Abstract
Caudal brainstem viscerosensory nuclei convey information about the body's internal state to forebrain regions implicated in feeding behavior and responses to immune challenge, and may modulate ingestive behavior following immune activation. Illness-induced appetite loss might be attributed to accentuated “satiety” pathways, activation of a distinct “danger channel” separate from satiety pathways, or both. To evaluate neural substrates that could mediate the effects of illness on ingestive behavior, we analyzed the pattern and phenotypes of medullary neurons responsive to consumption of a preferred food, sweetened milk, and to intraperitoneal lipopolysaccharide challenge that reduced sweetened milk intake. Brainstem sections were stained for c-Fos, dopamine-beta-hydroxylase, phenylethanolamine-N-transferase, and glucagon-like peptide-1 (GLP-1) immunoreactivity. Sweetened milk intake activated many neurons throughout the nucleus of the solitary tract (NTS), including A2 noradrenergic neurons in the caudal half of the NTS. LPS challenge activated a similar population of neurons in the NTS, in addition to rostral C2 adrenergic and mid-level A2 noradrenergic neurons in the NTS, many C1 and A1 neurons in the ventrolateral medulla, and in GLP-1 neurons in the dorsal medullary reticular nucleus. Increased numbers of activated of GLP-1 neurons in the NTS was only associated with sweetened milk ingestion. Evidence for parallel processing was reflected in the parabrachial nucleus, where sweetened milk intake resulted in activation of the inner external lateral, ventrolateral and central medial portions, whereas LPS challenge induced c-Fos expression in the outer external lateral portions. Thus, signals generated in response to potentially dangerous physiological conditions seem to be propagated via specific populations of catecholaminergic neurons in the NTS and VLM, and likely include a pathway through the external lateral PBN. The data indicate that immune challenge engages multiple ascending neural pathways including both a distinct catecholaminergic “danger” pathway, and a possibly multimodal pathway derived from the NTS.
1. Introduction
One of the most common consequences of immune activation is alteration in ingestive behavior. Reduction in drinking or eating (anorexia), are observed in many clinical syndromes and are found in animals treated with experimental immune challenge as well. Because reduced food intake is associated with poorer outcomes of chronic illness (Hauser et al., 2006; Strassburg & Anker, 2006), understanding of the neurobiological substrates impacted by immune challenges and inflammation could contribute to clinically important strategies for intervention.
Ingestive behavior is controlled by interactions between peripheral “bottom up” signals related to physiological states (including immune challenge), and “top-down” cognitive and affective influences related to learning, arousal and hedonics (Berthoud, 2004). We have previously shown (Park et al., 2008) that an intraperitoneal challenge with lipopolysaccharide (LPS) that inhibited ingestion of a palatable sweetened milk solution influenced activity of a network of brain regions that included the paraventricular thalamus, the rostral nucleus accumbens (NAc), (which has been implicated in negative hedonics and the inhibition of food intake; Reynolds and Berridge, 2002; Reynolds and Berridge, 2003; Zheng et al., 2003) and the orexin neurons of the lateral hypothalamus (LH). Neurons in the LH act as a nodal point where bottom-up and top-down regulatory signals converge to modulate hypothalamic drive on the brainstem neurons that control motor aspects of ingestive behavior. The findings from Park et al. (2008) support the idea that one way illness inhibits food intake is by influencing “top-down” basal forebrain and diencephalic neurocircuitry that integrate noxious or aversive stimuli with control of ingestive behavior.
A missing part of the known neurocircuitry involved in immune effects on behavior involves the identity of the “bottom-up” neural pathways that relay incoming sensory information signaling immune challenges and that impinge upon the forebrain neural networks that control ingestive behavior. Candidates include the immune-sensitive caudal brainstem neurons in the nucleus of the solitary tract (NTS) and ventrolateral medulla (VLM) that project to the paraventricular (Rinaman, 1999; Gaykema et al., 2007) and possibly adjacent hypothalamic areas involved in ingestive behavior. These neurons receive immune-related signals from sensory terminations of the vagus nerve, and from the area postrema (a circumventricular organ in which the blood barrier is weak). Caudal brainstem catecholamine neurons drive the HPA axis in the context of immune challenge (Rinaman, 2007; Dayas et al., 2001; Ericsson et al., 1994), and have been proposed to signal “physiological” or interoceptive stress. In addition, glucagon-like peptide-1 (GLP-1) containing neurons in the NTS have been reported to respond to peripheral LPS challenge (Rinaman, 1999) and may contribute to LPS-related anorexia (Grill et al. 2004). Thus, caudal brainstem-derived neuronal projections make plausible candidates as conduits from immunosensory interfaces to brain regions controlling ingestive behavior.
Ascending projections from the caudal brainstem carry a diverse range of signals related to normal viscerosensory stimuli (e.g. presence of food in stomach) as well as information indicating physiological challenges (e.g. pathogenic bacteria in the gastro-intestinal tract). The extent to which these different types of information, which likely induce somewhat different physiological or behavioral responses, are coded differently is not known. For instance, the nucleus of the solitary tract (NTS) is responsive to both satiety (Rinaman et al., 1998; Emond et al., 2001; Faipoux et al., 2008) and immune-related signals (Bienkowski and Rinaman, 2008; Elmquist et al., 1996; Gaykema et al., 2004; Goehler et al., 2005; Rinaman and Dzmura, 2007; Wan et al., 1994), and both types of stimuli serve to reduce further food intake. An immune challenge could activate a selective sickness-mediating (“danger”) channel, or it could act via enhancement of ascending projections that carry signals related to satiety, which would also lead to a reduction in ingestive behavior, or activate both types of pathways.
If information related to immune challenge is conveyed to the forebrain by a selective “danger-related” pathway, a discernable difference in the pattern of activated neurons in the caudal brainstem should be evident following immune challenge compared to nonthreatening viscerosensory stimuli such as a satiating meal. On the other hand, if immune challenge utilizes the same pathway as do, for instance, satiety-related signals, the same neurons should be active irrespective of type of stimulus. Thus, to determine whether sickness and satiety pathways can be distinguished in the brainstem, we compared the pattern of neuronal activation in brainstem regions between animals that were offered either sweetened milk or water, and received either LPS or saline injections. The animals used were the same as those used for the description of activation patterns in the forebrain associated with LPS-induced reduction of sweetened milk ingestion (Park et al., 2008). We analyzed c-Fos expression throughout the rostrocaudal extent of the NTS (the first order gustatory and visceral sensory relay nucleus) and the VLM. To identify specific populations of projection neurons, we assessed c-Fos protein induction in specific populations, i.e., adrenergic, noradrenergic, and glucagon-like peptide (GLP)-containing neurons. Animals challenged with LPS consume much less sweetened milk than saline-treated controls, which could be reflected in differences in neuronal activation patterns independent of immune challenge (especially due to differences in gastric distension, see Rinaman et al., 1998). To distinguish between possible contributions of ingestion of sweetened milk per se from c-Fos activation due to immune challenge (in LPS-treated rats that had sweetened milk access) we included two groups of “yoked controls”. These animals were allowed access to a rationed amount of sweetened milk, corresponding to the average amount consumed by the LPS-treated rats given ad libitum access.
One of the primary targets for ascending viscerosensory projections is the pontine parabrachial nucleus (PBN). The PBN constitutes a major of node of immune-responsive neural networks (Buller et al., 2004; Gaykema et al., 2007), although its functional contribution to sickness responses is not clear. Neurons in this nucleus seem to be organized into regions that respond selectively to functional aspects of the stimulus (Yamamoto et al., 1994). Thus, if LPS activates a unique pathway, distinct patterns of immune challenge-related and sweetened milk-induced c-Fos induction should be observable in the PBN. To acquire further evidence regarding the organization of ascending neural projections carrying categorically different viscerosensory modalities, we therefore assessed c-Fos in the PBN.
2. Results
For these experiments we used six groups of rats, of which four groups were accustomed to receiving ad libitum access to either sweetened milk (non-threatening viscerosensory stimulus) or water (to control for arousal associated with disturbance of the cage) for 30 minutes, and who were given i.p. injections of either LPS (threatening viscerosensory stimulus) or saline. Because LPS-treated animals consumed much less sweetened milk than controls (down to 5.0 ml), two additional groups were included that were rationed (“yoked”) to the same amount of drinking solution that was consumed by the LPS-treated animals given ad libitum access to sweetened milk. Data on the ingestive behavior of the animals were previously published (Park et al., 2008). The saline-treated animals in the ad libitum milk group consumed approximately 20 ml of sweetened milk, which led to dramatically distended stomachs filled with milk observable at the time of perfusion.
Both ad libitum (but not limited) sweetened milk consumption (in saline treated rats) and LPS treatment (in rats with access to water) significantly increased the number of c-Fos positive neurons in the caudal brainstem, but with distinct, albeit overlapping patterns. Increases in c-Fos staining occurred throughout the NTS in response to both stimuli (see Table 1). c-Fos expression also increased in DBH neurons throughout the VLM column in response to LPS, however milk consumption was associated with c-Fos induction only in DBH-labeled cells of the caudal VLM. In the PBN, stimulus-distinct distribution patterns of c-Fos staining emerged in specific subregions, with strong LPS-induced induction of c-Fos in the external lateral subnucleus and sweetened milk-associated c-Fos induction in other portions of the PBN, including the dorsolateral and central medial subnuclei. The distribution of c-Fos positive neurons within the NTS, VLM and PBN is described in detail in the following sections.
Table 1.
| Effect: Region/cells |
treatment LPS/saline |
drinking solution sw milk ad lib/water |
interaction treatm × drink sol |
interaction r-c level × treatm × drink sol |
|---|---|---|---|---|
| NTS c-Fos (Fig. 2A,B) |
F(1,168) = 18.3 p = 0.0008 |
F(1,168) = 8.3 p = 0.01 |
F(1,168) = 17.5 p = 0.0009 |
F(12,168) = 11.76 p < 0.0001 |
| NTS c-Fos & DBH (Fig. 4A,B) |
F(1,285) = 13.5 p = 0.0016 |
F(1,285) = 5.06 p = 0.036 |
F(1,285) = 6.2 p = 0.023 |
F(15,285) = 1.83 p = 0.03 |
| VLM c-Fos & DBH (Fig. 7A) |
F(1,260) = 202.6 p < 0.0001 |
F(1,260) = 8.1 p = 0.031 |
F(1,206) = 1.1 (p = 0.09) |
F(13,260) = 1.11 (p = 0.35) |
Effects of LPS treatment and sweetened milk consumption on the rostrocaudal distribution of c-Fos-positive and c-Fos-DBH double-labeled cells in the NTS and the VLM
2.1. NTS c-Fos expression
2.1.1. Sweetened milk intake activates neurons throughout the rostrocaudal extent of the NTS
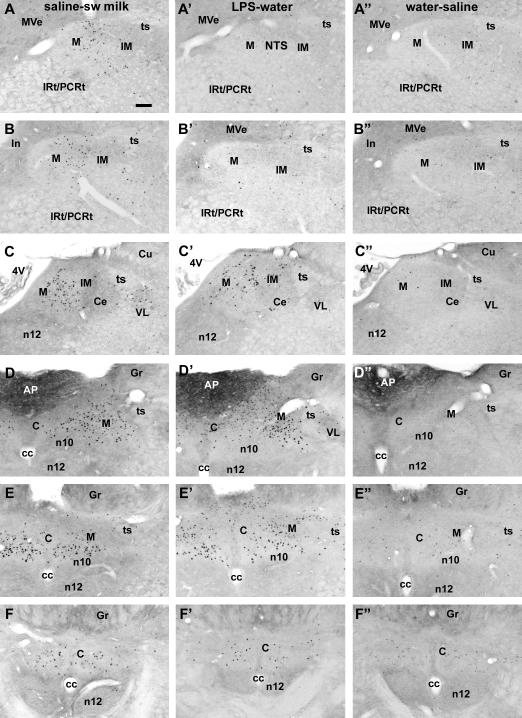
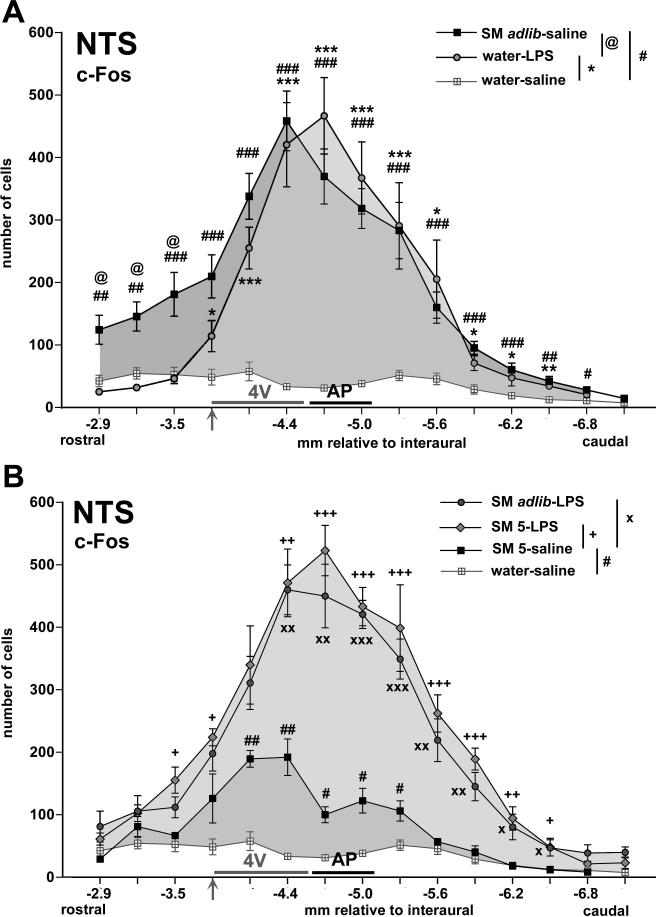
Unrestricted (satiating) sweetened milk consumption induced c-Fos expression in neurons throughout the NTS, from the extreme rostral (gustatory) into caudal (general visceral) portions (Fig.1A-F, Fig. 2A). In animals that received saline injections and were allowed to drink sweetened milk ad libitum, c-Fos positive cells were observed at the caudal levels of the central/commissural subnuclei (Fig. 1E,F), in the medial subnucleus at the level of the area postrema (Fig 1C,D), and in the intermediate subnuclei as far rostral as the rostral pole of the NTS close to the level of the entry of the eighth nerve (Fig. 1A,B). Restricted consumption of sweetened milk led to significantly fewer numbers of c-Fos-ir cells than unrestricted consumption in the saline-treated groups (ad libitum vs. “yoked” comparison: F(1,84) = 19.1, p = 0.0033). In the milk-restricted animals, there was relatively small increase in c-Fos, as compared to the water-offered saline control group (main effect F(1,84) = 14.8, p = 0.0063), mainly in the mid-portion of the NTS (Fig. 2B).
Fig. 1.
Series of photomicrographs showing the rostrocaudal distribution (from top to bottom with the rostral-most image at the top) of c-Fos staining in the NTS following saline injection and sweetened milk consumption (left column A-F), after LPS injection (0.1 mg/kg i.p.) and offered water (middle column A’-F’), and following saline injection and water offer (right column, A”-F”). Milk consumption (ad libitum) induced large numbers of c-Fos-labeled cells in the rostral-most NTS ventral to the medial vestibular nucleus (A,B), in which region LPS injection resulted in few c-Fos cells (A’,B’). At mid- and caudal levels where the NTS borders the fourth ventricle, the area postrema and the gracile nucleus, both stimuli gave rise to comparable levels of c-Fos expression (C-F and C’-F’), but the distribution patterns within these parts of the NTS are not identical. Small numbers of c-Fos-labeled cells were present following saline injection and presentation of water (A”-F”). Scale bar in A = 100 μm. Abbreviations: 4V, fourth ventricle; C, commissural subnucelus (NTS); Ce, central subnucleus (NTS); cc, central canal; Gr, gracile nucleus; IM, intermediate subnucleus (NTS); IRt/PCRt, intermediate/parvicellular reticular nucleus; M, medial subnucleus (NTS); n10, motor nucleus of the vagus; n12, hypoglossal nucleus; ts, tractus solitarius; VL, ventrolateral subnucleus (NTS).
Fig. 2.
Graphs summarizing the rostrocaudal distribution of c-Fos-positive cells in the NTS, displaying group means (+/− SEM) of the cell counts in sections corresponding with atlas coordinates −2.9 mm to −7.1 mm relative to interaural with 0.3 mm increments. The grey horizontal bar along the X-axis levels indicates where the NTS abuts the floor of the fourth ventricle (4V) with the arrow indicating where the NTS recedes from the 4V. Caudally, the black horizontal bar indicates the level of the area postrema (AP). Panel A depicts the sweetened milk (ad libitum)-saline and water-LPS groups (with the water-saline group as reference) for comparison between the two different stimuli. # p < 0.05, ## p < 0.005, ### p < 0.0005: sweetened milk ad lib-saline vs. water-saline; * p < 0.05, ** p < 0.005, *** p < 0.0005: water-LPS vs. water-saline; @ p < 0.01: sweetened milk ad lib-saline vs. water-LPS. Panel B depicts the LPS-treated groups that had access to ad libitum sweetened milk (SM adlib) and to 5.0 ml milk (SM 5) for comparison with the saline-treated group that were offered 5.0 ml sweetened milk solution. These three groups consumed comparable non-satiating amounts. # p < 0.05, ## p < 0.005: sweetened milk 5 ml [SM 5]-saline vs. water-saline; + p < 0.05, ++ p < 0.005, +++ p < 0.0005: SM 5-LPS vs. SM 5-saline; x p < 0.05, xx p < 0.005, xxx p < 0.0005: sweetened milk ad lib-LPS vs. SM 5-saline.
2.1.2 Sweetened milk activates noradrenergic but not adrenergic neurons
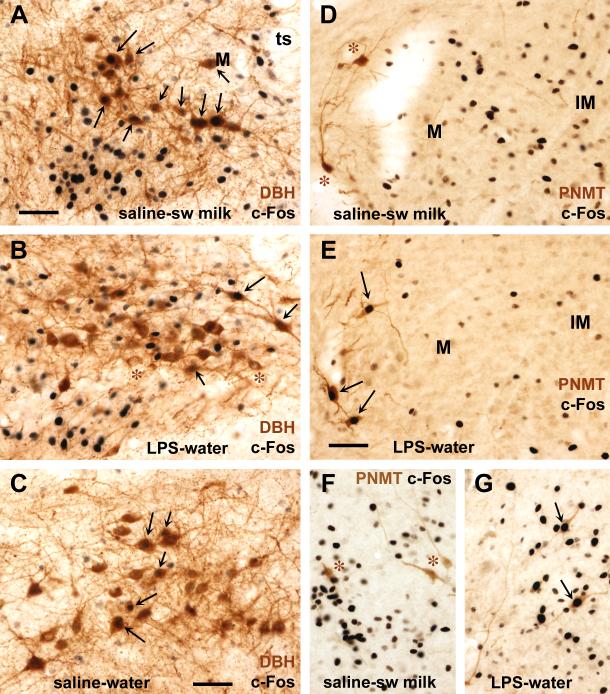
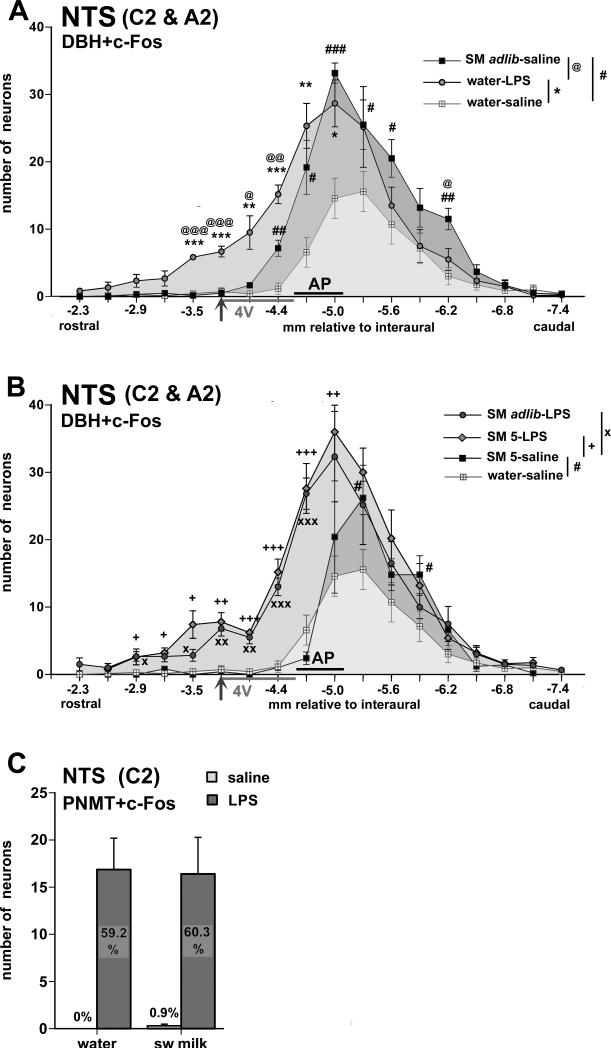
In the saline-treated rats with unrestricted (ad libitum) consumption of sweetened milk, many neurons that stained for both c-Fos and DBH immunoreactivity were located at the level of the area postrema (AP) and further caudally within the commissural NTS (Fig. 3A, 4A), whereas in rats with access to a rationed amount, c-Fos-DBH double-labeled cells were predominant in the commissural NTS caudal to the AP (Fig 4B). The saline-treated controls that were offered water also harbored double-labeled neurons with 17.4% (+/− 3.6%) and 33.4% (+/− 4.7%) of DBH neurons showing nuclear stain for c-Fos at the level of the obex-AP and caudal to the AP, respectively (Fig. 4A, Table 2). In comparison with these saline-water controls, restricted sweetened milk consumption led to significantly increased Fos staining in DBH neurons caudal to the AP (up to 56.6 +/− 7.5 %, p = 0.01), but not at the level of the obex-AP (19.2 +/− 5.9%). Unrestricted (ad libitum) sweetened milk consumption, in contrast, increased nuclear c-Fos staining among DBH neurons both at the level of the obex-AP (up to 50.4 +/− 4.6%, p = 0.0001) and caudal to the AP (up to 64.2 +/−5.2%, p = 0.001). Indeed, the comparison between rationed and ad libitum consumption led to a difference in the proportion of DBH neurons showing c-Fos staining only at the obex and AP level (19.2 vs. 50.4%, p = 0.0003).
Fig. 3.
Sweetened milk consumption and LPS challenge induced c-Fos expression in NTS noradrenergic and adrenergic neurons. Photomicrographs depict dual immunostaining for c-Fos (black cell nuclei) and either DBH or PNMT (brown cytoplasmic staining) at three different rostrocaudal levels. Panels A-C depict DBH-immunoreactive A2 cells in the medial subnucleus of the NTS at the level of the caudal AP (left hemisphere). Panels D-G depict PNMT-immunoreactive C2 cells in the rostral medial-intermediate subnuclei of the NTS ventral to the medial vestibular nucleus (D,E), and in the medial NTS just deep to the floor of the fourth ventricle (F,G), all in the right hemisphere. Black nuclear c-Fos labeling appeared most abundantly in DBH-labeled A2 cells in saline-treated rats following sweetened milk consumption (A), followed by those treated with LPS (B), and few such double-labeled neurons were present in the saline-water control group (C). In contrast, PNMT-labeled C2 cells lacked black nuclear staining for c-Fos following ad libitum sweetened milk consumption (indicated with asterisks in D,F), as c-Fos positive neurons were present in large numbers but separate from the PNMT-labeled neurons. Instead, PNMT-labeled C2 cells endowed with black nuclear c-Fos staining occurred exclusively following LPS challenge (E,G). Scale bar = 50 μm.
Fig. 4.
A,B. Graphs summarizing the rostrocaudal distribution of c-Fos- and DBH double-labeled cells in the NTS displaying group means (+/− SEM) of the cell counts in sections that correspond with atlas coordinates −2.3 mm to −7.2 mm relative to interaural with 0.3 mm increments. The grey horizontal bar along the X-axis levels indicates where the NTS abuts the floor of the fourth ventricle (4V) with the arrow indicating where the NTS recedes from the 4V. Caudally, the black horizontal bar indicates the level of the area postrema (AP). Panel A includes the sweetened milk (ad libitum)-saline and water-LPS groups with the water-saline control group as reference. # p < 0.05, ## p < 0.005, ### p < 0.0005: sweetened milk ad lib-saline vs. water-saline; * p < 0.05, ** p < 0.005, *** p < 0.0005: water-LPS vs. water-saline; @ p < 0.05, @@ p < 0.005, @@@ p < 0.0005: sweetened milk ad lib-saline vs. water-LPS. Panel B includes the LPS-treated groups that had access to ad libitum sweetened milk (SM adlib) and 5.0 ml milk (SM 5) as well as the saline-treated group offered restricted amount of sweetened milk (5.0 ml). These three groups consumed comparable non-satiating amounts of sweetened milk. For reference, the water-saline group is also included. # p < 0.05: sweetened milk 5 ml [SM 5]-saline vs. water-saline; + p < 0.05, ++ p < 0.005, +++ p < 0.0005: SM 5-LPS vs. SM 5-saline; x p < 0.05, xx p < 0.005, xxx p < 0.0005: sweetened milk ad lib-LPS vs. SM 5-saline. C. LPS challenge, but not sweetened milk consumption, leads to c-Fos induction in PNMT-immunoreactive adrenergic neurons in the rostral NTS. Bar graphs summarize the numbers of c-Fos-PNMT double-labeled cells (group mean +/− SEM in groups offered water or sweetened milk and treated with either saline or LPS) counted and summed across evenly spaced sections (0.3 mm apart) rostral to the AP (between −2.3 and −4.4 mm relative to interaural). Mean percentages of the total number of PNMT cells that express c-Fos are indicated in the bar graphs. Total counts of PNMT neurons did not differ between groups. In all instances, LPS challenge dramatically increased c-Fos staining in PNMT-labeled cells (p < 0.0001).
Table 2.
| Region | NTS-rostral | (−2.9 – −4.1)a | NTS-mid portion | (−4.4 – −5.0) | NTS-caudal | (−5.3 – −7.1) |
|---|---|---|---|---|---|---|
| saline | LPS | saline | LPS | saline | LPS | |
| water | 3.5 +/− 2.2 | 59.6 +/− 3.6 ** | 17.4 +/− 3.6 | 67.8 +/− 3.6 ** | 33.4 +/− 4.6 | 46.1 +/− 6.4 |
| sw milk (5 ml) | 2.1 +/− 1.0 | 52.4 +/− 4.7 ** | 19.2 +/− 6.0 | 65.8 +/− 3.4 ** | 56.6 +/− 7.5 # | 57.5 +/− 5.2 |
| sw milk ad lib | 6.3 +/− 2.0 | 46.1 +/− 3.1 ** | 50.4 +/− 4.6 ## | 62.3 +/− 4.2 | 64.2 +/− 5.2 ## | 50.7 +/− 6.6 |
| Region | VLM-rostral | (−3.2 – −4.4 IA) | VLM-mid portion | (−4.7 – −5.3) | VLM-caudal | (−5.6 – −7.4) |
|---|---|---|---|---|---|---|
| saline | LPS | saline | LPS | saline | LPS | |
| water | 0.4 +/− 0.2 | 47.5 +/− 5.8 ** | 0.7 +/− 0.4 | 76.6 +/− 4.3 ** | 1.7 +/− 0.9 | 61.8 +/− 4.9 ** |
| sw milk (5 ml) | 0.8 +/− 0.6 | 65.4 +/− 0.6 ** | 0.9 +/− 0.6 | 90.4 +/− 2.8 ** | 8.3 +/− 2.0 | 77.4 +/− 5.0 ** |
| sw milk ad lib | 0.1 +/− 0.1 | 57.3 +/− 2.9 ** | 1.3 +/− 0.3 | 84.1 +/− 3.1 ** | 12.0 +/− 3.0 # | 68.3 +/− 4.4 ** |
Percentages of DBH-labeled cells that are c-Fos positive in rostral, mid, and caudal parts of the NTS and the VLM: Effects of LPS treatment and consumption of rationed (5 ml) and unrestricted (ad lib) sweetened milk solution
p < 0.001 LPS vs. saline treatment
p < 0.01
p < 0.001 sweetened milk vs. water
atlas coordinates in mm relative to interaural (Paxinos and Watson, 1998)
In the NTS rostral to the obex, where it abuts the floor of the fourth ventricle, DBH-c-Fos double-labeled neurons were rather sparse in both water and sweetened milk groups (2.1-6.3%, all of the double-labeled cells were close the obex). Most of the rostrally located subset of catecholaminergic neurons that comprise the adrenergic C2 neurons can be visualized by PNMT immunostaining. Further evaluation of c-Fos-PNMT-stained sections revealed that, in saline-treated rats, none of the PNMT-positive neurons were c-Fos-positive following presentation of water or after sweetened milk consumption (Fig. 3D,F, 4C).
2.1.3. LPS treatment activates NTS neurons at mid and caudal levels
In LPS-treated rats that were offered water, c-Fos expression increased dramatically in the NTS subdivisions medial to the solitary tract along most of its rostrocaudal axis, with the exception of the rostral part that is receded (ventrolateral) from the floor of the 4th ventricle (Fig. 1A’-F’; interaction rostrocaudal level and LPS treatment: F(12,168) = 18.4, p < 0.0001). In the rostral-most portion of the NTS, c-Fos expression was present in smaller numbers and located along the medial edge (Fig. 2A). In contrast to the more clustered pattern of sweetened milk-associated c-Fos positive nuclei in the mid central portion of the intermediate subnucleus, LPS-induced c-Fos staining was distributed across a wider range of subnuclei, including the medial, dorsomedial, and commissural subdivisions (Fig. 1C’,D’). LPS treatment gave rise to much larger numbers of Fos in the NTS than seen after saline treatment among the yoked groups with restricted access to sweetened milk (F(1,105) = 144.2, p < 0.0001), with a distribution of c-Fos-labeled cells across the rostrocaudal axis highly comparable to the other LPS-treated groups (Fig. 2B).
2.1.4. LPS activates both NTS noradrenergic and adrenergic neurons
Sections double-stained for DBH and c-Fos showed a large proportion of double-labeled somata after LPS challenge of water-offered rats, present from the level of the posterior end of the AP caudally (Fig. 3B) to the rostral-most portion, where the NTS is receded from the floor of the 4th ventricle. In the rostral, but not the caudal NTS, numbers of double-labeled cells were much higher in LPS-treated rats than saline-treated ones that were offered water (Fig. 4A, Table 2; interaction rostrocaudal level and LPS treatment: F(15,285) = 3.88, p < 0.0001). Among the DBH neurons that are caudal to the AP, the proportion of those expressing c-Fos increased only slightly after LPS challenge as compared to saline-treated water-offered controls (from 33.4 +/− 4.7% to 46.1 +/− 6.4%, p = 0.13). This limited (non-significant) effect of LPS treatment contrasts with the large effect of sweetened milk consumption seen in the DBH-labeled neurons at this caudal level (see 2.1.2). Along the extent of the AP, LPS treatment dramatically increased the proportion of DBH neurons that showed c-Fos staining (from 17.4 +/− 3.6 % to 67.8 +/− 3.6 %). A similar strong effect of LPS treatment was seen in the more rostral portion of the NTS, among DBH neurons abutting the floor of the fourth ventricle (from 3.5 +/− 2.2 % to 59.6 +/− 3.6 %). Strong LPS-induced c-Fos expression in DBH neurons was especially prominent in the rostral third of the NTS (compare Fig. 4A with Fig. 2A). Staining for PNMT confirmed a large proportion of PNMT-labeled cells expressed c-Fos after LPS treatment (Fig. 3E,G; up to 60%), while none were c-Fos-positive after saline treatment (Fig. 4C; F(1, 20) = 41.31, p < 0.0001). This strong effect of LPS on the rostral population of catecholaminergic neurons contrasts the lack of effect of sweetened milk consumption on these cells (see 2.1.2).
2.1.5. Combined LPS treatment and sweetened milk consumption does not increase total numbers of activated cells
In the rats that were offered sweetened milk and received LPS challenge, the pattern of c-Fos expression in the NTS generally resembled that of LPS-treated rats that were offered water (Fig. 2A,B). The pattern included the induction of c-Fos in rostral and mid-level DBH neurons (Table 2) as well as rostral PNMT-labeled neurons (Fig. 4B). Comparisons among the LPS-treated groups reveal no statistical differences (p > 0.05). However, abundance of c-Fos expression in the DBH-labeled cells in the caudal NTS (57.5%) was similar to that seen in the saline-treated rats that were offered either restricted or ad libitum sweetened milk (56.7 and 64.2%, Table 2).
Because animals administered LPS consume much less sweetened milk than saline-treated controls, which could be reflected in differences in neuronal activation patterns, independent of immune challenge, we included two groups of “yoked controls” that were allowed access to an amount of sweetened milk that corresponded to the average amount consumed by the LPS-treated rats given ad libitum access. The yoked control procedure allowed us to assess possible contributions to c-Fos expression from ingestion of sweetened milk per se from that due to immune challenge in LPS-treated rats. This comparison revealed that an increase in c-Fos positive cells in a large portion of the NTS can be attributable to LPS treatment (Fig. 2B), as well as a significant increase in c-Fos expression due to LPS in the DBH-labeled cell population at the level of the obex and AP (from 19.2% to 65.8%) and further rostrally (from 2.1% to 52.4%). However, a discernable difference (i.e., different from the saline-treated rationed/yoked milk group, depicted in Fig. 4B) was not evident in the caudal NTS (from 56.6% to 57.5%; interaction rostrocaudal level and LPS treatment: F(15,60) = 2.06, p = 0.025). Again (as described in 2.1.4), this indicates that LPS challenge activates DBH neurons at the level of the AP and further rostrally, but not caudally.
2.1.6. c-Fos expression in NTS GLP-1-labeled neurons was related to sweetened milk ingestion but not LPS treatment
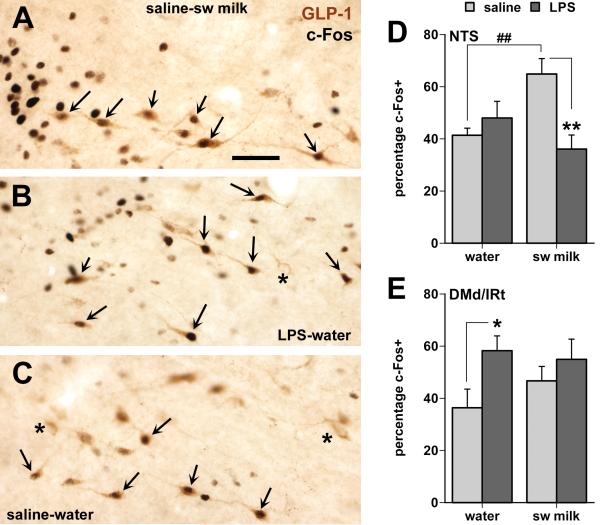
GLP-1-positive neurons were present in the ventrolateral portions of the medial NTS extending caudally from the level of the posterior AP. In all treatment groups, single-labeled GLP-1 immunoreactive cell bodies intermingled with c-Fos-GLP-1 double-labeled ones (Fig. 5). In animals that received sweetened milk with saline injections, the percentage of GLP-1 and c-Fos double labeled neurons was statistically significantly elevated compare to the other three groups (Fig 5D; interaction F(1, 20) = 9.50, p = 0.006).
Fig. 5.
Effects of LPS treatment and sweetened milk consumption on c-Fos expression in GLP-1-immunoreactive neurons in the caudal NTS (A-D) and in those distributed within the dorsal and intermediate medullary reticular nuclei (DMd/IRt in E) in between the caudal-lateral NTS and the VLM. A-C: Photomicrographs of GLP-1 neurons in the NTS (situated just ventral to the tractus solitarius at the level of the caudal tip of the area postrema) many of which show black nuclear staining for c-Fos irrespective of treatment (arrows point at double-labeled cells, asterisks at GLP-1 neurons without c-Fos staining). Note that in the water-saline controls most of the c-Fos labeling in this region colocalizes with GLP-1 neurons (C), whereas many c-Fos nuclei in the sweetened milk (A) and LPS groups (B) are also present in neurons other than the GLP-1-labeled cells. Scale bar = 50 μm. D: Only the unrestricted consumption of sweetened milk by the saline-treated rats increased c-Fos expression in the GLP-1 neurons of the caudal NTS (##, ** p < 0.01). E: LPS treatment caused a moderate increase in c-Fos expression among the GLP-1 neurons within the DMd/IRt (* p < 0.05). The total number of GLP-1 neurons in the NTS and the DMd/IRt did not differ between groups.
2.2. VLM c-Fos expression
2.2.1. Sweetened milk intake consumption does not activate neurons in the VLM
There was little or no c-Fos expression in the VLM in the saline-treated rats that consumed either the restricted or satiating amounts of sweetened milk, except for a small increase in the caudal-most third of the VLM. Concordantly, there was no induction of c-Fos staining in either DBH- or PNMT-labeled neurons in response to sweetened milk intake (Figs. 6,7), with the exception of a small, but significant, increase in the proportion of DBH neurons that stain for c-Fos in the caudal VLM (Fig. 7A; table 2).
Fig. 6.
LPS-induced c-Fos staining in VLM catecholaminergic neurons. Photomicrographs depict dual immunolabeling for c-Fos (black nuclear staining) and either DBH or PNMT (brown cytoplasmic staining) in the caudal (A,B) and rostral (C,D) VLM, respectively. Nuclear c-Fos staining appeared in many of the VLM A2 and C2 cells after LPS challenge (B,D), but not ad libitum sweetened milk consumption (A,C). Scale bar = 50 μm.
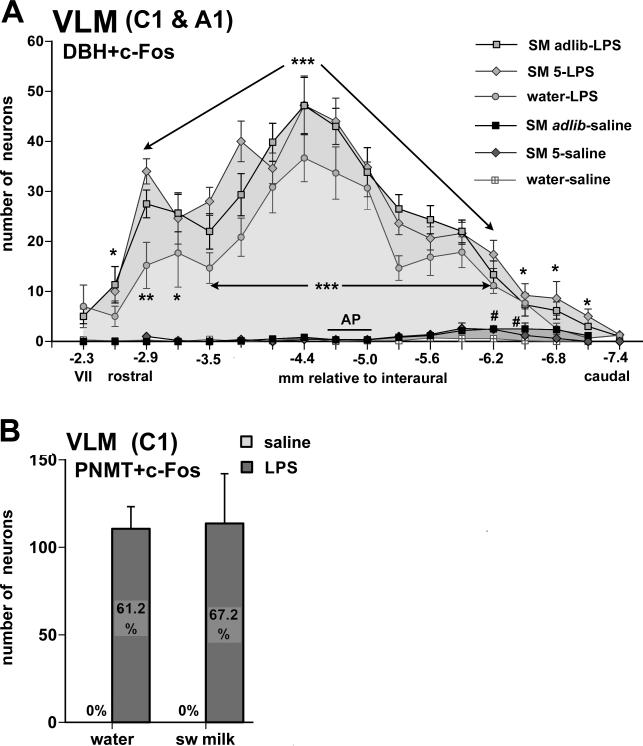
Fig. 7.
Panel A depicts the distribution of c-Fos-DBH double-labeled cells in the VLM of all six experimental groups, with the three LPS-treated groups giving rise to large numbers of double-labeled cells across the entire rostro-caudal extent, with few if any DBH cells expressing c-Fos after saline injections. * p < 0.05, ** p < 0.005, *** p < 0.0005: LPS vs. saline treatment; #, p < 0.05: sweetened milk [SM] ad lib-saline vs. water-saline. The total number of DBH-labeled cells did not differ between groups. Panel B depicts the number of PNMT-immunoreactive adrenergic neurons in the rostral VLM that express c-Fos following LPS challenge and/or sweetened milk consumption. Bar graphs summarize the numbers of c-Fos-PNMT double-labeled cells (mean +/− SEM) in groups offered water or sweetened milk and treated with either saline or LPS, representing cumulative counts from evenly spaced sections (0.3 mm apart, between −2.3 and −4.4 mm relative to interaural). Mean percentages of the total number of PNMT cells that express c-Fos are indicated in the bar graphs. Total counts of PNMT neurons did not differ between groups. In all instances, LPS challenge dramatically increased c-Fos staining in PNMT-labeled cells (p < 0.0001).
2.2.2. LPS induces c-Fos protein in both catecholaminergic and GLP neurons in the VLM and dorsal medullary reticular nucleus
LPS challenge, irrespective of sweetened milk consumption, induced c-Fos expression in many DBH-labeled neurons throughout the rostro-caudal extent of the VLM that make up the rostral adrenergic C1 and caudal noradrenergic A1 populations, which overlap in an area close to the level of the obex, Fig. 6B,D, Fig. 7). The proportion of DBH-labeled cells that were c-Fos-positive in the LPS-treated groups varied between 47.5 and 65.4% in the rostral third of the VLM, between 76.6% and 90.4% in the mid-portion of the VLM and between 61.8 and 77.4% in the caudal third of the VLM (table 2). In the rostral VLM, c-Fos induction following LPS treatment was indeed strong in the adrenergic C1 cell group as observed in the series of sections stained for both c-Fos and PNMT immunoreactivity, as 61-68% of the PNMT-labeled neurons were double-stained for c-Fos, whereas none of the PNMT cells showed c-Fos staining in the saline-treated groups (Fig. 7B). The percentage of activated GLP-1 neurons in the dorsal medullary reticular nucleus (and occasionally in the VLM) was significantly increased in LPS-treated rats (Fig. 5E; main effect of treatment F= 5.34, p. = 0.03).
2.3. Parabrachial nucleus (PBN)
2.3.1. Sweetened milk consumption activates multiple populations of PBN neurons
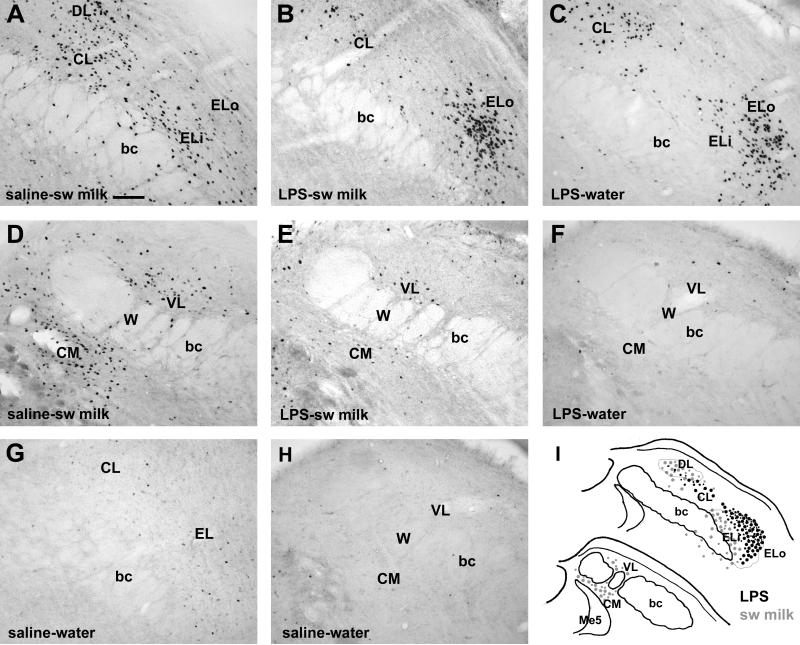
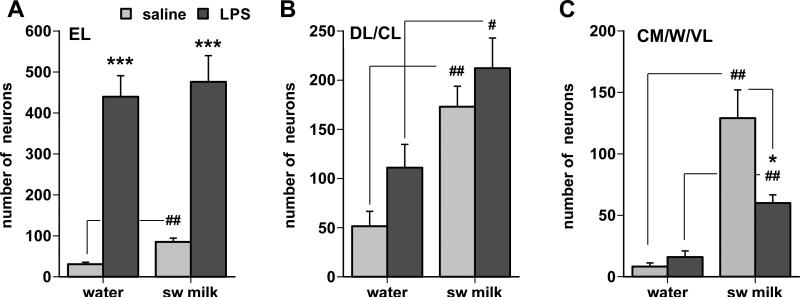
Sweetened milk consumption induced a distinctive pattern of c-Fos in the parabrachial nucleus (Fig. 8). This pattern was characterized by a prominent cluster of c-Fos-positive cells in the dorsolateral subnucleus (PB-DL, Fig. 8A), as well as scattered cells more caudally in the central medial and ventrolateral subnuclei (CM and VL, Fig. 8D) with additional cells interspersed in the waist area (W) interspersed with the adjacent brachium conjunctivum (bc). Although less consistently, scattered c-Fos-labeled cells were present close to, or inside, the rostral lateral portion of the bc including the inner division of the external lateral (Eli) and medial subnuclei of the PBN (Fig. 8A). The water-offered controls showed little or no c-Fos expression throughout the PBN, although in a few cases, a small but distinct cluster of c-Fos-positive cells was present in the PBDL (not shown). Analysis of cell counts (Fig. 9) reveals main sweetened milk consumption effects in the PB-DL/CL (F(1,21) = 22.23, p = 0.0001) and central medial-waist-ventrolateral (CM/W/VL) regions of the caudal PBN (F(1,21) = 44.4, p < 0.0001). In the PB-EL, comparison between saline-treated water and sweetened milk groups indicates an increase in c-Fos as well, mainly contributed by the inner division (p = 0.0002).
Fig. 8.
Differential effects of sweetened milk intake and LPS challenge on the pattern of c-Fos staining in the parabrachial nucleus. Representative photomicrographs depict the rostral lateral (A-C,G) and caudal portions of the PB (E-F,H) from saline-treated rats that ingested sweetened milk ad libitum (A,D), LPS-treated rats offered sweetened milk (B,E), those that received LPS injections and were offered water (C,F), and those that received saline injection and had access to water (G,H). Scale bar in A = 100 μm. Abbreviations: bc: brachium conjunctivum, CL: central lateral subnuceus, CM: central medial subncleus, DL: dorsolateral subnucleus, ELi: inner part of the external lateral subnucleus, ELo: outer part of the external lateral subnucleus, W: waist region, VL: ventral lateral subnucleus. Panel I depicts a summary diagram of the distribution of c-Fos-labeled cells following LPS challenge (black dots) and after ingestion of a satiating amount of sweetened milk (grey dots).
Fig. 9.
Bar graphs summarizing the effects of sweetened milk consumption and of LPS challenge on the number of c-Fos cells (group mean +/− SEM) in different subnuclei of the parabrachial nucleus, the external lateral (EL in A), the dorsal-lateral and central lateral (DL/CL in B), and the central medial, waist, and ventral lateral regions (CM/W/VL in C). * p < 0.05, *** p < 0.0001, saline vs. LPS treatment; # p < 0.05, ## p < 0.005, water vs. sweetened milk.
2.3.2. LPS activates neurons primarily in the external lateral PBN
In all LPS-treated cases, a strong increase in c-Fos expression occurred in the external lateral subnucleus (PB-EL) with a dense cluster prominent in the outer division of the PB-EL (ELo, Fig.8C). In addition, c-Fos cells were scattered throughout the central lateral subnucleus, but c-Fos staining was minimal in the PB-DL or in the central-medial region (Fig. 8F). A strong main effect of LPS treatment on numbers of c-Fos+ cells was seen in the PB-EL (F(1,21) = 92.4, p < 0.0001), and more moderately in the PB-CL/DL (F(1,21) = 4.3, p = 0.05).
2.3.3. Combined LPS treatment and sweetened milk consumption produces a pattern similar to LPS treatment alone
The pattern of c-Fos expression in LPS-treated rats that also consumed sweetened milk was highly similar to the LPS-treated rats that were offered water, in that the predominant subnucleus in which c-Fos positive neurons increased greatly in numbers was the PB-EL, with most c-Fos staining in the outer division (Fig. 8C, 9A). There were additional scattered c-Fos postive cells throughout the PB-CL and DL (Fig. 9B) as well as the PB-CM and VL (Fig. 8E, 9C). The numbers of c-Fos positive cells that occurred in the PB-CM/W/VL were significantly fewer, however, than seen in the saline-treated animals with ad libitum access to sweetened milk (interaction LPS treatment and drinking solution: F(1,21) = 9.63, p = 0.0056).
3. Discussion
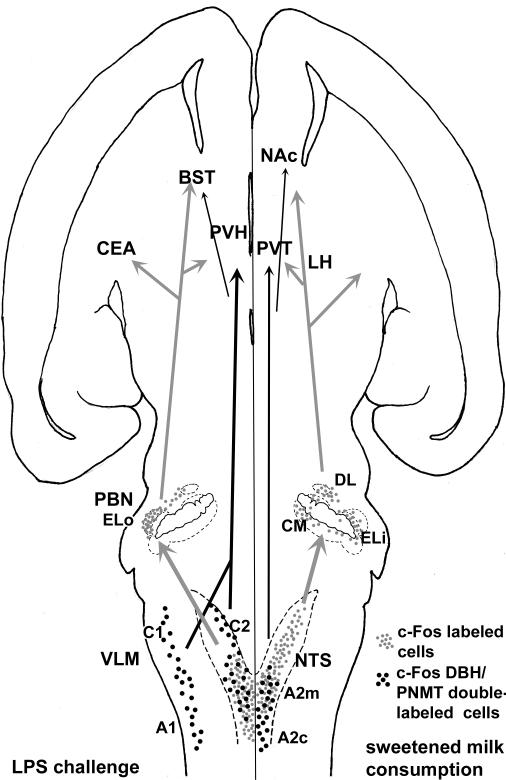
The findings from this study are consistent with the idea that immune challenge inhibits ingestive behavior by activating both “danger-related” channels and satiety-related mechanisms. LPS treatment exclusively activated catecholamine neurons in the VLM and the adrenergic neurons of the rostral NTS, that also respond selectively to other dangerous physiological conditions. However, the overall patterns of c-Fos induction at the midlevel of the NTS were nearly identical between LPS treated animals and saline treated animals that ingested satiating amounts of sweetened milk. This observation implies that gastrointestinal consequences of LPS treatment, such as gastric distension and stasis could drive neuronal population that respond to “satiety-related” stimuli, and thereby inhibit ingestive behavior. Evidence for separate (parallel) processing of the two stimuli was clearly evident in the parabrachial nucleus, where sweetened milk intake resulted in activation of the dorsal lateral and central medial portions and LPS challenge induced c-Fos expression in the external lateral portion. The findings indicate that inhibitory effects of immune challenge on palatable food intake may follow from the engagement of multiple ascending pathways from the brainstem to the diencephalic and telencephalic areas controlling feeding (summarized in Fig. 10). These pathways constitute a dedicated catecholaminergic “danger” pathway, but likely include satiety-related signaling as well. The multiplicity of ascending pathways may contribute to the complexity of immune-related effects on ingestive behavior.
Fig. 10.
Model diagram summarizing the effects of the two different stimuli: LPS and sweetened milk. Cells and pathways on the left indicate LPS-responsive pathways and on the right sweetened milk-engaged pathways. These pathways target forebrain regions including (but not exclusively) the central amygdala (CEA), paraventricular and lateral hypothalamus (PVH and LH), paraventricular thalamus (PVT), bed nucleus of the stria terminalis (BST) and nucleus accumbens (NAc). Catecholaminergic pathways are in black; projections of other phenotypes are in grey.
3.1. Methodological considerations
Sweetened milk was chosen for use in this experimental paradigm because it is nutritionally complex and approximates a palatable meal. It is a strong motivator in that animals voluntarily consume a large volume of sweetened milk, likely inducing satiety signals (hormonal and mechanoreceptors) in a naturalistic way. Sweetened milk has been used in previous studies to demonstrate the effects of sickness on ingestive behavior (Dunn and Swiergiel, 2001). However, because the stimulus is complex, interpretations regarding precise functional coding (e.g., gustatory, sweet taste, protein and fat levels, mechanoreception along the alimentary tract) are limited to issues related to potential channeling of modalities of general visceral sensory information: immune-related (LPS) compared to food-related (sweetened milk) information.
Although the use of c-Fos protein as a neuronal activation marker is well established, there are a number of limitations to the technique that impact interpretation of the findings. The approach may not identify all functionally activated neurons, nor does it reveal the inhibitory links in the neurocircuitry. Nonetheless, c-Fos analysis reveals patterns of activated ensembles of neurons throughout the neuraxis with a high degree of spatial resolution, and analyses of these patterns can provide a rationale for mechanistic studies of specific brain regions or populations of neurons.
Finally, the principal goal of the study reported here was to determine whether or not there are differences in populations of medullary neurons that propagate signals related to physiological challenges and those that convey non-threatening homeostatic signals. Ideally, we would be able to phenotype all known neurons in the NTS and VLM, many of which target the hypothalamus and other forebrain regions, but this is most feasible for catecholaminergic and GLP-1 neurons. However, many other neurons in the NTS that express c-Fos in response to a satiating meal or to LPS challenge do not express either type of marker and thus the results of this study do not reveal to what extent these groups of neurons are separate or overlapping populations. Interestingly, a recent paper (Hermann and Rogers, 2009) reports that a considerable proportion of c-Fos positive nuclei in the NTS following local TNF-α treatment belonged to astrocytes, rather than neurons. Given that systemic LPS induces TNF-α, many of the c-Fos positive cells in the NTS in our studies may in fact be astrocytes. The functional implications of this situation are not known, but because astrocytes control neuronal excitability, synaptic functions and coordination of adjacent neurons (Barres, 2008), activation of astrocytes along with neurons in the context of peripheral LPS treatment suggests that immune challenge may lead to a coordination of NTS responses via activation of astrocytes.
3.2. LPS treatment activates neurons that respond to physiological challenge: a “danger” pathway
A prominent finding from this study was that catecholamine neurons in the VLM and rostral NTS responded selectively to administration of LPS. In the NTS these neurons expressed PNMT, identifying them as adrenergic neurons (also labeled as the C2 cell group). In the VLM, the great majority of C1 and A1 catecholaminergic neurons responded only to LPS. Previous studies have demonstrated that VLM catecholaminergic neurons, and especially the rostrally situated C1 adrenergic neurons, respond to potentially dangerous physiological challenges. These neurons respond to hypovolemia and hypotension (Dun et al., 1993; Murphy et al., 1994), hypoxia (Smith et al. 1995) glucoprivic challenge (Ritter and Dinh, 1994; Ritter et al., 2006), intragastric gavage of bitter taste receptor ligands, (Hao et al., 2009), yohimbine (Myers et al., 2005), joint inflammation (Pinto et al., 2007), opiate withdrawal (Laorden et al., 2002), seizures (Kanter et al., 1996; Silviera et al., 2000), and pharmacologic levels of CCK (Myers and Rinaman, 2002). Notably, many of these catecholaminergic neurons project directly to the paraventricular nucleus of the hypothalamus (PVN; Buller and Day, 1996; reviewed in Rinaman, 2007), which coordinates autonomic and neuroendocrine response to stress. This arrangement allows for a rapid response to physiological stressors, which could be critical during immune challenges.
In addition to the catecholaminergic neurons of the VLM, a subset of GLP-1-positive neurons in the dorsal medullary and intermediate reticular nuclei (in between the caudalolateral NTS and the VLM) responded to LPS treatment, as evidenced by the small but statistically significant increase in the percentage of activated GLP-1 neurons seen in the LPS/water group. The findings echo those of Rinaman (1999), although the activation of GLP neurons in response to LPS treatment was less extensive. Like the VLM catecholaminergic neurons, some of these GLP-1 neurons project directly to the hypothalamic PVN, and thus contribute to the immune challenge-related drive on forebrain stress systems.
3.3 Satiety-related neurons in the NTS respond to both sweetened milk and LPS treatment
The findings from this study also support the idea that additional brainstem derived pathways propagate signals influencing ingestive behavior during illness. Populations of c-Fos positive neurons driven by LPS and sweetened milk were found to overlap in the middle portion of the NTS. This population of neurons receives input from gastric and hepatic vagal afferent nerve fibers, which also contribute to the anorexigenic effects of cholecystokinin (Rinaman, 2003), and gastric volume/distension-related signaling (Rinaman et al., 1998). Both satiating consumption of sweetened milk and LPS treatment activated DBH-labeled A2 noradrenergic neurons at the level of the AP. These cells seem to respond to satiety-related signals, as they also respond to other stimuli associated with inhibition of food intake, including cholecytokinin (Schreihofer et al., 1997) and intragastric lipid (Monnikes et al., 1997), and they contribute to noradrenergic projections to the paraventricular hypothalamus (Buller and Day, 1996; Rinaman et al., 1995). Thus, A2 cells constitute a plausible connection between peripheral metabolic signals related to nutrient status and forebrain neurocircuitry involved in the regulation of feeding behavior (Berthoud, 2006). Because this particular population of A2 noradrenergic neurons at the obex-AP level also responded to LPS treatment (see Fig. 10), they may also provide a pathway by which peripheral immune challenge inhibits food intake, as noted previously.
3.4 Functional segregation in the parabrachial nucleus
The partial segregation of sweetened milk- and LPS-related Fos induction in the PBN suggests that the danger-related pathway may the PBN as well as the hypothalamus. In contrast to the NTS, in which there was substantial overlap in the distribution of c-Fos positive cells between groups, LPS treatment and sweetened milk consumption activated rather different regions in the PBN. Even within the PB-EL, which has been established to process general viscerosensory information (Cechetto and Saper, 1987; Yamamoto and Sawa, 2000) and to contain neurons that respond to both gastric and gustatory signals (Hermann and Rogers, 1985; Karimnamazi et al., 2002), sweetened milk ingestion and LPS treatment activated neurons in distinct spatial regions, and there were dramatic differences in the level of response. Whereas sweetened milk ingestion activated scattered neurons within the internal regions of the PB-EL, LPS treatment resulted in a more dramatic increase in the numbers of c-Fos positive neurons in the external parts of the subnucleus. Immune-responsive PBN projections target forebrain regions other than the hypothalamus, including the amygdala, bed nucleus of the stria terminalis (BST) and nucleus accumbens (Gaykema et al., 2007; Gaykema and Goehler, unpublished data). Thus, at the level of the PBN, there appears to be evidence for an additional channel of ascending viscerosensory information (via the PB-ELo) specifically related to dangerous situations, which could influence “limbic” and motivational aspects of ingestive behavior.
Interestingly, in animals that received both sweetened milk and LPS administration, c-Fos expression was reduced in those PBN subnuclei that responded to sweetened milk consumption alone (the posterior central medial/waist region), that have been previously shown to contain neurons that may integrate taste and visceral signals (Karimnamazi et al., 2002). This finding could reflect the reduced consumption of sweetened milk by LPS-treated animals (reduced sensory drive), or could alternatively reflect inhibition of taste responses by gastric stimuli, as has been reported in other experimental paradigms (e.g. Baird et al., 2001). This inhibition has been suggested to contribute to mechanisms of satiety (Baird et al., 2001), and may constitute another mechanism by which illness-inducing substances such as LPS reduce food intake.
3.5 Forebrain targets of immune-responsive brainstem neurons contribute to changes in eating behavior
Both LPS and satiating amounts of sweetened milk inhibit feeding behavior, and activate neurons that innervate brain regions, including the lateral hypothalamus (LH), involved in feeding. However, the precise ensembles of neurons providing satiety-related (non-danger) input to these regions have not yet been identified using tract-tracing and functional activation studies, as has been done for immune-responsive projection neurons (Ericsson et al., 1994; Buller and Day, 2002; Gaykema et al., 2007). Such studies have shown that immune-responsive neurons in the VLM and NTS target the PVN, central nucleus of the amygdala, bed nucleus of the stria terminalis, and the PBN, all of which have been implicated in the modulation of feeding behavior (Baldo et al., 2005; Petrovich and Gallagher, 2003). Although tract-tracing studies investigating immune-sensitive VLM and NTS neurons have not focused on the LH as a target specifically, the catecholaminergic projections to the hypothalamus display a high degree of collateralization (Bienkowski and Rinaman, 2008), and it is quite possible that these neurons target the LH directly as well. Notably, adrenergic and noradrenergic terminals in the hypothalamus are derived exclusively from the caudal brainstem VLM and NTS (Cunningham et al., 1990, Palkovitz et al., 1992). Alternatively, they may target other regions that can modulate LH activity, such as the nucleus accumbens (Kelley et al., 2005); or paraventricular nucleus of the thalamus (Kirouac and Ganguly, 1995; Parsons et al., 2006), which contain noradrenergic and/or adrenergic terminals that derive from caudal brainstem neurons (Delfs et al., 1998; Phillipson and Bohn, 1994). As noted previously, immune-responsive projection neurons could derive from the VLM, the adrenergic neurons of the NTS, and the neurons of the PB-EL that seem to signal physiological challenges, and/or they could derive from the metabolically sensitive DBH neurons implicated in satiety.
In addition to the possibility that brainstem projection neurons might interface directly with forebrain feeding circuits, the effects of immune challenge on feeding behavior could also follow from suppression of arousal networks that are necessary for manifestation of the behavior (Valdes et al., 2005). In particular, LPS has been shown to suppress the histaminergic neurons of the posterior hypothalamus (Gaykema et al., 2008) and orexin neurons in the lateral and perifornical hypothalamus (Gaykema and Goehler, 2009).
3.6 Sickness vs. satiety: how might the brain distinguish pathological from physiological conditions?
Both sweetened milk ingestion and LPS administration lead to gastric distension, but only LPS leads to activation of the catecholaminergic and PB-EL “danger” pathways, and consequently the induction of brain-mediated host defense responses. This raises the question as to how the brainstem distinguishes similar sensory stimuli that have rather different physiological implications. Based on the work of Hermann, Rogers and their colleagues (Emch et al., 2000; Rogers et al., 2006), we suggest that for the case of gastric-related stimuli, the cytokine TNF-α may serve as a sort of conditional signal that imbues input from the stomach, in the context of illness, with a “danger signal”. LPS treatment induces TNF-α rapidly after systemic administration. Acting in the dorsal vagal complex, TNF-α induces gastric relaxation leading to stasis and distension (reviewed in Hermann & Rogers 2008). Gastric vagal afferents express TNF receptors, which when activated potentiate incoming signals (Hermann and Rogers, 2008). That is: TNF-α enhances calcium mobilization and glutamate release in response to normal gastric signals, such as distension. In addition to producing a feed-forward situation of gastric relaxation, further stasis, and prolonged vagal stimulation, TNF-α signaling in the NTS could also, via projection of NTS neurons to the VLM and PBN, initiate a trajectory of viscerosensory information to danger-related pathways. The enhancement of gastric stasis and likely resulting nausea (Hermann and Rogers, 2008) can also be expected to inhibit ingestive behavior based on e.g. discomfort, and activate ascending pathways to forebrain regions contributing to motivational adjustments to food-related stimuli, such as sweetened milk. In this way, TNF-α could act as an illness-induced danger signal, or switch, that influences both local, brainstem mediated responses to immune challenge and, potentially, leads to the induction of the activation of ascending danger-related neuronal projections operating through the VLM, rostral NTS and PB-EL.
3.7 Conclusions and perspectives
Appropriate responses to internal and external challenges require that the brain differentiate between dangerous and non-dangerous situations. Sensory systems are characterized by orderly representation of sensory stimuli within the brain, but this has been less clear for viscerosensory systems, especially for the general visceral systems that carry information about bodily states. Such information is parcellated in a general way, e.g., gustatory vs. “general visceral”, or by organ of origin (viscerotopic; e.g., Cechetto and Saper, 1987), but these stimuli are not represented in as clear a faithful spatial manner as are the visual, somatosensory, and auditory systems. Rather, by the level of the PBN, general visceral sensory systems seem to be organized around functional modalities (Chamberlin and Saper, 1994; Yamamoto et al., 1994; Yamamoto and Sawa, 2000). The findings presented here are consistent with the idea that different categories of viscerosensory stimuli (e.g., “dangerous” vs. “physiological”) are propagated along distinct, multiple ascending pathways (Gaykema et al., 2007), providing a mechanism for parcellating appropriate responses to different bodily conditions.
4. Experimental procedures
4.1 Animals
Thirty-four male Sprague-Dawley rats (240-250 g) were obtained from Taconic Laboratories, (Germantown, NY, USA) and housed in pairs in propylene boxes on a barrier cage rack (Allentown, Caging Smart Bio-Pak, Allentown, NJ, USA) in a temperature and humidity controlled vivarium room. (lights on at 7:00 AM and off at 7:00 PM). A week before the experiments, the rats were singly housed with food with water provided ad libitum at all times. Animals were handled on a daily basis at least one week before the experiment started to minimize possible stress. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Virginia Animal Care and Use Committee.
4.2 Lipopolysaccharide challenge
LPS (serotype 0111:B4; derived from Escherichia coli; Sigma, St Louis, L-2013) was dissolved in sterile pyrogen-free saline (0.9% NaCl) and diluted to a concentration of 0.1mg/ml before use. On the experiment day rats were injected intraperitoneally (i.p.) with either LPS or saline at a volume of 1 ml/kg (LPS dose of 0.1 mg/kg) and returned to their home cage two hours before their regular water bottles were switched out with drinking tubes containing the sweetened milk solution or water.
4.3 Behavioral procedures
4.3.1 Cohort 1: Ad libitum access to sweetened milk
The day following the transfer to single housing, twenty-six rats were divided randomly in two groups and offered 30 ml of a drinking solution made of sweetened condensed milk (Nestle Carnation Brand, containing milk and sugar, approximately 7.5 % protein, 7.5 % fat, 85% carbohydrate) diluted with equivolume deionized water (n = 12) or deionized water alone (n =14). The drinking solutions were presented in polypropylene tubes with ball-tipped drinking spouts by switching out the standard water bottles in their home cages beginning between 12:00 and 13:00h for 30 minutes for six consecutive days before the experiment day, during which each animal received the drinking solution at the same time of day. The presentation was staggered among the animals such that the timing of milk or water presentation would allow for precise timing of transcardial perfusion. To avoid confounding effects of food deprivation on c-Fos induction, standard laboratory chow was available at all times, i.e. the animals were not food deprived. After 30 min, the drinking tubes were removed from the cage and the regular water bottles returned.
On the experiment day, rats were injected with LPS (i.p.) or with sterile, non-pyrogenic saline between 10:00 and 11:00 h. Two hours after injection, each of them was offered with either sweetened milk or water as during the previous days. One hour after removal of the drinking tubes, the rats were deeply anesthetized with pentobarbital (i.p) and sacrificed by perfusion fixation for the analysis of c-Fos protein induction in the brain. In order to maintain the procedures within the limited time windows, this cohort of 24 rats was split into 4 groups of six animals, which were started and finished on different days.
4.3.2. Cohort 2: Restricted access to sweetened milk (“yoked” condition)
Ten rats were rationed to drink each day approximately 5 grams of sweetened milk solution (the average amount of sweetened milk solution consumed by LPS-treated animals in the first cohort) using the same procedure. On the experiment day, 5 rats were injected with saline and 5 rats with LPS, as above. They were presented with the sweetened milk solution 2 hours later for the duration of 30 min, and anesthetized in preparation for transcardial perfusion another hour later.
4.4 Tissue preparation, immunohistochemistry, and data analysis
4.4.1 Perfusion fixation and tissue preparation
The rats were anesthetized with Pentobarbital (60 mg/kg, i.p.) and transcardially perfused with saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB) with 15% saturated picric acid. The brains were removed and post-fixed overnight with the same fixative, then placed in 0.1 MPB until sectioning. The brains were blocked in the coronal plane into 4 parts. The parts containing the pontine tegmentum were assembled in pairs, whereas the caudal brainstems were separated from the cerebellum, assembled in groups of 4-6, and arranged in cryomolds. The cryomolds were filled with warm aqueous 10% gelatin solution, chilled to solidify the gelatin, and immersed in cold fixative (4% paraformaldehyde) overnight. The fixed gelatin blocks were removed from the molds, trimmed, and sectioned on a Vibratome (Model 1500) so that the brainstems were cut in the coronal plane at a thickness of 50 μm. The gelatin sections containing the 4-6 brainstems were collected serially into 6-well cell culture plates (yielding a representative set in each well containing every sixth section) containing in 0.1 M PB-0.1% sodium azide, and stored at 4 °C.
4.4.2 Immunohistochemical staining for c-Fos
To avoid variations in staining quality and intensity between groups, the sets of sections were processed simultaneously although the brains from the cohorts were at different times. Prior to incubation in the primary antibody solution, the sections were treated with a mixture of hydrogen peroxide (0.3%) and sodium azide (0.1%) in phosphate buffered saline (PBS, 0.154 M NaCl and 0.01 M PB, pH 7.4) for 30 minutes to block endogenous peroxidase activity. All blocking, primary and secondary antibodies solutions were diluted in PBS containing 0.5% Triton X-100 (PBS-T), to which also 2 % normal goat serum and 0.1% sodium azide were added. The sections were rinsed three times in phosphate buffered saline (PBS, 0.154 M NaCl and 0.01 M phosphate buffer, pH 7.4) between all incubation steps. To reduce nonspecific staining, the sections were immersed in PBS-T containing 4% normal goat serum (Jackson immunoResearch Laboratories, West Grove, PA) and Fab’ fragment of goat anti-rat igG (1:500) overnight at 4°C.
The first and fourth sets of sections through the pontine region, and four of the six sets of caudal brainstem sections were stained for for nuclear c-Fos protein using the avidin-biotin peroxidase method. The gelatin sections were incubated for 72 h under gentle agitation at 4°C in rabbit anti-Fos (Ab5, Oncogene, Cambridge, MA, 1:50,000), followed by biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch; 1:1,000 overnight at 4°C), and avidin–biotin peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA, 1:500 in PBS-T; overnight at 4°C). After rinses in PBS and Tris-HCl buffer (0.05M, pH 7.6), sections were immersed in 0.02% 3’3-diaminobenzidine (DAB), 0.15% nickel ammonium sulfate, 0.04% ammonium chloride, and 0.02% glucose oxidase in Tris-HCl buffer. The staining was initiated by adding β-D-glucose into the wells (10% solution to obtain a final concentration 0.1%), which process was visually guided and, upon the appearance of strong black nuclear staining, terminated by rinses in PBS. The sets of pontine sections and one set of sections through the caudal brainstem were mounted on gelatin coated microscope slides, dehydrated in graded ethanol solutions, cleared in Citrosolve, and coverslipped with DPX (BDH Laboratory Supplies, England).
4.4.3 Double-labeling immunohistochemistry
After completing the Fos staining the remaining three of the four sets of sections through the medulla oblongata were subsequently processed to visualize the immunoreactivity for each of the following markers: dopamine β-hydroxylase (DBH, the noradrenaline synthesizing enzyme), phenylethanolamine-N-methyltransferase (PNMT, the adrenaline synthesizing enzyme), and glucagon-related peptide-1 (GLP-1), which are all phenotpyes expressed by neurons in the NTS and VLM that project to the hypothalamus and other forebrain regions implicated in food-motivated behavior. After immersion in 0.3% hydrogen peroxide for 30 min, the sections were successively incubated for 48 h in solutions containing either mouse monoclonal anti-DBH (1: 10,000; MAB308, Millipore/Chemicon Int., Temecula, CA), rabbit anti-PNMT (1:5,000; #22572, Immunostar Inc., Hudson, WI), or rabbit anti-GLP-1(7-36) (1:5,000; T-4057; Peninsula Laboratories LLC./Bachem AG, Torrance, CA 90505). This was followed by the appropriate secondary antibody solution (biotinylated goat anti-rabbit IgG or goat anti-mouse IgG (1:1,000, Jackson immunoResearch Laboratories), and Vectastain Elite ABC kit (1: 500), and finally reacted with DAB (0.04%) yielding a brown cytoplasmic staining. After staining, all sections were mounted, dehydrated, and coverslipped on gelatin-coated microscope slides.
4.5 Microscopy
The sections were examined with an Olympus BX51 microscope and digital images were captured using 4x, 10x, and 20x objectives and a Magnafire digital camera (Optronics, Goleta, CA) that was linked to a G4 Apple Power Mac (Mac OS 9.2). The NIH Image software (version 1.61) was used for counting the c-Fos-immunoreactive (ir) profiles in the areas of interest (parabrachial nucleus, nucleus of the solitary tract). Digital photomicrograph images were taken in either grey scale (single Fos staining) or in color (double staining), cropped, resized to 300 pixels/inch, and only slightly adjusted in brightness and contrast in Adobe Photoshop 9.0 (CS2, Adobe Systems, Mountain View, CA).
Quantitative analysis of Fos immunoreactivity
In the series stained for only Fos ir, numbers of immunopositive neurons in selected brain regions were counted using NIH Image (v. 1.61). The regions analyzed were outlined based on landmark features with the aid of the Rat Brain Atlas (Paxinos and Watson, 1998). Counts were performed bilaterally in every third section through the PBN (by combining the first and fourth sets) and in every sixth section through the NTS. The PBN was divided into the external lateral, central, dorsal and ventral lateral, and central medial portions, the latter including the cells interspersed in the brachium conjunctivum (“waist” area). In each section the NTS was outlined in a fashion such that the entire area medial to the tractus solitarius was included. The counts in each hemisphere were combined and, for the PBN subdivisions, the counts collected from four sections spaced 300 um apart, were summed to a total for each animal. In the VLM, c-Fos expression was predominantly associated with the catecholaminergic cell groups, and only analyzed using the double-stained set of sections as described below.
Analysis of DBH-, PMNT-, and GLP-1-labeled cell populations in the NTS and VLM
Using the series of sections with dual staining for c-Fos and either DBH, PNMT, or GLP-1, the activation of catecholaminergic and GLP neurons along the entire rostro-caudal axis of the VLM, NTS, and dorsal medullary reticular nucleus (mostly GLP-1 cells) was determined by the presence or absence of black nuclear staining for c-Fos in DBH- PNMT- or GLP-1-labeled somata. For this purpose, double-labeled neurons (harboring both brown cytoplasmic and black nuclear reaction products) as well as single-labeled DBH-, PNMT- and GLP-1-positive perikarya lacking the black nuclear staining were counted manually. Sections through the medulla were examined using a 20X objective lens and a 10X eyepiece equipped with 10×10 reference grid. Perikarya and dendrite fragments that lacked a visible nucleus were not counted. The counts from both hemispheres were combined to yield total counts per section. To yield specific differences in the distribution of DBH-c-Fos double-labeled cells along the rostrocaudal extent of the NTS and the VLM, section-specific counts were plotted at 0.3 mm intervals as a function of rotstrocaudal axis coordinate. To determine the percentage of DBH neurons that express Fos expression in the different experimental conditions, the DBH- and DBH-c-Fos-double labeled cell counts were summed from three combined sets of sections along rostrocaudal axis of the NTS and VLM, those caudal to the AP (−5.3 to −7.2 mm caudal to interaural, 7 sections), at the level of the obex and AP (−4.4 to −5.0 mm, 3 sections), and rostral to the obex (−2.3 to −4.0 mm, 5 sections). For the PNMT-labeled cell population that is exclusively present in the rostral half of the NTS and VLM, counts from the 7 rostral-most sections were combined to yield a total number of single- and double-labeled cells for each individual rat. Because DBH is a marker for both noradrenergic and adrenergic neurons and PNMT a marker specific for adrenergic neurons, the PNMT cells were taken to be C1 and C2 adrenergic cells (in the VLM and NTS, respectively), whereas the DBH-labeled cells that were situated caudal to the obex were taken to be A1 ad A2 noradrenergic cells as few or no PNMT-labeled neurons were detected in the VLM or NTS at the level of the AP and further caudal. For the GLP-1-labeled cell population, which is present in the caudal half of the NTS and dorsal medullary reticular nucleus, counts from the 7 caudal-most sections (−5.0 through −6.8 mm caudal to interaural) were combined to yield a total number of single- and double-labeled cells for each individual rat.
Statistics
The total numbers of neurons from individual animals are expressed as group mean +/− SEM for each experimental group (n = 5-7 per group for all data sets). Data were analyzed by separate two-way analyses of variance (ANOVA) for each brain region with drinking solution and i.p. injection as independent variables. If the ANOVA revealed statistically significant main effects or interaction (probability of 0.05 or less), Fisher's protected least significant difference or t-tests were conducted for comparisons between pairs of groups. The rostrocaudal distribution of DBH-c-Fos double-labeled cells in the NTS and VLM were analyzed using 3-way ANOVA with injection and drinking solution as between subject and rostrocaudal level as within-subject variables, followed by separate between-group comparisons at each rostrocaudal level. For analysis of the numbers and percentage of DBH neurons that are c-Fos-positive in the different rostral, mid and caudal portions of the NTS and VLM (by combining serial sections into 3 sets of cumulative counts, see above) and of the series double-stained for Fos and PNMT or GLP-1, the total numbers of PNMT- or GLP-1 labeled neurons and the number and percentages of double-labeled neurons were analyzed with 2-way ANOVA and post-hoc comparisons using Fisher's protected least significant difference tests.
Acknowledgements
The authors thank Mary E. Tyler and Meghan Jones for their expert technical assistance in the behavioral testing and immunohistochemistry procedures. This work was supported by the NIH grant MH068834.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baird J-P, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am. J. Physiol. 2001;281:R1581–R1593. doi: 10.1152/ajpregu.2001.281.5.R1581. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Alsene KM, Negron A, Kelley AE. Hyperphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: Dependence on intact neural output from the central amygdaloid region. Behav. Neurosci. 2005;119:1195–1206. doi: 10.1037/0735-7044.119.5.1195. [DOI] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: A perspective of their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Mind versus metabolism in the control of food intake and energy balance. Physiol. Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol. Behav. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neurosci. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM, Day TA. Involvement of medullary catecholamine cells in neuroendocrine responses to systemic cholecystokinin. J. Neuroendocrinol. 1996;8:819–824. doi: 10.1046/j.1365-2826.1996.05252.x. [DOI] [PubMed] [Google Scholar]
- Buller KM, Day TA. Systemic administration of interleukin-1beta activates select populations of central amygdala afferents. J. Comp. Neurol. 2002;452:288–296. doi: 10.1002/cne.10389. [DOI] [PubMed] [Google Scholar]
- Buller KM, Allen T, Wilson LD, Munro F, Day TA. A critical role for the parabrachial nucleus in generating central nervous system responses elicited by a systemic immune challenge. J. Neuroimmunol. 2004;152:20–32. doi: 10.1016/j.jneuroim.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidnce for a viscerotopic sensory respresentation in the cortex ad thalamus of the rat. J. Comp. Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr., Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp. Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur. J. Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Chiaia NL. Hemorrhage induces Fos immunoreactivity in rat medullary catecholaminergic neurons. Brain Res. 1993;608:223–232. doi: 10.1016/0006-8993(93)91462-2. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The reductions in sweetened milk intake induced by interleukin-1 and endotoxin are not prevented by chronic antidepressant treatment. Neuroimmunomodul. 2001;9:163–169. doi: 10.1159/000049021. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammel,l TE, Jacobson CD, Saper CB. Distribution of fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp. Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am. J. Physiol. 2000;279:G582–586. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz G, Moran TH. Meal-related stimuli differentially induce c-Fos activation in the nucleus of the solitary tract. Am. J. Physiol. 2001;280:R1315–R1321. doi: 10.1152/ajpregu.2001.280.5.R1315. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faipoux R, Tome D, Gougis S, Darcel N, Fromentin G. Proteins activate satiety-related neuronal pathways in the brainstem and hypothalamus of rats. J. Nutr. 2008;138:1172–1178. doi: 10.1093/jn/138.6.1172. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Camplyobacter jejuni: analysis with Fos immunohistochemistry. Brain, Behav Immun. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Chen C-C, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130:130–145. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Park S-M, McKibbin CR, Goehler LE. Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways. Neurosci. 2008;152:273–282. doi: 10.1016/j.neuroscience.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE. Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: their potential role in sickness behavior. Brain Behav. Immun. 2009 doi: 10.1016/j.bbi.2009.03.005. in press, 10.1016/j.bbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr NA, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Carmody JS, Sadacca LA, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brainstem but not forebrain GLP-1-R. Am. J. Physiol. 2004;287:R1190–R1193. doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J. Physiol. 2009;296:R528–R536. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser CA, Stockler MR, Tattersall MHN. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support. Care Cancer. 2006;14:999–1011. doi: 10.1007/s00520-006-0079-9. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J. Auton. Nerv. Syst. 1985;13:1–17. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNF-α: A trigger of autonomic dysfunction. Neuroscientist. 2008;14:53–67. doi: 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: Implications for autonomic control. Brain Res. 2009 doi: 10.1016/j.brainres.2009.03.059. doi.1016/j.brainres.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter RK, Strauss JA, Sauro MD. Comparison of neurons in rat medulla oblongata with fos immunoreactivity evoked by seizures, chemoreceptor, or baroreceptor stimulation. Neurosci. 1996;73:807–816. doi: 10.1016/0306-4522(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamicstriatal axis for the integration of energy balance, arousal, and food reward. J. Comp. Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neurosci. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Nunez C, Almela P, Milanes MV. Morphine withdrawal-induced c-Fos expression in the hypothalamic paraventricular nucleus is dependent on the activation of catecholamine neurons. J. Neurochem. 2002;83:132–140. doi: 10.1046/j.1471-4159.2002.01123.x. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R. Pathways of Fos expression in locus coeruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am. J. Physiol. 1997;273:R2059–R2071. doi: 10.1152/ajpregu.1997.273.6.R2059. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Ennis M, Shipley MT, Behbehani MM. Directionally specific changes in arterial pressure induce differential patterns of fos expression in discrete areas of the rat brainstem: a double-labeling study for fos and catecholamines. J. Comp. Neurol. 1994;349:36–50. doi: 10.1002/cne.903490104. [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol. Behav. 2002;77:723–729. doi: 10.1016/s0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J. Comp. Neurol. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Skirboll LR, Hokfelt T. Adrenergic projections for the lower brainstem to the hypothalamic paraventricular nucleus, the lateral hypothalamic area and the central nucleus of the amygdala in rats. J. Chem. Neuroanat. 1992;5:407–415. doi: 10.1016/0891-0618(92)90057-w. [DOI] [PubMed] [Google Scholar]
- Park S-M, Gaykema RPA, Goehler LE. How does immune challenge inhibit ingestion of palatable food? Systemic lipopolysaccharide modulates key nodal points of feeding neurocircuitry. Brain, Behav. Immun. 2008;22:1160–1172. doi: 10.1016/j.bbi.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Ed. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann. N.Y. Acad. Sci. 2003;985:251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Bohn MC. C1-3 adrenergic medullary neurons project to the paraventricular thalamic nucleus in the rat. Neurosci. Lett. 1994;176:67–70. doi: 10.1016/0304-3940(94)90873-7. [DOI] [PubMed] [Google Scholar]
- Pinto M, Lima D, Tavares I. Neuronal activation at the spinal cord and medullary pain control centers after joint stimulation: A c-fos study in acute and chronic articular inflammation. Neurosci. 2007;147:1076–1089. doi: 10.1016/j.neuroscience.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: Rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am. J. Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J. Neurosci. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front. Neuroendrocrinol. 2007;28:40–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am. J. Physiol. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J. Comp. Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am. J. Physiol. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-D-glucose induce Fos-like immunoreactivity in the rat brain. Brain Res. 1994;641:111–120. doi: 10.1016/0006-8993(94)91822-8. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li A-J. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol. Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J. Neurosci. 2006;26:12642–12646. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer DA, Cameron JL, Verbalis JG, Rinaman L. Cholecystokinin induces Fos expression in catecholaminergic neurons of the macaque monkey caudal medulla. Brain Res. 1997;770:37–44. doi: 10.1016/s0006-8993(97)00732-4. [DOI] [PubMed] [Google Scholar]
- Silveira DC, Schacter SC, Schomer DL, Holmes GL. Flurothyl-induced seizures in rats activate Fos in brainstem catecholamine neurons. Epilepsy Res. 2000;39:1–12. doi: 10.1016/s0920-1211(99)00106-0. [DOI] [PubMed] [Google Scholar]
- Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J. Neurosci. 1995;15:7979–7988. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg S, Anker SD. Metabolic and immunologic derangements in cardiac cachexia: where to from here? Heart Fail. Rev. 2006;11:57–64. doi: 10.1007/s10741-006-9193-5. [DOI] [PubMed] [Google Scholar]
- Valdes JL, Farias P, Ocampo-Garces A, Cortes N, Seron-Ferre M, Torrealba F. Arousal and differential Fos expression in histaminergic neurons of the ascending arousal system during a feeding-related motivated behaviour. Eur. J. Neurosci. 2005;21:1931–1942. doi: 10.1111/j.1460-9568.2005.04013.x. [DOI] [PubMed] [Google Scholar]
- Wan W, Wetmore L, Sorensen CM, Greenberg AM, Nance DM. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol. Behav. 1994;56:1197–1202. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sawa K. Comparison of c-Fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res. 2000;866:144–151. doi: 10.1016/s0006-8993(00)02242-3. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud H-R. Peptides that regulate food intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am. J. Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]