drugs (n = 518) did so for 13.08 ± 9.77 years. Among those who injected, mean ± SD years of injecting in their arms were 9.08 ± 8.15; years injecting in their groin, legs, and/or feet, M±SD=9.19 ± 8.40.
The groups were distinguished by sited of injection/noninjection drug use: Group 1 consisted of noninjection drug users (n = 195); Group 2 consisted of participants who injected drugs in their arms and/or upper body only (n = 178). Group 3 consisted of participants with a history of injecting in their legs (n = 340); all but 14 (4.1%) of persons in this group had a history of both arm and leg injection. For this sample, 1,144 persons were screened (see Figure 1). Since we did not release information about the screening variables to potential subjects, the primary reason for subject exclusion was that the study cell was closed (326/431 = 75.6%).
Figure 1.
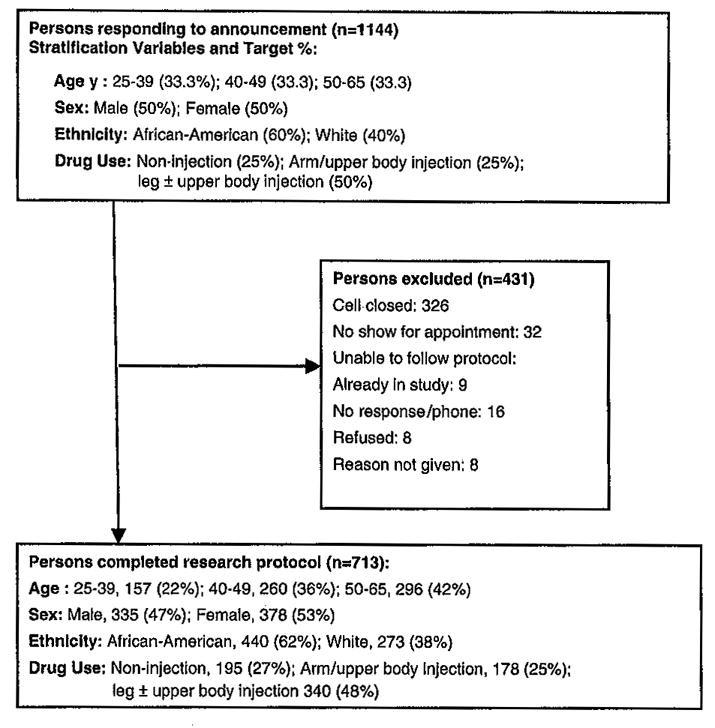
Flowchart showing participant screening, selection and allocation to stratification variables.
Stratification by drug group (3), age (3), gender (2), and race/ethnicity (2) resulted in a 36-cell design (see Figure 1); 28 (77.8%) of the cells were completely filled with the number of participants needed. The remaining eight cells on average were 43% complete (range 7–92%). Six (75%) of these eight cells were in the 25–39-year-old age group reflecting the lower numbers of younger persons in methadone treatment. Volunteers who were turned away for any reason were slightly older (54.4% vs. 41.5% in the 50–65-year-old category, contingency coefficient = 0.15, p < 0.01), more likely to be White than African American (45.2% vs. 38.3%, respectively, contingency coefficient = 0.07, p = 0.02), but not different in gender.
As shown in Table 1, stratification resulted in groups that were not significantly different in gender, ethnicity, marital status, completion of high school or BMI. Significant differences were found in age, number of co-morbid health conditions, and full time employment. Although the differences were relatively small, participants with a history of leg injection were older, had more co-morbid health conditions, and were less likely to be full-time employed.
Table 1.
Background variables by site of injection/noninjection drug use
| Injection drug use group |
||||||
|---|---|---|---|---|---|---|
| No injection drugs |
Arms only |
Legs ± arms |
||||
| Variables | M | SD | M | SD | M | SD |
| Age (M)a | 45.73 | 9.55 | 44.46 | 9.53 | 47.50 | 8.33 |
| Gender (%Male) | 42.6 | 49.6 | 47.2 | 50.1 | 49.4 | 50.1 |
| Ethnicity (% Black) | 65.1 | 47.8 | 55.1 | 49.9 | 63.2 | 48.3 |
| Married (%) | 28.2 | 45.1 | 20.2 | 40.3 | 25.0 | 43.4 |
| HS graduate (%) | 66.2 | 47.4 | 64.0 | 48.1 | 63.8 | 48.1 |
| Working (%)b | 31.8 | 46.7 | 36.7 | 48.3 | 25.4 | 43.6% |
| BMI (M) | 28.15 | 6.72 | 28.45 | 7.48 | 28.28 | 6.67 |
| No. comorbidities (M)a | 2.25 | 1.81 | 2.40 | 1.81 | 3.21 | 2.31 |
One way analysis of variance found significant differences among groups for the age, and number of comorbidities (p < 0.05).
Chi-square tests of association were significant for percent working (p < 0.05).
CVD distribution
The right and left leg distributions of the clinical CEAP were highly similar with a rank order correlation of 0.93. Because of this high similarity, we classified individuals by the clinical CEAP classification of their worst leg. Using this classification, only 7.7% (n = 55) of the sample did not exhibit clinical changes; 56.8% had mild disease (Classes 1–3); 18.5% had moderate disease (Classes 4a and 4b) and 17.8% had severe disease (Classes 5 and 6). The most common classification was Class 3, edema without skin changes (24.8%).
There was a strong relationship between the distribution of clinical CEAP scores and site of injection/noninjection group [χ2 (14, N = 713) = 168.5, p < 0.001] (see Figure 2). More than 39% of the leg ± arm injection group (134/340} were in clinical CEAP Class 4b or higher compared with 4.2% (16/373) in the other two groups. The distributions did not differ between the no injection and arm-only injection groups [χ2 (7, N = 373) = 6.46, p = 0.49].
Figure 2.
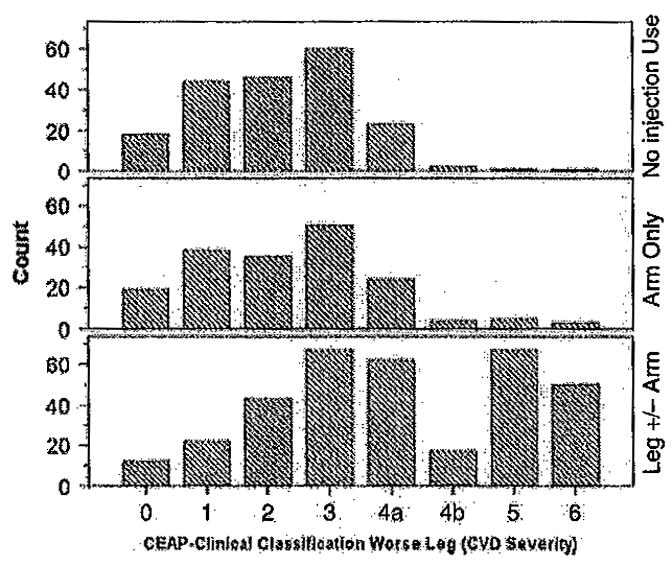
Distribution of clinical CEAP score of the worse leg by site of injection/noninjection drug use. The distribution of clinical CEAP scores and site of injection/noninjection group was significant [χ2(14, N = 713) = 168.5, p < 0.001]. The distributions did not differ between the no injection and arm-only injection groups [χ2(7, N = 373) = 6.46, p = 0.49].
Severity of leg disease
A 3×3×2×2 [Age ×Drug Use ×Gender × Ethnicity] analysis of covariance, ANCOVA, was performed using the clinical CEAP as a severity of CVD. Number of co-morbid health conditions was entered as a covariate. The analysis showed a strong effect of injecting in the legs: participants who injected in the legs ± arms had significantly more severe CVD than either those who never injected or those who injected in the arms/upper body only, F(2, 676) = 44.12, p < 0.001. Age and number of co-morbid conditions were also significant: Older age and more co-morbid conditions were associated with worse CVD (p < 0.05). While it appears that severity increased more with age for the leg injectors, this interaction was not significant. A small three-way interaction between race, age and type of injection drug use was also found (p = 0.02). This snowed that injecting in the legs was associated with worse CVD for middle-age White participants and for older-age Black participants. This difference might be attributed to the fact that White participants began injecting at a younger age (27.18 vs. 29.57, p = 0.01) and injected in the groin, legs, and/or feet more times per day (2.55 vs. 2.01, p < 0.001).
Specific CEAP clinical disorders
Figure 3 shows the occurrence of 10 specific clinical disorders of CVD associated with either leg. The relative frequency of each clinical disorder is shown by site of injection/noninjection use and age groups. The analyses revealed that two of the least severe disorders, telangiectasis and reticular veins, did not differ by site of injection/noninjection use. These disorders occurred in one or both legs with a relative frequency of 0.24 and 0.46, respectively. The other eight disorders were significantly associated with injection drug use and with leg injection consistently associated with the highest odds of disease occurrence (see Table 2). The leg injecting vs. no injecting compared with the leg injecting vs. arm injecting groups was 1.5–5 times more likely to show pigmentation, eczema, lipodermatatosclerosis, scars from healed ulcers, and active ulcer. After controlling for age and co-morbid conditions, persons who injected in the legs ± arms were 9.14 (p < 0.001) times more likely to develop venous ulcers than those that injected in the arms and upper body only and 34.64 (p < 0.001) times more likely as those who never injected (see Table 2).
Figure 3.
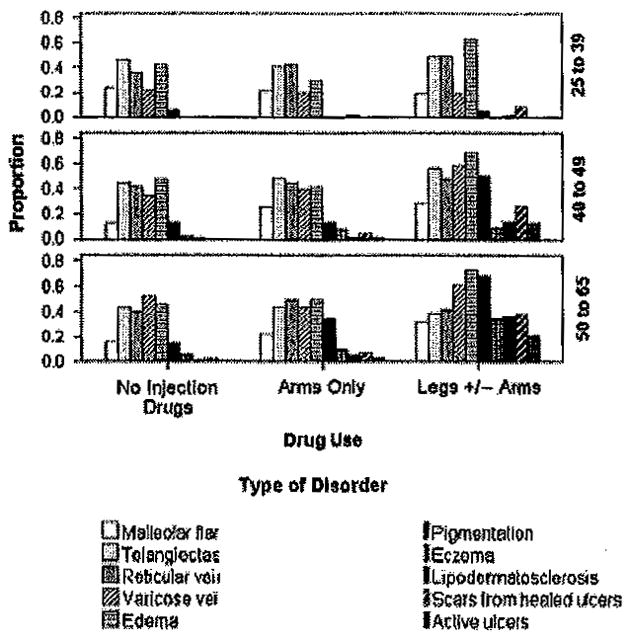
Proportions are based on occurrence of disorder in either leg. Malleolar flare was more frequent in the legs ± arms group than in the no injection group (p < 0.05). Each of the other disorders, except for telangiectasis, and reticular veins, differed significantly (p < 0.01) by injection group (see Table 2) with the highest proportions in the legs ± arms group.
Table 2.
Logistic regression results showing odds of disease (specific disorder) when injecting in the legs vs. injecting in the arms (3 vs. 2) and odds of disease when injecting in the legs vs. noninjecting (3 vs. 1)
| Hypothesis test |
95% Wald’s confidence interval for odds |
||||||
|---|---|---|---|---|---|---|---|
| Specific disorder of the legs | Comparison | Wald’s Chi-square | df | Significance | Odds | Lower | Upper |
| Malleolar flare | 3 vs. 1 | 5.15 | 1.00 | 0.02 | 1.68 | 1.07 | 2.63 |
| 3 vs. 2 | 0.51 | 1.00 | 0.48 | 1.17 | 0.76 | 1.80 | |
| Telangiectasis | 3 vs. 1 | 0.33 | 1.00 | 0.57 | 1.11 | 0.78 | 1.59 |
| 3 vs. 2 | 0.44 | 1.00 | 0.51 | 1.13 | 0.78 | 1.64 | |
| Reticular veins | 3 vs. 1 | 1.98 | 1.00 | 0.16 | 1.30 | 0.90 | 1.87 |
| 3 vs. 2 | 0.00 | 1.00 | 0.98 | 1.01 | 0.69 | 1.46 | |
| Varicose veins | 3 vs. 1 | 7.51 | 1.00 | 0.01 | 1.69 | 1.16 | 2.46 |
| 3 vs. 2 | 9.09 | 1.00 | < 0.001 | 1.82 | 1.23 | 2.68 | |
| Edema | 3 vs. 1 | 20.56 | 1.00 | < 0.001 | 2.37 | 1.63 | 3.44 |
| 3 vs. 2 | 30.04 | 1.00 | < 0.001 | 2.94 | 2.00 | 4.33 | |
| Pigmentation | 3 vs. 1 | 57.01 | 1.00 | < 0.001 | 7.35 | 4.38 | 12.34 |
| 3 vs. 2 | 35.06 | 1.00 | < 0.001 | 4.21 | 2.62 | 6.78 | |
| Eczema | 3 vs. 1 | 16.94 | 1.00 | < 0.001 | 5.77 | 2.51 | 13.30 |
| 3 vs. 2 | 6.74 | 1.00 | < 0.001 | 2.44 | 1.25 | 4.79 | |
| Lipodermatosclerosis | 3 vs. 1 | 20.97 | 1.00 | < 0.001 | 29.11 | 6.88 | 123.23 |
| 3 vs. 2 | 18.40 | 1.00 | < 0.001 | 6.82 | 2.84 | 16.40 | |
| Scars from healed ulcers | 3 vs. 1 | 24.77 | 1.00 | < 0.001 | 36.86 | 8.90 | 152.57 |
| 3 vs. 2 | 26.80 | 1.00 | < 0.001 | 7.39 | 3.46 | 15.75 | |
| Active ulcers | 3 vs. 1 | 12.10 | 1.00 | < 0.001 | 34.64 | 4.70 | 255.16 |
| 3 vs. 2 | 13.26 | 1.00 | < 0.001 | 9.14 | 2.78 | 30.06 | |
Logistic regression was performed on each leg disorder separately while controlling for age and comorbidities. The predictor, site of injection/noninjection drug use group, was coded with Category 3 as the reference category. Because the odds ratio is symmetric, reversing the reference category of the outcome resulted in the contrasts indicated.
The covariance among the clinical disorders was examined with categorical principle components. Two clusters of disorders were identified; these loaded on nearly independent dimensions. The first cluster (Dimension 1) consisted of pigmentation, eczema, and lipodermatosclerosis, active, and/or healed ulcers. A second cluster (Dimension 2) consisted of telangiectasis, reticular veins, malleolar flare, and varicose veins. These were not associated with the first cluster. Edema was equally associated with both symptom clusters (see Figure 4). Dimension 1 appears to represent the cluster of clinical disorders known to be associated with chronic venous insufficiency as the underlying pathology.
Figure 4.
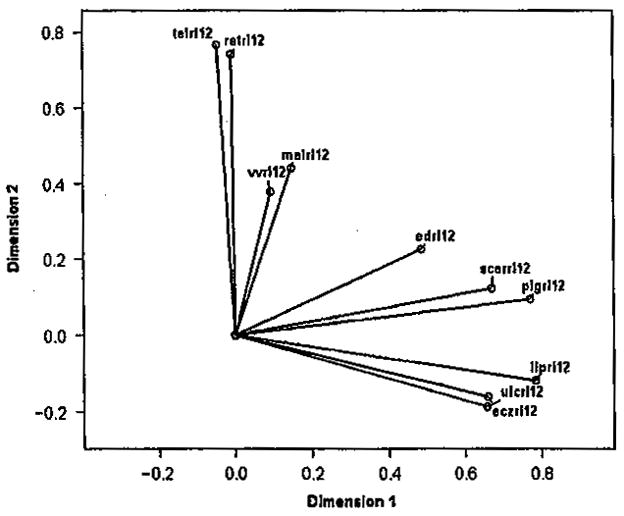
Loadings on two primary dimensions obtained from categorical principal components analysis (malrl12, malleolar flare; telrl12, telangiectasis; retrl12, reticular veins; vvrl12, varicose veins; edrl12, edema; pigrl12, pigmentation: eczrl12, eczema; liprl12, lipodermatosclerosis; scarrl12, scars from healed ulcers; ulcrl12, active ulcers).
Years of injecting in the lower extremities and severity of CVD
Using only the persons in the legs ± arms injection group (n = 340) with years of leg injection data (n = 336), years of injecting in the legs were categorized to six levels with approximately 16.6% of cases (n = 57) in each interval. The mean severity (clinical CEAP score) ranged from 2.91 (SD = 1.45) for clinical CEAP category 0–4.82 (SD = 1.83) for clinical CEAP category 6. Severity of disease increased with increasing years of injection use; the greatest increase occurred in the first 6 years of injecting. The quadratic regression of severity on years injecting in the legs accounted for 11.2% of the variance in disease severity [F(2, 333) = 15.62, p < 0.001].
DISCUSSION
The major finding in this study was the strong relationship between clinical CEAP score and site of injection/noninjection group. Thirty-nine percent of the legs ± arms injection group had high clinical CEAP scores as compared with an average of 4.2% of the other two groups. In fact, after controlling for age and co-morbid conditions, ulcers were 34.64 times more likely to occur in persons who injected in the legs ± arms than in persons who never injected. These findings suggest that (1) leg injection rather than drug use is causal in the high risk for venous disease, (2) persons with a history of leg injection should be monitored and treated for venous disease, and (3) these patients represent a model to better study advanced venous disease.
In our previous study, 84.3% of participants had injected in the veins of the lower extremities and severe (Classes 4–6) CVD occurred in 57.8%.4 Because general CVD risk factors (i.e., older age, co-morbid health problems, injuries, family history of varicose veins, pregnancy, obesity, prolonged standing, etc.),12,13 it is understandable that it will occur across drug use categories. Our finding showed older age and more co-morbidities were associated with worst CVD. The Edinburgh Veins Study showed CVD rose significantly with age.14 Injecting in the legs resulted in worse CVD for middle-aged whites and older-aged Blacks. Racial differences in CVD related to drug use needs further study.
Specific clinical leg changes/disorders were associated with drug use. We found scar, ulcer, pigmentation, lipodermatosclerosis, and eczema occurred as a symptom cluster and associated the most with injection drugs. Our findings support Bergan et al.’s15 definition where chronic venous insufficiency was restricted to disease of greater severity, namely Classes 4–6.
The severity of CVD gradually increased with increasing years of injecting in the legs. Given the high prevalence of CVD in leg injectors, the socioeconomic impact of these changes after a few years of leg injecting is great. Considering the young age that injecting begins in the legs and the few leg injecting years associated with advanced CVD, venous ulcers develop at a younger age.16 Persons with venous ulcers have impaired ability to participate in social and occupational activities which lead to reduced quality of life and economic constraints.13 CVD leg changes may be a critical factor for disability associate with drug use.
The findings from this study support the need of ongoing leg assessment and early, conservative CVD-management for persons who inject drugs in the legs. As part of harm reduction, safe injection practices should discourage leg injecting although it is not known if these practices will impact occurrence of CVD because of the risk of deep vein thrombosis and damage from repeated trauma. Leg management should include wearing compression stockings, leg elevation when sitting, and avoiding leg trauma. If conservative prevention and treatment is not successful, noninvasive testing to determine the extensiveness of venous damage and the potential for invasive venous procedures may need to be done.
This study had limitations. Doppler studies examining venous damage were not done. Future work to correlate these Doppler values with clinical manifestations would strengthen the findings. A longitudinal study would provide additional evidence as to CVD changes over time. Because of the study’s stratified design, persons 50–65 years of age were often not allowed to participate because of the study’s cell being closed; yet this was the group that expressed the most interest in participating. Since injection drug users are aging,17 research needs to focus on these older adults, their leg problems and care strategies.
In summary, persons who injected in the groin, legs, and feet had significantly worse CVD than those who never injected and those who injected in the arms/upper body. Older age and more co-morbid conditions were associated with worst CVD. Clustering of clinical manifestations identified pigmentation, eczema, and lipodermatosclerosis most highly associated with active or healed ulcers. Venous leg changes need to be assessed and monitored lifelong in persons who injected in the lower extremities.
Acknowledgments
The authors gratefully acknowledge Terri Gibbons, BS, Valerie Grech, ADN, RN, and Joyce Peck, BSN, RN as research assistants. The authors acknowledge the invaluable contributions of the following methadone treatment centers: Department of Human Services, Building 5 & Gratiot, Detroit; Metropolitan Rehabilitation Clinics, Oak Park; Millennium Treatment Services, Madison Heights & Warren; Nardin Park Recovery Center, Detroit; New Light Recovery Center, Detroit; Parkview Counseling Centers Detroit, Dearborn Heights, & Pontiac; STAR Center Inc., Detroit; University Psychiatric Centers—Jefferson, Detroit.
Grant Support: National Institute of Nursing Research/National Institute of Health (NINR/NIH), Effect of Drug Use on the Legs: Chronic Venous Insufficiency, Mobility and Pain, R01 NR009264.
References
- 1.Schoener EP, Hopper JA, Pierre JD. Injection drug use in North America. Infect Dis Clin N Am. 2002;16:535–51. doi: 10.1016/s0891-5520(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 2.NIDA research report series heroin: Abuse and addiction. Washington, DC: National Institute on Drug Abuse; 1997. [Google Scholar]
- 3.Finnie A, Nicolson P. Injecting drug use: implications for skin and wound management. Br J Nurs. 2002;11:S17–28. doi: 10.12968/bjon.2002.11.Sup1.12246. [DOI] [PubMed] [Google Scholar]
- 4.Pieper B, Templin T. Chronic venous insufficiency in persons with a history of injection drug use. Res Nurs Health. 2001;24:423–32. doi: 10.1002/nur.1042. [DOI] [PubMed] [Google Scholar]
- 5.Kirchenbaum SE, Midenberg ML. Pedal and lower extremity complications of substance abuse. J Am Podiatr Assoc. 1982;72:380–7. doi: 10.7547/87507315-72-8-380. [DOI] [PubMed] [Google Scholar]
- 6.Pieper B, Kirsner RS, Templin TN, Birk TJ. Injection drug use – an understudied cause of venous disease. Arch Dermatol. 2007;143:1305–9. doi: 10.1001/archderm.143.10.1305. [DOI] [PubMed] [Google Scholar]
- 7.McColl MD, Tait RC, Greer IA, Walker ID. Injecting drug use is a risk factor for deep vein thrombosis in women in Glasgow. Br J Haematol. 2001;112:641–3. doi: 10.1046/j.1365-2141.2001.02633.x. [DOI] [PubMed] [Google Scholar]
- 8.Pieper B, Templin TN, Birk TJ, Kirsner RS. Effects of injection-drug injury on ankle mobility and chronic venous disorders. J Nurs School. 2007;39:312–8. doi: 10.1111/j.1547-5069.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 9.Pieper B, Templin TN, Birk TJ, Kirsner RS. Reliability and clinical validity of a technique to assess lifetime illicit drug us. Ostomy Wound Manage. 2008;54:16–34. [PubMed] [Google Scholar]
- 10.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–52. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Eifell RK, Ashour HY, Lees TA. Comparison of new continuous measurements of ambulatory venous pressure (AVP) with conventional tiptoe exercise ambulatory AVP in relation to the CEAP clinical classification of chronic venous disease. J Vasc Surg. 2007;44:794–802. doi: 10.1016/j.jvs.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.White JV, Ryjewski C. Chronic venous insufficiency. Perspect Vasc Endovasc Ther. 2005;17:319–27. doi: 10.1177/153100350501700406. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111:2398–409. doi: 10.1161/01.CIR.0000164199.72440.08. [DOI] [PubMed] [Google Scholar]
- 14.Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Commun Health. 1999;53:149–53. doi: 10.1136/jech.53.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergan JJ, Schmid-Schonbein GW, Coleridge Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488–98. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 16.Pieper B. A retrospective analysis of venous ulcer healing in current and former users of injected drugs. J Wound Ostomy Continence Nurs. 1996;23:291–6. doi: 10.1016/s1071-5754(96)90048-0. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong GL. Injection drug users in the United States, 1979–2002. Arch Intern Med. 2007;167:166–73. doi: 10.1001/archinte.167.2.166. [DOI] [PubMed] [Google Scholar]


