Abstract
Objective
Survival of arteriovenous fistulas (AVFs) in hemodialysis patients is associated with both far infrared (FIR) therapy and length polymorphisms of the heme oxygenase-1 (HO-1) promoter. In this study, we evaluated whether there is an interaction between FIR radiation and HO-1 in regulating vascular inflammation.
Methods and Results
Treatment of cultured human umbilical vein endothelial cells (ECs) with FIR radiation stimulated HO-1 protein, mRNA, and promoter activity. HO-1 induction was dependent on the activation of the antioxidant responsive element/NF-E2-related factor-2 complex, and was likely a consequence of heat stress. FIR radiation also inhibited tumor necrosis factor-α-mediated expression of E-selectin, vascular cell adhesion molecule-1, intercellular cell adhesion molecule-1, monocyte chemoattractant protein-1, interleukin-8, and the cytokine-mediated adhesion of monocytes to ECs. The anti-inflammatory action of FIR was mimicked by bilirubin, and was reversed by the HO inhibitor, tin protoporphyrin-IX, or by the selective knockdown of HO-1. Finally, the anti-inflammatory effect of FIR was also observed in patients undergoing hemodialysis.
Conclusions
These results demonstrate that FIR therapy exerts a potent anti-inflammatory effect via the induction of HO-1. The ability of FIR therapy to inhibit inflammation may play a critical role in preserving blood flow and patency of AVFs in hemodialysis patients.
Keywords: endothelium, far infrared therapy, inflammation, leukocyte adhesion
Introduction
A well-functioning vascular access is necessary for achieving adequate hemodialysis (HD) in patients with end stage renal failure. Approximately 17-25% of HD patient hospitalizations in the United States arise from vascular access complications, with an annual cost of more than 1 billion US dollars, which continues to grow (1). Nearly 85% of vascular access failures arise from thromboses, more than 80% of which are associated with stenoses (2), which usually presents with inadequate blood flow (3,4). The causative factors of vascular access stenosis include small diameter of artery and vein, surgical technique, previous venipunctures, hemodynamic stress, and the presence of accessory veins (5). Moreover, many factors leading to endothelial dysfunction, such as oxidative stress, hyperhomocysteinemia, platelet activation, and inflammation are associated with arteriovenous fistula (AVF) failure (5).
The histological features of vascular access stenosis comprise vascular inflammation, intimal hyperplasia, and excessive accumulation of extracellular matrix (5). Despite these findings, the cause of stenoses remains unknown in a significant proportion of HD patients. This inter-individual variation may relate to differences in the genetic background that may influence susceptibility to vascular inflammation and neointima formation following endothelial and smooth muscle injury (6,7). Consistent with this notion, AVF survival was reported to be associated with specific genetic polymorphisms of transforming growth factor β1 (8) and methylene tetrahydrofolate reductase (9). In addition, we recently identified a length polymorphism in the heme oxygenase-1 (HO-1) gene that is associated with patency of AVFs (10). HO-1 catalyzes the oxidative degradation of heme into equimolar amounts of biliverdin, free iron, and carbon monoxide (CO) (see 11-14). Biliverdin is subsequently metabolized to bilirubin by the enzyme biliverdin reductase. Induction of HO-1 elicits potent anti-inflammatory, anti-proliferative, anti-thrombotic, and anti-oxidant effects in the circulation via the generation of CO and bilirubin. Interestingly, we found that a long guanidine thymidine dinucleotide repeat [(GT)n≧30] in the HO-1 promoter, which is linked to impaired inducibility (11), is associated with a higher frequency of access failure and diminished patency of AVFs in HD patients, suggesting a critical role for HO-1 in governing the survival of AVFs (10).
Far infrared (FIR) radiation is an invisible electromagnetic wave with a characteristic wavelength between 5.6 and 1000μm that can be perceived as heat by thermo-receptors in the skin (15,16). Recent studies indicate that FIR therapy exerts beneficial effects in the cardiovascular system. FIR radiation improves ventricular arrhythmias and endothelial function in patients with heart disease (17,18). In addition, FIR radiation promotes microvascular blood flow and angiogenesis in various animal models (19,20). Moreover, we recently demonstrated that FIR therapy improves access flow and patency of AVFs in HD patients (21). However, the mechanism by which FIR radiation exerts these favorable effects is not known.
In the present study, we investigated the effect of FIR radiation on HO-1 gene expression and inflammation in human endothelial cells (ECs), In particular, we determined whether FIR radiation modulates the expression of inflammatory proteins and the adhesion of monocytes to the surface of ECs, and the potential involvement of HO-1 in mediating the anti-inflammatory action of FIR therapy. In addition, we explored the effect of FIR therapy on circulating inflammatory markers in patients following a single HD session.
Methods
Human umbilical vein ECs (HUVEC) were exposed to FIR radiation using a WS™ TY101 FIR emitter (WS Far Infrared Medical Technology Co., Ltd., Taipei, Taiwan). This device generates electromagnetic waves with wavelengths between 3 and 25 μm (peak 5-6 μm). The radiator was set at a height of 25 cm above the bottom of the tissue culture plates and cells were exposed to FIR radiation for various times (0-40 minutes). The effect of FIR radiation on HO-1 or NF-E2-related factor-2 (Nrf2) protein and mRNA expression were quantified by western and northern blotting, respectively, while the action of FIR radiation on HO-1 promoter activity was determined using wild-type and mutant HO-1 promoter/firefly luciferase constructs. Vascular inflammation was assessed by measuring the expression of endothelial adhesion receptor molecules, the endothelial production of chemokines, and the adhesion of monocytes to HUVEC. Finally, the anti-inflammatory effect of FIR was also investigated in patients undergoing HD. For complete details of Material and Methods please see Supplemental Materials and Methods (available online at http://atvb.ahajournals.org).
Results
FIR therapy stimulates HO-1 expression via the Nrf2/ARE complex in human ECs
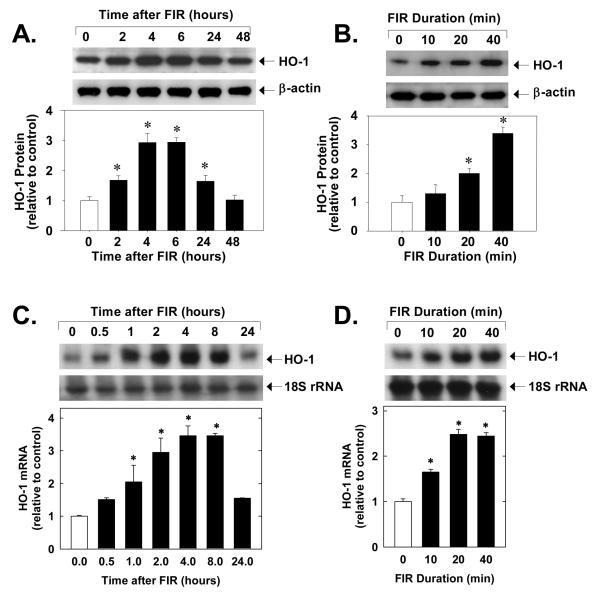
Treatment of HUVEC with FIR radiation for 40 minutes stimulated a time-dependent increase in HO-1 protein. A significant increase in HO-1 protein was observed after 2 hours of FIR radiation and protein levels returned to basal levels by 48 hours (Figure 1A). The induction of HO-1 protein by FIR radiation was also dependent on the duration of FIR exposure. Increasing the duration of FIR exposure from 10 to 40 minutes resulted in a progressive increase in HO-1 protein (Figure 1B). In addition, application of FIR radiation for 40 minutes stimulated a time-dependent increase in HO-1 mRNA beginning 2 hours after exposure (Figure 1C). The induction of HO-1 mRNA by FIR radiation was also dependent on the exposure interval (Figure 1D). Application of FIR radiation for 40 minutes had no effect on cell viability (data not shown) and did not stimulate the production of reactive oxygen species (please see supplemental Figure I). However, administration of FIR radiation for 40 minutes resulted in a significant elevation in temperature of the culture media (36.8±0.3° C in control media versus 39.6±0.4°C immediately after FIR radiation; n=3). Interestingly, raising the temperature from 36.8 to 39.6°C also evoked a significant increase in HO-1 expression in HUVEC (please see supplemental Figure IIA).
Figure 1.
FIR radiation for 40 minutes stimulates HO-1 protein (A) and mRNA (C) expression in HUVEC. Duration-dependent effect of FIR radiation on HO-1 protein (B) and mRNA (D) expression in HUVEC six hours after FIR exposure. Results are means ± SD (n=3-5). *Statistically significant effect of FIR therapy.
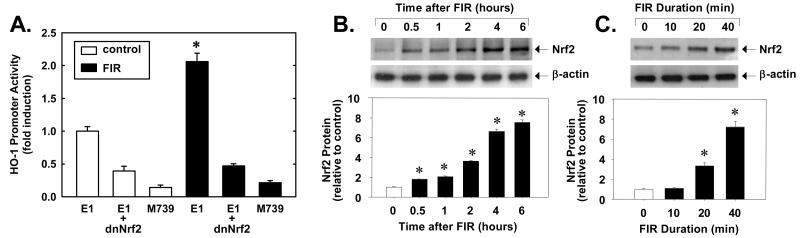
To determine whether the increased expression of HO-1 in response to FIR therapy involves the transcriptional activation of the gene, HUVEC were transiently transfected with a wild-type HO-1 promoter construct and promoter activity was monitored. Treatment of HUVEC with FIR therapy for 40 minutes stimulated more than a 2-fold increase in HO-1 promoter (E1) activity (Figure 2A). However, mutation of the ARE (M739) markedly attenuated basal HO-1 promoter activity and abolished the response to FIR radiation, suggesting that FIR exposure activates HO-1 transcription via the ARE. Since the transcription factor Nrf2 appears crucial for ARE-mediated gene expression, we determined whether Nrf2 was involved. Indeed, transfection of HUVEC with a dominant-negative Nrf2 mutant (dnNrf2) suppressed basal activity by more than 50% and blocked the induction of HO-1 promoter activity by FIR (Figure 2A). Furthermore, 40 minutes of FIR therapy resulted in a rapid time-dependent increase in Nrf2 protein (Figure 2B). A significant increase in Nrf2 protein was observed only after 30 minutes after FIR exposure and Nrf2 levels progressively increased for up to 6 hours after FIR exposure. In addition, the induction of Nrf2 protein expression was dependent on the duration of FIR exposure, with longer exposures demonstrating a progressive increase in Nrf2 protein (Figure 2C). Interestingly, raising the temperature of the culture media from 36.8 to 39.6°C also stimulated a time-dependent increase in Nrf2 protein (please see supplemental Figure IIB).
Figure 2.
FIR radiation for 40 minutes stimulates HO-1 promoter activity in HUVEC six hours after FIR exposure (A). FIR radiation for 40 minutes stimulates Nrf2 protein expression (B). Duration-dependent effect of FIR radiation on Nrf2 protein expression (C). Results are means ± SD (n=3-6). *Statistically significant effect of FIR therapy.
FIR therapy inhibits TNFα-stimulated inflammatory protein expression
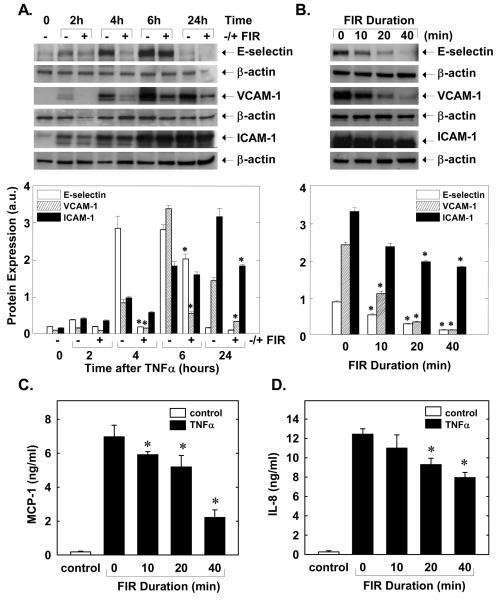
Treatment of HUVEC with TNFα (100ng/ml) stimulated a time-dependent increase in the expression of the adhesion molecules: E-selectin, vascular cell adhesion molecule -1, (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) (Figure 3A). Application of FIR therapy for 40 minutes suppressed the cytokine-mediated induction of the adhesion molecules (Figure 3A). Optimal inhibition of adhesion molecules by FIR was observed at 4 hour for E-selectin, at 6 hour for VCAM-1, and at 24 hour for ICAM-1. The effect of FIR therapy duration on individual adhesion proteins was examined at their optimally inhibited times and revealed that protein expression for all three adhesion molecules was maximally inhibited by 40 minutes of FIR radiation (Figure 3B). In addition, treatment of HUVEC with TNFα (100ng/ml) for 6 hours stimulated an increase in the production of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) that was blocked by FIR therapy in a duration-dependent manner (Figure 3C and D).
Figure 3.
FIR radiation for 40 minutes inhibits TNFα (100ng/ml)-stimulated adhesion receptor expression in HUVEC (A). Duration-dependent effect of FIR radiation on adhesion receptor expression (B). FIR radiation inhibits TNFα (100ng/ml for 6 hours)-stimulated MCP-1 (C) and IL-8 (D) production. Results are means ± SD (n=3-4). *Statistically significant effect of FIR radiation.
FIR therapy suppresses TNFα-stimulated VCAM-1 protein expression and monocyte adhesion to human ECs via the induction of HO-1
In subsequent experiments, we explored whether the induction of HO-1 is involved in mediating the inhibitory effects of FIR therapy on adhesive protein expression. Since FIR radiation markedly inhibited VCAM-1 expression at nearly all time points studied, we focused on this particular adhesion molecule. Consistent with our earlier results, we found that FIR therapy for 40 minutes markedly suppressed the TNFα (100ng/ml for 6 hours)-mediated induction of VCAM-1 (please see supplemental Figure III). However, treatment of HUVEC with the HO inhibitor, SnPP (10 or 20μM), reversed the inhibitory effect of FIR radiation in a concentration-dependent manner. Significantly, SnPP did not induce the expression of VCAM-1 in the absence of TNFα and slightly, but not significantly, increased the induction of VCAM-1 by TNFα
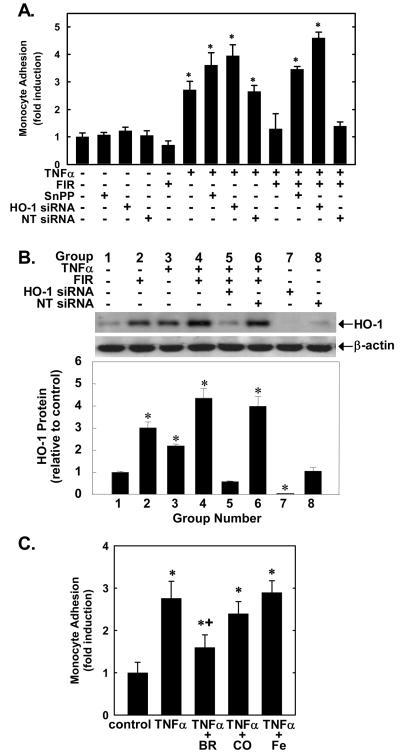
The physiological significance of FIR therapy mediated inhibition of HUVEC adhesion molecule expression was examined by determining the effect of FIR radiation on monocyte adhesion. FIR therapy, SnPP, HO-1 siRNA, or the control non-targeting oligonucleotide minimally affected monocyte adhesion in the absence of TNFα. Treatment of HUVEC with TNFα (100ng/ml) for 6 hours stimulated a nearly 3-fold increase in monocyte adhesion which was potentiated by SnPP (20μM) or by treating HUVEC with the HO-1 siRNA (0.1μM) for two days but unaffected by the non-targeting siRNA (NT siRNA; 0.1μM) (Figure 4A). Application of FIR therapy for 40 minutes significantly blocked cytokine-stimulated monocyte adhesion to HUVEC (Figure 4A). Similarly, the TNFα-mediated adhesion of monocytes to human aortic endothelial cells (HAEC) was blocked by FIR radiation, indicating that the anti-inflammatory effect of FIR was not unique to venous endothelium (please see supplemental Figure IV). However, treatment of ECs with SnPP (20 μM) or pretreatment of cells with the HO-1 siRNA (0.1 μM) fully reversed the inhibitory effect of FIR radiation (Figure 4A). In contrast, the NT siRNA (0.1μM) failed to block the anti-adhesive effect of FIR therapy. Figure 4B shows relative HO-1 protein expression in HUVEC exposed to various treatment regimens. In comparison with control cells, HO-1 expression was elevated approximately 3-fold in HUVEC treated with FIR radiation for 40 minutes and 2-fold in cells treated with TNFα (100ng/ml for 6 hours). Treatment of cells with the combination of FIR and TNFα resulted in an additive increase (approximately 4-fold) in HO-1 protein. In addition, treatment of HUVEC with the HO-1 siRNA (0.1μM) suppressed the increase in HO-1 protein expression evoked by the combined treatment of cells with FIR radiation and TNFα. In contrast, the NT siRNA (0.1μM) had no effect on HO-1 expression in response to FIR therapy and TNFα. The HO-1 siRNA also abolished the basal expression of HO-1 whereas the NT siRNA had no effect on HO-1 expression, confirming the selectivity of our HO-1 knockdown approach.
Figure 4.
Monocyte adhesion (A, C) and HO-1 protein (B) in HUVEC treated with HO-1 or NT siRNA, FIR radiation, or untreated HUVEC exposed to TNFα in the presence of SnPP, BR, CO, or Fe. Results ar means ± SD (n=3-6). *Statistically significant effect versus untreated cells. †Statistically significant effect of BR.
Exogenous administration of bilirubin mimics the anti-inflammatory action of FIR therapy
We also determined which of the HO-1 products mediates the anti-inflammatory action of HO-1. Incubation of HUVEC with bilirubin (BV; 20 μM) significantly suppressed TNFα (100ng/ml for 6 hours)-stimulated monocyte adhesion (Figure 4C). In contrast, the addition of CO (500 ppm) or free iron (Fe; 20 μM) failed to block cytokine-mediated monocyte adhesion (Figure 4C). In the absence of TNFα, the HO-1 products had no effect on monocyte adhesion (data not shown).
FIR therapy exerts an anti-inflammatory effect in HD patients
Finally, we examined whether FIR radiation could also evoke an anti-inflammatory effect in vivo. A prospective, randomized, controlled clinical trial involving twenty Chinese HD patients was conducted. Patient characteristics were as follows: 13 males and 7 females, average age of 66.5±9.1 years, HD duration of 91.2±63.8 months. The distribution of the underlying cause of end stage renal disease for these patients includes diabetes mellitus for 8, chronic glomerulonephritis for 6, hypertension for 4, IgA nephropathy for 1, and unknown for 1 patient.
Circulating levels of inflammatory markers before and after a single HD session as well as changes in inflammatory marker concentration are shown in Table 1. Consistent with previous reports showing that HD can provoke an inflammatory response (22-24), a significant increase in hypersensitive C-reactive protein (hsCRP), soluble ICAM-1 (sICAM-1), and soluble VCAM-1 (sVCAM-1) was observed in patients following a single HD session that lasted for 4 hours. However, significant elevations in only sICAM-1 and sVCAM-1 were noted in patients that were exposed to 40 minutes of FIR therapy. Interestingly, the concentration of sVCAM-1 following HD was significantly lower in patients treated with FIR therapy. Moreover, the incremental change in serum concentration of all three inflammatory markers following a single HD session with FIR therapy was significantly lower than that without FIR.
Table 1.
Serum concentrations of inflammatory markers for 20 HD patients before and after a single HD session with or without FIR therapy.
| HD session without FIR | HD session with FIR | |
|---|---|---|
| hsCRP (mg/L)-BHD | 4.10±4.07 | 4.63±4.32 |
| hsCRP (mg/L)-AHD | 4.34±4.26* | 3.98±2.93 |
| Δ (AHD-BHD) hsCRP (mg/L) | 0.24±0.43 | −0.65±1.73† |
| sICAM-1 (ng/mL)-BHD | 690±225 | 728±218 |
| sICAM-1 (ng/mL)-AHD | 886±281* | 823±320* |
| Δ (AHD-BHD) sICAM (ng/mL) | 196±128 | 95±190† |
| sVCAM-1 (ng/mL)-BHD | 1135±664 | 1164±676 |
| sVCAM-1 (ng/mL)-AHD | 1461±716* | 1243±667*† |
| Δ (AHD-BHD) sVCAM-1 (ng/mL) | 326±249 | 79±107† |
hsCRP, hypersensitive C-reactive protein; BHD, before hemodialysis; AHD, after hemodialysis; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1
Values are expressed as the mean ± SD.
Statistically significant effect of HD.
Statistically significant effect of FIR therapy.
Discussion
In the present study, we identified FIR therapy as a novel inducer of HO-1 gene expression in human vascular endothelium. The induction of HO-1 is dependent on the duration of FIR exposure and requires the activation of the Nrf2-ARE signaling pathway. In addition, we found that FIR therapy inhibits the expression of pro-inflammatory adhesion receptors and chemoattractant molecules in human ECs, and blocks the adhesion of monocytes to ECs. Moreover, we found that FIR therapy blunts the increase in circulating inflammatory markers in patients following a single HD session. These potent anti-inflammatory effects of FIR therapy are dependent on the induction of HO-1 and are mimicked by the exogenous administration of bilirubin. Thus, the ability of FIR therapy to inhibit inflammation via the HO-1-catalyzed production of bilirubin may provide an important mechanism by which FIR radiation promotes the survival of AVFs in HD patients.
Treatment of ECs with FIR radiation results in a time-dependent increase in HO-1 protein and mRNA expression that correlates with the duration of FIR exposure. Transient transfection experiments indicate that FIR radiation stimulates the transcriptional activation of the HO-1 gene. The induction of HO-1 by FIR radiation is likely thermally-mediated. FIR radiation results in a modest degree of heat stress, and application of a similar amount of heat is sufficient to induce HO-1 expression in vascular endothelium. Interestingly, the activation of the HO-1 gene by FIR radiation does not occur via classical heat shock responsive elements since these elements are inactive in most mammalian species, including the mouse and human HO-1 gene (25). Instead, we found that the induction of HO-1 requires the presence of AREs in the HO-1 promoter since mutation of these sites abolishes the stimulation of promoter activity by FIR radiation. While many transcription factors are capable of binding to the ARE, Nrf2 appears to play a predominant role in ARE-dependent HO-1 expression (25,26). In support of this, we found that FIR radiation stimulates the expression of Nrf2 in a manner that precedes the rise of HO-1 mRNA. In addition, we observed that transient transfection of ECs with a dominant-negative mutant of Nrf2 abrogates the activation of HO-1 promoter activity by FIR radiation. The mechanism by which FIR radiation activates Nrf2 is not known. Activation of Nrf2 is regulated by the cytosolic protein Keap1 that negatively modulates the nuclear translocation of Nrf2 and facilitates the degradation of Nrf2 via the proteasome. Interestingly, heat has been shown to inhibit proteasomal activity (27), which can lead to the activation of Nrf2 (28). Thus, it is possible that FIR radiation activates Nrf2 via a thermally-induced decrease in proteasomal function. Consistent with this notion, we found that a similar degree of heat stress that is evoked by FIR radiation is able to increase Nrf2 protein levels.
Emerging evidence from numerous laboratories indicate that FIR therapy exerts beneficial effects in the cardiovascular system (17-21,29,30). In the present study, we extend these findings to show that FIR radiation exerts a potent anti-inflammatory effect on vascular endothelium. We observed that FIR radiation inhibits the TNFα-mediated expression of the adhesion molecules VCAM-1, ICAM-1, and E-selectin, as well as the chemoattractants MCP-1 and IL-8. The expression of adhesion receptors and chemoattractant molecules is crucial in recruiting inflammatory cells to sites of active inflammation and therefore important for influencing the outcome of the inflammatory reaction. Each adhesion molecule plays a different role in modulating leukocyte-endothelial interactions. While E-selectin is deemed to participate in the initial phases of rolling and attachment of leukocytes to ECs, VCAM is mainly responsible for establishing firm leukocyte adhesion to ECs, and ICAM functions to promote the transmigration of the leukocytes to the extravascular space (31). Our finding that FIR radiation inhibits the expression of all three adhesive proteins, MCP-1, and IL-8 indicates the FIR therapy may modulate multiple components of the inflammatory response. The physiological significance of FIR radiation-mediated inhibition of adhesion molecule expression is underscored by our finding the FIR therapy completely suppresses the cytokine-mediated adhesion of monocytes to cultured human ECs. Interestingly, the intensity and duration of FIR radiation (40 minutes) employed to inhibit endothelial adhesion molecule expression and monocyte adhesion is similar to what we recently used to improve access blood flow and patency of AVFs in hemodialysis patients (21). Moreover, in the present study, we found that HD-induced inflammatory stress is reduced in patients treated with FIR therapy. Circulating levels of sVCAM-1 were significantly lower following a single HD session in patients exposed to FIR radiation. In addition, the increments in hsCRP, sICAM-1, and sVCAM-1 following a single HD session are reduced in patients treated with FIR therapy. Given that stenotic AVFs are associated with marked inflammatory activity consisting of abundant leukocyte infiltration and augmented ICAM-1 and VCAM-1 expression (5,32), the ability of FIR radiation to exert potent anti-inflammatory effects may contribute to the beneficial effects observed with FIR therapy in HD patients.
The anti-inflammatory effect of FIR radiation is mediated via the induction of HO-1. We found that pharmacological inhibition of HO-1 activity completely reverses the ability of FIR radiation to suppress cytokine-mediated VCAM-1 expression. Furthermore, inhibition of HO-1 activity abolishes the anti-adhesive action of FIR radiation. Similarly, the selective knockdown of HO-1 expression by HO-1 siRNA prevents the FIR radiation-mediated induction of HO-1 protein and the inhibition of monocyte adhesion. Interestingly, TNFα also stimulates HO-1 protein expression. Moreover, the cytokine-mediated adhesion of monocytes is potentiated by inhibiting HO-1 expression or activity, suggesting that the induction of HO-1 functions in a negative feedback manner to limit cytokine-stimulated monocyte adhesion. Finally, the anti-inflammatory effect of HO-1 is likely mediated via the release of bilirubin because the exogenous administration of bilirubin mimics the anti-adhesive action of HO-1 whereas the other HO-1 products had no effect on monocyte adhesion. Our finding the FIR therapy inhibits the expression of EC adhesion molecules and monocyte adhesion to endothelium via the HO-1-derived formation of bilirubin is consistent with studies demonstrating that overexpression of HO-1 or the exogenous application of bilirubin exerts an anti-inflammatory effect by blocking the activation of the transcription factor NF-κB, which is strictly required for cytokine-mediated induction of endothelial adhesion receptors (33-36).
The ability of FIR radiation to stimulate vascular HO-1 gene expression may contribute to its therapeutic effect in maintaining the patency of AVFs in HD patients (10). Aside from its potent anti-inflammatory action, HO-1 may also preserve blood flow through the AVF by inhibiting vascular smooth muscle cell proliferation, platelet aggregation, and vasospasm (see 12-14). Furthermore, HO-1 can stimulate EC regrowth at sites of endothelial injury which further inhibits vascular stenosis by reestablishing a non-thrombogenic surface and retaining the underlying vascular smooth muscle cells in a quiescent state. Thus, the induction of HO-1 by FIR may promote the viability of AVFs by counteracting many of the causative factors that lead to AVF failure.
The induction of HO-1 may also contribute to other vasoprotective effects associated with FIR therapy. In particular, the ability of non-ablative infrared laser therapy to inhibit neointimal hyperplasia after percutaneous coronary angioplasty in hypercholesterolemic rabbits (29) may also occur through HO-1 since this enzyme protects against intimal thickening in various animal models of arterial injury (12-14). In addition, the induction of HO-1 may contribute to the antioxidant effect of FIR (30). This latter effect may be particular relevant for uremic patients which suffer from excessive oxidative stress that is further aggravated by dialysis (37).
In conclusion, the present study demonstrates that FIR therapy induces HO-1 gene expression in human ECs via the activation of the Nrf2/ARE complex. In addition, this study found that the induction of HO-1 contributes to the ability of FIR therapy to inhibit the expression of EC adhesion molecules and the adhesion of monocytes to vascular endothelium. The ability of FIR to inhibit endothelial inflammation through the induction of HO-1 may play a critical role in maintaining the patency of AVFs in HD patients.
Acknowledgments
Sources of Funding
This work was supported by grants from the Taipei Veterans General Hospital (V95-ER2-003, V96-ER2-004, V96-B2-009), the National Science Council of Taiwan (NSC95-2314-B-075-070, NSC96-2314-B-010-045), and the National Institutes of Health of the USA (NIH R01 HL59976, NIH R01 HL74966, and NIH R01 HL62467).
References
- 1.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 2.Windus DW. Permanent vascular access: A nephrologist's view. Am J Kidney Dis. 1993;21:457–471. doi: 10.1016/s0272-6386(12)80391-x. [DOI] [PubMed] [Google Scholar]
- 3.Paun M, Beach K, Ahmad S, Hickman R, Meissner M, Plett C, Strandness DE., Jr New ultrasound approaches to dialysis access monitoring. Am J Kidney Dis. 2000;35:477–481. doi: 10.1016/s0272-6386(00)70201-0. [DOI] [PubMed] [Google Scholar]
- 4.Lin CC, Chang CF, Chiou HJ, Sun YC, Chiang SS, Lin MW, Lee PC, Yang WC. Variable pump flow-based doppler ultrasound method: a novel approach to the measurement of access flow in hemodialysis patients. J Am Soc Nephrol. 2005;16:229–236. doi: 10.1681/ASN.2004040266. [DOI] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 6.Heine GH, Ulrich C, Kohler H, Girndt M. Is AV fistula patency associated with angiotensin-converting enzyme (ACE) polymorphism and ACE inhibitor intake? Am J Nephrol. 2004;24:461–468. doi: 10.1159/000080464. [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int. 2002;62:1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 8.Heine GH, Ulrich C, Sester U, Sester M, Kohler H, Grindt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003;64:1101–1107. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Fukasawa M, Matsushita K, Kamiyama M, Mikami Y, Araki I, Yamagata Z, Takeda M. The methylenetetrahydrofolate reductase C677T point mutation is a risk factor for vascular access thrombosis in hemodialysis patients. Am J Kidney Dis. 2003;41:637–642. doi: 10.1053/ajkd.2003.50125. [DOI] [PubMed] [Google Scholar]
- 10.Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, Lee PC, Lee SD, Su TS, Fann CS, Chung MY. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69:165–172. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Chau LY, Lin MW, Chen LC, Yo MH, Chen JW, Lin SJ. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J. 2004;25:39–47. doi: 10.1016/j.ehj.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 13.Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J Cell Physiol. 2003;195:373–382. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]
- 14.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J. Cell. Mol. Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyokawa H, Matsui Y, Uhara J. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med. 2003;228:724–729. doi: 10.1177/153537020322800612. [DOI] [PubMed] [Google Scholar]
- 16.Capon A, Mordon S. Can thermal lasers promote skin wound healing? Am J Clin Dermatol. 2003;4:1–12. doi: 10.2165/00128071-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kihara T, Biro S, Ikeda Y. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J. 2004;68:1146–1151. doi: 10.1253/circj.68.1146. [DOI] [PubMed] [Google Scholar]
- 18.Imamura M, Biro S, Kihara T. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–1088. doi: 10.1016/s0735-1097(01)01467-x. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, Orihara K, Yu B, Kihara T, Miyata M, Hamasaki S, Otsuji Y, Minagoe S, Tei C. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ J. 2005;69:722–729. doi: 10.1253/circj.69.722. [DOI] [PubMed] [Google Scholar]
- 20.Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, Wu CW. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed. 2006;22:78–86. doi: 10.1111/j.1600-0781.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin CC, Chang CF, Lai MY, Chen TW, Lee PC, Yang WC. Far Infrared Therapy: a Novel Treatment Improving Access Blood Flow and Unassisted Patency of Arteriovenous Fistula in Hemodialysis Patients. J Am Soc Nephrol. 2007;18:985–992. doi: 10.1681/ASN.2006050534. [DOI] [PubMed] [Google Scholar]
- 22.Korevaar JC, Van Manen JG, Dekker FW, De Waart DR, Boeschoten EE, Krediet RT, for the Necosad Group Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J. Am. Soc. Nephrol. 2004;15:2916–2922. doi: 10.1097/01.ASN.0000143744.72664.66. [DOI] [PubMed] [Google Scholar]
- 23.Musial K, Zwolinska D, Polak-Jonkisz D, Berny U, Szprynger K, Szczpepanska M. Soluble adhesion molecules in children and young adults on chronic hemodialysis. Pediatric. Nephrol. 2004;19:332–336. doi: 10.1007/s00467-003-1353-4. [DOI] [PubMed] [Google Scholar]
- 24.Memoli B. Cytokine production in haemodialysis. Blood Purif. 1999;17:149–158. doi: 10.1159/000014387. [DOI] [PubMed] [Google Scholar]
- 25.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 26.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AMK, Burrow ME, Tou J. Mechanism of Heme Oxygenase-1 Gene Activation by Cadmium in MCF-7 mammary epithelial cells. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 27.Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K. The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J. 2003;22:3557–3567. doi: 10.1093/emboj/cdg349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of the transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 29.Kipshidze N, Nikolaychik V, Muckerheidi M, Keelan MH, Chekanov V, Maternowski M, Chawla P, Hernandez I, Iyer S, Dangas G, Sahota H, Leon MB, Roubin G, Moses JW. Effect of short pulsed nonablative infrared laser irradiation on vascular cells in vitro and neointimal hyperplasia in a rabbit balloon injury model. Circulation. 2001;104:1850–1855. doi: 10.1161/hc3901.096101. [DOI] [PubMed] [Google Scholar]
- 30.Masuda A, Miyata M, Kihara T, Minagoe S, Tei C. Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2alpha) Jpn Heart J. 2004;45:297–303. doi: 10.1536/jhj.45.297. [DOI] [PubMed] [Google Scholar]
- 31.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 32.Chang C-J, Ko Y-S, Ko P-J, Hsu L-A, Chen C-F, Yang C-W, Hsu T-S, Pang J-HS. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68:1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 33.Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT. 3-Hydroxyanthranilic acid, one of l-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187:274–284. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui T-Y, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 35.Banning A, Brigelius-Flohe R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression. Anti. Redox. Signal. 2005;7:889–899. doi: 10.1089/ars.2005.7.889. [DOI] [PubMed] [Google Scholar]
- 36.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 37.Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr. Opin. Nephrol. Hypertens. 2003;12:593–598. doi: 10.1097/00041552-200311000-00004. [DOI] [PubMed] [Google Scholar]