Abstract
Sexual differentiation of the mammalian nervous system has been studied intensively for over 25 years. Most of what we know, however, comes from work on relatively non-social species in which direct reproduction (i.e., production of offspring) is virtually the only route to reproductive success. In social species, an individual’s inclusive fitness may include contributions to the gene pool that are achieved by supporting the reproductive efforts of close relatives; this feature is most evident in eusocial organisms. Here, we review what is known about neuroendocrine mechanisms, sexual differentiation, and effects of social status on the brain and spinal cord in two eusocial mammals: the naked mole-rat and Damaraland mole-rat. These small rodents exhibit the most rigidly organized reproductive hierarchy among mammals, with reproduction suppressed in a majority of individuals. Our findings suggest that eusociality may be associated with a relative lack of sex differences and a reduced influence of gonadal hormones on some functions to which these hormones are usually tightly linked. We also identify neural changes accompanying a change in social and reproductive status, and discuss the implications of our findings for understanding the evolution of sex differences and the neuroendocrinology of reproductive suppression.
Keywords: naked mole-rat, Damaraland mole-rat, sex difference, social status, reproductive hierarchy, eusociality, social system
1. Introduction
Charles Darwin was intrigued by the type of social system seen in ants, termites and many bees and wasps where only a few individuals within a large colony engage in direct reproduction, while other members are sterile and act to support the reproductive efforts of the colony as a whole. Darwin pointed out that these “eusocial” societies, as they came to be called, posed a potential threat to his theory of evolution through natural selection, which emphasized the relative fitness of individuals in determining traits that would be transmitted across generations. It was difficult to understand how the trait of sterility could be transmitted to future generations. The dilemma has been resolved largely through the insights of W. D. Hamilton and others who analyzed the factors that contribute to inclusive fitness, emphasizing the fitness of individual genes. In short, genes that lead to sterility in some individuals can enhance fitness if those individuals support the reproductive efforts of their close relatives, who are likely to carry copies of the same genes [62].
While perhaps the most impressive degrees of sociality based on a reproductive hierarchy have been achieved in the numerous species of eusocial insects, a few mammals have similarly complex social structures. Eusociality occurs in at least two species of bathyergid rodents, naked mole-rats (Heterocephalus glaber) and Damaraland mole-rats (Cryptomys damarensis). In this review, we consider the evolution and neuroendocrine bases of the reproductive hierarchy associated with mammalian eusociality. We also describe our ongoing studies examining sexual differentiation and effects of social and reproductive status on the nervous systems of naked and Damaraland mole-rats. We review data showing that eusociality may be accompanied by a reduction of sex differences in behavior and in neural anatomy. Moreover, in the most social of mammals, neuroanatomy may be influenced more by an individual’s breeding status than by its sex.
2. Cooperative Breeding and Eusociality
Cooperative breeding is defined as any social system in which members of a group assist in rearing young that are not their own. Cooperative breeders may exhibit plural breeding, where more than one adult female is actively reproducing within the cooperative group, or singular breeding, where only one female (at a given time) breeds within the social group despite the presence of other females of breeding age [57]. Cooperative breeding has been especially well studied in avians and is estimated to occur in about 3% of extant bird species. In mammals, cooperative breeding has been reported for 35 species in the order Rodentia [116] as well as for several species of callitrichid primates [57, 117], canids [5, 93] and in the dwarf mongoose and the meerkat [35].
Eusociality (literally, ‘good sociality’) is a term coined for the highest level of social organization based on reproductive castes [92] and can be understood as an extreme form of cooperative breeding. Eusociality probably evolves from cooperative breeding, and a survey of a variety of social species suggests a continuum from cooperative breeding to eusociality [83, 113]. For a species to be considered eusocial, individuals must live in large groups including multiple adult generations in which only a few individuals participate in breeding, while other members are either sterile or do not reproduce for other reasons, such as behavioral inhibition. Some authors add the requirement of the existence of a caste system [37]. Eusociality has been a highly successful strategy within insects, with eusocial species constituting 75% of the insect biomass in some ecosystems [53]. Just two mammalian species are generally agreed to be eusocial.
2.1 Eusociality in Mammals
The first claim of eusociality in a mammal was the description of the social/reproductive system observed in naked mole-rats [72], which are small (approx. 30–60 g), nearly hairless rodents native to Africa (Figure 1a). Colonies of naked mole-rats collected in the field typically contain 60–80, and in one instance at least 295, individuals. There is typically just one breeding female (the queen) and 1–3 breeding males in each colony [11, 82]. The reproductive hierarchy appears to be quite stable, and once an animal becomes a breeder, it generally remains so for life, which can be over 20 years in this species [22]. Reproductive animals are reported to be socially dominant to non-reproductive colony members [44, 82].
Figure 1.
Colonies of naked mole-rats (A) and Damaraland mole-rats (B). The youngest naked mole-rats shown are 6 weeks old. In the foreground, one of these juveniles is about to be picked up by an older sibling. The breeding pair is not included in the photograph. Photography by Virge Kask.
Of five genera of the family Bathyergidae, or African mole-rats, naked mole-rats are the sole extant member of the genus Heterocephalus. Three of the bathyergid genera contain only solitary-living species, whereas the genus Cryptomys includes several species of varying sociality. At least one of these, the Damaraland mole-rat (Cryptomys damarensis), has also been claimed to be eusocial, although Damaraland mole-rats exhibit a smaller reproductive skew than do naked mole-rats, as each colony contains an average of about 16 animals with a single breeding pair (Figure 1b).1 All bathyergids are fossorial and are rarely observed above ground in the field [9]. Because the African mole-rats exhibit perhaps the widest range of sociality of any phylogenetically similar group of vertebrates, they offer a wonderful opportunity to test hypotheses regarding evolved associations between sociality, reproductive skew and particular aspects of physiology, anatomy and behavior.
2.2 Evolutionary Origins of Eusociality
In the Hymenoptera (ants, bees and wasps) eusociality appears to have originated independently approximately 12 times [129]. The preponderance of eusocial species may be related to haplodiploid sex determination in this group; that is, males possess a haploid chromosome number whereas females are diploid. One important consequence of haplodiploidy is that females are more closely related to their sisters (3/4 related, since all sisters share an identical set of chromosomes from their father) than they are to their daughters (1/2 related). This increases the value of helping sisters to reproduce. However, the existence of eusociality in termites and other diploid organisms, including certain species of aphids, thrips, beetles, and spiders raises doubts regarding the importance of haplodiploidy in the origins of hymenopteran eusociality [3, 36, 78, 124].
Indeed, some have suggested that the multiple, independent origins of eusociality among hymenopterans may be related to the prior appearance in the group of a habit of selecting sheltered, enclosed nesting sites in which to deposit eggs and, in many cases, raise the young [1]. The protracted presence of a female and her offspring at a well protected nest site may set the stage for the origin of extended sociality, with a key step being delayed dispersal of offspring, perhaps because similarly protected sites are not readily available so that dispersal becomes relatively risky as compared to remaining at the natal site. In fact, before eusociality was known in any mammal, R.D. Alexander predicted that if a eusocial mammal did exist it would likely be a species of fossorial habit, living in a circumscribed and protected site [112].
Phylogenetic studies as well as comparative studies of colony dynamics suggest that eusociality evolved independently in naked mole-rats and Damaraland mole-rats [2, 74]. Haplodiploidy cannot account for eusociality in these species, as both naked mole-rats and Damaraland mole-rats are strictly diploid. Instead, two hypotheses have been invoked to explain the evolution of cooperative breeding and eusociality in mole-rats, borrowing from ideas developed in the avian literature. First, the ecological constraints hypothesis suggests that ecological factors such as a shortage of suitable breeding territories, high mortality risk of dispersal, and low probability of finding a mate leads to delayed dispersal of young and, hence, overlapping generations of adults living in a single group [63]. Second, the life history hypothesis emphasizes the role of life history traits such as litter size and longevity in the origins of cooperative breeding. The two hypotheses are not mutually exclusive [63].
In support of the ecological constraints hypothesis, a major cost associated with fossorial life is the extraordinary amount of energy required to move to new territory during foraging. This is particularly significant for fossorial species that inhabit environments where food is patchily distributed, as is the case for both naked and Damaraland mole-rats [1, 10]. In addition, both species consume underground tubers and bulbs only exposed by burrowing, and burrowing is quite difficult during the dry season, when the soil is hard packed. A large number of animals working together may be required for success in this niche. By contrast, most of the solitary-living African mole-rats are found in mesic habitats with a more uniform distribution of food resources [74, 75].
The longevity of eusocial mole-rats is consistent with the life history hypothesis. Naked mole-rats are remarkably long-lived as compared to other small rodents; individuals have survived for at least 28 years in the laboratory, the longest life span recorded for any rodent [22]. Damaraland mole-rats have achieved an age of at least 15 years in our laboratory (B. D. Goldman, unpublished). It should be noted, however, that longevity is characteristic of a number of solitary-living fossorial mammals and of bats as well [128]; therefore, whereas longevity may predispose to eusociality, it is not exclusively associated with this form of social life.
3. Eusociality: castes versus social roles
The eusocial insect societies are frequently characterized by clearly defined castes. Among the eusocial mammals, however, the behavioral profile of individuals, and thus their role within the colony, may change over a lifetime. In addition, there is overlap among the presumptive “castes” of non-reproductive members of the colony, and differences in behaviors among the groups are quantitative rather than qualitative. For these reasons, “social roles” may be a more apt term than castes. Roles within a naked mole-rat colony were initially reported to include not just breeder versus nonbreeder, but three castes among the nonbreeders: frequent workers, infrequent workers and nonworkers, with the latter being mainly involved with colony defense against predators [72]. However, later studies questioned the existence of discrete castes among subordinate naked mole-rats [82]. Similarly, nonbreeding Damaraland mole-rats may fall into two categories (frequent and infrequent workers) [108], although it is not clear whether there are two distinct groups or a continuum, or whether individuals may transition from one type of worker to the other as they age.
As mentioned above, the reproductive hierarchy of naked mole-rats is quite rigid. Once an animal becomes a breeder, it is rarely dethroned, and it has been claimed that only a very small percentage of naked mole-rats in nature ever achieve breeding status [75]. Crucially, however, nonbreeding naked mole-rats and Damaraland mole-rats are not sterile. They can become reproductive if removed from their natal colonies and paired with an individual of opposite sex, and this is our routine procedure for initiating new colonies in the laboratory. The importance of this observation for our understanding of neural and endocrine mechanisms of mammalian eusociality is that it implies that the attainment of distinct roles (i.e., breeding vs. nonbreeding) is the result of social experience and environmental factors rather than genetic differences between the two groups.
In the sections that follow, we describe neuroendocrine differences between breeders and nonbreeders. We also examine sexual differentiation as it relates to social structure and discuss our findings of neural changes that are seen in naked mole-rats following the experimental manipulation of breeding status. Within the naked mole-rat literature, as well as for the purposes of this review, “breeder” refers to an animal that has at one time produced a litter but is not necessarily actively breeding (naked mole-rat queens, for example, can sometimes go for several years without producing a litter, while still maintaining their status). The terms “nonbreeder” and “subordinate” are often used interchangeably in reference to the other members of the colony, i.e., those animals that have never reproduced [44].
3.1 Hormones and reproductive status
The reproductive suppression of nonbreeding females within both naked and Damaraland mole-rat colonies is profound. Female nonbreeders have small uteri as well as small ovaries without corpora lutea, indicative of complete failure to ovulate. Thus, despite achieving adult body size, the non-reproductive females remain in a pre-pubertal state throughout life. Not surprisingly, levels of gonadal hormones are also relatively low in these animals. Concentrations of urinary and plasma progesterone are consistently low in subordinate females [30, 45, 48, 90] (M. M. Holmes, M. B. Lovern, M. L. Seney, B. D. Goldman, and N. G. Forger, unpublished), a pattern mirrored in Damaraland mole-rats [13]. While nonbreeding female Damaraland mole-rats have low levels of urinary estradiol [8], little is known about estradiol levels in naked mole-rats. We have recently measured estradiol in plasma of naked mole-rats and find low, and in many cases undetectable, levels in both breeding and nonbreeding females (M. M. Holmes, M. B. Lovern, M. L. Seney, B. D. Goldman, and N. G. Forger, unpublished). In other rodents, estradiol levels typically peak around the time of ovulation and are considerably lower throughout the remainder of the cycle. Since our breeding females were sampled randomly with respect to phase of the reproductive cycle, it is quite possible that we missed a window of elevated estradiol.
In both naked mole-rats and Damaraland mole-rats, breeding and nonbreeding females can produce a surge of luteinizing hormone (LH) from the pituitary in response to injection of gonadotropin releasing hormone (GnRH) [12, 46, 94], demonstrating that the pituitaries of subordinate females are responsive to GnRH. However, breeding females show a greater LH response at lower doses of GnRH as compared to subordinates. The relative insensitivity of nonbreeding females to low doses of GnRH is reversible; four consecutive injections of GnRH administered at one hour intervals results in similar LH profiles between breeder and subordinate naked mole-rats [46]. These observations suggest that pituitary insensitivity to GnRH in subordinate females is due to insufficient chronic stimulation by endogenous GnRH, and that the primary site of reproductive hormone inhibition is at the level of the brain rather than the pituitary [46]. In this sense, the endocrine status of subordinate, fully-grown female naked mole-rats seems to resemble that of juvenile mammals of other species [99] or of mammals in which reproductive activity is suspended during seasonal anestrus [21].
Although nonbreeding males do not normally exhibit reproductive behavior, physiological suppression is less profound than in nonbreeding females. Nonbreeding male naked mole-rats generally have lower levels of urinary testosterone compared to breeders [27, 28, 29, 47], but the difference is not huge (on the order of 2-fold) and is not always found [30]. Plasma (as opposed to urinary) testosterone has not been reported, but in preliminary studies we find lower plasma testosterone in subordinate male naked mole-rats than in breeder males (M. M. Holmes, M. B. Lovern, M. L. Seney, B. D. Goldman, and N. G. Forger, unpublished). Mean testis weights and numbers of spermatozoa are greater in breeding male naked mole-rats as compared to subordinates but, again, some nonbreeding males do have active sperm [50].
In Damaraland mole-rats, no differences in mean urinary testosterone, plasma LH, or the LH response to a GnRH challenge were seen between breeding and nonbreeding males [8, 14]. Testis weights were somewhat greater in breeding males as compared to nonbreeders, but no differences in numbers of spermatozoa were detected [50]. This suggests that reproductive inhibition in subordinate male Damaraland mole-rats may be strictly behavioral and independent of circulating concentrations of sex hormones. Even for naked mole-rats, the hormonal differences between breeders and subordinates may not be important for behavioral differences because treating male subordinates in a colony with testosterone does not cause them to display the genital nuzzling behavior characteristic of breeders (D. Ciszek and B. D. Goldman, unpublished; see below).
3.2 Mechanisms of reproductive inhibition
It has been repeatedly suggested that the queen in a naked mole-rat colony is responsible for the suppression of reproductive activity in nonbreeding females, and perhaps also in nonbreeding males. If this is true, however, it is still not known how she achieves this. Two studies have failed to reveal a role of urinary pheromones in the inhibition of reproductive activity in nonbreeding naked mole-rats. In the first study [43], nonbreeders of both sexes were removed from their natal colonies and housed singly or paired with an individual of the opposite sex. About half of the animals in each group received a daily transfer of soiled bedding from their parent colony. Females showed a rise in urinary progesterone within about 7–9 days following separation from the natal colony; this endocrine response was seen in both single-housed and paired females and was not affected by exposure to soiled bedding from the natal colony. In males, concentrations of urinary testosterone and plasma LH increased in both control and bedding transfer groups within a few days following removal from the home colony [43]. In another study, nonbreeding naked mole-rats were removed from their colonies and exposed either to soiled bedding (daily) from the parent colony or to physical contact (for 2–day periods) with other nonbreeders from the parent colony [114]. All of the experimental females exhibited increased urinary progesterone within 3 days after isolation from their natal colonies, and this was interpreted to indicate that neither urinary odors from the entire colony nor social contact with nonbreeding siblings prevented reproductive activation [114].
These studies suggest (but see below) that urinary pheromones are not the source of reproductive inhibition, although it remains possible that volatile chemicals that are not present in urine could be involved. When naked mole-rats meet head-on in tunnels they briefly sniff each other’s facial region, suggesting that a pheromone or some other odoriferous material used for individual identification may be present on the face. It has recently been shown that mice secrete sex-specific peptides from extraorbital lacrimal glands [79]. Thus, odors present on the skin of the reproductive mole-rats might be involved in the inhibition of reproductive activity in the nonbreeders. Alternatively, it has been suggested that the queen inhibits reproduction by behavioral intimidation, specifically by repeated prodding and pushing of nonbreeders [103, 114].
Regardless, we believe there may be reason to be cautious of the interpretation that an increase in urinary progesterone in recently isolated female naked mole-rats indicates reproductive activation. First, progesterone is normally high only during the ovulatory and luteal phases in rodents, and naked mole-rats are reported to have a cycle length of approximately 34 days [48]. Thus, a rise in progesterone after just a few days of isolation seems almost too rapid to indicate initiation of ovulatory cycles. Second, in our experience, when nonbreeding mole-rats of opposite sexes are removed from their colonies and paired, litters are rarely produced in less than 5–6 months (and often only after considerably longer) even though the gestation period is just over 2 months. If ovulatory cycles are activated within a few days following removal from the natal colony, why the time lag of several months before the appearance of litters? Finally, data from Syrian hamsters cast doubt on the validity of assuming that increased progesterone is a reliable indicator of reproductive activation. Female hamsters become completely anovulatory for about 16 weeks during prolonged exposure to short photoperiods, as would normally occur during fall and winter months. During this extended anovulatory phase, serum progesterone is chronically elevated to concentrations approaching those seen during the progesterone peak at the periovulatory time of the estrous cycle. Essentially identical patterns of serum progesterone were observed in lactating hamsters, which are also anovulatory [20, 84, 109, 118]. Thus, in this species, progesterone is chronically elevated in females that are reproductively inhibited as well as during specific phases of reproduction. In summary, while the elevation of urinary progesterone shortly following removal of nonbreeding female naked mole-rats from their colonies is an indication of some change in the reproductive axis, it may be premature to assume this is an indication of reproductive activation per se.
In laboratory colonies of Damaraland mole-rats, incest avoidance may be sufficient to prevent reproduction among nonbreeders. When foreign males were introduced into laboratory colonies, females that previously had been nonbreeders sometimes solicited matings with the unfamiliar males. In about half of the colonies where foreign males were introduced, fighting between the queen and a high-ranking daughter resulted in the previously nonbreeding female usurping the position of breeding female within 1–14 days; former queens became submissive, indicating their change in status [33]. Perhaps when the introduction of a foreign male leads to stimulation of reproductive activity in a previously nonbreeding female, she is able to compete with the established breeding female for exclusive breeding status. Our ongoing laboratory observations of sex behaviors in pairs of non-reproductive Damaraland mole-rats following removal from their natal colonies also suggest a strict incest avoidance in this species (see Section 7.2). However, in a field study of Damaraland mole-rat colonies, genetic analysis revealed the presence of immigrants of both sexes in established colonies, despite the fact that morphological examination indicated just a single breeding female in each colony at a given time [23]. This suggests that in addition to incest avoidance some type of inhibition, probably from the breeding female, must be responsible for maintaining reproductive suppression among the nonbreeding Damaraland mole-rat females [23], as is the case in naked mole-rats.
3.3 Succession of breeders and founding of new colonies
Following the death or removal of a breeder, subordinate naked mole-rats may achieve breeding status within their natal colony. Loss of the queen is often followed by fighting (in many cases leading to deaths) among a subset of subordinate females before a new queen becomes established [27, 90]. In any event, since a breeder is most likely replaced by a daughter or son, genetic relatedness of colony members is likely to be perpetuated. Indeed, immigration of foreign animals into an established colony is probably a very rare event [18] and genetic analyses demonstrate a very high degree of inbreeding in wild naked mole-rat colonies, unprecedented in any mammal [49].
Once a queen is established, she becomes morphologically distinct from subordinates. Breeding female naked mole-rats develop an elongated body associated with an actual lengthening of individual vertebrae [96]. Most of the vertebral lengthening occurs during successive pregnancies, which suggests that the hormones of pregnancy are involved, but this has not been directly established [65]. Lengthening of the body may represent an adaptation that allows the breeding female to maneuver through narrow tunnels during the gestation of large litters; one litter of 29 was born in captivity [11].
As might be predicted from the strict incest avoidance of Damaraland mole-rats, new colonies of this species are founded by nonbreeders that disperse from their natal colonies [64]. Naked mole-rats can also form new colonies by dispersing, and this may be more common than was originally appreciated. In field studies, Stanton Braude captured 54 entire colonies in Kenya, marked all the animals, and returned them to their burrows [18]. Over the next few years, 16 previously marked animals were captured at distances ranging from .02 – 2.4 km from their natal colonies. Most of the nascent colonies contained adults from at least two different natal colonies, suggesting that dispersal usually leads to outbreeding [18]. Similarly, in a laboratory study, when nonbreeding naked mole-rats of both sexes were housed communally with animals from their own colony and a foreign colony, the breeding pairs that formed revealed that outbreeding was favored [26]. Thus, although inbreeding is extremely high in established naked mole-rat colonies [49], in those instances where the opportunity arises, outbreeding may actually be preferred.
Indeed, there may be a ‘disperser morph’ in naked mole-rats, first inferred from a laboratory study in which animals were found that had large deposits of subcutaneous adipose tissue in the neck region (possibly to provide energy reserves during dispersal), a tendency to leave the confines of the natal colony when the opportunity was available, and a propensity to solicit matings when exposed to individuals from a different colony [97]. Eighteen of the 19 individuals identified as putative dispersers in this study were males. Similarly, in our laboratory we have occasionally identified animals with the adipose accumulations characteristic of dispersers, and these animals have been almost exclusively males (Figure 2). This is somewhat surprising since in Braude’s field studies, mentioned above, approximately equal numbers of animals of both sexes were trapped at a distance from their natal burrows [18]. It may be that female dispersers do not exhibit the anatomical characteristics of male dispersers, or that the laboratory environment does not provide the conditions needed to evoke dispersal attempts by females. In a laboratory study, disperser morph males exhibited a circadian pattern of locomotor activity that was different from the relatively non-rhythmic pattern observed in all other colony members. The presumptive dispersers displayed robust rhythms of nocturnal locomotor activity, with activity beginning at about the time of lights-off [104]. The activity rhythms seen in these individuals continued when they were transferred to continuous darkness, suggesting control by the endogenous circadian system. The expression of a nocturnal activity rhythm in dispersers might represent an adaptation to ensure that above-ground dispersal would occur at night when the threat of predation would be minimized [104].
Figure 2.
The two naked mole-rats shown in this photograph are non-breeding males from the same colony. Note the prominent accumulation of fat around the neck region of the animal on top (arrow), characteristic of the disperser morph. Photography by Sharry Goldman.
3.4 Induction of ovulation
Two means of induction of ovulation are common among mammals. In spontaneous (or cyclic) ovulators, ovulation occurs at regular intervals and usually does not require the presence of a male. In induced (or reflex) ovulators, females maintain a relatively continuous supply of mature ovarian follicles during the breeding season, but they ovulate only following mating. It has been speculated that induced ovulation might be a particularly adaptive mechanism for solitary-living species where encounters with the opposite sex are relatively infrequent [127].
We are aware of three studies of ovulation mechanisms in African mole-rats. The first study involved a solitary species, the Cape mole-rat (Georychus capensis) [122]. Females captured in the field were housed either (a) separately and without the presence of a male, (b) singly, but separated from a male by a metal grid to allow only non-physical interaction, or (c) periodically housed with a vasectomized male, allowing physical interactions. After several weeks, only females that were actually housed in physical contact with males and thus permitted to mate had corpora lutea in their ovaries, suggesting that the species is an induced ovulator [122]. In a similarly designed study, a highly social bathyergid, the Natal mole-rat (Cryptomys hottentotus natalensis), also was found to be an induced ovulator since only females housed in physical contact with males exhibited corpora lutea [69]. However, a study in Damaraland mole-rats yielded somewhat more ambiguous results [115]. One of 3 previously nonbreeding females that were housed in isolation had corpora lutea after 6 weeks. None of the 6 females separated from a male by a metal grid had corpora lutea, and 5 of 6 females housed in physical contact with vasectomized males exhibited corpora. The authors suggest that Damaraland mole-rats are capable of spontaneous ovulation, but that the act of coitus (females housed in contact with males) may advance the onset of ovulation [115].
It is possible that Damaraland mole-rats may be capable of both spontaneous and coitus-induced ovulation. This possibility would be particularly intriguing for a species where females encounter two very different types of reproductive opportunities: first, established breeders mate and reproduce in a colony setting, where they are in relatively continuous proximity to a mate, and second, previous nonbreeders that disperse from the natal colony may encounter a disperser of opposite sex and establish a new colony. The details of this encounter are not known, but it seems likely that it would be adaptive for the reproductive process to be initiated soon; the gestation period is more than two months in those bathyergids that have been examined, allowing time for the new pair to establish a burrow. In this situation, induced ovulation might be a useful mechanism, as females would be able to respond rapidly to first encounter with a male. In contrast, for established breeders in a colony setting, it is not clear that induced ovulation would provide any advantage as compared to spontaneous ovulation.
Measures of serum LH under appropriate conditions could either confirm or refute the hypothesis that Damaraland mole-rats are capable of both spontaneous and induced ovulation. Since the distribution of the two modes of ovulation among mammals does not follow a strict phylogenetic pattern, it appears that the two have been evolutionarily at least somewhat flexible. Female laboratory rats, though spontaneous ovulators, do release LH following coitus, though the amount released is much less than during the preovulatory surge [42, 95]. This response may indicate some degree of retention of the coitus-induced mechanism in a spontaneous ovulator.
4. Behavioral specializations
Naked mole-rats exhibit a complex behavioral repertoire that has been described in detail [81]. A total of 72 nonvocal behaviors were grouped into 17 categories based on functional similarity. The majority of these behaviors can be performed by all members of the colony, with frequency of performance often dictated by whether a given animal is a breeder or nonbreeder. The most conspicuous specialization of behavior is seen in reproductive behaviors, as mating is restricted to the established breeders in the colony. A copulatory episode typically consists of the breeding female “backing” up to a male in a tunnel while exhibiting a lordosis-like posture, followed by the male mounting the female and “pedaling” his hind legs in an effort to establish genital contact [73, 81]. Copulation occurs when the male and female genitals contact each other and pelvic thrusting by the male is observed. Parturition and nursing are exclusive to the queen while other aspects of pup care, such as carrying and grooming, are shared among all colony members.
In contrast to copulation, which is rarely observed since it probably occurs on only one day of the 34-day ovulatory cycle, genital nuzzling behavior is frequently performed by breeders. This behavior is stereotyped and can be intense, and is almost never performed by subordinate members of naked mole-rat colonies; thus, genital nuzzling is the most convenient day-to-day way to identify the breeders in a naked mole-rat colony (Figure 3). Genital nuzzling occurs at all times of the female’s ovulatory cycle and during pregnancy. The frequency of genital nuzzling in male breeders may be modulated to some extent by testosterone, but the behavior continues to be expressed even after gonadectomy of both members of the breeding pair [59]. Remarkably, although nonbreeders rarely exhibit nuzzling within their natal colonies, they may nuzzle within minutes when removed from the colony and paired on neutral territory with a nonbreeder from a different colony (S. L. Goldman, personal observation). This observation further suggests that nuzzling is not under strict endocrine control but is strongly influenced by social environment.
Figure 3.
Genital nuzzling by a breeding pair of naked mole-rats. When nuzzling is performed by a breeder, it is almost always directed toward another breeder. This is often a mutual behavior in which the animals nuzzle each other simultaneously. Nonbreeding mole-rats rarely exhibit nuzzling behavior unless they are removed from the colony. Photography by Sharry Goldman.
The behavioral specialization of breeders extends to vocal behaviors. Naked mole-rats have at least 17 distinct vocalizations, and four of these are restricted to breeders [101]. Interestingly, only one, the V-trill, is associated with copulatory behavior, suggesting the others may be involved in maintaining the social hierarchy, although more work on this is needed.
4.1 Lack of sex differences in behavior and genitalia of subordinates
Strikingly, for the many behaviors displayed by subordinate naked mole-rats, no sex differences are observed. Subordinate males and females participate equally in all sub-categories of grooming, resting, thermoregulation (i.e., crouching, huddling, shivering and basking), feeding, elimination, coprophagy, locomotion, orientation, transport of food and nesting material, digging, neonate tending, interactive behaviors, agonistic behaviors, and alarm reactions [81, 82]. In addition, there is no sex difference in overall body size or weight [83, 102], and even the external genitalia are nearly identical in subordinate males and females (Figure 4). The penis or clitoris is immediately adjacent to the anus in both sexes and ano-genital distance does not differ by sex or is, if anything, greater in females [102] (M. L. Seney, unpublished). All males are non-scrotal, the external penis is tiny and virtually identical to the clitoris (or female genital mound), and the vagina is imperforate in subordinate females [72, 102]. The relative lack of sex differences in physical and behavioral parameters raised the question of whether, or to what degree, the nervous system of naked mole-rats is sexually differentiated.
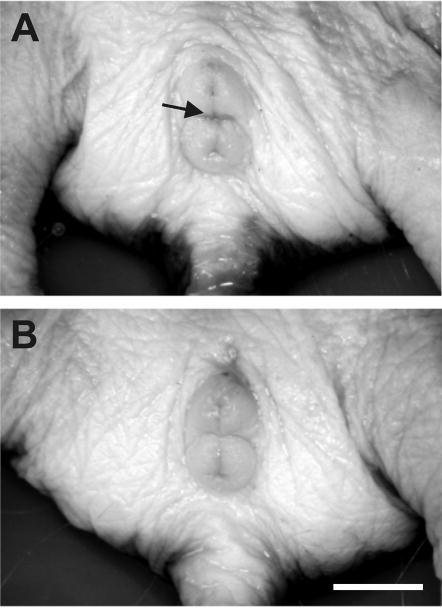
Figure 4.
External genitalia of a female (A) and male (B) nonbreeding naked mole-rat. The genital mound is anterior to the anal mound and has a very similar appearance in both sexes. The vagina is not perforate in nonbreeding females; the arrow denotes where the vagina opens in breeders. Scale bar = 5 mm. Reprinted from [102], copyright Wiley-Blackwell.
5. Sexual differentiation of the nervous system
Sexual dimorphisms have been reported in the nervous system in all vertebrate classes and are thought to underlie sex differences in reproductive physiology and behavior. These neural sex differences are seen at multiple levels of analyses, ranging from gross morphological to molecular. An exhaustive review is beyond the scope of this article, but a brief introduction to a selection of well-studied neural dimorphisms will serve to set the stage for our recent work using the naked mole-rat as a model for sexual differentiation of the nervous system. Three of the best characterized sex differences in the nervous system are found in the spinal nucleus of the bulbocavernosus (SNB) [19], the sexually dimorphic nucleus of the preoptic area (SDN-POA) [61], and vasopressin innervation of the brain [41]. The SNB (also called Onuf’s nucleus in some species, including naked mole-rats) is a population of motoneurons in the lumbosacral spinal cord that innervate striated perineal muscles involved in copulation, including the bulbocavernosus and levator ani. SNB motoneurons are larger and more numerous in male mice and rats and the target muscles are absent or vestigial in females [19, 126]. A similar sex difference is seen in many species, including humans [55, 56, 119, 120, 121]. The volume of the SDN-POA is also dimorphic, favoring males, and is approximately 5–7 times larger in male than in female rats [61]. While the precise function of this sex difference is not known, lesioning this brain region modestly impairs the acquisition of copulatory behaviors in males [39]. Finally, the lateral septum has a higher density of vasopressin innervation in males than females in a wide variety of vertebrate species [41]. Central vasopressin has been linked to many social behaviors, including pair-bonding, parenting, social recognition, and aggression (see review by Young, this issue). In all three cases, the masculine pattern results from testosterone or its metabolites acting early in development in males [4]. Not all neural sex differences, however, are the result of developmental actions of hormones. For example, the posterodorsal medial amygdala of rats is approximately 1.5 times larger in males than females and this is largely due to sex differences in circulating levels of testosterone in adulthood [32].
Sex differences in the nervous system have presumably evolved as specializations that support the different reproductive strategies of the sexes. For example, male mammals typically mount, intromit, and ejaculate while females exhibit species typical receptive behaviors (e.g. lordosis); thus both sexes have evolved the morphological and neural adaptations that facilitate and permit their respective behaviors. As mentioned above, subordinate naked mole-rats do not mate or show sex differences in other behaviors. We therefore reasoned that the nervous system of nonbreeding naked mole-rats might not be sexually differentiated; more generally, a reduction of behavioral and neural sex differences may evolve in conjunction with a lifestyle in which inclusive fitness is achieved indirectly, by supporting the reproductive efforts of breeding individuals.
5.1 Onuf’s nucleus is sexually monomorphic in naked mole-rats
Inspired by the nearly identical appearance of the external genitalia in males and females, we first examined the naked mole-rat homologue of the SNB neuromuscular system, (i.e., the perineal muscles and the motoneurons that innervate them). We used retrograde tracing to identify motoneurons innervating perineal muscles in naked mole-rats and found a single pool, in a position similar to Onuf’s nucleus found in primates and canines. We therefore refer to this cluster as Onuf’s nucleus in naked mole-rats. Initially, perineal muscle morphology and motoneuron number and size were examined only in subordinate males and females. In contrast to other mammals examined to date, we found all features of this neuromuscular system to be sexually monomorphic in subordinate naked mole-rats [102]. The absence of sex differences in the perineal musculature was particularly surprising as these muscles often differ quite dramatically between the sexes. Interestingly, the levator ani and bulbocavernosus muscles attach exclusively to the base, or “bulb” of the penis in males of other species, and male naked mole-rats seem to entirely lack a penile bulb.
We speculated that sexual differentiation might be “on hold” in subordinate naked mole-rats, and that sex differences might emerge only in those few individuals that become breeders. To test this, we compared the perineal muscles and motoneurons in breeding and subordinate animals of both sexes. Contrary to our prediction, sex differences did not emerge in breeders. We again found no sex differences in Onuf’s nucleus of subordinates, but also found no sex differences in the breeders [110]. We did, however, find an effect of social status whereby breeders had approximately 30% more Onuf’s nucleus motoneurons than subordinates (Figure 5) [110]. This is likely a result of motoneuron recruitment and not due to adult-generated motoneurons per se, because the increase in motoneuron number corresponds with a decrease in “small cells” in the region. These small cells are about 1/3 the size of motoneurons and do not exhibit motoneuron-typical morphology. We are currently using motoneuron-specific markers to determine whether the small cells in Onuf’s nucleus are immature or atrophied motoneurons, or some other cell type.
Figure 5.
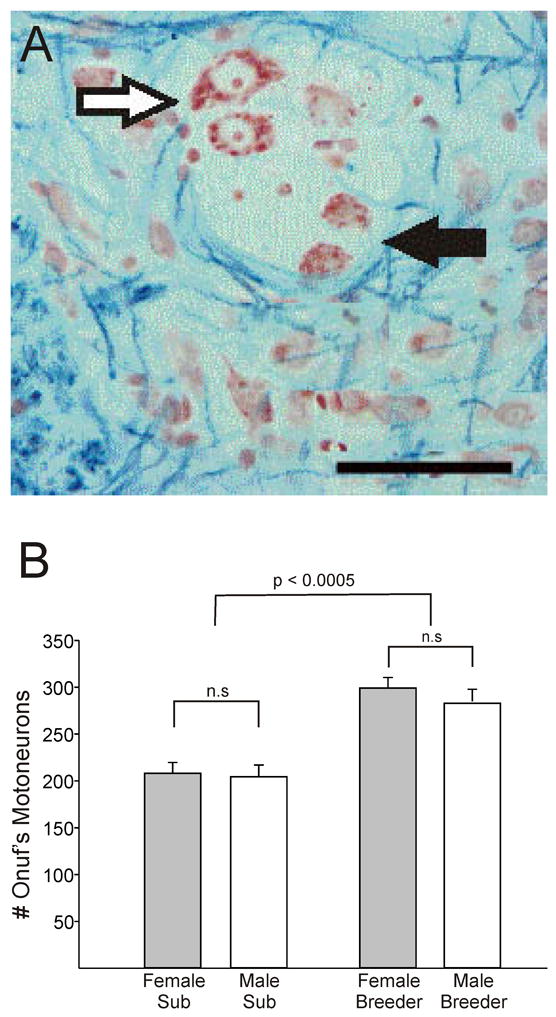
(A) Photomicrograph of a section through Onuf’s nucleus stained with Kluver-Barrera. (B) Mean (+ SEM) number of motoneurons in Onuf’s nucleus of subordinate (sub) and breeder naked mole-rats. There are no sex differences, but cell number is significantly increased in breeders of both sexes. Scale bar = 50 μm. Adapted from [110], copyright Wiley-Blackwell.
5.2 Perineal muscles in mole-rats of varying social structure
We wondered whether the lack of sex differences seen in naked mole-rats is a feature common to African mole-rats, or is instead related to social structure. To address this, we recently compared the genitalia and perineal muscles in three African mole-rat species: the naked mole-rat, the silvery mole-rat (Heliophobius argenteocinereus), and the Damaraland mole-rat. The silvery mole-rat is a strictly solitary species and, as described above, the Damaraland mole-rat is considered to be eusocial, but has a smaller colony size and less reproductive skew than naked mole-rats. Our findings demonstrate that the lack of sex differences in naked mole-rats is not an attribute of all African mole-rats. Naked mole-rats lack sex differences in the external genitalia and perineal morphology, silvery mole-rats exhibit sex differences, and Damaraland mole-rats are intermediate (specifically, the external genitalia and one of three perineal muscles are sexually dimorphic in Damaraland mole-rats; M. L. Seney, D. Kelly, R. Sumbera, B. D. Goldman and N. G. Forger, unpublished). These findings support a relationship between social structure and degree of sexual differentiation.
Most neural sex differences described in other rodents are caused by testosterone acting during early development (see Section 5). No developmental studies have been performed in any African mole-rat species to determine, for example, when the gonads differentiate or whether there is a perinatal testosterone surge in males, as is seen in other mammals. We have recently treated Damaraland mole-rats with testosterone during approximately the last third of gestation. We find that the external genitalia and the only sexually dimorphic perineal muscle in this species are masculinized in the female offspring of androgenized mothers [M.L. Seney, B.D. Goldman and N.G. Forger, unpublished]. Thus, for those features that are dimorphic, a prenatal period of sexual differentiation is indicated (see further discussion, Section 7.1).
5.3 Effects of sex and breeding status on the brain
We have also begun to examine the brains of naked mole-rats [66, 67]. Although previous published studies had examined somatosensory cortex [24] and a naked-mole rat brain atlas has been published [132, 133], no study had compared subordinates and breeders; indeed, no study had examined any aspect of the brain in breeders. Using unbiased stereological procedures we compared the morphology of brain regions related to reproduction and shown to be dimorphic in other species: the bed nucleus of the stria terminalis (BNST), the paraventricular nucleus (PVN), the medial amygdala (MeA), and the ventromedial nucleus of the hypothalamus (VMH) (Figure 6). We also looked for a sex difference in the preoptic area but did not see a densely packed cluster of cells in a position equivalent to the rat SDN-POA. The results of these analyses were striking: we found no sex differences in overall volume, cell number or cell size in any region examined [67]. This was true for both breeders and subordinates, and demonstrates that sexual monomorphism in the naked mole-rat nervous system is not limited to the perineal motoneurons. We did, however, find effects of social status on neural morphology: overall volumes of the BNST, PVN, and MeA were larger in breeders than subordinates and breeders had more cells in the VMH than subordinates (Figure 7) [67]. Taken together, these data indicate that social status is a more important factor than sex for determining neural morphology in naked mole-rats. Because our experimental animals were randomly assigned to remain subordinate in their natal colony or to become breeders (i.e., removed and paired with an opposite sex mate), we conclude that the change in social status caused the neural changes.
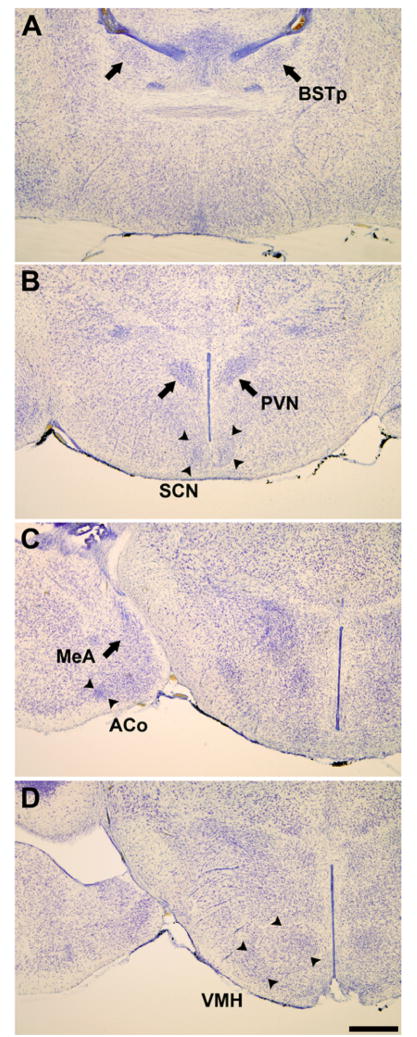
Figure 6.
Photomicrographs of Nissl-stained coronal sections of the naked mole-rat brain illustrating the location of the: (A) principal nucleus of the bed nucleus of the stria terminalis (BSTp); (B) paraventricular (PVN) and suprachiasmatic (SCN) nuclei; (C) medial amygdala (MeA) and anterior cortical amygdaloid nucleus (ACo); and (D) ventromedial nucleus of the hypothalamus (VMH). Scale bar = 500 μm. Reprinted from [67], copyright National Academy of Sciences.
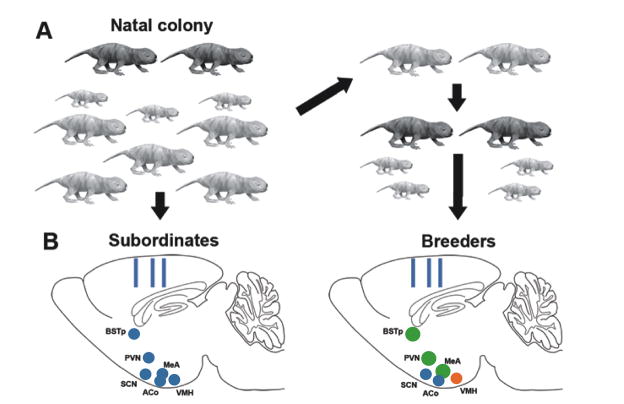
Figure 7.
(A) Experimental design of study by Holmes et al., 2007 [67]. All subjects were initially adult subordinates (light grey) within the natal colony, which also contains breeders (dark grey) and pups of various ages. Half of the animals were randomly assigned to remain as colony subordinates (Left). The other half were removed from the colony and housed with an opposite sex subordinate (Right). These paired animals were defined as breeders when they produced at least one litter. (B) Schematics of the sagittal plane of the naked mole-rat brain illustrating the approximate location of the brain regions examined. On the left is the brain of a subordinate: hypothalamic and limbic nuclei are indicated by blue circles; rectangles indicate sites of cortical thickness measurements. On the right is the brain of an animal that has transitioned to breeding status. Green circles indicate regions that are significantly larger in breeders (BSTp, PVN, MeA); orange indicates more cells in breeders (VMH). Regions shaded blue did not differ between subordinates and breeders (SCN, ACo, cortical thickness). Adapted from [67], copyright National Academy of Sciences.
We do not claim that no sex differences exist in the nervous systems of naked mole-rats. We examined only a few brain regions and our sample sizes were relatively small. However, if sex differences in morphology do exist, they may be more subtle than in other mammals, where differences in the regions examined are often large enough be detected even with very small sample sizes. This study also looked only at morphology (volume, cell number, and cell size) in a Nissl stain, and many other types of sex differences are possible (e.g., synapse number, neurochemistry, or gene expression).
As a follow-up to this initial morphological investigation, we therefore looked at the distribution of neuropeptides that have been linked to social behaviors in other animals (Section 5.3) and of androgen receptor (AR) protein in the brains of naked mole-rats. The brain regions that changed in volume or cell number in response to a change in breeding status [67] express AR and/or estrogen receptor in other species. Immunocytochemical analysis of AR in subordinates and breeders of both sexes revealed the first sex difference in the brain of naked mole-rats [66]: we find a higher percentage of AR-immunoreactive cells in the premammillary nucleus (PMv), MeA, and VMH of males compared to females, regardless of social status [66]. This is similar to findings in other species where males often exhibit increased AR expression relative to females (e.g., [52, 88, 105, 131]). Interestingly, however, the effect of social status is still more robust than sex: subordinates had a greater percentage of AR immunoreactive cells than did breeders in all brain regions examined (BNST, PVN, MeA, PMv, and VMH) [66]. The pattern of results was surprising and defies a simple hormonal explanation. The sex difference (male > female) in percentage of AR-immunoreactive cells in several brain regions suggests a positive relationship between androgen titers and AR expression. However, male breeders have higher androgen levels than do subordinates [44], yet AR-immunoreactivity was uniformly lower in breeders. The animals in this first study of AR expression in naked mole-rats were gonad-intact. By experimentally controlling circulating hormones, we may be able to better understand the interactions between hormone levels, social status, and brain AR in this species.
5.3 Vasopressin and oxytocin in naked mole-rats
Social recognition memory (the ability to remember a previously-encountered conspecific) may be the foundation of all mammalian social relationships [17], and is critically controlled by the neuropeptides, vasopressin (VP) and oxytocin (OT) (see review by Young, this issue). These closely related nonapeptides are produced by magnocellular neurons in the PVN and supraoptic nucleus (SON) and released into the bloodstream at the posterior pituitary. In addition, VP produced extrahypothalamically (specifically at the BNST, MeA, and suprachiasmatic nucleus (SCN)) and OT produced by parvocellular neurons of the PVN are released in specific regions throughout the brain [40]; it is these centrally-projecting cells that appear to modulate social functions.
In other rodents, social recognition memory is primarily based on olfactory cues, and is generally examined by recording the amount of time a test animal spends investigating a familiar versus a novel conspecific. Experiments involving pharmacological manipulations (i.e., the central administration of agonists and antagonists), antisense knockdown of vasopressin receptors, viral over-expression of vasopressin receptors, and transgenic animals lacking the peptide or its receptors all support the premise that centrally-released VP is involved in the ability of a rodent to recognize conspecifics (for reviews see [17, 125, 134]). The role of VP in social cognition is also well conserved, appearing in species ranging from fish, to birds, to mammals [60]. Although the evidence is not quite as thorough for OT, this peptide also clearly plays a role in the distinct form of memory employed in social recognition [51].
Members of a naked mole-rat colony work cooperatively and sleep together in large huddles. They exhibit very low levels of aggression to other colony members, yet are fiercely xenophobic and will mount vigorous attacks against conspecific intruders in the laboratory [98]. It is not known whether colony members recognize scents of individuals or a more generalized colony scent, but given that the queen is thought to suppress reproduction in subordinates and that she consistently recognizes her consorts [72, 82], individual social recognition likely plays a crucial role in naked mole-rat sociality. There is direct evidence that individual recognition is the basis for both xenophobia and incest avoidance in Damaraland mole-rats [70, 71].
In addition to social recognition, both VP and OT have been implicated in pair-bond formation in monogamous prairie voles; these peptides enhance pair-bond formation even in the absence of mating and antagonists prevent pair-bonding [25, 130]. Although naked mole-rats are not necessarily monogamous (the queen sometimes mates with up to three males), the breeders do form life-long reproductive bonds. In many species VP or its evolutionary antecedent, arginine vasotocin, also is associated with parental behavior, and dominance-subordinance [60]. For all these reasons, the VP and OT systems are logical places to look in the quest to identify neural mechanisms underlying the unusual sociality of naked mole-rats.
Using immunohistochemistry, we mapped the distribution of VP in the brains of subordinates and breeders [106] and find that, as in other mammals, naked mole-rats have VP immunoreacitve (VP-ir) cell bodies in the PVN and SON that project to the neurohypophysis. In several other ways, however, VP-ir in the naked mole-rat brain is unusual. Naked mole-rats lack VP cell bodies in the SCN and BNST (Figure 8a, b). The network of VP-ir fibers usually seen in the lateral septum (a projection site of the BNST and SCN) is also missing, and instead we find a dense cluster of large-caliber VP-ir fibers in the dorsomedial septum. We also find VP-ir cells in the dorsomedial hypothalamus, but only in breeders [106]. It is not yet clear how this suite of features affects naked mole-rat social behavior. The absence of suprachiasmatic VP may be related to the subterranean life style of naked mole-rats and the fact that these animals rarely display circadian rhythms in a colony setting [38, 104]. The large caliber VP fibers seen in the dorsomedial septum [106] could be related to social recognition memory: although VP receptors are not normally found in the medial septum of other species, forced expression of the VP1a receptor throughout the septum, including the dorsomedial septum, is sufficient to restore social recognition memory in V1a receptor knockout mice [16].
Figure 8.
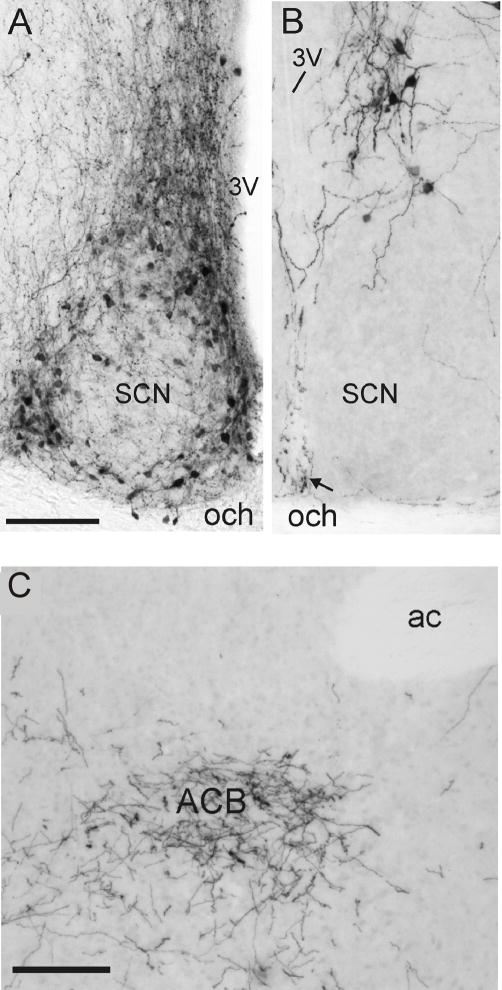
Photomicrographs of vasopressin immunoreactive (VP-ir) cells and fibers in the SCN of the (A) mouse, and (B) naked mole-rat. The mouse SCN contains numerous VP-ir somata and fibers; the naked mole-rat SCN lacked VP-ir somata, although the region between the right and left SCN contained a few fine VP-ir fibers (arrow). VP-ir cell bodies in the ventral PVN can be seen above the SCN in (B). (C) Photomicrograph of oxytocin immunoreactive fibers in the nucleus accumbens (ACB) of a naked mole-rat. Scale bar = 100 μm in A and B and 150 μm in C. Abbreviations: och, optic chiasm; 3V, third ventricle; ac, anterior commissure. (A) and (B) reprinted from [108], copyright Wiley-Liss, Inc.; (C) reprinted from [107], copyright Elsevier.
In voles, species differences in sociality correlate not with differences in the presence of VP or OT, but in the expression patterns of their receptors. For example, monogamous voles have more VP receptors in the ventral pallidum than do promiscuous species [135]. Moreover, when a viral vector is used to over-express the VP1a receptor in the ventral pallidum of normally promiscuous meadow voles, they develop partner preferences [86]. Monogamous voles also express more OT receptors in the nucleus accumbens than do promiscuous voles [68]. Intriguingly, a preliminary report suggests that receptors for OT are found in the nucleus accumbens of naked mole-rats but not of Cape mole-rats, a solitary species with no communal care of the young [76]. These two mole-rat species also differ with respect to the distribution of receptors for corticotrophin releasing factor [77]. We recently examined oxytocin immunoreactivity in the brains of subordinate naked mole-rats [107] and found a dense innervation of the nucleus accumbens (Figure 8c). As far as we are aware, a similarly robust innervation of the accumbens has not been seen in any other rodent species, although there is some OT innervation of this region in prairie voles [85].
Blockade of oxytocin receptors in the accumbens of voles prevents pairbond formation and inhibits maternal behavior [87], and OT receptor densities are correlated with individual differences in the expression of parental behaviors [100]. Thus, the dense OT innervation of the accumbens in subordinate naked mole-rats might be related to the fact that most members of the colony actively participate in pup care. Although only the breeding female lactates, nonbreeders provide care including nest building, retrieval of pups, and provisioning of fecal material that is consumed by pups. The role of OT in the nucleus accumbens of naked mole-rats could be tested in future studies by examining the effects of central administration of an OT antagonist on pup care. A similar approach could test whether OT or VP are important for maintenance of the reproductive hierarchy in naked mole-rats or for the genital nuzzling seen exclusively between breeders. Additional studies comparing the neuroanatomy and neurochemistry of the naked mole-rat with that of solitary mole-rat species will also be important for untangling the role of neuropeptides in the various behaviors required for eusocial life.
6. What are the mechanisms by which social status affects the eusocial brain?
Our findings thus far suggest that reproductive status is more important than sex for determining some aspects of neural morphology. What is it about the change in reproductive status that triggers the changes we see in breeders? Several possibilities occur to us, and they are not mutually exclusive. The areas that change are all hormone-sensitive so one possible mechanism is gonadal hormones. As mentioned above, levels of gonadal steroids are higher in breeders than subordinates (see Section 2 above). However, the switch to breeder involves much more than changes in hormones. Breeders have the experience of being socially dominant in addition to experiencing sexual behavior and birth of a litter. Importantly, our method of creating new breeders removes them from the colony environment (i.e., removal from established breeders and/or the presence of other subordinates); thus the removal of suppressive cues must also be considered and could by itself be sufficient.
We are currently attempting to tease apart hormonal and social factors by comparing the brains and spinal cords of naked mole-rats in four different groups: 1) gonad-intact paired animals that have not produced a litter, 2) gonad-intact pairs that have produced a litter, 3) breeders that produced at least one litter and were subsequently gonadectomized, and 4) subordinates within a colony. We hope to determine whether removal from the colony and pairing is enough to trigger the neural plasticity previously seen or, alternatively, whether actual reproduction (as in groups 2 and 3) may be required. The gonadectomized breeders will allow us to ask whether gonadal hormones are required to maintain the neural changes associated with social dominance/reproductive status.
While preliminary, our data suggest that different elements of becoming a breeder are responsible for specific brain changes. For example, we find that PVN volume is greater in all three groups of paired animals compared to subordinates (M. M. Holmes, B. D. Goldman, and N. G. Forger, unpublished). This suggests that removal from the colony and pairing with an individual of opposite sex may be enough to trigger this neural change and, once established, increased PVN volume is not reversed by gonadectomy. We do not know whether breeder-like changes in the PVN would be seen in animals gonadectomized prior to pairing, but the preliminary results suggest that some effects of social status on the brain may be independent of the gonads. In contrast, production of young may be important for the change in volume seen in other brain regions. For example, volume of the BNST is larger in gonad-intact and gonadectomized breeders compared to subordinates and paired animals that never produced a litter (M. M. Holmes, B. D. Goldman, and N. G. Forger, unpublished). Determining the time course of neural changes, which subpopulations of cells are responsible for the volume changes we report, as well as the specific mechanisms via which status or reproductive experience act to change the brain are among our future challenges.
7. Conclusions
7.1 Sexual Differentiation and Social Systems
We suggest that the reduction of morphological and behavioral sex differences in subordinate naked mole-rats, despite sex-typical gonadal differentiation, may be a feature that has evolved in relation to the reproductive hierarchy and associated reproductive skew in this species. Most individual naked mole-rats will never become reproductive and a large proportion of the animals may never engage in attempts to breed, either via dispersal or via competition to replace breeders within the natal colony. Thus, the majority of naked mole-rats achieve reproductive fitness strictly through their roles as helpers within their natal colony. We suggest that this would not require sexual specializations, since all helping roles are accomplished by both sexes. In addition, some features of sexual specialization, such as male-male aggression, might hinder the smooth functioning of the colony and actually reduce a subordinate’s inclusive fitness.
While we have suggested an evolutionary explanation for the reduced degree of sex differences in eusocial rodents, the proximate explanation for this phenomenon is unknown. Two possibilities might be considered for the considerable reduction in sex differences reported for naked mole-rats. First, sexually monomorphic tissues might be unresponsive to the presence of androgen during critical developmental periods. The features described in this review that have been found to be sexually monomorphic in naked mole-rats include various behaviors, and the morphologies of Onuf’s nucleus of the spinal cord, perineal muscles and brain regions. The critical period for early organizing actions of androgens for masculinization of these parameters encompasses the perinatal period in rodents with short gestations, such as rats, mice and hamsters, but masculinization is completed well in advance of birth in mammals with longer gestations such as guinea pigs, macaques and humans. Naked mole-rats and Damaraland mole-rats are reported to have gestation lengths of 65–74 days and 78–92 days, respectively [9]. Thus, it seems likely that critical developmental periods for these species would occur during fetal life. As mentioned briefly above (Section 5.2), we have obtained preliminary data that suggest a critical period for differentiation of the external genitalia occurring approximately 2–4 weeks prior to the end of gestation in Damaraland mole-rats. A second possibility is that androgens are not available during the time when a variety of tissues might have the potential for masculinization. Since, in contrast to Damaraland mole-rats, naked mole-rats do not exhibit sex differences in the external genitalia, it is difficult to predict the effect of prenatal hormone treatments in this species. If testosterone treatments did create more “traditional” male-like genitalia, this might suggest that there is normally no prenatal surge (or a reduced surge) in androgens in this species, which might account for the reduction in sex differences.
Another issue that relates to differences in the degree of sexual dimorphism seen across species is that of the mating system and its implications for male-male competition. Males are able to produce extremely large numbers of sperm, enabling them to mate with many females, whereas females are far more limited with respect to the number of potential mates. In polygamous species with a 1:1 sex ratio, this leads to intense male-male competition. In monogamous species, there may still be some competition for access to the best mates, but overall male-male competition is reduced. It is generally thought that high inter-male competition accounts for the observation that sexual dimorphisms in body size are often greater in polygamous species as compared to closely related monogamous species; thus, competition among males may provide a selective force for the evolution of larger body sizes, with larger males gaining an advantage in aggressive encounters. Notably, among anthropoids, species that are monogamous (such as gibbons) tend to be monomorphic with respect to body size and dentition, whereas in polygamous species (such as baboons) males tend to have greater body mass and larger canines than females [54]. A relationship between mating system and sexual dimorphism may also extend to the brain. The SDN-POA and anteroventral periventricular nucleus are less sexually dimorphic in monogamous than in polygamous voles [111], but this type of observation has not been extended to other structures or to comparisons of other groups of monogamous and polygamous species.
In naked mole-rat colonies, male-male competition is likely to be minimal, since once a male becomes a breeder, nonbreeding males do not appear to challenge his status. While one might evoke the principle elaborated above to explain the lack of detectable sex difference in body size in naked mole-rats, the reduction of male-male competition does not seem adequate for explaining the wider range of sexual monomorphism in this species, extending to the behavioral and neural features discussed earlier. We feel that the high degree of reproductive skew, with most individuals of both sexes not engaged directly in reproduction, provides a more promising explanation for the much reduced sexual dimorphism in naked mole-rats.
7.2 Independence from hormones?
Several observations cited in this review indicate that behaviors and neural changes in eusocial naked mole-rats may be relatively independent from gonadal hormones. For example, some of the morphological changes seen in the brains of breeders (e.g., in the PVN) may be independent of hormones, at least in terms of maintenance. Furthermore, breeder naked mole-rats continue genital nuzzling for at least many weeks after both members of the pair have been gonadectomized [59]. It is possible that in these highly social animals, social cues can substitute for some “traditional” roles of gonadal hormones.
Recently, we have been investigating the role of gonadal hormones in the sex behavior of Damaraland mole-rats and find that both males and females of this species exhibit robust sex behavior for at least five months following gonadectomy. In one case, animals that were removed from their colonies and gonadectomized prior to pairing (i.e., these individuals presumably had no sexual experience prior to gonadectomy) exhibited repeated sex behaviors over a period of months. Sex behaviors were observed only when a test animal was paired with an animal from a different colony; non-breeders from the same colony (i.e., brother-sister pairs), isolated from each other for a few days, did not exhibit sex behaviors when paired. Persistence of sex behavior following gonadectomy has been reported for other male mammals, although this is usually seen only in sexually experienced males [7, 31, 89, 91]. It has been reported that 25% of sexually inexperienced male Siberian hamsters exhibit copulatory behavior for weeks post-castration, although this did not occur in males castrated prior to puberty [34]. Our preliminary observations of sexual behavior in ovariectomized female Damaraland mole-rats are even more surprising than the parallel observations in males, as we are not aware of any other rodent in which females continue to exhibit sex behavior in the absence of ovarian sex hormones. In fact, this is rare for female mammals in general, with the exception of some individual rabbits, some primate species (including humans), and species with dissociated reproductive patterns, whereby the season of gametogenesis is separated from the time when mating occurs [6, 15, 58].
8. Summary
The study of sexual differentiation of the mammalian nervous system has focused on a limited number of relatively non-social species where reproductive success is almost exclusively obtained through direct reproductive efforts. However, while social factors (e.g., identifying a suitable mate) are key regulators of reproduction in diverse vertebrate species, little is known about the “rules” of sexual differentiation in mammals with alternative reproductive strategies. Our work with two species of African mole-rats suggests that gonadal hormones may have relatively little influence on behavioral and anatomical parameters in these highly social species. Naked mole-rats lie at one extreme of the spectrum of sociality displayed by mammals, with larger colony sizes, greater genetic relatedness of colony members, and more marked reproductive skew than seen in any other social mammals. Yet many mammalian species, including humans, exhibit some degree of reproductive division of labor, in which some members of the social group reproduce while others assist in rearing young that are not their own. Extending our understanding of sexual differentiation to include more social species may illuminate the diverse mechanisms by which gonadal hormones and social factors, acting independently or in concert, sculpt the brain and behavior of mammals.
Figure 9.
Sex behavior in gonadectomized Damaraland mole-rats. In (A), the female (right) exhibits soliciting behavior as the male (left) sniffs her genital region. In (B), the male mounts and the female displays lordosis. The female had been ovariectomized for 7 months when this photograph was taken; the male had been castrated for 5 months. Both animals were nonbreeders when they were removed from different colonies, gonadectomized, and housed singly except during repeated behavioral tests. Photography by Virge Kask.
Acknowledgments
Studies performed in the authors’ laboratories were supported by the National Science Foundation (IOS-0642050 and IOB-0344312 to NGF and BDG), the National Institute of Mental Health (K02 MH072825 to NGF), the National Institute of Neurological Disorders and Stroke (F31 NS058258 to MLS), and the Canadian Institutes of Health Research (FRN 76508 to MMH).
Footnotes
Recent taxonomy studies suggest that Damaraland mole-rats might more properly be considered Fukomys damarensis [80, 123]. We use Cryptomys here to be consistent with the majority of the existing literature on this species.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander RD, Noonan KM, Crespi BJ. The evolution of eusociality. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; Princeton, NJ: 1991. pp. 3–44. [Google Scholar]
- 2.Allard MW, Honeycutt RL. Nucleotide sequence variation in the mitochondrial 12S rRNA gene and the phylogeny of African mole-rats (Rodentia: Bathyergidae) Mol Biol Evol. 1992;9:27–40. doi: 10.1093/oxfordjournals.molbev.a040706. [DOI] [PubMed] [Google Scholar]
- 3.Aoki S. Evolution of sterile soldiers in aphids. In: Ito Y, Brown JL, Kikkawa J, editors. Animal Societies: Theories and Facts. Japan Science Society Press; Tokyo: 1987. pp. 53–65. [Google Scholar]
- 4.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 5.Asa CS. Hormonal and experiential factors in the expression of social and parental behavior in canids. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 129–149. [Google Scholar]
- 6.Baum MJ, Slob AK, de Jong FH, Westbroek DL. Persistence of sexual behavior in ovariectomized stumptail macaques following dexamethasone treatment or adrenalectomy. Horm Behav. 1978;11:323–347. doi: 10.1016/0018-506x(78)90036-3. [DOI] [PubMed] [Google Scholar]
- 7.Beach FA. Coital behavior in dogs. VI. Long-term effects of castration upon mating in the male. J Comp Physiol Psychol. 1970;70:1–32. [PubMed] [Google Scholar]
- 8.Bennett NC. Reproductive suppression in social Cryptomys damarensis colonies: a lifetime of socially-induced sterillity in males and females. J Zool. 1994;234:25–39. [Google Scholar]
- 9.Bennett NC, Faulkes CG. In: Introduction to the Bathyergidae. Bennett NC, Faulkes CG, editors. Cambridge UP; Cambridge, UK: 2000. pp. 1–28. [Google Scholar]
- 10.Bennett NC, Faulkes CG. The evolution of sociality in African mole-rats. In: Bennett NC, Faulkes CG, editors. African Mole-Rats: Ecology and Eusociality. Cambridge UP; Cambridge, UK: 2000. pp. 211–245. [Google Scholar]
- 11.Bennett NC, Faulkes CG. Social organisation in African mole-rats. In: Bennett NC, Faulkes CG, editors. African Mole-Rats: Ecology and Eusociality. Cambridge UP; Cambridge, UK: 2000. pp. 87–126. [Google Scholar]
- 12.Bennett NC, Faulkes CG, Molteno AJ. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility. Proc Biol Sci. 1996;263:1599–1603. doi: 10.1098/rspb.1996.0234. [DOI] [PubMed] [Google Scholar]
- 13.Bennett NC, Jarvis JUM. The social structure and reproductive biology of colonies of the mole-rat, Cryptomys damarensis (Rodentia, Bathyergidae) J Mamm. 1988;69:293–302. [Google Scholar]
- 14.Bennett NC, Jarvis JUM, Faulkes CG, Millar RP. LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats, Cryptomys damarensis. J Reprod Fertil. 1993;99:81–86. doi: 10.1530/jrf.0.0990081. [DOI] [PubMed] [Google Scholar]
- 15.Beyer C, Cruz ML, Rivaud N. Persistence of sexual behavior in ovariectomized-adrenalectomized rabbits treated with cortisol. Endocrinology. 1969;85:790–793. doi: 10.1210/endo-85-4-790. [DOI] [PubMed] [Google Scholar]
- 16.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Braude S. Dispersal and new colony formation in wild naked mole-rats: evidence against inbreeding as the system of mating. Behav Ecol. 2000;11:7–12. [Google Scholar]
- 19.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 20.Bridges RS, Goldman BD. Diurnal rhythms in gonadotrophins and progesterone in lactating and photoperiod induced acyclic hamsters. Biol Reprod. 1975;13:617–622. doi: 10.1095/biolreprod13.5.617. [DOI] [PubMed] [Google Scholar]
- 21.Bronson FH. Seasonal regulation of reproduction in mammals. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; NY: 1988. pp. 1831–1871. [Google Scholar]
- 22.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 23.Burland TM, Bennett NC, Jarvis JU, Faulkes CG. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol Ecol. 2004;13:2371–2379. doi: 10.1111/j.1365-294X.2004.02233.x. [DOI] [PubMed] [Google Scholar]
- 24.Catania KC, Remple MS. Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain. Proc Natl Acad Sci U S A. 2002;99:5692–5697. doi: 10.1073/pnas.072097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 26.Ciszek D. New colony formation in the "highly inbred" eusocial naked mole-rat: outbreeding is preferred. Behav Ecol. 2000;11:1–6. [Google Scholar]
- 27.Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1998;265:1391–1399. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke FM, Faulkes CG. Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Anim Behav. 2001;61:311–324. [Google Scholar]
- 30.Clarke FM, Miethe GH, Bennett NC. Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis: dominant control or self-restraint? Proc Biol Sci. 2001;268:899–909. doi: 10.1098/rspb.2000.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemens LG, Wee BE, Weaver DR, Roy EJ, Goldman BD, Rakerd B. Retention of masculine sexual behavior following castration in male B6D2F1 mice. Physiol Behav. 1988;42:69–76. doi: 10.1016/0031-9384(88)90262-4. [DOI] [PubMed] [Google Scholar]
- 32.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96:7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooney R, Bennett NC. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc Biol Sci. 2000;267:801–806. doi: 10.1098/rspb.2000.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantini RM, Park JH, Beery AK, Paul MJ, Ko JJ, Zucker I. Post-castration retention of reproductive behavior and olfactory preferences in male Siberian hamsters: role of prior experience. Horm Behav. 2007;51:149–55. doi: 10.1016/j.yhbeh.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Creel SR, Waser PM. Variation in reproductive suppression among dwarf mongooses: Interplay between mechanisms and evolution. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 150–170. [Google Scholar]
- 36.Crespi BJ. Eusociality in Australian gall thrips. Nature. 1992;359:724–726. [Google Scholar]
- 37.Crespi BJ, Yanega D. The definition of eusociality. Behav Ecol. 1995;6:109–115. [Google Scholar]
- 38.Davis-Walton J, Sherman PW. Sleep arrhythmia in the eusocial naked mole-rat. Naturwissenschaften. 1994;81:272–275. doi: 10.1007/BF01131581. [DOI] [PubMed] [Google Scholar]
- 39.De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- 40.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 41.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erskine MS, Kornberg E. Acute luteinizing hormone and prolactin responses to paced mating stimulation in the estrous female rat. J Neuroendocrinology. 1992;4:173–179. doi: 10.1111/j.1365-2826.1992.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 43.Faulkes CG, Abbott DH. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1993;99:225–230. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- 44.Faulkes CG, Abbott DH. The physiology of a reproductive dictatorship: Regulation of male and female reproduction by a single breeding female in naked mole-rats. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 302–334. [Google Scholar]
- 45.Faulkes CG, Abbott DH, Jarvis JU. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- 46.Faulkes CG, Abbott DH, Jarvis JU, Sherriff FE. LH responses of female naked mole-rat, Heterocephalus glaber, to single and multiple doses of exogenous GnRH. J Reprod Fertil. 1990;89:317–23. doi: 10.1530/jrf.0.0890317. [DOI] [PubMed] [Google Scholar]
- 47.Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. Reprod Fert. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- 48.Faulkes CG, Abbott DH, Liddell CE, George LM, Jarvis JUM. Hormonal and behavioral aspects of reproductive suppression in female naked mole-rats, Heterocephalus glaber. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-rat. Princeton University Press; Princeton, NJ: 1991. [Google Scholar]
- 49.Faulkes CG, Abbott DH, O'Brien HP, Lau L, Roy MR, Wayne RK, Bruford MW. Micro- and macrogeographical genetic structure of colonies of naked mole-rats Heterocephalus glaber. Mol Ecol. 1997;6:615–628. doi: 10.1046/j.1365-294x.1997.00227.x. [DOI] [PubMed] [Google Scholar]
- 50.Faulkes CG, Trowell SN, Jarvis JU, Bennett NC. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of two eusocial African mole-rats, the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis) J Reprod Fertil. 1994;100:411–6. doi: 10.1530/jrf.0.1000411. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Fittkau OH, Klinge H. On biomass and trophic structure of the central Amazonian rain forest ecosystems. Biotropica. 1973;5:2–14. [Google Scholar]
- 54.Fleagle JG. Primate Adaptation and Evolution. Academic Press; 1999. [Google Scholar]
- 55.Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: role of early androgen. Proc Natl Acad Sci U S A. 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol. 1996;375:333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 57.French JA. Proximate regulation of singular breeding in Callitrichid primates. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 34–75. [Google Scholar]
- 58.Goldfoot DA, Wiegand SJ, Scheffler G. Continued copulation of ovariectomized adrenal-suppressed stumptail macaques (Macaca arctoides) Horm Behav. 1978;11:89–99. doi: 10.1016/0018-506x(78)90060-0. [DOI] [PubMed] [Google Scholar]
- 59.Goldman SL, Forger NG, Goldman BD. Influence of gonadal sex hormones on behavioral components of the reproductive hierarchy in naked mole-rats. Horm Behav. 2006;50:77–84. doi: 10.1016/j.yhbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 61.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton WD. The genetical evolution of social behaviour I and II. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 63.Hatchwell BJ, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. [DOI] [PubMed] [Google Scholar]
- 64.Hazell RWA. Adult dispersal in the co-operatively breeding Damaraland mole-rat (Cryptomys damarensis): a case study from the Waterbury region of Namibia. J Zool. 2000;252:19–25. [Google Scholar]
- 65.Henry EC, Dengler-Crish CM, Catania KC. Growing out of a caste--reproduction and the making of the queen mole-rat. J Exp Biol. 2007;210:261–268. doi: 10.1242/jeb.02631. [DOI] [PubMed] [Google Scholar]
- 66.Holmes MM, Goldman BD, Forger NG. Social status and sex independently influence androgen receptor expression in the eusocial naked mole-rat brain. Horm Behav. 2008;54:278–285. doi: 10.1016/j.yhbeh.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci U S A. 2007;104:10548–10552. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson CR, Bennett NC. Is the natal mole-rat (Cryptomys hottentotus natalensis) a spontaneous or induced ovulator? J Mamm. 2005;86:1–6. [Google Scholar]
- 70.Jacobs DS, Kuiper S. Individual recognition in the Damaraland mole-rat, Cryptomys damarensis (Rodentia: Bathyergidae) J Zool Lond. 2000;251:41–45. [Google Scholar]
- 71.Jacobs DS, Reid S, Kuiper S. Out-breeding behaviour and xenophobia in the Damaraland mole-rat, Cryptomys damarensis. S Afr J Zool. 1998;33:189–194. [Google Scholar]
- 72.Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 73.Jarvis JUM. Reproduction in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-rat. Princeton University Press; Princeton, NJ: 1991. pp. 384–425. [Google Scholar]
- 74.Jarvis JUM, Bennett NC. Eusociality has evolved independently in two genera of Bathyergid mole-rats -- but occurs in no other subterranean mammal. Behav Ecol Sociobiol. 1993;33:253–260. [Google Scholar]
- 75.Jarvis JUM, O'Riain MJ, Bennett NC, Sherman PW. Mammalian eusociality: a family affair. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 76.Kalamatianos T, Hart L, Faulkes CG, Bennett NC, Coen CW. Telencephalic binding sites for oxytocin reflect social organization: evidence from eusocial naked mole-rats and solitary Cape mole-rats. Society for Neuroscience; San Diego: 2007. [Google Scholar]
- 77.Kalamatianos T, Poorun R, Faulkes CG, Bennett NC, Coen CW. Species differences in telencephalic binding sites for corticotrophin-releasing factor receptor subtypes 1 and 2: a study on eusocial naked mole-rats and solitary Cape mole-rats. Society for Neuroscience; Washington, DC: 2008. [Google Scholar]
- 78.Kent DS, Simpson JA. Eusociality in the beetle Austroplatypus incomperus (Coleoptera: Curculiodae) Naturwissenschaften. 1992;79:86–87. [Google Scholar]
- 79.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 80.Kock D, Ingram CM, Frabotta LJ, Honeycutt RL, Burda H. On the nomenclature of Bathyergidae and Fukomys n. gen. (Mammalia: Rodentia) Zootaxa. 2006;1142:51–55. [Google Scholar]
- 81.Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JUM. An ethogram for the naked mole-rat: Nonvocal behaviors. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-rat. Princeton University Press; Princeton, NJ: 1991. pp. 209–242. [Google Scholar]
- 82.Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: evidence for division of labor. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-rat. Princeton UP; Princeton: 1991. pp. 275–336. [Google Scholar]
- 83.Lacey EA, Sherman PW. Cooperative breeding in naked mole-rats: Implications for vertebrate and invertebrate sociality. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 267–301. [Google Scholar]
- 84.Libersky EA, Boatman DE. Progesterone concentrations in serum, follicular fluid, and oviductal fluid of the golden hamster during the periovulatory period. Biol Reprod. 1995;53:477–482. doi: 10.1095/biolreprod53.3.477. [DOI] [PubMed] [Google Scholar]
- 85.Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- 86.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 88.Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- 89.Manning A, Thompson ML. Postcastration retention of sexual behaviour in the male BDF1 mouse: the role of experience. Anim Behav. 1976;24:523–533. doi: 10.1016/s0003-3472(76)80065-6. [DOI] [PubMed] [Google Scholar]
- 90.Margulis SW, Saltzman W, Abbott DH. Behavioral and hormonal changes in female naked mole-rats (Heterocephalus glaber) following removal of the breeding female from a colony. Horm Behav. 1995;29:227–47. doi: 10.1006/hbeh.1995.1017. [DOI] [PubMed] [Google Scholar]
- 91.McGill TE, Manning A. Genotype and retention of the ejaculatory reflex in castrated male mice. Anim Behav. 1976;24:507–518. doi: 10.1016/s0003-3472(76)80063-2. [DOI] [PubMed] [Google Scholar]
- 92.Michener CD. Comparative social behavior of bees. Annu Rev Entomol. 1969;14:299–342. [Google Scholar]
- 93.Moehlman PD, Hofer H. Cooperative breeding, reproductive suppression, and body mass in canids. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 76–128. [Google Scholar]
- 94.Molteno AJ, Bennett NC. Anovulation in non-reproductive female Damaraland mole-rats (Cryptomys damarensis) J Reprod Fertil. 2000;119:35–41. doi: 10.1530/jrf.0.1190035. [DOI] [PubMed] [Google Scholar]
- 95.Moss RL, Cooper KJ. Temporal relationship of spontaneous and coitus-induced release of luteinizing hormone in the normal cyclic rat. Endocrinology. 1973;92:1748–1753. doi: 10.1210/endo-92-6-1748. [DOI] [PubMed] [Google Scholar]
- 96.O'Riain MJ, Jarvis JU, Alexander R, Buffenstein R, Peeters C. Morphological castes in a vertebrate. Proc Natl Acad Sci U S A. 2000;97:13194–13197. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Riain MJ, Jarvis JU, Faulkes CG. A dispersive morph in the naked mole-rat. Nature. 1996;380:619–21. doi: 10.1038/380619a0. [DOI] [PubMed] [Google Scholar]
- 98.O'Riain MJ, Jarvis JUM. Colony member recognition and xenophobia in the naked mole-rat. Anim Behav. 1997;53:487–498. [Google Scholar]
- 99.Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; NY: 1988. pp. 1699–1737. [Google Scholar]
- 100.Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 101.Pepper JW, Braude SH, Lacey EA, Sherman PW. Vocalizations of the naked mole-rat. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-rat. Princeton University Press; Princeton, NJ: 1991. pp. 243–274. [Google Scholar]
- 102.Peroulakis ME, Goldman B, Forger NG. Perineal muscles and motoneurons are sexually monomorphic in the naked mole-rat (Heterocephalus glaber) J Neurobiol. 2002;51:33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- 103.Reeve HK. Queen activation of lazy workers in colonies of the eusocial naked mole-rat. Nature. 1992;358:147–9. doi: 10.1038/358147a0. [DOI] [PubMed] [Google Scholar]
- 104.Riccio AP, Goldman BD. Circadian rhythms of locomotor activity in naked mole-rats (Heterocephalus glaber) Physiol Behav. 2000;71:1–13. doi: 10.1016/s0031-9384(00)00281-x. [DOI] [PubMed] [Google Scholar]
- 105.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 106.Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J Comp Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- 107.Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–817. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature. 2006;440:795–797. doi: 10.1038/nature04578. [DOI] [PubMed] [Google Scholar]
- 109.Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol Reprod. 1975;12:223–231. doi: 10.1095/biolreprod12.2.223. [DOI] [PubMed] [Google Scholar]
- 110.Seney M, Goldman BD, Forger NG. Breeding status affects motoneuron number and muscle size in naked mole-rats: recruitment of perineal motoneurons? J Neurobiol. 2006;66:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- 111.Shapiro LE, Leonard CM, Sessions CE, Dewsbury DA, Insel TR. Comparative neuroanatomy of the sexually dimorphic hypothalamus in monogamous and polygamous voles. Brain Res. 1991;541:232–240. doi: 10.1016/0006-8993(91)91023-t. [DOI] [PubMed] [Google Scholar]
- 112.Sherman PW, Jarvis JUM, Alexander RD. Preface. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; Princeton, NJ: 1991. pp. vii–xii. [Google Scholar]
- 113.Sherman PW, Lacey EA, Reeve HK, Keller L. The eusociality continuum. Behav Ecol. 1995;6:102–108. [Google Scholar]
- 114.Smith TE, Faulkes CG, Abbott DH. Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole-rats (Heterocephalus glaber) Horm Behav. 1997;31:277–88. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- 115.Snyman PC, Jackson CR, Bennett NC. Do dispersing non-reproductive female Damaraland mole-rats, Cryptomys damarensis (Rodentia: Bathyergidae) exhibit spontaneous or induced ovulation? Physiol Behav. 2006;87:88–94. doi: 10.1016/j.physbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Solomon NG, Getz LL. Examination of alternative hypotheses for cooperative breeding in rodents. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 199–230. [Google Scholar]
- 117.Tardif SD. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; UK: 1997. pp. 11–33. [Google Scholar]
- 118.Terranova PF, Greenwald GS. Steroid and gonadotropin levels during the luteal-follicular shift of the cyclic hamster. Biol Reprod. 1978;18:170–175. doi: 10.1095/biolreprod18.2.170. [DOI] [PubMed] [Google Scholar]
- 119.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 120.Ueyama T, Mizuno N, Takahashi O, Nomura S, Arakawa H, Matsushima R. Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol. 1985;232:548–56. doi: 10.1002/cne.902320411. [DOI] [PubMed] [Google Scholar]
- 121.Ulibarri C, Popper P, Micevych PE. Motoneurons dorsolateral to the central canal innervate perineal muscles in the Mongolian gerbil. J Comp Neurol. 1995;356:225–37. doi: 10.1002/cne.903560207. [DOI] [PubMed] [Google Scholar]
- 122.van Sandwyk JHT, Bennett NC. Do solitary, seismic signalling Cape mole-rat (Georychus capensis) demonstrate spontaneous or induced ovulation? J Zool. 2005;267:75–80. [Google Scholar]
- 123.Vandaele P, Verheyen E, Brunain M, Adriaens D. Cytochrome b sequence analysis reveals differential molecular evolution in African mole-rats of the chromosomally hyperdiverse genus Fukomys (Bathyergidae, Rodentia) from the Zambezian region. Mol Phylogenetics Evol. 2007;45:142–157. doi: 10.1016/j.ympev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Vollrath F. Eusociality and extraordinary sex ratios in the spider Anelosimus eximius (Araneae: Theridiidae) Behav Ecol Sociobiol. 1986;18:283–287. [Google Scholar]
- 125.Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 126.Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- 127.Weir BJ, Rowlands IW. Reproductive strategies of mammals. Ann Rev Ecol Syst. 1973;4:139–163. [Google Scholar]
- 128.Wilkinson GS, South JM. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 129.Wilson EO. The Insect Societies. Belknap Press of Harvard University Press; Cambridge, MA: 1971. [Google Scholar]
- 130.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 131.Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999;39:359–370. doi: 10.1002/(sici)1097-4695(19990605)39:3<359::aid-neu3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 132.Xiao J. A new coordinate system for rodent brain and variability in the brain weights and dimensions of different ages in the naked mole-rat. J Neurosci Methods. 2007;162:162–170. doi: 10.1016/j.jneumeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Xiao J, Levitt JB, Buffenstein R. A stereotaxic atlas of the brain of the naked mole-rat (Heterocephalus glaber) Neurosci. 2006;141:1415–1435. doi: 10.1016/j.neuroscience.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 134.Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–7. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
- 135.Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]