Background and Significance
Sleep-related disorders are common in the general adult population, and as the population ages, the prevalence of these disorders increases. A common misconception among clinicians and the public is that this increased prevalence is a normal and expected phenomenon of aging. However, this higher prevalence of sleep disruption is often the result of the increased presence of medical, and psychosocial comorbidities in this population. The complicated multifactorial interactions that generate sleep disorders in older individuals pose important challenges to clinicians. Furthermore, many clinicians are unaware of the seriousness and potential morbidity associated with sleep problems in older people, distinct from the morbidity of concurrent disorders. As a result, these issues are often underinvestigated, or completely ignored.1
Because of the high prevalence, complexity, and health implications associated with sleep-related disorders in older individuals, increasing attention is now being focused on this topic. For example, a recent publication has recommended that sleep problems be approached as a “multifactorial geriatric syndrome.”2
Of major clinical concern is the strong bidirectional relationship between sleep disorders and serious medical problems in older persons. Individuals with sleep disorders are more likely to develop hypertension, depression, cardiovascular, and cerebrovascular disease. Conversely, individuals with any of these diseases are at higher than normal risk of developing sleep problems.3,4
Older individuals consider quality sleep to be an essential part of good health. A Gallup survey of over 1000 Americans age 50 and older (43% of whom were age 65 or older) found that 80% answered “a great deal” when asked whether sleep was important for healthy aging. In the same survey, and contrary to the myth that older adults need less sleep, 45% believed they required more sleep now than when they were younger and 25% believed they had a sleep “problem.”5
The goal of this paper is not to present an exhaustive and comprehensive review of sleep and sleep disorders in older persons. Rather, we present an overview of sleep disorders and suggest appropriate evidence-based recommendations for assessing and treating sleep disorders in the older adult population. These recommendations have been developed by professionals with expertise in sleep disorders and in the clinical care of older people.
As in many areas of clinical research, older persons are often poorly represented (or specifically excluded) in clinical sleep studies. Thus, there are less data available from randomized controlled trials for this population compared to the general adult population. Nevertheless, given the importance of the subject, and the opportunity for successful intervention, we believe it is prudent and timely to propose recommendations based upon expert consensus of current evidence. While there have been a number of publications aimed at clinicians concerning sleep and sleep disorders in the adult and older adult populations, there are currently no recommendations for systematically approaching the assessment, treatment and follow-up of sleep disorders in the older adult population. 2,6-11
In developing these recommendations we are cognizant of a number of important themes: 1) the tremendous heterogeneity of the older adult population, and thus the critical importance for individualization of assessment and therapy; 2) the limited amount of time clinicians have to spend with each patient, making lengthy assessments for sleep problems unrealistic; 3) the body of knowledge regarding the approach to assessment and treatment of sleep disorders that clinicians need to possess; 4) the role of sleep specialists in this process, and the importance of recognizing when and where to refer; and 5) the frequent presence of comorbidities and multiple medication usage in this population of patients, requiring a careful approach and meticulous follow-up.
Sleep-related problems in the acute care hospital setting will not be addressed in this paper. Such problems have received little attention in research studies compared to sleep problems in outpatients, and the state of knowledge concerning these conditions is inadequate to make recommendations with a reasonable level of confidence. In this paper, therefore, we focus on chronic problems with sleep in older persons in the outpatient and long term care setting.
General Review of Sleep
Major physiologic changes occur in the context of aging. One such change that can be quite problematic for many older adults is the often profound change of the daily sleep-wake cycle. Sleep is composed of 2 very different physiologic states: rapid eye movement sleep (REM) and non-rapid eye movement sleep (NREM). NREM is further divided into 4 stages. Stage 1 is the lightest stage of sleep. Stage 2 sleep has a higher arousal threshold and is the stage in which most time sleeping is spent. Stages 3 and 4 are collectively referred to as “deep sleep,” “delta sleep,” or “slow wave sleep,” (based upon their characteristic EEG profiles) and are associated with a high arousal threshold. Recently, the American Academy of Sleep Medicine (AASM) has revised this classification slightly, into 3 NREM stages (Stages N1, N2, and N3, where N3 represents the traditional stages 3 and 4 combined).12 Sleep typically occurs in approximately 90-minute cycles of NREM/REM. However, more stage 3/4 sleep occurs in the first half of the night, while more REM sleep takes place in the last half. This sleep pattern can be interrupted by awakenings which may be extremely brief or of prolonged duration.
The sleep-wake pattern is regulated by a complex interaction between a time-awake-dependent increase in homeostatic sleep drive and a circadian wakefulness drive which typically reaches its maximum in the evening. Normally, homeostatic sleep drive and circadian wakefulness drive are both high in the evening, but as homeostatic sleep drive continues to build and circadian wakefulness drive declines sleep is initiated.
This normal sleep-wake process can be influenced by a wide variety of physiologic, psychologic and environmental factors. The most striking change in sleep patterns in older adults is the repeated and frequent interruption of sleep by long periods of wakefulness, possibly the result of an age-dependent intrinsic change in the interaction of the sleep homeostatic and circadian arousing processes that control sleep.13 Other age-dependent changes in sleep include decreased total sleep time (TST), reduced sleep efficiency (time asleep as a percentage of time in bed) and decreased slow wave and REM sleep, as well as increased stages 1 and 2 sleep. These age-dependent changes in nocturnal sleep are accompanied by an increased incidence of napping or falling asleep during the day.14-16 Aging is also associated with a tendency to both fall asleep and awaken earlier and to be less tolerant of phase shifts in the sleep-wake schedule such as those associated with jet lag and shiftwork.17 These changes also suggest age-dependent alterations in regulation of the circadian sleep-wake cycle.
When the sleep of individuals who may be considered to be “optimally aging” is examined and age-related medical and psychiatric comorbidities controlled for, it appears that most age-dependent sleep changes occur in early and middle adulthood (years 19-60).13 Further age-dependent sleep changes after age 60 are, at most, modest, assuming the individual is in good health.13 The presence of medical and psychiatric illnesses, however, is associated with exacerbations of age-dependent sleep disruption.13,18-20 Nevertheless, it is important to recognize that such sleep disturbance in the presence of comorbidities is not necessarily simply a symptom of the comorbid condition(s), but may represent an independent problem that may benefit from treatment.21,22
In addition to the impact of age-dependent sleep changes and age-associated comorbidities, the sleep of older adults can be further adversely impacted by common primary sleep disorders, such as insomnia, obstructive sleep apnea (OSA) and restless leg syndrome (RLS). Epidemiologic studies have consistently shown that the prevalence of sleep complaints and sleep disorders grows steadily with advancing age.23 As many as 57% of older adults complain of significant sleep disruption, 45% have periodic limb movements (PLMs) during sleep, 29% suffer from insomnia, 24% have obstructive sleep apnea (OSA), 19% complain of early morning awakening, and 12% have restless legs syndrome.24-28
Other factors such as prescription and over-the-counter medications, social drug use, and psychosocial, behavioral, and environmental factors including poor sleep hygiene (the behaviors and environmental factors that can improve or worsen sleep), can further contribute to sleep problems.29,30 Indeed, the sleep of an older adult can be adversely impacted by any, or several of these factors.30 In older adults, these sleep disturbances and increases in daytime sleepiness can have a significant negative impact, not only on quality of life, but also on morbidity and mortality.29,31,32
While sleep disturbances can have profound health implications, they may also be quite situation-specific. For example, sleep disturbances in a community-dwelling older adult who is aging optimally are likely to differ from those experienced by older adults in an acute hospital setting or in long term care, particularly in those with dementia who can neither describe their symptoms nor engage as actively in treatment.
In 2006, the Institute of Medicine (IOM) released a report entitled, “Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem” which recognized the wide range of deleterious health and safety consequences of disturbed and inadequate sleep.33 The report called for increased awareness among health care professionals about the physiology of healthy sleep and sleep disorders across the lifespan, as well as for the development and implementation of programs to promote the early diagnosis and treatment of sleep disorders.
The last 10 years have been marked by significant and rapid advances in our ability to diagnose and treat sleep disorders in the general and the older adult population utilizing both behavioral and pharmacologic approaches. The recommendations for the effective diagnosis and treatment of sleep disorders in this population presented below are offered in the spirit of the IOM report and the considerable recent progress made in the effective recognition and treatment of sleep disorders in older adults.
Recommendations Development Process and Methods
Two 2-day conferences involving a multidisciplinary group of sleep experts and clinicians representing major geriatric interest groups and societies, were held at the International Longevity Center (ILC-USA, New York) in November, 2005 and December, 2006. Participants in those conferences uniformly agreed that the time was appropriate to bring together representatives form national sleep organizations, geriatric clinical organizations, and other clinical organizations with an interest in geriatric care in order to develop and publish evidence and expert consensus-based recommendations for the assessment and management of sleep disorders in older persons. The development of such recommendations seemed especially important given the recent increasing attention in the literature to sleep problems in older adults and the absence of existing recommendations for this population.
A broad national group of 13 such organizations was assembled in 2007. In December of that year, a third conference at the ILC brought together representatives from these organizations, as well as other sleep experts and expert clinicians. Prior to the meeting, thought leaders in the field were asked to prepare presentations on the major disorders related to sleep disturbances in older adults, based upon their prevalence, potential morbidity and mortality, and possibility for response to therapy. The attendees subsequently identified the sleep disorders to be included and the authors were chosen because of their internationally recognized expertise in each particular sleep disorder. They determined that the recommendations paper should be a multi-authored document that would be submitted to a peer-review journal for publication and that participating organizations would not be asked to provide review prior to recommendations publication.
In addition to the references cited by the authors of the individual sections, a formal literature search and review was performed for each of the sleep disorders, and for the section concerning the specific sleep problems encountered in the long term care setting. The search focused on randomized controlled trials (RCTs), metaanalyses, and systematic reviews. Non-randomized clinical trials and controlled clinical trials were also included given the low volume of RCTs in older adults with certain sleep disorders (eg, parasomnias, hypersomnias). More than 11,600 citations were identified from sources including PubMed, the Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse and Centre for Reviews and Dissemination/Health Technology Assessment databases using key word searches for each condition and/or intervention of interest. Approximately 1,700 abstracts for these citations were selected and screened by panel members for evidence-based content. Selected full text, English language papers were summarized in evidence tables for review by all of the primary authors. The number of evidence based studies on patients over age 65 is limited in some conditions and consensus can vary on whether studies of younger subjects can be extrapolated to older subjects.
The quality and strength of evidence for each recommendation was initially assigned by the primary author proposing that specific recommendation (Table 1). All evidence designations were then reviewed by the coalition panel and final designations were decided by consensus. This assessment methodology has been widely used in previous guidelines.34
Table 1.
| Quality of Evidence | |
|---|---|
| Level I | Evidence from at least 1 properly designed randomized, controlled trial |
| Level II | Evidence from at least 1 well-designed clinical trial without randomization, from cohort or case-controlled analytic studies, from multiple time-series studies, or from dramatic results in uncontrolled experiments |
| Level III | Evidence from respected authorities, based on clinical experience, descriptive studies, or reports of expert committee |
| Strength of Evidence | |
| A | Good evidence to support the use of a recommendation; clinicians should do this all the time |
| B | Moderate evidence to support the use of a recommendation; clinicians should do this most of the time |
| C | Poor evidence to support or reject the use of a recommendation; clinicians may or may not follow the recommendation |
| D | Moderate evidence against the use of a recommendation; clinicians should not do this |
| E | Good evidence against the use of a recommendation; clinicians should not do this |
Detecting Sleep Disorders in an Ambulatory Setting: General Approach
The best method for detecting sleep-wake problems in ambulatory older people is simply to inquire about sleep on a regular basis.
The clinician may do this initially during the patient visit. An alternative is to allow a staff member to administer a brief sleep questionnaire before or during routine vital signs assessments, perhaps prior to the first visit in all new patients and then at least semi-annually in returning patients. The answers to these questions will then be immediately available to the clinician to review and or expand upon if necessary. If a bed partner is with the patient, he or she should assist with the answers.
The following 12 questions can serve as the initial assessment regarding sleep:
What time do you normally go to bed at night? What time do you normally wake up in the morning?
Do you often have trouble falling asleep at night?
About how many times do you wake up at night?
If you do wake up during the night, do you usually have trouble falling back asleep?
Does your bed partner say (or are you aware) that you frequently snore, gasp for air or stop breathing?
Does your bed partner say (or are you aware) you kick or thrash about while asleep?
Are you aware that you ever walk, eat, punch, kick, or scream during sleep?
Are you sleepy or tired during much of the day?
Do you usually take 1 or more naps during the day?
Do you usually doze off without planning to during the day?
How much sleep do you need to feel alert and function well?
Are you currently taking any type of medication or other preparation to help you sleep?
If symptoms of a sleep complaint are suggested in this initial screening, further questions may be appropriate to ask when taking a sleep history35:
Do you have the urge to move your legs or do you experience uncomfortable sensations in your legs during rest or at night?
Do you have to get up often to urinate during the night?
If you nap during the day, how often and for how long?
How much physical activity or exercise do you get daily?
Are you exposed to natural outdoor light most days?
What medications do you take, and at what time of day and night?
Do you suffer any uncomfortable side effects from your medications?
How much caffeine (eg, coffee, tea, cola) and alcohol do you consume each day/night?
Do you often feel sad or anxious?
Have you suffered any personal losses recently?
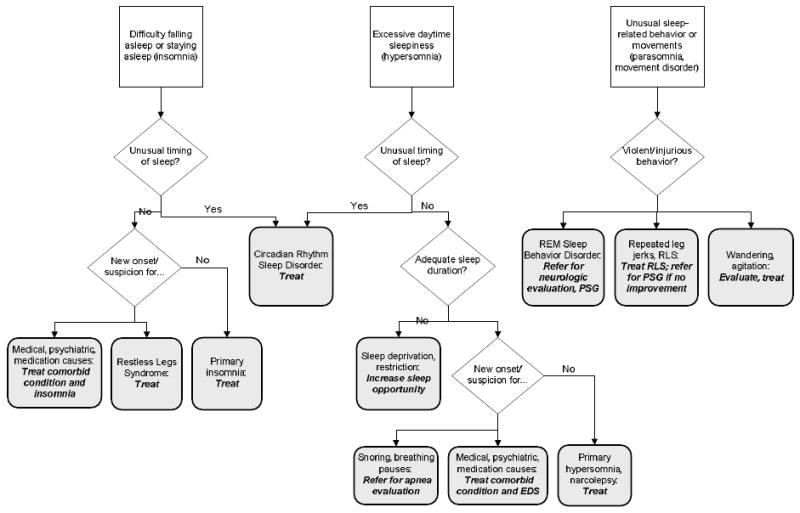
The patient's responses should indicate how to proceed with any further history, focused physical examination or laboratory investigations. Specific questions, examinations, laboratory tests, procedures, and possible referrals are discussed in more detail in the sections concerning the particular sleep disorders. A flow diagram (Figure 1) may be helpful in identifying and treating sleep complaints in older ambulatory individuals.
Figure 1. Diagnostic algorithm for sleep disorders in older persons.
Insomnia
Definition
Insomnia is defined as a complaint of disturbed sleep in the presence of an adequate opportunity and circumstance for sleep. The complaint may consist of difficulty initiating sleep, difficulty maintaining sleep, waking up too early and/or nonrestorative or poor quality sleep. For the diagnosis of an insomnia disorder to be made, the difficulty with sleep must have a negative impact on daily function.36
Insomnia is classified as either primary or comorbid. Primary insomnia implies that no other cause of sleep disturbance has been identified. Comorbid insomnia is more common and is most often associated with psychiatric disorders (eg, depression, anxiety, or substance use disorders), medical disorders (eg, cardiopulmonary disorders, neurologic disorders, or chronic somatic complaints that result in sleep disruption), medications, and other primary sleep disorders (eg, obstructive sleep apnea or restless legs).4,24 Comorbid insomnia does not suggest that other condition(s) “cause” insomnia, but rather that insomnia and the other condition(s) co-occur, and may each warrant clinical attention and treatment.
Prevalence
While the prevalence of insomnia in the general population has been estimated at 10-20%, studies in older adults have found higher frequencies. In a study of more than 9000 adults over the age of 65 years, 42% of participants had difficulty both falling asleep and staying asleep with a higher prevalence found in those older adults with poor health and who were taking medications for a variety of medical problems.24 Participants who were depressed were 2.5 times more likely to report insomnia, and those with respiratory symptoms were 40% more likely to do so. The finding that a considerable proportion of sleep complaints among older people may be associated with chronic disease and other health problems is corroborated in other reports.19,20,37
Consequences of insomnia
Insomnia in the older adult is associated with significant morbidity and mortality. Compared to controls, those with difficulty sleeping report decreased quality of life and increased symptoms of depression and anxiety.38-41 Napping during the day and sleeping less than 7 hours a night have been associated with an increased risk of falls.42 Cognitive decline, difficulty ambulating, difficulty with balance, and difficulty seeing are also associated with poor sleep, even after controlling for medication use.43-47 The relative risk for increased mortality in the older adult has been associated with taking more than 30 minutes to fall asleep and with a sleep efficiency (time asleep as a percentage of time in bed) of less than 80%.48
Comorbidities
As mentioned, much of the insomnia seen in older adults is likely to be comorbid with psychiatric illness. It has long been known that depression and insomnia are associated, and that the presence of depressed mood may predict insomnia. Many studies have suggested that untreated insomnia is a risk factor for recurrent and a new onset of depression.49-53
Older adults with medical conditions are also more likely to complain of difficulty sleeping. In the 2003 National Sleep Foundation survey of adults aged 65 years and over, more sleep complaints and more dissatisfaction with sleep were reported by those with more medical conditions, including cardiac and pulmonary disease.4 Pain associated with osteoarthritis, cancer, or diabetes mellitus, shortness of breath due to chronic obstructive pulmonary disease or congestive heart failure, nocturia due to an enlarged prostate, and neurologic deficits related to cerebrovascular accidents or Parkinson's disease have all been associated with sleep complaints and insomnia.54-59 Not only do older adults with medical and psychiatric problems have more insomnia, but those with insomnia are also more likely to have medical problems, including heart disease, cancer, high blood pressure, neurologic disease, breathing problems, urinary problems, diabetes, chronic pain and gastrointestinal problems, even after controlling for depression and anxiety.3
Medications
Many older adults regularly take multiple medications. Medications used to treat various underlying chronic medical and psychiatric conditions also contribute to sleep disruptions, including β-blockers, bronchodilators, corticosteroids, decongestants and diuretics, as well as other cardiovascular, neurologic, psychiatric, and gastrointestinal medications. Medications used to treat depression, such as selective serotonin reuptake inhibitors (SSRIs) and serotonergic and noradrenergic reuptake inhibitors (SNRIs) may also cause or exacerbate insomnia.60 In addition to prescription medications, older adults often take over the counter preparations which can cause or exacerbate sleep disturbances. Examples include cough and “cold” medications, especially those containing pseudoephedrine or phenylpropanolamine, any caffeine containing drugs (e.g. acetaminophen/aspirin/caffeine combinations), and drugs containing nicotine (e.g. nicotine gum or transdermal (patches).
Of course, cigarette smoking and coffee consumption themselves can impair sleep as well.
Assessment of insomnia
The diagnosis of insomnia in the older adult requires that the patient has difficulty falling asleep or staying asleep for at least 1 month and that impairment in daytime functioning results from difficulty sleeping. The differential diagnosis of chronic insomnia is broad, especially in older adults with many medical and psychosocial comorbidities who are also taking multiple medications. Therefore, a thorough clinical history is essential, especially with regard to prescription and nonprescription drugs and remedies, as well as any comorbid conditions or diseases. It is important to establish whether the individual's insomnia is primary or comorbid. However, it is not uncommon for older people to have more than 1 etiologic contributing factor responsible for the insomnia.
A focused physical examination, based upon the responses from the clinical history, is also essential. Any laboratory evaluation should follow logically from the results of the history and physical exam.
Treatment of insomnia
Behavioral treatment
Behavioral treatments have been shown to be highly effective in the treatment of insomnia in all age groups.36,61 Cognitive behavioral therapy for insomnia (CBT-I) has been shown to be most effective. CBT-I combines different behavioral treatments, including sleep hygiene instruction, stimulus control and/or sleep restriction, with cognitive restructuring.62-64 In CBT-I trials in older adults, insomnia not only resolved, but the effect was sustained for up to 2 years.62
A number of single-modality behavioral and other nonpharmacologic approaches have been utilized to treat and manage insomnia in all age groups. These include relaxation therapy and imagery, stimulus control, sleep restriction, sleep compression, improved sleep hygiene and sleep education, and cognitive therapies. Exercise and physical activity, massage therapy, chronotherapy and light therapy are also used. While any of these may be beneficial for an older adult with insomnia, 2 approaches have met evidence-based criteria for efficacy: sleep restriction-sleep compression therapy and multicomponent cognitive-behavioral therapy.65
Sleep hygiene and sleep education
Sleep hygiene and sleep education can be useful when used together with other modalities, but are usually not adequate by themselves for the treatment of severe, chronic insomnia. Addressing sleep hygiene entails examining sleep habits, behaviors and environmental factors that can have an effect upon sleep. A practitioner can educate patients about common habits or practices that may interfere with their sleep, and implement strategies for avoiding them.66 Clinicians must be aware that, as often occurs in this population, older adults may not voluntarily offer information about sleep practices unless specifically asked about them.
Behaviors and habits that may impair sleep include66:
Frequent daytime napping
Spending too much time in bed
Insufficient daytime activities
Late evening exercises
Insufficient bright light exposure
Excess caffeine
Evening alcohol consumption
Smoking in the evening
Late heavy dinner
Watching television or engaging in other stimulating activities at night
Anxiety and anticipation of poor sleep
Clock watching
Environmental factors, such as the room being too warm, too noisy, or too bright. Pets on the bed or in the bedroom, and/or active or noisy bed partners.
Sleep restriction- sleep compression
Sleep restriction therapy entails limiting time in bed to consolidate actual time sleeping. The patient is counseled to reduce the amount of time in bed to correlate closely with actual time sleeping. The recommended sleep times are based upon sleep logs kept for 2 weeks before sleep restriction therapy is begun. Thus, an individual who reports spending 8.5 hours in bed, but sleeping only 5.5 of those hours, would be counseled to limit his or her time in bed to 5.5 to 6 hours. Time allowed in bed is gradually increased by 15-20 minute increments (approximately once every 5 days if improvement is sustained) as sleep efficiency increases, until the individual's optimal sleep time is obtained.64
In sleep compression, a variant of sleep restriction, patients are counseled to decrease their time in bed gradually to match total sleep time rather than making an immediate substantial change, as is the case in sleep restriction therapy.64 A number of studies support the efficacy of sleep restriction-sleep compression therapy as a treatment for older patients with chronic insomnia.67-69 These approaches can also be combined with other modalities.
Stimulus control
People suffering from chronic insomnia may adopt coping strategies that exacerbate the problem. Watching television or reading in bed, worrying about falling asleep, or using the bedroom for vigorous discussions or arguments are examples of behaviors that can impair sleep by producing associations between the bed and bedroom and those activities; the bedroom should be associated only with sleeping and sex. Stimulus control therapy attempts to eliminate these behaviors in the bedroom and thereby strengthen the association between sleep and the bed and bedroom.
The following are helpful instructions for utilizing stimulus control and practicing good sleep hygiene.7,35,66
Develop a sleep ritual such as maintaining a 30-minute relaxation period before bedtime or taking a hot bath 90 minutes before bedtime.
Make sure the bedroom is restful and comfortable.
Go to bed only if you feel sleepy.
Avoid heavy exercise within 2 hours of bedtime.
Avoid sleep-fragmenting substances, such as caffeine, nicotine, and alcohol.
Avoid activities in the bedroom that keep you awake. Use the bedroom only for sleep and sex; do not watch television from bed, or work in bed.
Sleep only in your bedroom.
If you cannot fall asleep leave the bedroom and return only when sleepy.
Maintain stable bed times and rising times. Arise at the same time each morning, regardless of the amount of sleep obtained that night.
Avoid daytime napping. If you do nap during the day, limit it to 30 minutes and do not nap, if possible, after 2 pm.
Relaxation therapy
The goal of relaxation therapy is to guide individuals to a calm, steady state when they wish to go to sleep. The methods used include progressive muscle relaxation (tensing and then relaxing each muscle group), guided imagery, diaphragmatic breathing, meditation and biofeedback.70
Cognitive behavioral therapy for insomnia (CBT-I)
This treatment combines multiple behavioral approaches, usually incorporating sleep restriction, stimulus control and cognitive therapy, with or without relaxation therapy. Sleep hygiene and sleep education are frequently included. Protocols for older adults may vary somewhat from those used for younger patients. All approaches, however, aim to correct the common misperceptions regarding normal aging and sleep by providing information about how much sleep is necessary to maintain health, and the physical and psychologic consequences of sleep loss. Motivational strategies to increase compliance are also emphasized.36 A number of studies have demonstrated the efficacy of multicomponent CBT among older adults.71-73
Exercise and complementary and alternative treatment modalities
Some studies report that walking, Tai Chi, acupressure, and weight training improve sleep for some individuals.74-77 However, how these approaches affect sleep is not well understood and is likely to be complex.65 Also, difficulties inherent in these studies preclude their recommendation as evidence-based. Nevertheless, there are many good reasons to encourage regular physical activity in older individuals, given its positive effect on functional and cognitive status.
Pharmacologic treatment
Ten drugs have been approved by the FDA for the treatment of insomnia, including benzodiazepines, nonbenzodiazepines and a melatonin receptor agonist (Table 2). The selection of any given drug should depend on matching the characteristics of the particular drug with the patient's complaint. All should be started at the lowest available dose.
Table 2. FDA-Approved Hypnotics for Insomnia1.
| Generic Name | Trade Name | Indication | Geriatric Dose | Half life in older persons (hours) | Comments |
|---|---|---|---|---|---|
| Benzodiazepines2 | |||||
| flurazepam | Dalmane® | Short term treatment of insomnia | 15 mg | 126-158 | Should not be used in older adults because of very long half-life |
| quazepam | Doral® | 7.5 mg | 78 | Should not be used in older adults because of very long half-life | |
| estazolam | ProSom™ | 0.5-1 mg | 10-24 | Due to long half-life, residual CNS effects are likely. | |
| temazepam | Restoril® | 7.5-15 mg | 3.5-18.4 | ||
| triazolam | Halcion® | 0.0625-0.25 mg | 1.7-5 | Poor choice due to very short half life and high incidence of CNS adverse reactions | |
| Nonbenzodiazepines3 | |||||
| eszopiclone | Lunesta® | No short-term limitation for use; sleep onset and sleep maintenance insomnia | 1-2 mg | 9 | AEs>10%: headache, unpleasant taste |
| zolpidem ER | Ambien CR® | 6.25 mg | 1.9-7.3 | AEs>10%: dizziness, headache, somnolence | |
| zolpidem | Ambien® | 5 mg | 2.9-3.7 | AEs>10%: dizziness, headache, somnolence | |
| zaleplon | Sonata® | 5 mg | 1 | AEs: nausea (7%), myalgias (7%) | |
| Melatonin receptor agonist | |||||
| ramelteon | Rozerem™ | No short-term limitation for use; sleep onset insomnia | 8 mg | 1-2.6 | AEs: Headache (7%) Somnolence (5%) Dizziness (5%) Not a Class C-IV scheduled drug |
New interpretive guidelines (F329) from CMS also mandate quarterly review of sedative-hypnotic compounds for residents of long-term care facilities to assess continued need, dose and possible side-effects including possible decline in functional status or increased incidence of falls.
All are Class C-IV scheduled drugs, and may also be associated with amnesia and complex sleep-related behaviors such as sleepwalking or sleep-eating.
The nonbenzodiazepines have a fast onset of action (30-45 minutes).
The benzodiazepines are psychoactive drugs with varying hypnotic, sedative, anxiolytic, anticonvulsant, muscle relaxant and amnestic properties. Nonbenzodiazepines, also called benzodiazepine receptor agonists, are comparatively new drugs whose actions are similar to those of the benzodiazepines, although they are structurally unrelated. The one approved melatonin receptor agonist, also comparatively new, has a different mechanism of action. Melatonin receptors, acted upon by endogenous melatonin, are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle.
The NIH State-of-Science Conference on Insomnia concluded that the benzodiazepine receptor agonists are efficacious in the short-term management of insomnia and that the frequency and severity of any adverse effects are lower than those found in the older benzodiazepines.36 The NIH document was published prior to the availability of the melatonin agonist. However, while the nonbenzodiazepines may have less of a tendency for dependence and abuse, adverse effects can still become a problem with the newer drugs. To date, no significant effects indicative of potential for abuse or motor and cognitive impairment have been demonstrated for the melatonin receptor agonist.78
A metaanalysis that compared hypnotic use with placebo found that sleep quality improved, total sleep time increased and number of nocturnal awakenings decreased.79 However, adverse events were also more common with sedative-hypnotics than with placebo, although most adverse events were reported to be reversible and not severe. Older people may be at greater risk for adverse effects because of pharmacokinetic considerations, such as reduced clearance of certain sedative-hypnotics. There is also some evidence of pharmacodynamic differences such as increased sensitivity to peak drug effects. Impairment was shown to be dependent on dose and time since dosing.
Other classes of medications have also been used to treat insomnia in the elderly. The 2005 NIH State-of-the-Science Conference on Insomnia concluded that there is no systematic evidence for the effectiveness of many medications, including the antihistamines, antidepressants, antipsychotics or anticonvulsants used off-label for the treatment of insomnia and warned that the risks of use outweighed the benefits.36 Trazodone, a frequently prescribed antidepressant for insomnia in older persons, is very sedating, can cause orthostasis and has no published evidence of sustained efficacy.11
Combination therapy
Combining both behavioral and pharmacologic therapy may provide for better outcomes than use of either modality alone. Past studies in adults have shown that combination therapy has been efficacious, with medications providing short-term onset relief and behavioral therapy providing longer-term sustained benefit. Only 1 randomized, controlled clinical trial has evaluated combination therapy in older adults.61 In this study, combination therapy was not only more efficacious than placebo, it was more efficacious than either pharmacologic or behavioral therapy alone. The study concluded that, while combination therapy was effective for the short term management of insomnia in late life, sleep improvements were better sustained over time with behavioral treatment. While results from the few controlled studies that have been performed on combination therapy are encouraging, there is still enough of a paucity of data to caution against overgeneralization.80
Summary
Insomnia in the older adult is most often comorbid with medical and psychiatric illness and complicated by the polypharmacy conventionally associated with them. Treatment should include behavioral therapy whenever possible. Successful management of sleep in the older adult may result in significant improvement in quality of life and daytime functioning.
Future Research
Does improving insomnia in the older adult result in improvement in daytime functioning (including decreased risk of falls, decreased daytime sleepiness, improvement in memory and concentration, improved quality of life)?
Does improving insomnia in the older adult result in improvement in medical comorbidities (including fewer doctor office visits)?
Does improving insomnia in the older adult result in improvement in psychiatric comorbidities (particularly depression and anxiety)?
Will increasing slow wave sleep in the older adult result in improvements in overall quality of sleep as well as improvement in daytime functioning?
Sleep Apnea
Definition
Sleep apnea is a condition in which people stop breathing while asleep.86 Apneas (complete cessation of respiration) and hypopneas (partial decrease in respiration) both result in hypoxemia and changes in autonomic nervous system activity, resulting in increases in systemic and pulmonary arterial pressure and changes in cerebral blood flow.87,88 The episodes are generally terminated by an arousal (brief awakening) which results in fragmented sleep. These arousals are believed to be an important contributor to the symptoms of excessive daytime sleepiness (EDS) and the neurocognitive impairment seen in sleep apnea.
Two types of sleep apnea are recognized. In obstructive sleep apnea (OSA), the primary pathophysiologic event is obstruction of the upper airway, manifested by greatly diminished or absent airflow in the presence of an effort to breathe. Central sleep apnea (CSA) is characterized by recurrent episodes of apnea during sleep resulting from temporary loss of ventilatory effort, due to central nervous system or cardiac dysfunction.89,90 This latter type of apnea is commonly found in patients with congestive heart failure (CHF), particularly in those with Cheyne-Stokes respiration. In this guideline we will primarily focus on the much more common OSA, defined as sleep apnea associated with EDS.
Prevalence
OSA has been described in all age groups. In the adult population OSA (defined as 10 or more apneas and hypopneas per hour of sleep) occurs in about 15% of men and 5% of women.91 In older adults, OSA occurs in up to 70% of men and 56% of women.27 The syndrome is much more common in postmenopausal than premenopausal females, but the prevalence increases in both genders with aging.92
Assessment
Signs and symptoms
EDS and a history of snoring are by far the most common presenting symptoms in most patients with OSA. Other symptoms of OSA include observed apnea, choking or gasping on awakening, morning headache, and nocturia.93 While most younger OSA patients are obese, the elderly with OSA may not necessarily be obese.94
Risk factors
Risk factors for OSA include age, obesity, and anatomic abnormalities affecting the upper airway. In the older population, OSA is also more common among Asians compared to Caucasians.95 OSA has been associated with heart failure, atrial fibrillation and stroke, conditions that are more common in the older population.96 In women, OSA is often associated with a history of hypothyroidism.97
Morbidity and mortality
Studies show that older adults with OSA are excessively sleepy and that OSA likely contributes to decreased quality of life, increased neurocognitive impairment, and greater risk of nocturia and cardiovascular disease.98 Cardiovascular comorbidities particularly associated with OSA include arterial hypertension, heart failure and stroke.87 Often, the hypertension is difficult to control.99
Diabetes mellitus is also more common in this population, and there may be an association between apnea and insulin resistance. Depression has also been found as a common comorbidity in women with OSA.97 Although mortality is increased in untreated apnea patients under age 50, the impact of OSA on mortality in the older population is unclear.100
Management of OSA
OSA is managed via a 4-step approach: a) confirming the diagnosis; b) determining optimal treatment; c) general management measures; and d) ongoing, chronic follow-up.
a. Confirming the diagnosis
Taking the history
Because OSA is so common in older people, all older patients should be questioned to determine if OSA symptoms are present.91,101,102 The history should be obtained from the patient and a bed partner or caregiver, if possible, and should include questions covering the cardinal symptoms of OSA, specifically EDS, snoring, and observed apnea. Questions about nocturia and cognitive impairment, as well as any comorbidities should be included as well. Physicians should consider OSA syndrome in individuals who are overweight or have a history of heart disease, hypothyroidism, or stroke.
The Epworth Sleepiness Scale (ESS), although not validated for use in older persons, is useful for documenting daytime drowsiness.103 Nocturia is a surprisingly common finding in OSA patients. This symptom in males is commonly misinterpreted as being caused by prostatic hypertrophy.104 OSA should be suspected in all patients with hypertension, especially with hypertension that is resistant to treatment.99
Physical examination
The physical should focus on the upper airway, including the nasal and pharyngeal airways, to rule out anatomic obstruction. The skeletal structure of the face must be assessed to exclude the possibility of jaw abnormalities (retrognathia or micrognathia) which may cause OSA in the absence of obesity.105 Dental structures should be examined if a mandibular advancement device is being considered. Obesity often involves the trunk and neck, and documentation of neck collar size (>17 inches in men and >16 inches in women) may be helpful, especially in males.
Differential diagnosis
OSA needs to be distinguished from sleep deprivation, hypothyroidism, depression and the effects associated with using medications with sedating effects. These can all elicit the main symptom: EDS. Prescribed medications and over-the-counter products may also contribute to breathing difficulties during sleep or may produce daytime sleepiness. Inquiring about alcohol use, and obtaining a detailed list of all medications and other products, particularly sedative-hypnotics and opiate analgesics, are important.106-110
Polysomnography (PSG)
Patients suspected of having OSA based on historical features and physical examination will almost always require objective documentation by PSG to confirm the presence and severity of the apnea.111-114 The Center for Medicare Services (CMS) and most insurance carriers require PSG for reimbursement of CPAP therapy. Comprehensive PSG includes the measurement of variables to document sleep breathing disorders (oxygen saturation in arterial blood, rib cage and abdominal movement, nasal and oral airflow, and snoring sounds), data regarding sleep and stage of sleep (via electroencephalography, electrooculography, and electromyography), and electrocardiogram and leg electromyogram to document the presence of periodic leg movements. The PSG is usually followed by data obtained during continuous positive airway pressure (CPAP) titration. Although PSG is usually performed in a laboratory setting, home testing may be covered by CMS in selected patients.111
Quantification of OSA
The apnea hypopnea index (AHI) is the most widely used metric for characterizing the severity of the abnormalities of sleep respiration, and is based on the average number of apneas plus hypopneas per hour of sleep in a single night's study. A value >5 is considered diagnostic for OSA. CMS covers reimbursement for treatment when AHI is >15, or if AHI exceeds 5 and comorbidities (such as sleepiness and/or cardiovascular disease) are present.112,113
b. Determining optimal treatment
Determining treatment
When OSA in the older adult is associated with clinical symptoms, particularly hypertension, cognitive dysfunction, nocturia, high levels of sleep disordered breathing or cardiac disease, then it should be treated, regardless of the age of the patient. Most patients with OSA will probably require referral to or management by sleep specialists, including those with hypoventilation syndromes (eg, individuals suspected of having obesity-hypoventilation, impaired ventilation secondary to neuromuscular diseases, or CSA), those with significant respiratory disease (eg, chronic obstructive pulmonary disease [COPD], severe asthma, or restrictive diseases), and those with significant cardiac disease such as CHF. Such patients may require complex treatment.
Treatment
Currently there is no pharmacologic treatment for OSA. Continuous positive airway pressure (CPAP) is the best approach and first-line of treatment for most patients. CPAP works by stenting open the airway, increasing functional residual capacity of the lungs, possibly increasing pharyngeal dilator activity, and reducing afterload on the heart. Several studies have confirmed that older adults tolerate nightly CPAP use.111,112
The choice of interface type of headgear (nasal mask, or oronasal mask) for securing the mask to the head, and necessity for a chinstrap are determined objectively. Response to CPAP is usually assessed as part of comprehensive PSG either during the latter part of the diagnostic study night (split night study), or during an additional all night study. The CPAP titration is performed in a split night study after the patient has been asleep for at least 2 hours and the OSA diagnosis has been confirmed. This involves fitting the patient with an appropriate mask, educating him or her about what is to transpire, and then applying increased levels of pressure until OSA control is attained. Proper fit and education will help compliance and reduce claustrophobia.
A split night study may not be appropriate if there is insufficient time during the night to make a diagnosis and also determine optimal pressures. In addition, some patients may require a more complex device than a standard fixed-pressure CPAP machine. Only after a review of all diagnostic and therapeutic sleep studies can the optimal treatment approach be determined. Patients without teeth can sometimes present a challenge for CPAP treatment because of bone resorption in the upper and lower jaws. This situation presents difficulties for optimal mask fitting and makes oral appliances unfeasible.
c. General management measures
Although the following general measures have not been evaluated in rigorous randomized clinical trials, they are based on evidence from case series and general physiologic findings.
Avoidance of alcohol, sedative-hypnotics and opiates
Alcohol or other agents (eg, opiates, many anesthetic agents, and the sedative-hypnotics) can depress upper airway tone and may worsen OSA syndrome.107,108,110 Older patients about to undergo surgery should be screened for OSA, at least by history, since they might receive opiates during the perioperative period.
Weight loss
A great deal of evidence supports the strong positive correlation between weight and OSA risk. Weight reduction plays an important role in the management of the obese OSA patient.113-115 One study of older OSA patients monitored for 18 years found a reduction in the severity of the apnea with which the weight loss was associated.115
Treatment of CHF
Patients with CHF are at risk of developing Cheyne-Stokes respiration, a form of CSA. Cheyne-Stokes respiration can result in severe sleep onset and sleep maintenance insomnia, as well as daytime sleepiness.116, 117 Older patients with CHF and sleep apnea (particularly CSA) have a 2.7-fold greater risk of reduced survival than patients with CHF or apnea alone.118
CHF treatment may improve breathing abnormalities in CSA, but results from a recent randomized clinical trial indicate that CPAP may increase mortality in the first 2 years of treatment. Therefore, CPAP is not currently recommended as a first-line treatment in CHF.119 Small short-term clinical trials have suggested the effectiveness of oxygen and adaptive servoventilation, a ventilatory support mode specifically designed for CHF breathing abnormalities.120 No long term outcome studies are available.
General surgery in the OSA patient
Older people are more likely than younger people to have general surgery, and to require general anesthesia. All older patients, especially those with the risk factors for OSA, must be questioned about the possibility of OSA. If they are at high risk, an objective assessment should be done. If OSA is confirmed during the preoperative assessment, nasal CPAP should be initiated prior to hospital admission, and the equipment should be brought to the hospital at admission. The postsurgical period harbors significant risk for such patients because anesthetic agents and opiates can worsen OSA in unprotected individuals.110
Oral appliances
These devices, which move the lower jaw up and forward, can be effective, especially in mild to moderate cases.113 Guidelines for their use are identical to those in younger people. In older individuals, however, special attention must be paid to the examination of the jaws and teeth since at least 8 healthy teeth in each of the upper and lower jaws are required to anchor the appliances. At this time, patients without adequate dentition cannot be treated with such appliances.
d. Ongoing, chronic follow-up
OSA is a chronic illness and as such requires long term management. The main symptoms relate to neurocognitive function and daytime sleepiness. The Epworth Sleep Scale (ESS), while not specifically validated in the older population, is the most commonly utilized assessment instrument for daytime sleepiness. With CPAP treatment, an improvement in the ESS score of 2 or more points is expected, as well as an overall improvement in subjective sleepiness assessment. When CPAP is no longer effective or sleepiness returns, the patient should be reevaluated.
Cognitively-impaired patients may have difficulty mastering the steps involved in putting on their masks and cleaning their CPAP machines and headgear, although one study of patients with mild to moderate Alzheimer's disease living at home showed that these patients were compliant with CPAP treatment.121 Help either from a family member or caregiver is generally necessary. Compliance can be monitored by some of the newer CPAP systems, but the clinical utility of monitoring has not been rigorously determined.
Future research
What kind of alternatives to CPAP treatment in older people (who may have difficulty due to lack of dexterity in using CPAP) can be developed?
Will the treatment of OSA in the older adult result in improvements in nocturia and cognition?
What are the optimal diagnostic techniques for older people who are in skilled nursing facilities or long term care?
Restless Legs Syndrome and Periodic Limb Movements of Sleep
Restless legs syndrome (RLS)
Definition
RLS is a sleep disorder characterized by unpleasant leg sensations that disrupt sleep.90,126,127 The syndrome is classified as either primary or secondary. Primary, or idiopathic RLS is likely to develop at an earlier age, has no known associated or predisposing factors, and probably has a genetic basis. First and second degree relatives of patients with idiopathic RLS have a significantly increased risk of developing RLS compared with relatives of matched controls.128 Secondary RLS can result from a variety of medical conditions that have iron deficiency in common. These include iron-deficiency anemia, end-stage renal disease, and pregnancy.129
Prevalence
The prevalence of RLS symptoms is about 10% in most population-based surveys.130-132 While the rate of RLS may be lower in Asian compared to European populations, the prevalence is similar in African-Americans and Caucasians.133,134 Because the diagnosis of RLS is based on symptom report, prevalence rates for frequency and severity vary with different criteria. For example, in the Restless Legs Syndrome Prevalence and Impact (REST) study, RLS symptoms were reported by 7.2% of the survey population, but symptoms occurring at least twice per week were noted by only 5%, and were moderately or severely distressing in only 2.7%.132
The prevalence of RLS symptoms clearly increases with age for both men and women, at least until the seventh or eighth decade. Higher rates of symptoms are consistently reported in women compared to men.130-132 Some of the age-related increased risk is due to the fact that although RLS can develop at any age, it rarely remits. Increasing prevalence of RLS with age may also occur in association with the increasing presence of secondary causes in the aging population, such as iron deficiency and renal failure.
Typical signs and symptoms
RLS sensations are usually described as a compelling urge to move the lower extremities, but they may also be reported as a creepy-crawly, burning, itching or even painful feeling. The resultant sleep disruption may lead to insomnia and daytime sleepiness. Although symptoms most commonly involve the lower extremities, they have also been described in the upper extremities and even the trunk. RLS has a circadian pattern, with the intensity of the symptoms becoming worse at night and improving toward the morning. Symptoms also tend to worsen when the individual is at rest. They improve with movement such as walking, rubbing, or stretching. The diagnosis is made by history without the need for polysomnography (PSG) in the majority of cases.90,127
Risk factors
A clear familial risk exists for the development of both RLS and periodic limb movements of sleep (PLMS) (described in more detail below). In an Icelandic cohort of patients with RLS and PLMS, a significant association was found with a common variant on chromosome 6p21.2. The Icelandic investigators reported an association between the variant and PLMS without RLS and no association for RLS without PLMS, suggesting that the variant was a genetic determinant of PLMS.135
A variety of medications, including tricyclic antidepressants, SSRIs, lithium, and dopamine antagonists (antipsychotics) have been reported to exacerbate RLS.131 In addition, several social or lifestyle factors appear to contribute to RLS symptoms. These include increased body mass index (BMI) and caffeine intake, sedentary lifestyle, tobacco use, and lower income.131,136
Pathophysiology
The exact pathophysiology of RLS and PLMS remains unclear, but the spinal cord, peripheral nerves, and central dopamine and narcotic receptors may be involved.136,137 The impairment of dopamine transport in the substantia nigra due to reduced intracellular iron appears to play a critical role in most patients with this disorder.137
Assessment
Key questions to include in the history
Is there an urge to move the legs, and is this urge accompanied or caused by uncomfortable or unpleasant sensations in the legs?
Do the unpleasant sensations or the urge to move begin or worsen during periods of rest or inactivity, such as sitting or lying down?
Are the unpleasant sensations or the urge to move partially or totally relieved by movement, such as walking or stretching, for at least as long as the activity continues?
Do the unpleasant sensations or urge to move get worse or only occur in the evening or night?
The physical examination is usually unremarkable in primary RLS. Secondary causes such as peripheral neuropathy or radiculopathy may be elicited during an examination, however. Therefore a thorough neurologic exam is important.
There are no specific laboratory tests necessary to establish the diagnosis. However because iron deficiency states are often associated with RLS from secondary causes, obtaining a serum ferritin is recommended. Values less than 50 ng/mL are consistent with a diagnosis of RLS, and suggest the need for iron supplementation.
The differential diagnosis for RLS includes peripheral neuropathies, vascular disease (intermittent claudication), neuroleptic-induced akathesias, arthritides and venous varcosities. A careful history is usually sufficient to distinguish RLS from each of these.138
Assessing RLS in the cognitively impaired
Cognitively impaired individuals may require a broader approach when considering the diagnosis. The following are considered essential criteria to make the diagnosis of RLS in these patients127:
Signs of leg discomfort, such as rubbing or kneading the legs and/or groaning while holding the lower extremities
Excessive motor activity in the lower extremities, such as pacing, fidgeting, repetitive kicking, tossing and turning in bed, slapping the legs on the mattress, cycling movements of the lower limbs, repetitive foot tapping, rubbing the feet together, and the inability to remain seated
Signs of leg discomfort exclusively present or worse during periods of inactivity
Signs of leg discomfort are diminished with activity
Criteria 1 and 2 occur only in the evening or at night, or are worse at those times than during the day
Treatment
Pharmacologic approaches
The primary pharmacologic therapies are dopaminergic agents. Opioids, benzodiazepines and anticonvulsants are considered second-line agents. If pharmacologic therapy is required, evidence supports the use of dopaminergic agents as first-line treatment, especially the newer dopamine receptor agonists such as ropinirole or pramipexole (both FDA-approved for RLS).139 These agents are associated with less rebound and symptom augmentation than dopamine precursors such as levodopa-carbidopa. Side effects include nausea, orthostatic hypotension, sleepiness, headache, and compulsive behaviors. In the older patient, particular consideration should be given to drug interactions with other medications and the risk of orthostasis.
The beginning dose of ropinirole is 0.25 mg orally 1 to 3 hours before bedtime. The dose can be increased after 2 to 3 days to 0.5 mg, and to 1 mg after 7 days. Titration upwards by weekly 0.5 mg increments to a maximum of 4 mg at week 7 if needed.59 Pramipexole 0.125 mg orally should be administered 2 to 3 hours prior to bedtime. If needed, the dose can be doubled every 4 to 7 days to a maximum of 0.5 mg.140
Augmentation of RLS is characterized by worsening and earlier onset of symptoms in a patient whose leg discomfort was initially controlled on medication. Typical presentations are symptom onset earlier in the day, worsened intensity of symptoms, or spread of symptoms to other parts of the body, such as from the calves to the thighs. The frequency of augmentation with the newer FDA-approved dopamine agonists is unknown, but it is common in patients who are treated with levodopa-carbidopa.141 There is no standard approach to the treatment of augmentation with the newer dopaminergic agents, but options include taking a dose earlier in the day, splitting the existing doses into early evening and bedtime doses, or switching to a different class of medication, such as an anticonvulsant.
Nonpharmacologic approaches
Nonpharmacologic approaches to the management of RLS include modalities such as education, moderate exercise, smoking cessation, alcohol avoidance, caffeine reduction or elimination, and discontinuation of offending medications if appropriate.
Periodic limb movements of sleep (PLMS) and periodic limb movement disorder (PLMD)
Definition
This movement disorder of sleep, also sometimes called nocturnal myoclonus or periodic leg movements, consists of repeated rhythmical extensions of the big toe and dorsiflexions of the ankle with occasional flexions of the knee and hip. The movements may cause brief awakenings or arousals from sleep, of which the individual may or may not be aware.
Prevalence
As many as 90% of individuals with RLS have PLMS.142 PLMS associated with arousals are linked to disturbed sleep in older women.143 PLMS are more common with aging.25
Typical symptoms and signs
PLMS usually occur predominantly during the first part of the night. Each movement lasts approximately 2 to 4 seconds with a frequency of about 1 every 20-40 seconds.90
Risk factors
PLMS are usually associated with other sleep disorders, including sleep disordered breathing, but the most notable association occurs with RLS, suggesting a similar pathophysiology. The rate of PLMS correlates with subjective RLS severity.140,144 PLMS are also common in patients taking antidepressants.145 While the presence of PLMS supports the diagnosis of RLS, limb movements are neither necessary nor sufficient to make the diagnosis of RLS.
Assessment
The revised diagnostic criteria for PLMD (below) note that leg jerks occur with many medical conditions and in the presence of many medications. These criteria also “raise the bar” for the “abnormal” number of periodic limb movements in adults, from 5 to 15 as determined by the PLMS Index (the number of periodic limb movements per hour of total sleep time as determined by PSG).
Diagnostic Criteria for PLMD
Polysomnography demonstrates repetitive, highly stereotyped, limb movements.
The PLMS Index exceeds 15 per hour in most adult cases.
There is clinical sleep disturbance or a complaint of daytime fatigue.
The PLMS are not better explained by another current sleep disorder, medical or neurologic disorder, mental disorder, medication use, or a substance use disorder.
Note: If PLMS are present without clinical sleep disturbance, the PLMS can be reported as a polysomnographic finding, but criteria are not met for a diagnosis of PLMD.
Treatment
There is very little evidence to support pharmacologic treatment to suppress PLMS or PLMD, even in the face of insomnia or hypersomnia, particularly in older adults. No agent has been FDA-approved to treat PLMS or PLMD.
Future research
What medications are associated with an increased risk of RLS/PLMS in older people?
Does treatment of RLS affect outcomes such as blood pressure, depression, sleep measures, and health-related quality of life in older individuals?
What is the natural history of RLS in older individuals?
Circadian Rhythm Sleep Disorders (CRSD) in Aging
Definition
The hallmark of CRSD is the presence of relatively normal sleep that occurs at abnormal times. In the case of advanced sleep phase disorder (ASPD), sleep commences and ends at unusually early times; in the case of irregular sleep-wake disorder (ISWD), sleep is dispersed across the 24-hour day in bouts of irregular length. The combination of age-related changes in sleep and circadian rhythm regulation paired with decreased levels of light exposure and activity contribute to the development of circadian rhythm-based sleep disorders in older people.
Pathophysiology
Optimal sleep quality is achieved when the desired sleep time coincides with the timing of the endogenous circadian rhythm of sleep and wake propensity. CRSD arises from alterations of the central circadian clock or a misalignment between endogenous circadian timing and the external 24-hour social and physical environment. While the primary pathophysiology of CRSD is a disruption of circadian timing, the actual clinical presentation of CRSD is often influenced by a combination of physiologic, behavioral, and environmental factors. The CRSDs which are most prevalent in older people are ASPD and ISWD.
Significant changes in both sleep and circadian regulation occur with aging. Common sleep complaints among older adults include habitually earlier bedtimes and wake times, inability to maintain sleep through the night, undesired early morning awakening and frequent daytime sleepiness.26,147-149 These sleep disturbances may be caused in part by a change in the circadian timing system of older people and/or in the interaction between the circadian and homeostatic processes. Habitual wake time, the rise of hormone secretion and endogenous temperature nadir of older subjects occurs at an earlier clock hour, suggesting that the earlier wake time may be due to an advance of the circadian clock.150-153 There is also evidence that the interaction of a reduction in the homeostatic drive for sleep with a reduction in the strength of the circadian signal promoting sleep may be responsible for the impaired sleep of older individuals in the early morning.154
Assessment
For a diagnosis of CRSD, an accurate clinical history, sleep diary and/or actigraphy (a small motion sensor worn continuously, usually on the wrist) covering at least 7 days should be obtained. Other physiologic markers of the circadian phase such as dim light melatonin onset and nadir core body temperature are adjunctive tools to confirm the phase or amplitude of circadian rhythms, but are not widely available clinically. Polysomnography (PSG) is not routinely indicated. However, because of the age-related increase in the prevalence of other sleep disorders, a careful assessment for conditions such as sleep apnea, restless legs and REM sleep behavior disorder should be performed in all patients with CRSD.155 Furthermore, psychiatric conditions, including depression and anxiety disorders, are frequent comorbidities with CRSD and must be considered in the evaluation and differential diagnosis.
Advanced sleep phase disorder (ASPD)
Clinical presentation
The defining characteristic of ASPD (also known as advanced sleep phase syndrome or ASPS) is sleep-wake times that are earlier than desired or earlier than conventional. Sleep onset times may be as early as 6:00 pm to 9:00 pm, even if the patient attempts to delay sleep onset. These are coupled with wake times between 2:00 am to 5:00 am.
Excessive sleepiness during waking hours and sleep maintenance insomnia may occur in conjunction with abnormal sleep timing. Sleep is otherwise normal when individuals are permitted to sleep on their own particular sleep-wake schedule.
Diagnostic criteria require verification of the advanced sleep-wake phase through the use of at least 1 week of actigraphy or sleep log. Other sleep disorders, medical or psychological conditions (such as depression), medication factors or substance use disorders that may be causing the symptoms need to be ruled out.155 As expected, an earlier onset of increased melatonin levels and core body temperature minimum are seen and these can confirm the diagnosis, but are not required in the routine assessment.156 Not all individuals with an advanced sleep phase have ASPD, however. In fact, many older people are not particularly bothered by their sleep phase and have no consequent functional impairment. Such individuals can be considered “morning types” or “larks” rather than ASPD patients.
Prevalence
In middle- to older-aged adults, the prevalence of ASPD is estimated at 1%-7%.157,158 ASPD is much less common in the general adult population, with only a few reported cases of non-age-related ASPD.159-161
Pathophysiology
The pathogenesis of ASPD is thought to involve a combination of behavioral and genetic factors. For example, early sleep times and ophthalmologic conditions such as cataracts may decrease light exposure at a time that would delay the sleep phase (ie, evening hours), thereby perpetuating the advanced sleep phase. Intrinsic factors, such as a shortened endogenous circadian period (less than 24 hours) or alterations in the relationship of circadian timing and sleep homeostatic regulation may play a role in the development of ASPD.159,162 Furthermore, familial forms of ASPD have been reported in which the phenotype segregates in an autosomal dominant inheritance pattern159,161,163 and mutations in the circadian clock hPer2 and CK1 delta genes have been identified.164,165 Thus, decreased exposure or weakened responses to entrainment agents such as light and physical activity, together with intrinsic changes in circadian and sleep regulation and genetic predisposition may all contribute to the development of ASPD in older individuals.166,167
Treatment
A combination of good sleep hygiene practices and methods to delay the timing of sleep and wake times is often recommended for the treatment of ASPD. Chronotherapy has been used successfully in ASPD. In this approach, sleep times are advanced every 2 days until the desired sleep-wake time has been achieved. However, the need for rigorous compliance, the length of the treatment, and the necessity for close follow-up limit its overall clinical practicality. Therefore, use of evening light within the phase delay portion of the light phase response curve (PRC) is one approach used to treat. In addition to light and good sleep hygiene, other behavioral adjustments are also central to the effective treatment of the disorder.
Light
Successful phase delay with the use of evening light therapy has been reported in several studies. Light therapy in these patients may additionally improve sleep efficiency and total sleep time. 168,169 Bright light therapy used in the delay portion of the light PRC (that is, in the evening between 7:00-9:00 pm) can help normalize or delay circadian rhythms in patients with ASPD. Bright light therapy generally consists of broad spectrum light of 2500-10,000 lux for 1-2 hrs duration. Unfortunately, light at lower intensities may not delay sleep phase effectively. In addition, older subjects appear to have reduced response to the generally superior phase-shifting properties of short wavelength (blue) light compared to their younger counterparts, raising the question of the usefulness of this spectrum of light in the treatment of older subjects with ASPD.170, 171
Older adults may have difficulty tolerating bright light, however, and both compliance with and efficacy of light therapy may decline over time. Close follow-up is advised. The clinician should use the timing, intensity and duration of light exposure (7-9PM) as a general guideline to initiate therapy. If the initial therapy fails, a referral to a specialist to adjust the timing or duration of light therapy is recommended.
Side effects
Ultraviolet rays are filtered by light boxes. Therefore, they are considered to be safe. However, side effects have been reported, including hypomania, mild headache, nausea and vomiting, and self-limited visual problems.172 Patients with ophthalmologic disease should be evaluated by a specialist before beginning light therapy, in order to determine if this approach is appropriate. Additional caution is advised in subjects with preexisting mania, retinal photosensitivity, and migraine.
Dosing and duration of treatment
Although the exact length of treatment and dosing levels have yet to be clearly established, light therapy represents a potentially important instrument in the manipulation of circadian phase. The American Academy of Sleep Medicine has confirmed the potential usefulness of light therapy for CRSDs such as ASPD.173
Melatonin
Theoretically, melatonin delivered in the morning should result in a delay in sleep phase based on the melatonin PRC.152 However, data supporting the efficacy of melatonin in ASPD is lacking. Additionally, melatonin may produce soporific effects which may result in residual morning sleepiness.
Irregular sleep-wake disorder (ISWD)
Clinical presentation
ISWD, also known as irregular sleep-wake rhythm (ISWR), is characterized by the lack of a clearly identifiable circadian pattern of consolidated sleep and wake times. Although the total amount of sleep obtained over a 24-hour period is within the normal range, the time asleep is broken into at least 3 different periods of variable length. Erratic napping typically takes place during the day, whereas nighttime sleep is severely fragmented and shortened. Symptoms of chronic insomnia and/or daytime sleepiness may appear as a consequence. To confirm the diagnosis, the exclusion of other disorders which may better explain the patient's irregular sleep as well as at least 1 week of actigraphy or the use of a sleep log demonstrating 3 or more sleep bouts within the 24-hour day is required.155
Prevalence
ISWD is most commonly encountered in patients with dementia, particularly in those who are institutionalized. However, other disorders of the central nervous system (such as traumatic brain injury and mental retardation) can lead to an irregular sleep-wake pattern.153,174,175
Pathophysiology
The development and maintenance of an irregular sleep-wake rhythm likely result from dysfunctional central processes responsible for circadian rhythm generation as well as decreased exposure to external synchronizing agents such as light and social activities.176 The pathogenesis of the disease may be related to a loss of neurons or other deleterious changes within the suprachiasmatic nucleus (SCN).177 A few studies have demonstrated a decrease in the number of neurons within the SCN in patients with Alzheimer's disease.178,179 Also, residents of long term care facilities often lack exposure to adequate light and do not participate in regular daytime activities. This may contribute further to a decrease in the amplitude of circadian rhythms. In fact, lower daytime light levels are associated with an increase in nighttime awakenings, even after controlling for the degree of dementia.180
Treatment
The primary goal of treatment of ISWD is to consolidate the sleep-wake cycle. To this end, measures aimed at restoring or enhancing exposure to the various zeitgebers (“time-givers,” environmental cues that provide an estimate of time of day), are critical. Patients should be exposed to bright light during the day, while avoiding it in the evening181,182 Daytime physical and social activities should be strongly encouraged.177,183-185 A multicomponent approach using a variety of behavioral treatment options is recommended.
Light
The overall approach to light therapy for the treatment of the irregular sleep-wake type is to increase both the duration and intensity of light exposure throughout the daytime and avoid exposure to bright light in the evening. Bright light exposure delivered for 2 hours in the morning at 3,000 to 5,000 lux (a unit of light or illumination) over the course of 4 weeks has been found to decrease daytime napping and increase nighttime sleep in demented subjects.186 Light may further help consolidate nighttime sleep, decrease agitated behavior, and increase the amplitude of circadian rhythms.181,182,186
Melatonin
Studies evaluating the use of melatonin in ISWD have yielded inconsistent results. One trial involving patients with Alzheimer's disease found no statistically significant differences in actigraphy-derived sleep measures between control subjects and individuals taking 2.5 mg melatonin, although a trend towards improvement was seen with a 10 mg dose.187 A review of current evidence has found inconclusive evidence for the efficacy of melatonin treatment in circadian and sleep disorders.188,189 Melatonin may, however, be effective in patients with known melatonin deficiency.190
Other therapeutic approaches
Structured physical and social activity may help provide the temporal cues needed to increase the regularity of the sleep-wake schedule. A reduction in nighttime light and noise and improvement in incontinence care can encourage a favorable sleep environment that will minimize awakenings in nursing home residents.191 Furthermore, elderly subjects with disrupted sleep-wake patterns consistent with ISWD slept less during the day and increased participation in social and physical activities and social conversation when they followed a routine of reduced time in bed during the day, structured bedtime routine at night, 30 minutes or more of sunlight exposure a day and increased physical activity.192 A multidimensional, nonpharmacologic approach that includes increased sunlight exposure and social activity during the day, decreased time in bed during the day, and decreased nighttime noise may be particularly effective.
Recommendations
Recommendations for evaluation of CRSD: see Table 6
Table 6. Circadian Rhythm Sleep Disorders (CRSD).
| Recommendations for evaluation of CRSD | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. All older people with symptoms of insomnia and excessive daytime sleepiness (EDS) should be screened for the possibility of a circadian rhythm sleep disorder. | III 90 | A |
| 2. Diagnosis is made primarily by history. In addition, a sleep diary or actigraphy should be performed for at least 7 consecutive days to confirm the circadian sleep and wake pattern. | III 90 | A |
| 3. Circadian phase markers (eg, core body temperature, melatonin) are useful to confirm the diagnosis, but there is insufficient evidence to recommend their routine use in diagnosis and they are not available clinically. | III 90 | C |
| 4. PSG is indicated if other primary sleep disorders are suspected, but is not indicated for diagnosis. | III 90 | B |
Recommendations for management of ASPD see Table 7
Table 7. Advanced Sleep Phase Disorder (ASPD).
| Recommendations for management of ASPD | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. Chronotherapy (sleep-wake scheduling) is achieved by advancing sleep and wake times until the desired sleep and wake times are achieved. This approach, although possibly useful, is often clinically impractical. | III 160 | C |
| 2. Scheduled bright light in the evening delays circadian rhythms and improves sleep in patients with ASPD. | II 168,193 | B |
| 3. Melatonin should not be used in older persons with ASPD. | III 193 | B |
| 4. Overall, there is little scientific evidence to support the efficacy of behavioral interventions. However, because of the lack of alternative approaches and since the risks and relative costs are low, behavioral interventions are recommended. | III | B |
Recommendations for the management of ISWD: see Table 8
Table 8. Irregular Sleep-Wake Disorder (ISWD).
| Recommendations for the management of ISWD | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. For institutionalized ISWD patients in whom dementia is common, increasing exposure to bright light during the day can improve sleep-wake consolidation and circadian rest/activity rhythm. | II 193,194 | A |
| 2. Melatonin is not indicated for the treatment of ISWD in older adults with dementia. | I 187 | B |
| 3. Multimodal approaches that combine bright light exposure during the day, decreasing light exposure at night, physical activity, social activity And structured bedtimes and wake times and noise reduction can decrease nighttime awakenings, total wake time and daytime sleepiness. |
I 182 II 195,196 |
A A |
Future research
Research is needed to define the timing, duration and optimal light wavelength of bright light therapy for older adults with advanced sleep phase disorder.
Multicenter placebo-controlled randomized studies are necessary to determine the efficacy, safety, and tolerability of long term therapy with bright light in older adults.
Placebo-controlled randomized clinical trials of the efficacy and safety of melatonin receptor agonists are required in the treatment of ISWD in patients with dementia.
Basic research to understand the pathophysiology of circadian rhythm sleep disorders, including the role of genetics is also a priority.
Parasomnia: Rem Sleep Behavior Disorder
Definitions
Parasomnias are undesirable non-deliberate physical or emotional events that occur during sleep. They most often appear during entry into sleep or during arousals and may arise from specific sleep states, such as NREM or REM states. Parasomnias may include abnormal movements, behaviors, emotions, perceptions, dream enactment and autonomic activity that occur during sleep or are associated with arousal from sleep. Manifestations of parasomnias include enuresis, sleepwalking, night terrors, dream anxiety attacks, nocturnal complex seizures, and REM behavior disorder. The non-REM sleep parasomnias are more common in children, whereas REM sleep behavior disorder (RBD) is more common in older adults.
REM sleep behavior disorder
Signs and symptoms
REM sleep behavior disorder is one of the most dramatic and potentially injurious of the parasomnias. Patients report complex, often violent motor behaviors associated with dream enactment. However, approximately 10% of patients do not have dream recall.197 The potential for self- and bed-partner injury is high, especially during severe episodes. The majority of cases occur with advancing age, typically manifesting in the sixth or seventh decade.197
Pathophysiology
RBD is based on an underlying pathophysiology in which there is a lack of the normal atonia associated with REM sleep due to a dysfunction of motor neuron inhibition. The dream enactment is associated with loss of muscle atonia during REM sleep. Polysomnography (PSG) demonstrates intermittent loss of REM sleep-associated muscle atonia, with the patient manifesting complex, often violent motor activity associated with dream mentation.198
Assessment
Diagnosis of RBD is made by the history and PSG evidence of increased electromyographic activity during REM sleep (lack of atonia). The sleep study may also capture the actual episodes of limb jerking and other complex, vigorous and violent behaviors. If there is evidence of abnormal neurologic activity, a full neurologic workup, including a brain MRI, may be needed.198-200
RBD has been seen in association with various brainstem abnormalities, extrapyramidal neurologic disorders, and medical conditions (eg, Parkinson's disease, progressive supranuclear palsy, Shy–Drager syndrome, multiple systems atrophy, brainstem stroke, brainstem tumor, demyelinating disease, and medication toxicity or withdrawal). It may also be idiopathic. The differential diagnosis of RBD includes non-REM parasomnia, sleep apnea, periodic movements of sleep, nocturnal seizures, and nocturnal rhythmic movements. Medications such as tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and selective serotonin reuptake inhibitors (SSRIs) have been shown to induce or exacerbate RBD and RBD has also been described during alcohol and barbiturate withdrawal and with caffeine use.199-204
Treatment
Management of RBD involves pharmacologic treatment and interventions that address environmental safety. The most effective drug therapy is clonazepam at a dosage of 0.5-1 mg at bedtime. Clonazepam may be taken earlier (1 to 2 hours before bedtime) by patients who report sleep onset insomnia or morning drowsiness as a result of the medication. Clonazepam is effective in 90% of cases. There is little evidence of abuse, and only infrequent reports of tolerance in older patients. Beneficial effects are observed within the first week of clonazepam treatment, resulting in control of vigorous, violent sleep behaviors. However, mild to moderate limb movement, sleep-talking, and other complex behaviors may persist. Discontinuation of treatment usually results in recurrence of symptoms.198
Other medications that may be efficacious are levodopa, dopamine agonists and melatonin. Although there are studies reporting the efficacy of melatonin, it is a nutritional supplement that is not approved by the FDA, and in terms of pharmacologic preparation, is poorly regulated.205-207 It should probably not be used in older patients.
Environmental safety is an important issue in RBD management. Patients should be advised to remove potentially dangerous objects from the house, to pad hard and sharp surfaces around the bed, to cover windows with heavy draperies, and even to place the mattress on the floor to avoid falling out of bed, if necessary. The combination of drug therapy and implementation of safety precautions offers safe and effective management of RBD.
Future research
Multicenter trials are needed of the safety and efficacy of pharmacologic therapies in older adults with parasomnias, such as RBD. Trials should be conducted for clonazepam, melatonin and dopamine agonists.
Research is necessary to elucidate the relationship between RBD and Parkinson's disease.
Hypersomnias
Definition
The hypersomnias of central origin are a group of disorders characterized by a primary complaint of excessive sleepiness that is not caused by disturbed nocturnal sleep or misaligned rhythms. Excessive sleepiness is the inability to maintain wakefulness and alertness during the major waking episode of the day. This may result in unintentional episodes of falling asleep at inappropriate times or places, or in planned naps related to irresistible sleepiness. Patients occasionally use the word “sleepy” to describe times when they are “fatigued” or “tired.” However, the term “excessive sleepiness” is better reserved for cases characterized by an increased sleep propensity and falling asleep at inappropriate times.
Disorders that cause disturbed nocturnal sleep with subsequent sleepiness during the waking period may coexist with a hypersomnia. The nocturnal sleep disorder must be controlled prior to assigning a diagnosis of hypersomnia. One example would be the patient with hypersomnia and obstructive sleep apnea; the sleep apnea must be adequately treated before considering an independent diagnosis of a hypersomnia.
A number of disorders are included under the category of hypersomnias. The more common ones seen in older adults include narcolepsy with cataplexy, narcolepsy without cataplexy, narcolepsy due to a medical condition (secondary narcolepsy), idiopathic hypersomnia with long sleep time (sleep time longer than 10 hours), idiopathic hypersomnia without long sleep time (total sleep time of 6-10 hours), hypersomnia due to a medical condition and hypersomnia due to a drug or substance.
Prevalence
The prevalence and demographic characteristics for a number of the disorders in this category are unknown. Narcolepsy with cataplexy has an overall prevalence of 0.05% with a slight preponderance in males.90,209,210 Although all ages can be affected, the prevalence of narcolepsy with cataplexy and the other hypersomnias specifically in older adults is unknown although the medical conditions, comorbidities and medication usage typically associated with hypersomnia are very prevalent in this population.
Signs and symptoms
All the disorders in this category have several signs and symptoms in common. Each presents with excessive sleepiness during waking hours. Other symptoms are more specific to individual disorders.
Idiopathic hypersomnia
Idiopathic hypersomnia with long and short sleep times are defined by a 3 month history of excessive daytime sleepiness with a sleep time longer than 10 hours or a total sleep time between 6-10 hours, respectively.90,211-214 The daytime sleepiness may be shorter than 3 months in the case of hypersomnia due to a drug or substance.90 Some patients may experience symptoms associated with excessive sleepiness such as memory lapses, concentration problems, automatic behavior (an episode that occurs during a period of sleepiness and that is not remembered subsequently by the individual), ptosis, and hallucinations.215-222
Narcolepsy (with or without cataplexy)
To establish a diagnosis of narcolepsy with cataplexy, excessive daytime sleepiness and a definite history of cataplexy is required.90 Cataplexy is an episode of muscle weakness usually manifesting as weakness in the legs or arms, buckling at the knees, and/or dropping items from the hands in association with emotion (eg, laughter or anger). This symptom is absent in narcolepsy without cataplexy.90 Other features of narcolepsy include automatic behaviors, hypnagogic hallucinations, sleep paralysis, and disturbed nocturnal sleep. A hypnagogic hallucination is a hallucination, most often visual, that occurs at sleep onset, and sleep paralysis is an episode of immobility that occurs at sleep onset or upon awakening.
Hypersomnia associated with comorbidities
Patients with hypersomnia due to a medical condition have a complaint of excessive sleep present almost daily for at least 3 months that is secondary to a significant medical or neurologic condition90,223-225 Medical conditions include Parkinson's disease, post-traumatic brain injury, Niemann-Pick disease type C, myotonic dystrophy, Prader-Willi syndrome, Alzheimer's disease, stroke, multiple sclerosis, hypothyroidism, and hepatic encephalopathy.223,226-234
Patients with hypersomnia due to a drug or substance have a complaint of sleepiness or excessive sleep that is believed to be secondary to current use, recent discontinuation, or prior prolonged use of drugs or prescribed medications.90,235 Because older individuals often regularly take multiple medications, careful evaluation of an individual's drug regimen is an essential part of the assessment of hypersomnia in an older adult.
Risk factors
Many hypersomnias have both genetic and non-genetic risk factors, but usually they are not proven or definitively identified. For example, suggested precipitating factors for narcolepsy with or without cataplexy have included head trauma, sustained sleep deprivation, or non-specific viral illness.236
Morbidity and mortality
The morbidity and mortality associated with the hypersomnias of central origin are primarily related to excessive daytime sleepiness (EDS). Cognitive impairment is a common feature characterized by fatigue, tiredness, impaired memory, concentration, and coordination difficulties.237-240 Depression, problems at work (eg, loss of employment due to sleep-related errors), or with social life (eg, withdrawal from family and social activities because of sleepiness) are also common. Weight gain has also been linked to excessive sleep.241 There is also an increased risk for traffic accidents or work-related injury due to sleepiness and inattentiveness.242 Untreated narcolepsy with cataplexy can also be socially disabling, as the cataplexy attacks can lead to social withdrawal in addition to the increased risk of accidents.242,243
Assessment
Specific studies are lacking regarding the assessment or treatment of older patients with hypersomnias of central origin. Therefore, the data collection tools used to assess patients are not specifically validated for the older adult population. However, some extrapolations from existing studies can be made.
Key issues and questions to be addressed in obtaining a history
If possible, obtain the history from the bed partner as well as the patient.
Questions should address excessive daytime sleepiness, cataplexy, symptom response to napping (if any), presence of dreaming during naps, hypnagogic hallucinations, sleep paralysis, and automatic behaviors.
Establish onset, frequency, and duration of the sleepiness as well as any episodes of remission.
Include questions about the patient's medical, neurologic and psychiatric illnesses as well as use or recent discontinuation of recreational drugs, prescription drugs, and/or alcohol.
Questions about other comorbid sleep disorders such as obstructive sleep apnea (OSA) or restless leg syndrome (RLS) are also relevant.208,224,235,244-246 A number of subjective sleep questionnaires are available to assess sleep habits, sleep-wake schedules, and sleepiness (eg, the Epworth Sleepiness Scale [ESS] and Karolinska Sleep Scales [KSS]; sleep diaries are also useful assessment tools). The most commonly used questionnaire is the ESS. Such questionnaires should be part of the patient's evaluation.232,247
What is the duration of nighttime sleep?
Key areas to include in the physical exam
For patients suspected of having a hypersomnia of central origin, a thorough physical examination, including a neurologic evaluation, is important. An assessment of cognition is valuable and can be used to help make a diagnosis as well as to monitor treatment response.
Appropriate laboratory tests
For diagnosis, patients suspected of having hypersomnias of central origin usually require an overnight polysomnography (PSG) followed by a multiple sleep latency test (MSLT).248-250 The MSLT is an electrophysiologic test of sleep tendency that involves 4 or 5 daytime naps at 2 hourly intervals with assessment of the latency to sleep onset and the type of sleep that occurs. A mean sleep latency of 8 minutes or less and the presence of REM sleep on 2 or more naps are indicative of narcolepsy. The MSLT is also required to support a diagnosis of one of the other hypersomnias of central origin. Common medications used to treat chronic conditions in older adults may complicate the interpretation of these studies, however.
An MRI of the brain is useful to identify causes of hypersomnia or narcolepsy due to a neurologic disease (eg, tumors, multiple sclerosis, intracranial bleeds, or strokes). Additionally, blood work can help identify suspected medical conditions that may cause the patient's excessive sleepiness (eg, thyroid stimulating hormone, liver function tests, complete blood count, serum chemistry). Cerebrospinal fluid hypocretin levels can confirm a diagnosis of narcolepsy with cataplexy in the absence of a MSLT.
Treatment options
Initial management of hypersomnias of central origin requires treatment optimization of any underlying medical, neurologic or psychiatric disorder. Furthermore, careful withdrawal of sedating medications or substances, if possible, is prudent. Ensuring an adequate opportunity for nighttime sleep is important to exclude sleep deprivation as a cause of excessive sleepiness.
Excessive sleepiness is treated with one or more of the following: behavioral modification, modafinil, or other stimulants.251-256 Cataplexy is controlled with behavioral modification, antidepressants, or sodium oxybate.257
Behavioral
Some degree of behavioral modification is beneficial to most patients with excessive sleepiness. Good sleep hygiene techniques should be adopted, and a regular sleep-wake schedule allowing adequate time for nocturnal sleep should be maintained. Heavy meals throughout the day and alcohol use should be avoided. Two short 15-20 minute naps, 1 scheduled around noon and the other around 4:00-5:00 pm, may alleviate some sleepiness.
The older patient who is still employed may benefit from occupation counseling. These individuals should avoid shiftwork, on-call schedules, jobs that involve driving, or any other job that demands continuous attention for long hours without breaks, especially under monotonous conditions. Healthcare workers should assist the patient with occupational and social accommodation for disabilities due to excessive sleepiness. Referral for support services and to support groups such as the Narcolepsy Institute or the National Sleep Foundation is very helpful to many patients.210,229,248,258
Pharmacologic
Traditionally, the stimulant medications (amphetamines, methamphetamines, D-amphetamines and methylphenidate) are used to treat excessive daytime sleepiness.259 Recently, modafinil has gained favor for first-line use in the treatment of narcolepsy.259 Modafinil has also been increasingly used for the treatment of idiopathic hypersomnia as well as hypersomnias due to a medical or neurologic condition. For elderly patients, a starting dose of modafinil at 100 mg, once upon awakening in the morning, is recommended. This dose can be increased at weekly intervals as necessary. Typical doses range from 200 to 400 mg per day. The most common adverse reactions are nausea, headaches and nervousness.
Other medications used to treat excessive daytime sleepiness in patients with narcolepsy include sodium oxybate, selegiline, and ritanserin253,257,260-263 Judicious use of caffeine may also be beneficial. In patients with drug- or medication-induced sedation, the treatment is to reduce or remove the drug or substance. This therapy should preferably be instituted under the guidance of both a sleep specialist, who is familiar with these drugs, and the patient's primary care physician, who knows the patient's medical problems and the medications he or she is taking.
Treatment of cataplexy and REM sleep intrusion into wakefulness
Sodium oxybate improves daytime sleepiness as well as cataplexy.260,261 In addition, sodium oxybate may be used to treat the other symptoms of narcolepsy, including disrupted nocturnal sleep, hypnagogic hallucinations, and sleep paralysis. Sodium oxybate is a liquid that is given in 2 divided doses at night. The first dose is given at bedtime and the second 2.5- to 4 hours later. Sodium oxybate can cause headaches, nausea, unexpected neuropsychiatric effects and fluid retention. Selegiline, a MAOI rarely used in narcolepsy because of the potential for side effects, not only improves daytime sleepiness, but can also treat cataplexy. Other REM sleep suppressant medications, such as TCAs, SSRIs, venlafaxine and reboxetine, have all been used to treat cataplexy, hypnagogic hallucinations, and sleep paralysis, although adequate scientific evidence is lacking.
Follow-up
Most of the hypersomnias of central origin are long term or lifelong disorders and require ongoing management.
Monitoring medications
As in most clinical scenarios, more frequent follow-up is usually necessary when starting a medication or adjusting doses. For example, starting or adjusting the dose of a stimulant requires monitoring for adverse effects, including hypertension, palpitations or arrhythmias, irritability, or behavioral manifestations such as psychosis. Patients should be questioned about excessive stimulatory effects or nocturnal sleep disturbances.
Monitoring symptoms
As medications such as modafinil generally only improve sleepiness and do not eliminate it, frequent reassessment of impairments in functional ability due to residual sleepiness is necessary. The ESS is a useful tool for monitoring subjective sleepiness and its response to therapy at each patient visit. Once symptoms are stable, any future exacerbation of symptoms (sleepiness, cataplexy, sleep paralysis, hypnagogic hallucinations or behavioral abnormalities) needs to be evaluated formally by history, physical examination and/or repeat PSG. Healthcare workers should continue to assist the patient with occupational and social accommodation for disabilities due to excessive sleepiness.
Referral
Primary care physicians should refer a patient to a sleep specialist when narcolepsy or idiopathic hypersomnia is suspected or the cause of the sleepiness is unknown. In addition, complex patients who are unresponsive to initial or subsequent therapy may benefit from a sleep specialist consultation.
Future research
1. The basic pathophysiologic mechanism(s) of excessive sleepiness due to idiopathic hypersomnia, narcolepsy, and Alzheimer's disease needs to be further investigated.
2. There is a need for standardization and validation of MSLT studies in older adults.
3. The efficacy of treatment modalities, especially pharmacotherapy (eg, modafinil, lithium, sodium oxybate) in older adults for hypersomnia of central origin requires further study.
4. There is a need for better understanding the comorbidities that may be associated with hypersomnias of central origin in older adults (eg, cognitive deficits and obesity).
5. There is a need for better understanding of how increasing age and comorbidities may modify the symptoms and response to treatment in older adults.
Sleep Problems in Long Term Care Facilities
Background
Sleep disturbances are common among older people living in nursing homes, with many factors specific to this setting and population contributing to sleep difficulties. Medical conditions common to nursing home residents complicate these environmental factors. Frequently, residents experience pain, paresthesias, nighttime cough and dyspnea, gastroesophageal reflux and nocturia, all of which can interfere with sleep. Neurologic illnesses are also common in this population, particularly neurodegenerative disorders such as dementia and Parkinson's disease, which are also associated with sleep disturbance. Medications prescribed for nursing home residents may also interfere with sleep, including diuretics, stimulating agents (eg, sympathomimetics, bronchodilators, and stimulating antidepressants), anti-Parkinsonian agents, antihypertensives, and cholinesterase inhibitors taken near bedtime, or sedating medications (eg, antihistamines, anticholinergics, and sedating antidepressants) taken during the day. The latter group may contribute to daytime drowsiness and further disrupt the sleep-wake cycle.
Environmental factors may also play a role in sleep-wake problems in the nursing home. Many residents have limited interaction with the community outside of the nursing home due to physical and/or cognitive impairment. Most residents have very little, if any, bright light exposure, which impedes the coordination of the internal circadian clock to the external environment. Nursing home residents also spend extended periods in bed and are often physically inactive during the day, factors that contribute to sleep-wake and circadian rhythm abnormalities. Additionally, nighttime noise and light interruptions are also disruptive, and are often caused by staff providing personal care to either the resident or a roommate.
Assessment
A variety of measures have been utilized to assess sleep in nursing home residents. Most studies use wrist actigraphy for objective sleep measurement. Polysomnography (PSG) and portable sleep recording is used less frequently. Subjective sleep measures generally involve observations by research staff, resident questionnaires, nursing staff interviews or questionnaires, or medical record review.
Prevalence
Probably because of the different methodologies used for measurement, prevalence data regarding sleep problems in nursing homes vary. The lowest estimate, 6.3% of residents, was estimated using Minimum Data Set (MDS) documentation from 34,000 nursing home residents in the state of Michigan.43 However, given concerns about the accuracy of the MDS data for insomnia, this is likely to be a significant underestimation.270
Residents have been reported to have significant sleep problems, including long sleep onset latency (ie, time to fall asleep at night), long wake time after sleep onset (ie, amount of time awake after initially falling asleep), low sleep efficiency (total sleep time as a percentage of total time spent in bed), and a very high percentage of daytime napping.271
Structured daytime observations have shown in one study that nearly three-quarters of residents sleep excessively during the day. Compared to residents without this pattern, those with excessive daytime sleeping were observed more often in bed, were less likely to have any time outdoors, had less social and physical activity, and required greater mean level of assistance for personal care activities. Excessive daytime sleeping was accompanied by significantly reduced time asleep at night. Most residents also had evidence of nighttime sleep disturbance, although this was very rarely documented in their charts.272
Factors associated with sleep disturbances among nursing home residents
Studies using actigraphy in demented nursing home residents suggest an association between dementia and circadian rhythm disturbance, with more severely demented residents having more disturbed circadian rest-activity rhythms.180,273 Older people on a rehabilitation ward or nursing home have also described pain, discomfort and the need to go to the toilet as the most common causes of sleep disturbance.274
Nocturia, noise and/or light disruption, and pain have been reported by cognitively intact nursing home residents as the most common causes of subjective sleep disturbance. In addition, greater comorbidity and more depressive symptoms were both significant independent predictors of worse Pittsburgh Sleep Quality Index scores.275
Mild-to-moderate sleep apnea has been reported in one study in 32% of residents, with another 38% having evidence of severe sleep apnea. There was a strong relationship between sleep apnea and dementia in this sample.276 A later study in nursing home residents with dementia found that over 50% had evidence of severe sleep apnea.277
The evidence for a relationship between sleep and psychotropic medications in nursing home residents has been mixed. For example, one study found that sleep fragmentation (estimated by actigraphy) was not associated with sedative-hypnotic use while another found that residents on psychoactive medications had a dampening of the normal day/night variation in sleep and waking over 24-hours.278,279 Another study showed that use of psychotropic medications (eg, antipsychotics, sedative-hypnotics and/or antidepressant medications) was not associated with measures of daytime or nighttime sleep. However, residents on psychotropic medications did have less in-bed body movement at night, which may increase risk of skin breakdown.280
Institutional factors contribute to sleep disturbance in the nursing home setting. One study that used a bedside monitor in residents' rooms to measure noise and light levels found that half of nighttime awakenings were associated with noise and/or light.281
Many residents also have limited exposure to bright light, which is a key zeitgeber (“time-giver”) to time intrinsic biological rhythms to the external clock. Studies have shown that residents do not get much, if any, exposure to bright light, with nearly half of residents having no bright light exposure at all.170,273 Residents with higher light levels had fewer nighttime awakenings and a later rest-activity acrophase, a measure of circadian rhythm.170
Sleep disturbances in nursing home residents are associated with significant negative consequences. Sleep disturbance has been identified as a significant predictor of increased mortality among nursing home residents.282 Excessive daytime sleeping has also been associated with worse quality of life (less participation in social and physical activities, less social conversation) and more functional impairment (more nursing assistance for eating, drinking, bathing, dressing, grooming, and toileting).271
Although little evidence of a relationship between psychoactive medications and sleep has been found among nursing home residents, there is clinical concern for a potential relationship between sedating medications and the risk of falls. One study found that use of benzodiazepines was associated with increased risk of daytime and nighttime falls.283 Risk of daytime and nighttime falls was the same regardless of use of benzodiazepines with intermediate or long half-lives, although use of benzodiazepines with short half-lives were only predictive of falls during the night. Another large study suggested that it was insomnia, rather than the use of hypnotics, that was associated with greater risk of falls although the findings from this study remain controversial. 43,271
Interventional studies
Studies on sleep in nursing home residents have included interventions involving bright light therapy, exercise or physical activity, multicomponent nonpharmacologic programs, changes to nighttime nursing care, social and individualized activities, client-centered nursing care, medications, and discontinuation of antipsychotic medication.
Beneficial effects of morning bright light therapy have been reported.182,284-286 Demented nursing home residents receiving bright overhead lighting in the morning or all day long have been shown to have increased total sleep time at night, with the effect most pronounced in participants with severe dementia.287 Importantly, the most relevant dimensions of light therapy may be the timing, duration and intensity of the intervention.
The beneficial effects exercise and physical activity on sleep in nursing home residents has been demonstrated in a number of studies.280,288,289 Positive sleep effects have also been reported with use of a stationary bicycle and Tai Chi.290,291 The combination of daily social and physical activity among residents of a continuing care facility has also been shown to be associated with increased slow wave sleep (as assessed by PSG) and improvement in memory-oriented tasks.167
Efforts to improve the nighttime nursing home environment to make it more conducive to sleep have been difficult to implement. A nursing intervention that decreased nighttime noise and light disruption was shown to be associated with reduced nighttime arousals.191
Multicomponent nonpharmacologic interventions have also had mixed results. A study which combined efforts to increase daytime physical activity and sunlight exposure, decrease time in bed during the day, provide a bedtime routine and decrease nighttime noise and light levels found a decrease in duration of nighttime awakenings and daytime sleeping, and an increased participation in social activities, conversation, and physical activity during the day.192 A similar multicomponent nonpharmacologic intervention found no significant effects on nighttime sleep, but did show a modest but significant decrease in daytime sleeping.292
Few studies have tested sleep medications specifically in the nursing home setting. A randomized trial of temazepam, diphenhydramine or placebo in residents with sleep problems found that those who received diphenhydramine reported a shorter sleep latency than those given placebo, with some report of a longer duration of sleep compared with temazepam treatment, but those on medication performed more poorly on tests of neurologic function, and exhibited more daytime hypersomnolence.293 Another study in patients with Alzheimer's disease (including some participants in long term care facilities) comparing melatonin with placebo found no significant differences between groups in objective sleep measures (wrist actigraphy), and only an isolated finding of improved sleep quality with melatonin based on a caregiver rating.187
The American Medical Directors Association (AMDA) has developed a clinical practice guideline which offers a 16-step approach to managing sleep problems among nursing home residents. They divide their approach into 4 different categories: recognition, assessment, treatment and follow-up.294
Recommendations for future research
Future research should clarify the consequences of sleep disturbance among nursing home residents, particularly in terms of effects on quality of life.
The effectiveness of pharmacologic interventions to improve sleep among nursing home residents should be tested, with careful attention to the balance of potential risks and benefits in this vulnerable population.
More effective behavioral and other nonpharmacologic interventions must be identified that will help to improve disturbed sleep patterns among nursing home residents.
The relationship between insomnia, sedative-hypnotic medications and adverse events, including falls, among nursing home residents must be clarified.
Table 3. Insomnia.
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. The healthcare practitioner should periodically screen patients for symptoms of insomnia during health examinations. | III 81 | A |
| 2. An in-depth sleep history is essential in identifying the cause(s) and consequences of insomnia. Additionally, a physical examination is an important element in the evaluation of insomnia patients with medical symptoms. | III 81 | A |
| 3. CBT-I is an effective treatment for insomnia in the older adult. | I 36 | A |
| 4. Nonbenzodiazepines And melatonin receptor agonists are the safest and most efficacious hypnotic drugs currently available. |
II 36, 82 I 83 |
B B |
| 5. All FDA approved drugs for the treatment of insomnia can be associated with clinically significant adverse events. | III 79 | A |
| 6. Combining CBT-I and pharmacologic therapy can be helpful in some patients. | III 61,80 | A |
| 7. Antihistamines And antidepressants, anticonvulsants, and antipsychotics are associated with more risks than benefits in the treatment of insomnia, particularly in older persons. |
II 36,84 III 36,85 |
B B |
Table 4. Sleep Apnea.
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. OSA is very common in older people. All older people should be screened for the possibility of OSA by asking whether the 3 key features are present: daytime sleepiness, snoring, and observed apnea. | II 27,91,98,101,102,122,123 | A |
| 2. All older patients who are found to be at high risk of OSA should be assessed to document whether the condition is present. This will consist of either a referral to a sleep specialist, or by obtaining a PSG. | III 116 | A |
| 3. Older patients whose sleep apnea is associated with CHF or respiratory disease should be referred to a sleep specialist. | III | A |
| 4. CPAP is the most reliable treatment at this time for OSA (but not for CSA). Oral appliances may be appropriate for some patients with adequate dentition | I 111,112,118-120,124 | A |
| 5. All older patients prescribed CPAP should receive education on the rationale, methods of application, expectations, and follow-up. Bed partners should also be educated. This information improves adherence to treatment. | II 112,113,125 | B |
| 6. Since obstructive apnea is a chronic disease with associated chronic comorbidities, older patients should be followed up frequently, especially during the first 6 months following onset of treatment to assess response to treatment, and to monitor compliance. | III 113 | A |
| 7. Patients with OSA should take their CPAP equipment with them on trips, and to the hospital, particularly if they are expecting to undergo a surgical procedure. | III 110 | A |
| 8. A weight loss program should be part of the treatment plan in overweight patients with OSA. | II 114,115 | A |
| 9. The older sedative-hypnotics should not be prescribed for patients with confirmed OSA, and patients should be advised to avoid drinking alcohol within 2 hours of bedtime. | III 107,108,110 | A |
| 10. Patients with OSA require follow-up for their comorbidities, particularly patients with hypertension. Patients treated with CPAP may have a change in their requirements for antihypertensive medications. | III 99 | A |
Table 5. Restless Legs Syndrome and Periodic Limb Movement of sleep (RLS/PLMS).
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. Patients with sleep onset insomnia should be asked about uncomfortable leg sensations. | III 90,127,132 | A |
| 2. Patients with RLS should have a serum ferritin level checked. | III 137 | B |
| 3. Dopaminergic agents are the first-line treatment for RLS. | II 139 | B |
| 4. Patients who are treated with dopaminergic agents should be warned about the possibility of augmentation. | III 90,132 | A |
| 5. Periodic limb movements rarely need to be treated with medication in the absence of RLS symptoms. | III 144,146 | B |
Table 9. Parasomnias.
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. All older adults with a history of vigorous motor behaviors during sleep should be queried about prior episodes or the potential for injurious behavior associated with dream mentation. | III 90,198-200 | A |
| 2. PSG is indicated for the diagnosis of RBD. | III 90,198-200 | A |
| 3. In a patient with RBD and an abnormal physical examination, further neurologic evaluation is recommended. | III 201-204 | B |
| 4. Clonazepam has demonstrated efficacy and is indicated for the treatment of RBD. | III 198,208 | B |
| 5. Other medications, such as dopamine agonists may be indicated for the treatment of RBD. | II 207 | B |
Table 10. Hypersomnias.
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. A complaint of excessive sleepiness should be thoroughly evaluated by means of a detailed history and the appropriate use of subjective questionnaires. | III 16,221-224,230,231,244,245,247,264-267 | A |
| 2. An accurate diagnosis should be established in older patients with hypersomnia by means of a PSG and MSLT. | II 210,248-250 | B |
| 3. In patients with hypersomnia, management of any medical, neurologic, and psychiatric disorders should be optimized. | III 16,208 | A |
| 4. In patients with hypersomnia, medications or substances with sedating properties should be withdrawn when possible or the timing of the medications should be changed to minimize sedation during waking hours. | III 208,235,246 | A |
| 5. Behavioral modification of sleep-wake behavior is an effective treatment strategy that is useful for many patients with excessive sleepiness and should be advised. | II 208,229,246,259 | A |
| 6. Scheduled naps can be beneficial to relieve sleepiness with or without the use of pharmacologic agents. | II 259,268 | B |
| 7. Pharmacologic management should be considered for all patients who have a diagnosis of hypersomnia of central origin. | III 208,210,269 | A |
| 8. There is little evidence that sleepiness due to medications is improved with other medications that are used to counteract sleepiness. If possible, discontinue the offending medication or change the timing and/or dose. | III | A |
| 9. The following medications are effective treatments for narcolepsy with or without cataplexy: Modafinil: effective for excessive sleepiness due to narcolepsy. Sodium oxybate: may be effective for EDS due to narcolepsy in the older adult population And may be effective for cataplexy in the older adult. Methylphenidate and amphetamine derivatives: may be effective for excessive sleepiness due to narcolepsy in the older adult. Antidepressant medications: may be an effective treatment for cataplexy. |
I 210,251-253,269 I 257,261,269 I 257,260,261,269 II 208 II 208,210,262,263,269 |
B C B C B |
| 10. Modafinil, methylphenidate and amphetamine derivatives may be effective for the treatment of excessive sleepiness due to idiopathic hypersomnia or recurrent hypersomnia, as well as hypersomnia due to a medical condition. | II 208,229,253-256 | C |
| 11. Regular follow-up of patients with excessive sleepiness is necessary to monitor and ensure effective treatment. | III 210,230 | A |
| 12. Referral to a sleep specialist should be undertaken when narcolepsy or idiopathic hypersomnia is suspected or if the cause of the sleepiness is unknown | III | A |
Table 11. Long-term Care (LTC).
| Recommendations | Quality of Evidence (with references) | Strength of Evidence |
|---|---|---|
| 1. Given the high prevalence of sleep problems among nursing home residents, the clinician should consider sleep disturbance as a potential issue in every nursing home resident. | II 271,272 | A |
| 2. Excessive daytime sleeping is common among nursing home residents, and should be addressed. | II 272 | B |
| 3. Wrist actigraphy for the identification of sleeping problems in nursing home residents should be used if possible. Trained staff observations, resident self-report, or nursing staff report should be used if actigraphy is not available. | II 180,271-273,275,295 | B |
| 4. Clinicians should have a high index of suspicion for sleep disordered breathing among nursing home residents, particularly those with dementia. | II 275 | B |
| 5. Careful review of medical conditions and medications that may be causing or contributing to sleep disturbance is warranted in every nursing home resident with evidence of a sleep problem. | III | A |
| 6. Clinicians should encourage nursing home facilities to address nighttime environmental conditions and nursing care practices contributing to a nighttime environment that is not conducive to sleep. | II 281 | B |
| 7. Clinicians should encourage nursing home facilities to implement measures to decrease amount of time residents spend in bed during the daytime and increase daytime physical activity. | III 28 | B |
| 8. There is good evidence that daytime bright light exposure improves sleep-wake patterns among nursing home residents. Clinicians should encourage facilities to implement measures that increase bright light exposure via either commercially available light boxes or sunlight exposure. | I 182,285-288 | A |
| 9. There is essentially no evidence regarding the effectiveness of sedative-hypnotic medications in the nursing home population. Careful review of potential risks and benefits of these medications in nursing home residents is warranted, particularly concerns about risks of daytime sedation and falls. | III 284 | B |
| 10. Use of medications not FDA-approved for the treatment of insomnia (eg, sedating antidepressants and sedating antipsychotics) should not be used in nursing home residents, except in those for whom other indications for use of these agents are present (eg, depression, psychotic symptoms, agitation). | III | B |
| 11. There is essentially no evidence to support the use of melatonin for sleep disturbance among nursing home residents with dementia. | I 187 | B |
| 12. Any medication treatment for sleep disturbance among nursing home residents should be reviewed for effectiveness and adverse consequences, with frequent reevaluation to assess whether medication reduction or withdrawal is indicated. | III | A |
References
- 1.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: A marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 2.Vaz Fragoso CA, Gill TM. Sleep complaints in community-living older persons: A multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55:1853–1866. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DJ, Mallory LJ, Lichstein KL, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 4.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.The Gallup Organization; 2005. [July 21, 2008]. Gallup Poll: Sleep and Healthy Aging. (online). Available at: http://www.gallup.com/poll/20323/Older-Americans-Dream-Good-Nights-Sleep.aspx. [Google Scholar]
- 6.Gammack JK. Light therapy for insomnia in older adults. Clin Geriatr Med. 2008;24:139–149. doi: 10.1016/j.cger.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Laine C, Goldman D, Wilson JF. In the clinic: Insomnia. Ann Int Med. 2008;148(1):ITC13-1–ITC13-16. doi: 10.7326/0003-4819-148-1-200801010-01001. [DOI] [PubMed] [Google Scholar]
- 8.Folks DG. Emerging concepts in management of insomnia. J Am Geriatr Soc. 2005;53:S257–S257. [Google Scholar]
- 9.Krystal AD. The effect of insomnia definitions, terminology, and classifications on clinical practice. J Am Geriatr Soc. 2005;53:S258–S263. doi: 10.1111/j.1532-5415.2005.53391.x. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 11.McCall WV. Diagnosis and management of insomnia in older people. J Am Geriatr Soc. 2005;53:S272–S277. doi: 10.1111/j.1532-5415.2005.53393.x. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Chicago: AASM; 2007. [Google Scholar]
- 13.Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Browman KE, Monk TH, et al. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin JL, Ancoli-Israel S. Napping in older adults. Sleep Med Clin. 2006;1:177–186. [PubMed] [Google Scholar]
- 16.Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain and nocturia in older adults. Am J Ger Psych. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 17.Monk TH. Aging human circadian rhythms: Conventional wisdom may not always be right. J Biol Rhythm. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- 18.Vitiello MV. Sleep in normal aging. Sleep Med Clin. 2006;1:171–176. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: Clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–559. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 20.Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- 21.Stepanski EJ, Rybarczyk B. Emerging research on the treatment and etiology of secondary or co-morbid insomnia. Sleep Med Rev. 2006;10:7–18. doi: 10.1016/j.smrv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Harvey AG. Insomnia: Symptom or diagnosis? Clin Psychol Rev. 2001;21:1037–1059. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 23.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 24.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 26.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates Arch Gen Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 27.Ancoli-Israel S, Kripke DF, Klauber MR. Sleep disordered breathing in community dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: The MEMO study. Memory and Morbidity in Augsburg Elderly Neurology. 2000;54:1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 29.Kryger MH, Monjan A, Bliwise DL, et al. Sleep, health, and aging. Geriatrics. 2004;59:24–30. [PubMed] [Google Scholar]
- 30.Vitiello MV. Effective treatment of sleep disturbances in older adults. Clin Cornerstone. 2000;2:16–27. doi: 10.1016/s1098-3597(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 31.Zee PC, Turek FW. Sleep and health: Everywhere and in both directions. Arch Intern Med. 2006;166:1686–1688. doi: 10.1001/archinte.166.16.1686. [DOI] [PubMed] [Google Scholar]
- 32.Reid KJ, Marinovick Z, Finkel S, et al. Sleep: A marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 33.Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Institute of Medicine of the National Academy of Sciences. National Academies Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- 34.California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 35.Zee P, Bloom HG. Understanding and resolving insomnia in the elderly. Geriatrics. 2006;61(5) Special section1-12. [Google Scholar]
- 36.National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 37.Ganguli M, Reynolds CF, Gilby JE. Prevalence and persistence of sleep complaints in a rural older community sample: The MoVIES project. J Am Geriatr Soc. 1996;44:778–784. doi: 10.1111/j.1532-5415.1996.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 38.Barbar SI, Enright PL, Boyle P, et al. Sleep disturbances and their correlates in elderly Japanese American men residing in Hawaii. J Gerontol A Biol Sci Med Sci. 2000;55:M406–M411. doi: 10.1093/gerona/55.7.m406. [DOI] [PubMed] [Google Scholar]
- 39.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2008;29:1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S. Insomnia in the elderly: A review for the primary care practitioner. Sleep. 2000;23(suppl 1):S23–S30. [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–S353. [PubMed] [Google Scholar]
- 42.Stone KL, Ewing SK, Lui LY, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: The study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1177–1183. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 43.Avidan AY, Fries BE, James MC, et al. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53:955–962. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 44.Brassington GS, King AC, Bliwise DL. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64-99 years. J Am Geriatr Soc. 2000;48:1234–1240. doi: 10.1111/j.1532-5415.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 45.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 46.Stone KL, Ancoli-Israel S, Blackwell T, et al. Poor sleep is associated with increased risk of falls in older women. Arch Intern Med. 2007 doi: 10.1001/archinte.168.16.1768. in press. [DOI] [PubMed] [Google Scholar]
- 47.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–492. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 48.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 49.Buysse DJ. Insomnia, depression and aging: Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59:47–51. [PubMed] [Google Scholar]
- 50.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 51.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 52.Buysse DJ, Reynolds CF, Kupfer DJ, et al. Clinical diagnoses in 216 insomnia patients using the international classification of sleep disorders (ICSD), DSM-IV and ICD-10 categories: A report from the APA/NIMH DSM-IV field trial. Sleep. 1994;17:630–637. doi: 10.1093/sleep/17.7.630. [DOI] [PubMed] [Google Scholar]
- 53.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behavior Sleep Med. 2006;4:104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 54.Wilcox S, Brenes GA, Levine D, et al. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 55.Sridhar GR, Madhu K. Prevalence of sleep disturbance in diabetes mellitus. Diabetes Res Clin Pract. 1994;23:183–186. doi: 10.1016/0168-8227(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 56.Ancoli-Israel S, Moore P, Jones V. The relationship between fatigue and sleep in cancer patients: A review. European Journal of Cancer Care. 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klink ME, Quan SF, Kaltenborn WT, et al. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med. 1992;152:1634–1637. [PubMed] [Google Scholar]
- 58.Quan SF, Katz R, Olson J, et al. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: The cardiovascular health study. Am J Med Sci. 2005;329:163–172. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 59.García-Borreguero D, Larrosa O, Bravo M. Parkinson's disease and sleep. Sleep Med Rev. 2003;7:115–129. doi: 10.1053/smrv.2002.0229. [DOI] [PubMed] [Google Scholar]
- 60.Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 12th. Ohio: Lexi-Comp; 2007. [Google Scholar]
- 61.Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 62.Morin CM, Colecchi C, Stone J, et al. Behavioral and pharmacological therapies for late life insomnia. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 63.Bootzin RR, Nicassio PM. Behavioral treatments for insomnia. In: Hersen M, Eisler RM, Miller PM, editors. Progress in Behavior Modification. Vol. 6. New York: Academic Press Inc.; 1978. pp. 1–45. [Google Scholar]
- 64.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 65.McCurry SM, Logsdon RG, Terri L, et al. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22(1):18–27. doi: 10.1037/0882-7974.22.1.18. [DOI] [PubMed] [Google Scholar]
- 66.Joshi S. Non-pharmacological therapy for insomnia in the elderly. Clinics Geriatr Med. 2008;24:107–119. doi: 10.1016/j.cger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Riedel BW, Lichstein KL, Dwyer WD. Sleep compression and sleep education for older insomniacs: Self-help versus therapist guidance. Psychol Aging. 1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 68.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13:17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 69.Lichstein KL, Riedel BW, Wilson NM, et al. Relaxation and sleep compression for late-life insomnia: A placebo-controlled trial. J Consult Clin Psychol. 2001;69:227–239. doi: 10.1037//0022-006x.69.2.227. [DOI] [PubMed] [Google Scholar]
- 70.Manber R, Kuo TF. Cognitive-behavioral therapies for insomnia. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 177–185. [Google Scholar]
- 71.Morin CM, Bootzin PR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 72.Irwin M, Cole JC, Nicassio PM. Comparative metaanalysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 73.Pallesen S, Nordhus IH, Kvale G. Nonpharmocological interventions for insomnia in older adults: A meta-analysis of treatment efficacy. Psychother Theory Res Pract Train. 1998;35:472–482. [Google Scholar]
- 74.Chen ML, Lin LC, Wu SC, et al. The effectiveness of acupressure in improving the quality of sleep in institutionalized residents. J Gerontol A Biol Sci Med Sci. 1998;54:M389–M394. doi: 10.1093/gerona/54.8.m389. [DOI] [PubMed] [Google Scholar]
- 75.King AC, Oman RF, Brassington GS, et al. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 76.Li F, Fisher KJ, Harmer P, et al. Tai Chi and self-rated quality of sleep and daytime sleepiness in older adults: A randomized controlled trial. J Am Ger Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 77.Tsay S, Rong J, Lin P. Acupoints massage in improving the quality of sleep and quality of life in patients with end-stage renal disease. J Adv Nurs. 2003;42:134–142. doi: 10.1046/j.1365-2648.2003.02596.x. [DOI] [PubMed] [Google Scholar]
- 78.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149–1157. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 79.Glass J, Lanctot KL, Herrmann N, et al. Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. BMJ. 2005;331:1169–1176. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendelson WB. Combining pharmacologic and nonpharmacologic therapies for insomnia. J Clin Psychiatry. 2007;68 5:19–23. [PubMed] [Google Scholar]
- 81.Chesson A, Jr, Hartse K, Anderson WM, et al. Practice Parameters for the Evaluation of Chronic Insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2000;23:237–241. [PubMed] [Google Scholar]
- 82.Cotroneo A, Gareri P, Nicoletti N, et al. Effectiveness and safety of hypnotic drugs in the treatment of insomnia in over 70-year old people. Arch Gerontol Geriatr Suppl. 2007;1:121–124. doi: 10.1016/j.archger.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–318. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Agostini JV, Leo-Summers LS, Inouye SK. Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161:2091–2097. doi: 10.1001/archinte.161.17.2091. [DOI] [PubMed] [Google Scholar]
- 85.Cooke JR, Ancoli-Israel S. Sleep and its disorders in older adults. Psychiatr Clin North Am. 2006;29:1077–1093. doi: 10.1016/j.psc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Phillips B, Kryger MH. Management of obstructive sleep apnea hypopnea syndrome: Overview. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th. Philadelphia: Elsevier; 2005. pp. 1109–1121. [Google Scholar]
- 87.Banno K, Kryger MH. Sleep apnea: Clinical investigations in humans. Sleep Med. 2007;8:400–426. doi: 10.1016/j.sleep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Somers V, Javaheri S. Cardiovascular effects of sleep-related breathing disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th. Philadelphia: Elsevier; 2005. pp. 1180–1191. [Google Scholar]
- 89.White DP. Central sleep apnea. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th. Philadelphia: Elsevier; 2005. pp. 969–982. [Google Scholar]
- 90.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2nd. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 91.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 92.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: Effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 93.Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J Obstet Gynecol. 2006;194:1399–1403. doi: 10.1016/j.ajog.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 94.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;22:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 95.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–1364. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buchner NJ, Sanner BM, Borgel J, et al. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 97.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 98.Launois SH, Pepin JL, Levy P. OSA in the elderly: A specific entity? Sleep Med Rev. 2007;11:83–85. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 100.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea: Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 101.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 102.Tishler PV, Larkin EK, Schluchter MD, et al. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 103.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1999;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 104.Umlauf MG, Chasens ER, Greevy RA, et al. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–144. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 105.Lam B, Ip MS, Tench E, et al. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–510. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitler MM, Dawson A, Henriksen SJ, et al. Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res. 1988;12:801–805. doi: 10.1111/j.1530-0277.1988.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scanlan MF, Roebuck T, Little PJ, et al. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16:909–913. doi: 10.1183/09031936.00.16590900. [DOI] [PubMed] [Google Scholar]
- 108.Guilleminault C. Benzodiazepines, breathing, and sleep. Am J Med. 1990;88:25S–28S. doi: 10.1016/0002-9343(90)90282-i. [DOI] [PubMed] [Google Scholar]
- 109.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: Physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–371. [PubMed] [Google Scholar]
- 110.Heinzer R. Obstructive sleep apnea: A risk for general anesthesia? Rev Med Suisse. 2007;3:2670–2674. [PubMed] [Google Scholar]
- 111.Collop N. The effect of obstructive sleep apnea on chronic medical disorders. Cleve Clin J Med. 2007;74:72–78. doi: 10.3949/ccjm.74.1.72. Erratum in. [DOI] [PubMed] [Google Scholar]; Cleve Clin J Med. 2007;74:576. doi: 10.3949/ccjm.74.8.572. [DOI] [PubMed] [Google Scholar]
- 112.Gay P, Weaver T, Loube D, et al. Positive Airway Pressure Task Force; Standards of Practice Committee; American Academy of Sleep Medicine. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 113.Kushida CA, Littner MR, Hirshkowitz M, et al. American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 114.Pedro-Botet Pons J, Roca Montanari A. Efficacy of weight loss in the treatment of obstructive sleep apnea syndrome. Experience in 135 patients. Med Clin (Barc) 1992;98:45–48. [PubMed] [Google Scholar]
- 115.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med. 2001;2:511–516. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 116.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 117.Ancoli-Israel Sleep apnea in older adults – is it real and should age be the determining factor in the treatment decision matrix? Sleep Med Rev. 2007;11:83–85. doi: 10.1016/j.smrv.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasayama S, Izumi T, Seino Y, et al. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J. 2006;70:1–7. doi: 10.1253/circj.70.1. [DOI] [PubMed] [Google Scholar]
- 119.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 120.Morgenthaler TI, Gay PC, Gordon N, et al. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–475. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 121.Ayalon L, Ancoli-Israel S, Stepnowsky C, et al. Adherence to continuous positive airway pressure treatment in patients with Alzheimer's disease and obstructive sleep apnea. Am J Geriatr Psychiatry. 2006;14:176–180. doi: 10.1097/01.JGP.0000192484.12684.cd. [DOI] [PubMed] [Google Scholar]
- 122.Kezirian EJ, Harrison SL, Ancoli-Israel S, et al. Behavioral correlates of sleep-disordered breathing in older women. Sleep. 2007;30:1181–1188. doi: 10.1093/sleep/30.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–1738. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 124.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–342. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Russo-Magno P, O'Brien A, Panciera T, et al. Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc. 2001;49:1205–1211. doi: 10.1046/j.1532-5415.2001.49238.x. [DOI] [PubMed] [Google Scholar]
- 126.National Heart, Lung, and Blood Institute. Restless legs syndrome; detection and management in primary care NHLBI-NIH;March 2000. [July 27, 2008]; NIH Publication No. 00-3788. Also available at: http://www.nhlbi.nih.gov/guidelines/archives/rls_gde/rls_gde_archive.pdf. [PubMed]
- 127.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 128.Winkelmann J, Wetter TC, Collado-Seidel V, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23:597–602. [PubMed] [Google Scholar]
- 129.Lesage S, Hening WA. The restless legs syndrome and periodic limb movement disorder: a review of management. Semin Neurol. 2004;24:249–259. doi: 10.1055/s-2004-835066. [DOI] [PubMed] [Google Scholar]
- 130.Berger K, Luedemann J, Trenkwalder C, et al. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 131.Phillips BA, Young T, Finn L, et al. Epidemiology of restless legs syndrome in adults. Arch Int Med. 2000;160:2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 132.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 133.Tan EK, Seah A, See SJ, et al. Restless legs syndrome in an Asian population: A study in Singapore. Mov Disord. 2001;16:577–579. doi: 10.1002/mds.1102. [DOI] [PubMed] [Google Scholar]
- 134.Lee HB, Hening WA, Allen RP, et al. Race and restless legs syndrome symptoms in an adult community sample in east Baltimore. Sleep Med. 2007;7:642–645. doi: 10.1016/j.sleep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 135.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 136.Montplaisir J, Allen RP, Walter AS, et al. Restless Legs Syndrome and Periodic Limb Movements during Sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th. Philadelphia: Elsevier Saunders; 2005. pp. 839–852. [Google Scholar]
- 137.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 138.Hening WA, Allen PR, Tenzer P, et al. Restless legs syndrome: Demographics, presentations and differential diagnosis. Geriatrics. 2007;62:26–29. [PubMed] [Google Scholar]
- 139.Littner MR, Kushida C, Anderson WM, Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:557–559. doi: 10.1093/sleep/27.3.557. [DOI] [PubMed] [Google Scholar]
- 140.García-Borreguero D, Larrosa O, de la Llave Y, et al. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med. 2004;5:561–565. doi: 10.1016/j.sleep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 141.García-Borreguero D, Allen RP, Kohnen R, et al. International Restless Legs Syndrome Study Group. Diagnostic standards for dopaminergic augmentation of restless legs syndrome: report from a World Association of Sleep Medicine-International Restless Legs Syndrome Study Group consensus conference at the Max Planck Institute. Sleep Med. 2007;5:520–530. doi: 10.1016/j.sleep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 142.Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: A study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 143.Claman DM, Redline S, Blackwell T, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2:438–445. [PubMed] [Google Scholar]
- 144.Exar EN, Collop NA. The association of upper airway resistance with periodic limb movements. Sleep. 2001;24:188–192. doi: 10.1093/sleep/24.2.188. [DOI] [PubMed] [Google Scholar]
- 145.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 146.Chervin R. Periodic leg movements and sleepiness in patients evaluated for sleep-disordered breathing. Am J Respir Crit Care Med. 2001;164:1454–1458. doi: 10.1164/ajrccm.164.8.2011062. [DOI] [PubMed] [Google Scholar]
- 147.Dement WC, Miles LE, Carskadon MA. “White paper” on sleep and aging. J Am Geriatr Soc. 1982;30:25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 148.Middelkoop HA, Smilde-van den Doel DA, Neven AK, et al. Subjective sleep characteristics of 1,485 males and females aged 50-93: Effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol Ser A Biol SciMed Sci. 1996;51:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 149.Prinz PN. Sleep and sleep disorders in older adults. J Clin Neurophysiol. 1995;12:139–146. doi: 10.1097/00004691-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 150.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 151.van Coevorden A, Mockel J, Laurent E, et al. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–E661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- 152.Lewy AJ, Ahmed S, Jackson JM, et al. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 153.Hoogendijk WJ, van Someren EJ, Mirmiran M, et al. Circadian rhythm-related behavioral disturbances and structural hypothalamic changes in Alzheimer's disease. Int Psychogeriatr. 1996;8 3:245–252. doi: 10.1017/s1041610297003426. [DOI] [PubMed] [Google Scholar]
- 154.Dijk DJ, Duffy JF, Riel E, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thorpy MJ. Diagnostic Classification Steering Committee. Rochester, Minnesota: American Sleep Disorders Association; 2001. International classification of sleep disorders: Diagnostic and coding manual. Chairman. [Google Scholar]
- 156.Reid KJ, Chang AM, Turek FW, et al. Identification and characterization of familial advanced sleep phase. Sleep Research Online. 1999;2(Suppl 1):424. [Google Scholar]
- 157.Ando K, Kripke DF, Ancoli-Israel S. Estimated prevalence of delayed and advanced sleep phase syndromes. Sleep Res. 1995;24:509. [Google Scholar]
- 158.Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci. 2002;39:11–18. [PubMed] [Google Scholar]
- 159.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 160.Moldofsky H, Musisi S, Phillipson EA. Treatment of a case of advanced sleep phase syndrome by phase advance chronotherapy. Sleep. 1986;9:61–65. doi: 10.1093/sleep/9.1.61. [DOI] [PubMed] [Google Scholar]
- 161.Reid KJ, Chang AM, Dubocovich ML, et al. Familial advanced sleep phase syndrome. Arch Neurol. 2001;58:1089–1094. doi: 10.1001/archneur.58.7.1089. [DOI] [PubMed] [Google Scholar]
- 162.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 163.Satoh K, Mishima K, Inoue Y, et al. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep. 2003;26:416–417. doi: 10.1093/sleep/26.4.416. [DOI] [PubMed] [Google Scholar]
- 164.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 165.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 166.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback and self-regulation. 1991;16:349–359. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 167.Naylor E, Penev PD, Orbeta L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- 168.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 169.Lack L, Wright H, Kemp K, et al. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 170.Shochat T, Martin J, Marler M, et al. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 171.Herljevic M, Middleton B, Thapan K, et al. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 172.Terman M, Terman JS. Bright light therapy: Side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999;60:799–808. [PubMed] [Google Scholar]
- 173.Chesson AL, Jr, Littner M, Davila D, et al. Practice parameters for the use of light therapy in the treatment of sleep disorders. Standards of Practice Committee, American Academy of Sleep Medicine. Sleep. 1999;22:641–660. doi: 10.1093/sleep/22.5.641. [DOI] [PubMed] [Google Scholar]
- 174.Wagner DR. Disorders of the circadian sleep-wake cycle. Neurol Clin. 1996;14:651–670. doi: 10.1016/s0733-8619(05)70278-4. [DOI] [PubMed] [Google Scholar]
- 175.Wagner DR. Circadian rhythm sleep disorders. Curr Treat Options Neurol. 1999;1:299–308. doi: 10.1007/s11940-999-0020-x. [DOI] [PubMed] [Google Scholar]
- 176.Witting W, Kwa IH, Eikelenboom P, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 177.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 178.Swaab DF. Ageing of the human hypothalamus. Horm Res. 1995;43:8–11. doi: 10.1159/000184230. [DOI] [PubMed] [Google Scholar]
- 179.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 180.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 181.Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer's disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 182.Ancoli-Israel S, Martin JL, Kripke DF, et al. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Benloucif S, Orbeta L, Ortiz R, et al. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27:1542–1551. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- 184.Niggemyer KA, Begley A, Monk T, et al. Circadian and homeostatic modulation of sleep in older adults during a 90-minute day study. Sleep. 2004;27:1535–1541. doi: 10.1093/sleep/27.8.1535. [DOI] [PubMed] [Google Scholar]
- 185.Vitiello MV, Prinz PN, Schwartz RS. Slow wave sleep but not overall sleep quality of healthy older men and women is improved by increased aerobic fitness. Sleep Res. 1994;23:149. [Google Scholar]
- 186.Mishima K, Okawa M, Hishikawa Y, et al. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89:1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 187.Singer C, Tractenberg RE, Kaye J, et al. Alzheimer's Disease Cooperative Study. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 189.Gehrman PR, Connor DJ, Martin JL, et al. Melatonin fails to improve sleep or agitation in a double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer's disease. Am J Ger Psychiatry. 2008 doi: 10.1097/JGP.0b013e318187de18. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pandi-Perumal SR, Zisapel N, Srinivasan V, et al. Melatonin and sleep in aging population. Exp Gerontol. 2005;40:911–925. doi: 10.1016/j.exger.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 191.Schnelle JF, Alessi CA, Al-Samarrai NR, et al. The nursing home at night: Effects of an intervention on noise, light, and sleep. J Am Geriatr Soc. 1999;47:430–438. doi: 10.1111/j.1532-5415.1999.tb07235.x. [DOI] [PubMed] [Google Scholar]
- 192.Alessi CA, Martin JL, Webber AP, et al. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53:803–810. doi: 10.1111/j.1532-5415.2005.53251.x. [DOI] [PubMed] [Google Scholar]
- 193.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sack RL, Auckley D, Auger RR, et al. American Academy of Sleep Medicine. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schnelle JF, Cruise PA, Alessi CA, et al. Sleep hygiene in physically dependent nursing home residents: behavioral and environmental intervention implications. Sleep. 1998;21:515–523. [PubMed] [Google Scholar]
- 196.Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: Clinical features of sleep-wake rhythm disorders. Psychiatry Clin Neurosci. 1996;50:195–201. doi: 10.1111/j.1440-1819.1996.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 197.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behavior disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 198.Schenck CH, Bundlie SR, Patterson AL, et al. Rapid eye movement sleep behavior disorder: A treatable parasomnia affecting older adults. JAMA. 1987;257:1786–1789. [PubMed] [Google Scholar]
- 199.Schenck CH, Mahowald MW. REM sleep parasomnias. Neurol Clin. 1996;14:697–720. doi: 10.1016/s0733-8619(05)70281-4. [DOI] [PubMed] [Google Scholar]
- 200.Mahowald MW, Schenck CH. REM Sleep Behavior Disorder. Philadelphia: WB Saunders; 1994. pp. 574–588. [Google Scholar]
- 201.Boeve BF, Silber MH, Ferman T. REM sleep behavior disorder and degenerative dementia: An association likely reflecting Lewy body disease. Neurology. 1998;51:363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- 202.Ferini-Strambi L, Zucconi M. REM sleep behavior disorder. Clin Neurophysiol. 2000;111(Suppl 3):s136–s140. doi: 10.1016/s1388-2457(00)00414-4. [DOI] [PubMed] [Google Scholar]
- 203.Plazzi G, Corsinin R, Provini F. REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48:1094–1097. doi: 10.1212/wnl.48.4.1094. [DOI] [PubMed] [Google Scholar]
- 204.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 205.Schmidt MH, Koshal VB, Schmidt HS. Use of pramipexole in REM sleep behavior disorders: Results from a case series. Sleep Med. 2006;7:418–423. doi: 10.1016/j.sleep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 206.Boeve BF, Silber MH, Ferman J. Melatonin for treatment of REM sleep disorder in neurological disorders: Results in 14 patients. Sleep Med. 2003;4:281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 207.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–269. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 208.Wolkove N, Elkholy O, Baltzan M, et al. Sleep and aging: 2. Management of sleep disorders in older people. CMAJ. 2007;176:1449–1454. doi: 10.1503/cmaj.070335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ozturk L, Bulut E. Unexpectedly high prevalence of narcolepsy in Sivas. Tuberk Toraks. 2006;54:299–300. [PubMed] [Google Scholar]
- 210.Chakravorty SS, Rye DB. Narcolepsy in the older adult: epidemiology, diagnosis and management. Drugs Aging. 2003;20:361–376. doi: 10.2165/00002512-200320050-00005. [DOI] [PubMed] [Google Scholar]
- 211.Billiard M, Dauvilliers Y. Idiopathic hypersomnia. Sleep Med Rev. 2001;5:349–358. doi: 10.1053/smrv.2001.0168. [DOI] [PubMed] [Google Scholar]
- 212.Anderson KN, Pilsworth S, Sharples LD, et al. Idiopathic hypersomnia: A study of 77 cases. Sleep. 2007;30:1274–1281. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bassetti C, Aldrich MS. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120:1423–1435. doi: 10.1093/brain/120.8.1423. [DOI] [PubMed] [Google Scholar]
- 214.Billiard M, Merle C, Carlander B, et al. Idiopathic hypersomnia. Psychiatry Clin Neurosci. 1998;52:125–129. doi: 10.1111/j.1440-1819.1998.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 215.Naumann A, Bellebaum C, Daum I. Cognitive deficits in narcolepsy. J Sleep Res. 2006;15:329–338. doi: 10.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 216.Bartel P, Offermeier W, Smith F, et al. Attention and working memory in resident anaesthetists after night duty: group and individual effects. Occup Environ Med. 2004;61:167–170. doi: 10.1136/oem.2002.006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 218.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13:395–406. doi: 10.1111/j.1365-2869.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 219.Heuer H, Spikers W, Kiesswetter E, et al. Effects of sleep loss, time of day, and extended mental work on implicit and explicit learning of sequences. J Exp Psychol Appl. 1998;4:139–162. doi: 10.1037//1076-898x.4.2.139. [DOI] [PubMed] [Google Scholar]
- 220.Babkoff H, Sing HC, Thorne DR, et al. Perceptual distortions and hallucinations reported during the course of sleep deprivation. Percept Mot Skills. 1989;68:787–798. doi: 10.2466/pms.1989.68.3.787. [DOI] [PubMed] [Google Scholar]
- 221.Furuta H, Thorpy MJ, Temple HM. Comparison in symptoms between aged and younger patients with narcolepsy. Psychiatry Clin Neurosci. 2001;55:241–242. doi: 10.1046/j.1440-1819.2001.00841.x. [DOI] [PubMed] [Google Scholar]
- 222.Ohayon MM, Ferini-Strambi L, Plazzi G, et al. How age influences the expression of narcolepsy. J Psychosom Res. 2005;59:399–405. doi: 10.1016/j.jpsychores.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 223.Laberge L, Gagnon C, Jean S, et al. Fatigue and daytime sleepiness rating scales in myotonic dystrophy: A study of reliability. J Neurol Neurosurg Psychiatry. 2005;76:1403–1405. doi: 10.1136/jnnp.2004.043455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Piani A, Brotini S, Dolso P, et al. Sleep disturbances in elderly: A subjective evaluation over 65. Arch Gerontol Geriatr. 2004;(Suppl):325–331. doi: 10.1016/j.archger.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 225.Meindorfner C, Korner Y, Moller JC, et al. Driving in Parkinson's disease: Mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005;20:832–842. doi: 10.1002/mds.20412. [DOI] [PubMed] [Google Scholar]
- 226.Ciafaloni E, Mignot E, Sansone V, et al. The hypocretin neurotransmission system in myotonic dystrophy type 1. Neurology. 2008;70:226–230. doi: 10.1212/01.wnl.0000296827.20167.98. [DOI] [PubMed] [Google Scholar]
- 227.Wagner MH, Berry RB. An obese female with Prader-Willi syndrome and daytime sleepiness. J Clin Sleep Med. 2007;3:645–647. [PMC free article] [PubMed] [Google Scholar]
- 228.Oyama K, Takahashi T, Shoji Y. Niemann-Pick disease type C: Cataplexy and hypocretin in cerebrospinal fluid. Tohoku J Exp Med. 2006;209:263–267. doi: 10.1620/tjem.209.263. [DOI] [PubMed] [Google Scholar]
- 229.Happe S. Excessive daytime sleepiness and sleep disturbances in patients with neurological diseases: Epidemiology and management. Drugs. 2003;63:2725–2737. doi: 10.2165/00003495-200363240-00003. [DOI] [PubMed] [Google Scholar]
- 230.Hobson DE, Lang AE, Martin WR, et al. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: A survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 231.Hagell P, Broman JE. Measurement properties and hierarchical item structure of the Epworth Sleepiness Scale in Parkinson's disease. J Sleep Res. 2007;16:102–109. doi: 10.1111/j.1365-2869.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 232.Castriotta RJ, Lai JM. Sleep disorders associated with traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1403–1406. doi: 10.1053/apmr.2001.26081. [DOI] [PubMed] [Google Scholar]
- 233.Baumann CR, Werth E, Stocker R, et al. Sleep-wake disturbances 6 months after traumatic brain injury: A prospective study. Brain. 2007;130:1873–1883. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 234.Bjornstad B, Goodman SH, Sirven JI, et al. Paroxysmal sleep as a presenting symptom of bilateral paramedian thalamic infarctions. Mayo Clin Proc. 2003;78:347–349. doi: 10.4065/78.3.347. [DOI] [PubMed] [Google Scholar]
- 235.Schlesinger I, Ravin PD. Dopamine agonists induce episodes of irresistible daytime sleepiness. Eur Neurol. 2003;49:30–33. doi: 10.1159/000067022. [DOI] [PubMed] [Google Scholar]
- 236.Picchioni D, Hope CR, Harsh JR. A case-control study of the environmental risk factors for narcolepsy. Neuroepidemiology. 2007;29:185–192. doi: 10.1159/000111581. [DOI] [PubMed] [Google Scholar]
- 237.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8:733–741. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 238.Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99:S750–S756. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- 239.Vignatelli L, D'Alessandro R, Mosconi P, et al. Health-related quality of life in Italian patients with narcolepsy: The SF-36 health survey. Sleep Med. 2004;5:467–475. doi: 10.1016/j.sleep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 240.Dixon JB, Dixon ME, Anderson ML, et al. Daytime sleepiness in the obese: Not as simple as obstructive sleep apnea. Obesity (Silver Spring) 2007;15:2504–2511. doi: 10.1038/oby.2007.297. [DOI] [PubMed] [Google Scholar]
- 241.Bixler EO, Vgontzas AN, Lin HM, et al. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 242.Broughton WA, Broughton RJ. Psychosocial impact of narcolepsy. Sleep. 1994;17(8 Suppl):S45–S49. doi: 10.1093/sleep/17.suppl_8.s45. [DOI] [PubMed] [Google Scholar]
- 243.Kotterba S, Mueller N, Leidag M, et al. Comparison of driving simulator performance and neuropsychological testing in narcolepsy. Clin Neurol Neurosurg. 2004;106:275–279. doi: 10.1016/j.clineuro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 244.Marinus J, Visser M, van Hilten JJ, et al. Assessment of sleep and sleepiness in Parkinson disease. Sleep. 2003;26:1049–1054. doi: 10.1093/sleep/26.8.1049. [DOI] [PubMed] [Google Scholar]
- 245.Hoekert M, der Lek RF, Swaab DF, et al. Comparison between informant-observed and actigraphic assessments of sleep-wake rhythm disturbances in demented residents of homes for the elderly. Am J Geriatr Psychiatry. 2006;14:104–111. doi: 10.1097/01.JGP.0000192481.27931.c5. [DOI] [PubMed] [Google Scholar]
- 246.Koch S, Haesler E, Tiziani A. Effectiveness of sleep management strategies for residents of aged care facilities: Findings of a systematic review. J Clin Nurs. 2006;15:1267–1275. doi: 10.1111/j.1365-2702.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 247.Kumru H, Santamaria J, Belcher R. Variability in the Epworth sleepiness scale score between the patient and the partner. Sleep Med. 2004;5:369–371. doi: 10.1016/j.sleep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 248.Dauvilliers Y, Gosselin A, Paquet J. Effect of age on MSLT results in patients with narcolepsy-cataplexy. Neurology. 2004;62:46–50. doi: 10.1212/01.wnl.0000101725.34089.1e. [DOI] [PubMed] [Google Scholar]
- 249.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129:1609–1623. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 250.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: Failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 251.Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord. 2003;18:287–293. doi: 10.1002/mds.10390. [DOI] [PubMed] [Google Scholar]
- 252.Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson's disease: A double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep. 2002;25:905–909. [PubMed] [Google Scholar]
- 253.Ondo WG, Fayle R, Atassi F, et al. Modafinil for daytime somnolence in Parkinson's disease: Double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry. 2005;76:1636–1639. doi: 10.1136/jnnp.2005.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nieves AV, Lang AE. Treatment of excessive daytime sleepiness in patients with Parkinson's disease with modafinil. Clin Neuropharmacol. 2002;25:111–114. doi: 10.1097/00002826-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 255.Rammohan KW, Rosenberg JH, Lynn DJ, et al. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: A two centre phase 2 study. J Neurol Neurosurg Psychiatry. 2002;72:179–183. doi: 10.1136/jnnp.72.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Al-Adawi S, Burke DT, Dorvlo AS. The effect of methylphenidate on the sleep-wake cycle of brain-injured patients undergoing rehabilitation. Sleep Med. 2006;7:287–291. doi: 10.1016/j.sleep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 257.U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–35. [PubMed] [Google Scholar]
- 258.Monk TH, Buysse DJ, Carrier J, et al. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 259.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnia of central origin: An AASM report. Sleep. 2007;30:1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6:415–421. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 261.Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–397. [PubMed] [Google Scholar]
- 262.Sonka K, Kemlink D, Pretl M. Cataplexy treated with escitalopram--Clinical experience. Neuro Endocrinol Lett. 2006;27:174–176. [PubMed] [Google Scholar]
- 263.Vignatelli L, D'Alessandro R, Candelise L. Antidepressant drugs for narcolepsy. Cochrane Database Syst Rev. 2005:CD003724. doi: 10.1002/14651858.CD003724.pub2. [DOI] [PubMed] [Google Scholar]
- 264.Baldwin CM, Kapur VK, Holberg CJ, et al. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004;27:305–311. doi: 10.1093/sleep/27.2.305. [DOI] [PubMed] [Google Scholar]
- 265.Mazza M, Della Marca G, De Risio S, et al. Sleep disorders in the elderly. Clin Ter. 2004;155:391–394. [PubMed] [Google Scholar]
- 266.Morrish E, King MA, Smith IE, et al. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5:37–41. doi: 10.1016/j.sleep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 267.Wolkove N, Elkholy O, Baltzan M, et al. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep. 2001;24:385–391. doi: 10.1093/sleep/24.4.385. [DOI] [PubMed] [Google Scholar]
- 269.Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 270.Martin JL, Alessi CA. Limited validity of minimum data set items on sleep and hypnotic use in predicting falls and hip fracture in nursing home residents. J Amer Geriatr Soc. 2005;53:955–962. doi: 10.1111/j.1532-5415.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 271.Fetveit A, Bjorvatn B. Sleep disturbances among nursing home residents. Internat J Geriatr Psychiatry. 2002;17:604–609. doi: 10.1002/gps.639. [DOI] [PubMed] [Google Scholar]
- 272.Martin JL, Webber AP, Alam T, et al. Daytime sleeping, sleep disturbance and circadian rhythms in the nursing home. Am J Geriatr Psychiatry. 2006;14:121–129. doi: 10.1097/01.JGP.0000192483.35555.a3. [DOI] [PubMed] [Google Scholar]
- 273.Ancoli-Israel S, Clopton P, Klauber MR, et al. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ersser S, Wiles A, Taylor H, et al. The sleep of older people in hospital and nursing homes. J Clin Nursing. 1999;8:360–368. doi: 10.1046/j.1365-2702.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 275.Gentili A, Weiner DK, Kuchibhatil M, et al. Factors that disturb sleep in nursing home residents. Aging Clin Exper Research. 1997;9:207–213. doi: 10.1007/BF03340151. [DOI] [PubMed] [Google Scholar]
- 276.Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: Relation to sleep apnea. J Amer Geriatr Soc. 1991;39:258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 277.Gehrman PR, Martin JL, Shochat T, et al. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–433. [PubMed] [Google Scholar]
- 278.Ancoli-Israel S, Parker L, Sinaee R, et al. Sleep fragmentation in patients from a nursing home. J Gerontology. 1989;44:M18–M21. doi: 10.1093/geronj/44.1.m18. [DOI] [PubMed] [Google Scholar]
- 279.Bliwise DL, Bevier WC, Bliwise NG, et al. Systemic 24-hour behavior observations of sleep and wakefulness in a skilled-care nursing facility. Psychol Aging. 1990;15:16–24. doi: 10.1037//0882-7974.5.1.16. [DOI] [PubMed] [Google Scholar]
- 280.Alessi CA, Schnelle JF, MacRae PG. Does physical activity improve sleep in impaired nursing home residents? J Amer Geriatr Soc. 1995;43:1098–1102. doi: 10.1111/j.1532-5415.1995.tb07007.x. [DOI] [PubMed] [Google Scholar]
- 281.Schnelle JF, Ouslander JG, Simmons SF, et al. The nighttime environment, incontinence care, and sleep disruption in nursing homes. J Am Geriatr Soc. 1993;1:910–914. doi: 10.1111/j.1532-5415.1993.tb06754.x. [DOI] [PubMed] [Google Scholar]
- 282.Manabe K, Matsui T, Yamaya M, et al. Sleep patterns and mortality among elderly patients in a geriatric hospital. Gerontology. 2000;46:318–322. doi: 10.1159/000022184. [DOI] [PubMed] [Google Scholar]
- 283.Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Amer Geriatr Soc. 2000;48:682–685. doi: 10.1111/j.1532-5415.2000.tb04729.x. [DOI] [PubMed] [Google Scholar]
- 284.Lyketsos CG, Lindell Veiel L, Baker A, et al. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Internat J Geriatr Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 285.Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalized elderly – an open trial. Internat J Geriatr Psychiatry. 2003;18:520–526. doi: 10.1002/gps.852. [DOI] [PubMed] [Google Scholar]
- 286.Fetveit A, Bjorvatn B. The effects of bright-light therapy on actigraphical measured sleep last for several weeks post-treatment. A study in a nursing home population. J Sleep Research. 2004;13:153–158. doi: 10.1111/j.1365-2869.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 287.Sloane PD, Williams CS, Mitchell CM, et al. High-intensity environmental light in dementia: effect on sleep and activity. J Amer Geriatr Soc. 2007;55:1524–1533. doi: 10.1111/j.1532-5415.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 288.Alessi CA, Yoon EJ, Schnelle JF, et al. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: Do sleep and agitation improve? J Am Geriatr Soc. 1999;47:784–791. doi: 10.1111/j.1532-5415.1999.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 289.Landi F, Russo A, Bernabei R. Physical activity and behavior in the elderly: A pilot study. Arch Gerontol Geriatr Suppl. 2004;9:235–241. doi: 10.1016/j.archger.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 290.Buettner LL, Fitzsimmons S. AD-venture program: Therapeutic biking for the treatment of depression in long-term care residents with dementia. Amer J Alzheimer's Dis Other Dementias. 2002;17:121–127. doi: 10.1177/153331750201700205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen KM, Ci CH, Lin JN, et al. A feasible method to enhance and maintain the health of elderly living in long-term care facilities through long-term, simplified tai chi exercises. J Nurs Research. 2007;15:156–164. doi: 10.1097/01.jnr.0000387610.78273.db. [DOI] [PubMed] [Google Scholar]
- 292.Ouslander JG, Connell BR, Bliwise DL, et al. A nonpharmacological intervention to improve sleep in nursing home patients: Results of a controlled clinical trial. J Amer Geriatr Soc. 2006;54:38–47. doi: 10.1111/j.1532-5415.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 293.Meuleman JR, Nelson RC, Clark RL., Jr Evaluation of temazepam and diphenhydramine as hypnotics in a nursing-home population. Drug Intell Clin Pharmacy. 1987;21:716–720. doi: 10.1177/106002808702100908. [DOI] [PubMed] [Google Scholar]
- 294.American Medical Directors Association. AMDA Foundation; Columbia, MD: 2006. [July 21, 2008]. Clinical Practice Guidelines: Sleep Disorders in the Long Term Care Setting. Product Code CPG21. Available at: www.amda.com/tools/cpg/sleep.cfm. [Google Scholar]
- 295.Voyer P, Verreault R, Mengue PN, et al. Prevalence of insomnia and its associated factors in elderly long-term care residents. Arch Gerontol Geriatrics. 2006;42:1–20. doi: 10.1016/j.archger.2005.06.008. [DOI] [PubMed] [Google Scholar]


