Abstract
Background
This is the first ever evaluation of narrow band imaging (NBI), an innovative endoscopic imaging procedure, for the visualisation of pleural processes.
Methods
The pleural cavity was examined in 26 patients with pleural effusions using both white light and narrow band imaging during thoracoscopy under local anaesthesia.
Results
In the great majority of the patients narrow band imaging depicted the blood vessels more clearly than white light, but failed to reveal any differences in number, shape or size. Only in a single case with pleura thickened by chronic inflammation and metastatic spread of lung cancer did narrow band imaging show vessels that were not detectable under white light.
Conclusion
It is not yet possible to assess to what extent the evidence provided by NBI is superior to that achieved with white light. Further studies are required, particularly in the early stages of pleural processes.
Thoracoscopy is the standard diagnostic procedure for investigating exudative pleural effusions and leads to a conclusive diagnosis for 95% of patients when carried out under local anaesthesia [1]. Thoracoscopy can also be employed for staging primary thoracic malignancies, i.e. malignant pleural mesotheliomas or primary malignant pulmonary tumours with possible pleural dissemination. Despite the high diagnostic yield of thoracoscopy under local anaesthesia, some patients still remain without a conclusive diagnosis or have to undergo a surgical procedure under general anaesthesia. Apart from the conventional white light, other imaging procedures that are said to yield more information, especially at to the presence of a pleural tumour, have already been investigated, but the evidence has remained limited [2-4].
Narrow band imaging (NBI) is a new, alternative light-wavelength capture system that takes advantage of altered blood vessel morphology. Wavelengths of light in the visible spectrum are filtered from the illumination source, with the exception of narrow bands in the blue and green spectrum centered at 415 nm and 540 nm, coinciding with the peak absorption spectrum of oxyhemoglobin, making blood vessels more pronounced when viewed in NBI mode [5]. We present the first ever results with NBI in a series of patients with pleural processes.
Methods
Medical thoracoscopy was performed under local anaesthesia and conscious sedation, using a prototype OLYMPUS XLTF-160 pleuravideoscope in single hole technique [6]. Following removal of the pleural fluid, the pleural cavity was inspected at first under white light and then under NBI as described elsewhere for bronchoscopy [5,7]. Afterwards, biopsies were taken from macroscopically altered sites. We used the OLYMPUS EVIS EXERA II video system (CV-180 videoprocessor and CLV-180 light source) manufactured by Olympus Medical Systems Corp., Japan. The findings were analysed retrospectively.
Results
The results are summarised in Table 1. A total of 15 women (median age 66 years) and 11 men (median age 64 years) with pleural effusions were examined. Biopsies of the parietal pleura or diaphragm were taken for all but one of these patients. Only in patient #26 NBI showed more vessels than white light (fig. 1 and 2). In all other patients, there was either no difference, or blood vessels merely appeared more prominent (example in fig. 3 and 4).
Table 1.
Results of thoracoscopy in all patients (n = 26)
| Pat. | Gender | Age | Macroscopical findings | Histological diagnosis |
| 1 | female | 81 | chronic inflammation, pleural thickening (visceral and parietal) | chronic pleuritis (underlying disease: chronic renal failure) |
| 2 | male | 74 | multiple polyps (parietal) | small cell lung cancer |
| 3 | male | 69 | small nodes (parietal), adhesions | squamous-cell lung cancer |
| 4 | female | 62 | Adhesions, small nodes (parietal) | malignant mesothelioma |
| 5 | female | 58 | small confluent nodes (parietal) | breast cancer |
| 6 | male | 59 | pleural thickening (parietal) | lung cancer (adenocarcinoma) |
| 7 | female | 81 | large nodes (parietal and visceral), adhesions | malignant mesothelioma |
| 8 | male | 47 | large nodes (parietal), adhesions | malignant mesothelioma |
| 9 | female | 65 | small confluent nodes (parietal) | breast cancer |
| 10 | female | 63 | solitary node/polyp (parietal) | ovarian cancer |
| 11 | female | 68 | acute inflammation, adhesions, lymphangiectasis | breast cancer |
| 12 | male | 64 | small confluent nodes, polyps (parietal) | malignant mesothelioma |
| 13 | male | 85 | large nodes, polyps (parietal and visceral) | malignant mesothelioma |
| 14 | male | 82 | pleural plaques (parietal), adhesions | squamous-cell lung cancer |
| 15 | male | 64 | pleural plaques (parietal), small confluent nodes | malignant mesothelioma |
| 16 | female | 88 | multiple large nodes (parietal and visceral) | lung cancer (adenocarcinoma) |
| 17 | female | 37 | acute inflammation, adhesions, pleural thickening | tuberculous pleurisy |
| 18 | female | 46 | no abnormalities | inflammatory changes (underlying diease: squamous-cell lung cancer, effusion e vacuo) |
| 19 | female | 63 | subacute inflammation, pleural thickening | malignant mesothelioma |
| 20 | female | 63 | large nodes, polyps (parietal and visceral) | lung cancer (adenocarcinoma) |
| 21 | female | 82 | large confluent nodes, polyps (parietal and visceral) | malignant mesothelioma |
| 22 | male | 64 | multiple small nodes, polyps (parietal and visceral) | malignant mesothelioma |
| 23 | male | 72 | pleural thickening, solitary polyps | squamous-cell lung cancer |
| 24 | male | 66 | pleural thickening, adhesions | small cell lung cancer |
| 25 | female | 80 | large solitary nodes (parietal) | breast cancer |
| 26 | female | 67 | large solitary nodes (parietal) and chronic inflammatory changes | lung cancer (adenocarcinoma) |
Figure 1.
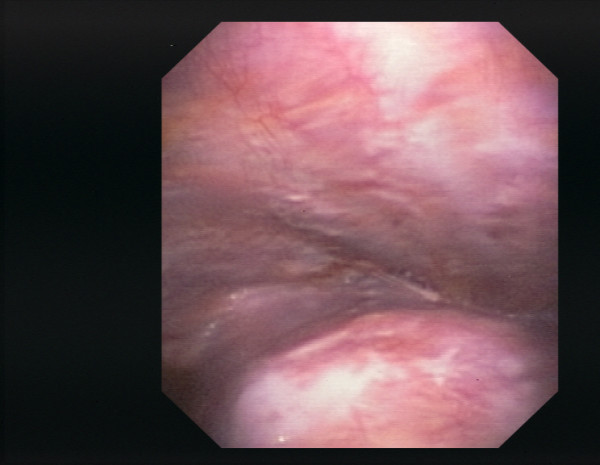
Pleural cavity of patient #26 (lung cancer (adenocarcinoma), chronic inflammatory changes), white light.
Figure 2.

Pleural cavity of patient #26, NBI.
Figure 3.
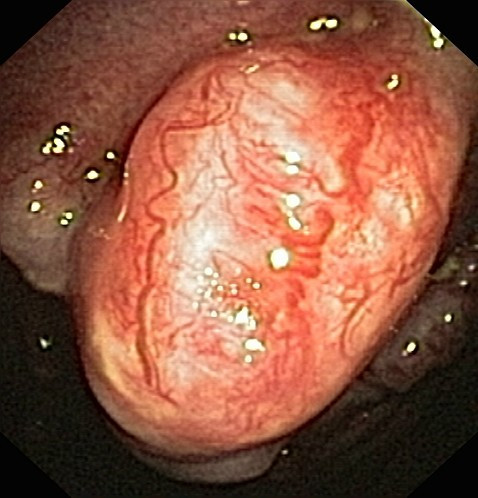
Pleural cavity of patient #2 (small cell lung cancer, large polyps), white light.
Figure 4.
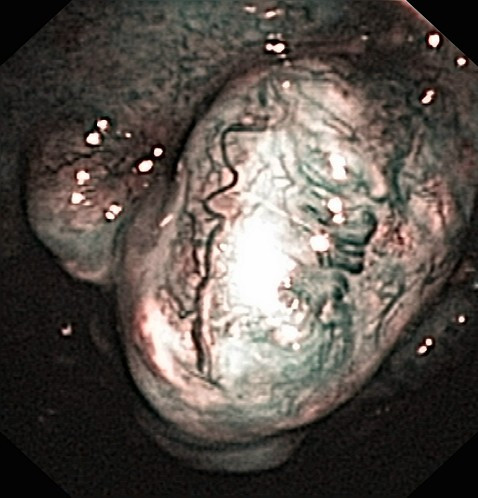
Pleural cavity of patient #2, NBI.
Discussion
Our first examinations of the pleural cavity with NBI have indicated that in cases with diffuse spread of malignant tumour no substantial improvement in diagnoses is to be expected. Whereas the blood vessels in the region of the tumour tissue that was already identifiable macroscopically were more clearly depicted, the number of changes rendered visible was no greater than with white light. This was also true for mesothelioma patients. In the two cases of non-specific pleuritis, in which the pleura did not appear to be essentially thickened, the visualisation of the blood vessels was similar under both white light and NBI.
As was to be expected, NBI also failed to demonstrate any blood vessels in the deeper layers of pleural plaques typical of asbestos-related disease. However, a different situation was found in a single patient with pleura showing chronic inflammatory changes, besides tumour polyps. In this case distinctly more deeper blood vessels were identifiable than under white light. To what extent this observation is indicative of an actual diagnostic advantage cannot, however, be ascertained on the basis of this initial series.
Other groups having used different procedures such as fluorescence techniques reported to have found more exact indications of spreading of malignant pleural mesotheliomas [3,4]. However, the numbers of patients participating in these studies were so small that it has not as yet been possible to produce reliable evidence. It is possible that the same applies to NBI, in so far as in some cases mesotheliomas are associated with the development of a considerable amount of fibrotic tissue [8] and may thus not be identifiable histologically in biopsies taken under medical thoracoscopy. In such cases imaging of vascular structures in deeper layers of a thickened pleura could give some indication of from where the biopsy should best be taken. However, until considerably larger numbers of patients have been examined with NBI this remains speculation.
A second interesting question that could be investigated in future clinical studies with NBI in pleural processes is whether NBI were to be used intra-operatively to inspect the pleura before planned resection of lung cancer [9]. This would facilitate detection of any previously unobserved pleural dissemination at other locations. It is already common in surgery for small effusions associated with primary pulmonary malignomas to begin the operation under thoracoscopy and only to perform thoracotomy and continue with the resection if there are no signs of pleural dissemination. If possible, studies of this kind should not – as is so often done with innovative techniques – be carried out at only a single centre, but be performed as prospective, multicentre studies. It would thus be possible to arrive at a more objective assessment of such innovative techniques.
Abbreviations
NBI: narrow band imaging.
Competing interests of authors
The authors declare that they have no competing interests.
Authors' contributions
All authors have taken part in the procedures (thoracoscopies) and, thus, the interpretation of clinical and endoscpical findings. Drs. Schönfeld, Bauer und Ott have in particular contributed to the retrospective analysis and interpretation of data.
Contributor Information
Nicolas Schönfeld, Email: schoenfeld.berlin@t-online.de.
Carsten Schwarz, Email: carri.schwarz@t-online.de.
Jens Kollmeier, Email: jens.kollmeier@helios-kliniken.de.
Torsten Blum, Email: torstenblum@t-online.de.
Torsten T Bauer, Email: torsten.bauer@helios-kliniken.de.
Sebastian Ott, Email: S.R.Ott@web.de.
References
- Loddenkemper R. Thoracoscopy – state of the art. Eur Respir J. 1998;11:213–221. doi: 10.1183/09031936.98.11010213. [DOI] [PubMed] [Google Scholar]
- Prosst RL, Winkler S, Boehm E, Gahlen J. Thoracoscopic fluorescence diagnosis (TFD) of pleural malignancies: experimental studies. Thorax. 2002;57:1005–1009. doi: 10.1136/thorax.57.12.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthidis MG, Janssen JP. Autofluorescence videothoracoscopy in exudative pleural effusions: preliminary results. Eur Respir J. 2005;26:989–992. doi: 10.1183/09031936.05.00027505. [DOI] [PubMed] [Google Scholar]
- Baas P, Triesscheijn M, Burgers S, van Pel R, Stewart F, Aaldres M. Fluorescence detection of pleural malignancies using 5-aminolaevulinic acid. Chest. 2006;129:718–724. doi: 10.1378/chest.129.3.718. [DOI] [PubMed] [Google Scholar]
- Vincent BD, Fraig M, Silvestri GA. A pilot study of narrow-band imaging compared to white light bronchoscopy for evaluation of normal airways and premalignant and malignant airways disease. Chest. 2007;131:1794–1799. doi: 10.1378/chest.06-2794. [DOI] [PubMed] [Google Scholar]
- Munavvar M, Khan MA, Edwards J, Wagaruddin Z, Mills J. The autoclavable semirigid thoracoscope: the way forward in pleural disease? Eur Respir J. 2007;29:571–574. doi: 10.1183/09031936.00101706. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Hoshino H, Chiyo M, Iyoda A, Yoshida S, Sekine Y, Iizasa T, Saitoh Y, Baba M, Hiroshima K, Ohwada H, Fujisawa T. High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax. 2003;58:989–995. doi: 10.1136/thorax.58.11.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury PM, Butcher DN, Corrin B, Nicholson AG. The use of histological and immunohistochemical markers to distinguish pleural malignant mesothelioma and in situ mesothelioma from reactive mesothelial hyperplasia and reactive pleural fibrosis. J Pathol. 1999;189:251–257. doi: 10.1002/(SICI)1096-9896(199910)189:2<251::AID-PATH412>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sebastián-Quetglás F, Molins L, Baldó X, Buitrago J, Vidal G, Spanish Video-assisted Thoracic Surgery Study Group Clinical value of video-assisted thoracoscopy for preoperative staging of non-small cell lung cancer. A prospective study of 105 patients. Lung Cancer. 2003;42:297–301. doi: 10.1016/j.lungcan.2003.06.001. [DOI] [PubMed] [Google Scholar]


