Abstract
The medial prefrontal cortex (mPFC) has been implicated as a site of dysfunction and abnormal morphology in major depressive disorder and post-traumatic stress disorder, two illnesses that can be brought on by exposure to stress. In animal models, stress has long been shown to induce impairments in tasks known to be mediated by the mPFC, and recent work has demonstrated that chronic stress can lead to morphological changes in mPFC pyramidal cells. This review explores the current literature on stress-induced dendritic remodeling in the mPFC, with particular focus on new findings that illuminate modulators of these effects.
Introduction
Stressful experiences can precipitate or exacerbate mental illnesses such as major depressive disorder (MDD; (Lechin et al., 1996) and post-traumatic stress disorder (PTSD, (Turner and Lloyd, 2004), but the mechanisms by which the effects of stress contribute to the development of clinical pathologies are not well understood. Both postmortem and functional imaging studies of patients with these disorders implicate the medial prefrontal cortex (mPFC) as a site of abnormal structure and function, suggesting a potential vulnerability to stress in this region. Specifically, the brains of suicide victims--who are likely to be depressed (Hovanesian et al., 2009)--are reported to have decreased neuron and glia density compared to healthy controls (Rajkowska et al., 2001), and mPFC activity is suppressed in patients with MDD (Liberzon and Phan, 2003; Pizzagalli et al., 2004). Common symptoms of MDD and PTSD—rumination, poor concentration and negative affect, for example—also suggest mPFC dysfunction (Arnsten, 1998). Together, these findings identify the mPFC as a key player in the manifestation of stress-related mental illnesses, and an understanding of the effects of stress in this region will be critical to our progress toward better treatments.
Stress-induced dendritic remodeling in the mPFC
Though the mechanisms by which stress exposure can lead to clinical pathology are not clear, much work has been done in animals in an attempt to identify possible contributing factors. Animal models of chronic stress commonly involve subjecting an animal to daily restraint or immobilization, a regimen that has been reliably shown to activate the hypothalamic-pituitary-adrenal (HPA) axis, induce corticosterone release and cause morphological changes in several brain regions (reviewed in(McEwen, 1999). In the rat mPFC, repeated restraint stress can cause retraction of the apical dendritic arbor in layer II/III pyramidal cells, an effect that has been demonstrated in the anterior cingulate (AC), prelimbic (PL; (Izquierdo et al., 2006; Radley et al., 2004) and infralimbic (IL; (Shansky et al., 2009) subregions (Fig 1). These changes in dendritic length have been shown to be accompanied by impairments in cognitive tasks selectively mediated by the mPFC while sparing non-mPFC-specific abilities (Liston et al., 2006), suggesting that stress-induced remodeling in the mPFC may have distinct functional consequences.
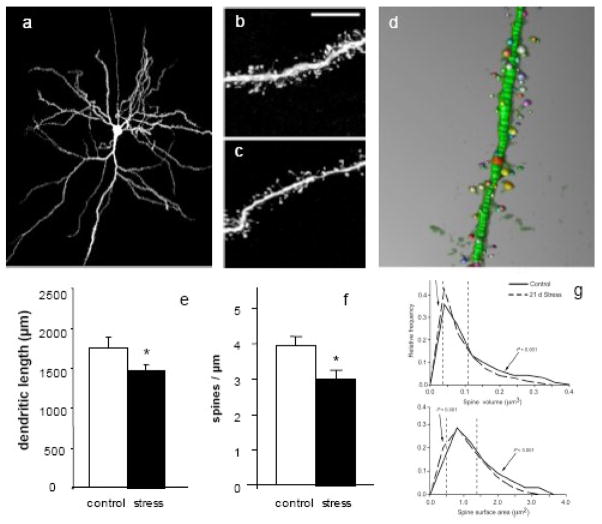
Figure 1. Chronic restraint stress causes dendritic remodeling in mPFC dendrites and spines.
(a) a layer II/III pyramidal mPFC neuron filled with Lucifer Yellow to allow for dendrite visualization. (b) and (c) dendrite segments from control and stress groups, demonstrating spine loss. (d) NeuronStudio software reconstruction of a dendritic segment, demonstrating spine size measurements. (e) and (f) 21 days restraint stress causes significant decrease in total apical dendrite length and in spine density. (g) Chronic restraint stress also causes a shift in spine size, with dendrites exhibiting a smaller percentage of large spines. Adapted from (Radley et al., 2004), (Radley et al., 2006) and (Radley et al., 2008). * p < 0.05
In addition to dendritic length changes, chronic stress has been shown to induce alterations in mPFC spines on the same neuronal populations that exhibit dendritic remodeling. In the AC and PL, spine density is reduced by 16% in chronically stressed animals (Fig 1), which, when combined with the decrease in dendritic arborization, translates into a 30% loss of spines on each neuron (Radley et al., 2006). Recent data suggest that spine morphology, e.g. size, may be a critical reflection of capacity for synaptic plasticity (Kasai et al., 2003); thus in addition to overall spine number, it is important to determine which spine classes are preferentially affected. More specifically, it has been proposed that small spines are highly motile and transient, capable of expansion and consolidation under certain circumstances or retraction, while large spines are viewed as more stable and likely to mediate long-term well established neural networks (Kasai et al., 2003). A recent analysis of spine volume and surface area in the mPFC neurons affected by stress revealed that the spine loss can be accounted for primarily by a reduction in large (> 1.0 μm2 and/or > 0.2 μm3) spines (Radley et al., 2008) (Fig 1). Since it is unlikely that these large stable spines would retract with stress, this shift in the size profile of spines was interpreted as a reflection of decreased capacity for the maturation and stabilization of small spines into large stable spines (Radley et al., 2008). This selective decrease in large spines is notably in opposition to age-related spine loss in PFC of non-human primates in which mainly small spines are vulnerable (Hao et al., 2006). Such considerable spine changes likely alter a neuron’s connectivity and functionality, and it has been speculated that while large spines are needed for memory consolidation, small spines are required for neural plasticity and active learning (Kasai et al., 2003). Consistent with this, aged non-human primates show impaired cognitive flexibility in a PFC-mediated task (Hao et al., 2007). In demonstrating the robust effects of chronic stress on the dendritic structure of neurons in the mPFC of rats, these studies lay the groundwork for investigations into the nuances and functional implications of spine dynamics as they relate to behavioral alterations.
Circuit-Specific Stress Effects
One avenue of recent exploration is the potential for circuit-specific responses to stress. The mPFC maintains extensive connections with the amygdala, a brain region known to mediate the expression of fear and memory for aversive stimuli (Hendler et al., 2003; Wright et al., 2003). Physiological studies have shown that the mPFC can suppress amygdala activity (Sotres-Bayon et al., 2004), and it is through this pathway that the mPFC likely regulates emotional behavior and inhibits inappropriate responses (e.g., an unwarranted stress response). A compromise in the integrity of this pathway may underlie some of the symptoms of stress-related mental illnesses, and thus an understanding of the effects of stress on mPFC-amygdala projection neurons could help illuminate the nature of these disorders.
Recent work has tested the idea that this pathway may have a unique response to stress (Shansky et al., 2009). Using a retrograde tracer to identify mPFC neurons that project to the basal nucleus of the amygdala (BLA) combined with Lucifer-Yellow cell loading (Fig 2a), the effects of chronic immobilization stress in this neuronal subset were compared with those in mPFC neurons that are selected at random. As has been demonstrated previously, randomly-selected neurons underwent dendritic retraction with stress (Fig 2d). Since most layer II/III neurons project inter-cortically, we assume that the majority of randomly selected pyramidal cells neurons are cortico-cortical. However, BLA-projecting neurons remained morphologically unchanged (Fig 2e), suggesting, somewhat surprisingly, that this pathway may be particularly resilient against the effects of stress. This resilience may reflect a “healthy” disposition; while MDD not uncommon, people endure stressful experiences without developing a clinical disorder. Thus, the mPFC-BLA pathway may be protected from the effects of stress under normal circumstances, but particularly vulnerable when predisposing factors are present.
Figure 2. The effects of chronic stress in the mPFC are circuit- and estrogen-dependent.
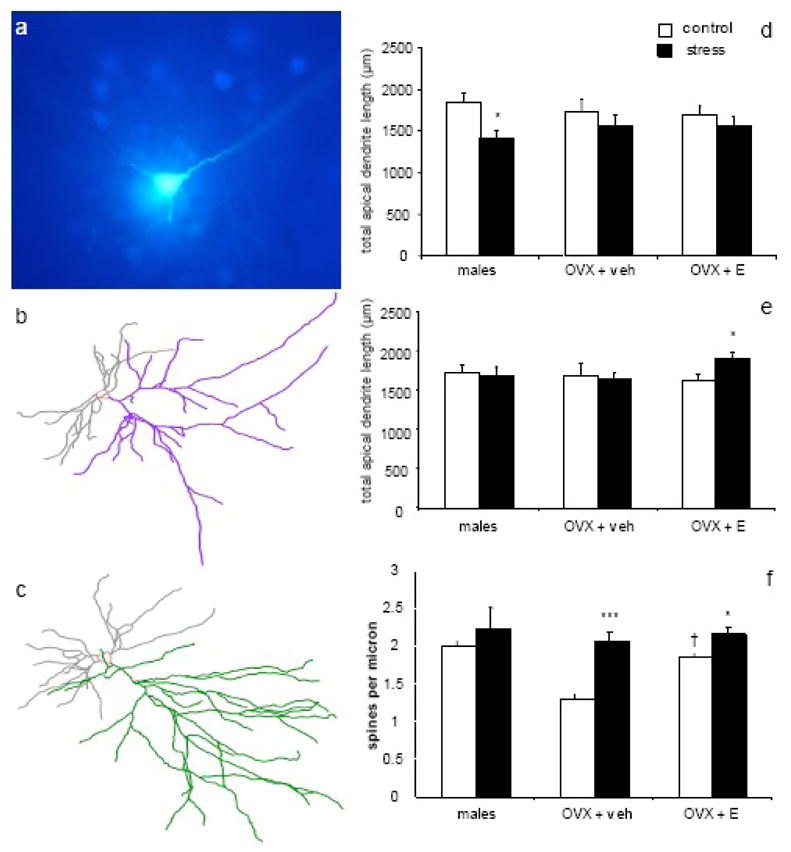
(a) A Lucifer Yellow-filled mPFC pyramidal cell surrounded by FastBlue-labeled cells, demonstrating the technique for selectively loading BLA-projecting neurons. Representative Neurolucida tracings of BLA-projecting neurons from OVX + E control (b) and stress (c) groups. In randomly-selected neurons (d), males, but not females, displayed stress-induced dendritic retraction. However, in BLA-projecting neurons (e), males and OVX + veh showed no stress-induced changes, while OVX + E exhibited stress-induced dendritic expansion. Stress-induced increases in spine density (f) were seen in BLA-projecting neurons of both OVX + veh and OVX + E but not males, and estrogen alone increased spine density in control animals. * p < 0.05 compared to same-group control; *** p < 0.0001 compared to same-group control; † p < 0.05 compared to OVX + veh control. Adapted from (Shansky et al., 2009) and Shansky et al, unpublished observations.
Estrogen-Stress Interactions
Women are twice as likely as men to develop both MDD and PTSD (Weissman et al., 1996), a discrepancy that is most robust during childbearing years (Bebbington et al., 2003). This phenomenon suggests that circulating estrogen may impart a sensitivity to the detrimental effects of stress, but this hypothesis has not been thoroughly tested. To date, experimental evidence indicates that estrogen’s influence on the stress response varies by brain region, conferring protection from stress-induced cognitive impairments (Conrad et al., 2004) (Bowman et al., 2002) and dendritic remodeling in some structures (Galea et al., 1997), but sensitivity in others (Shansky et al., 2004).
Very little is known about estrogen-stress interactions in the mPFC, but recent work suggests that this specificity is reflected at the level of the circuit as well (unpublished observations). When ovariectomized (OVX) female rats with and without estrogen treatment (OVX + E and OVX + veh, respectively) were tested in the retrograde tracer/chronic stress experiment described above, IL neurons responded in a pattern wholly opposite from that of male rats. While no dendritic remodeling was observed in OVX + veh animals regardless of neuron set (randomly-selected or BLA-projecting), OVX + E exhibited a stress-induced increase in dendritic arborization exclusively in the BLA-projecting neurons (Fig 2b–e). That no remodeling was observed in randomly-selected neurons in either treatment group (unlike males, whose randomly-selected neurons underwent significant retraction) suggests that the mPFC in females may be, in general, protected from the morphological effects of stress. However, the stress-induced increase in arborization in BLA-projecting neurons of OVX + E animals indicates that estrogen may confer a sensitivity to the effects of stress in a circuit that is particularly relevant to stress-related disorders.
These studies also revealed several novel findings regarding the effects of estrogen and stress on spine density in the mPFC. While stress had no effect on spine density in males, it caused an increase in spine density in OVX + veh in all neurons, and an increase in spine density in OVX + E in BLA-projecting neurons only, again indicating a circuit-specific interaction of estrogen and stress (Fig 2f). Because BLA-projecting neurons in stressed OVX + E animals not only have longer dendrites but also more spines per micron, each neuron then has substantially more total spines than control neurons. Together, these findings suggest that the subset of IL neurons that project to the BLA is particularly responsive to the effects of stress when estrogen is present. Though the functional implications of these phenomena remain to be explored, that estrogen can impart such a specific set of stress effects in females emphasizes the need to approach future studies with such potential hormonal interactions in mind. Given the gender discrepancy in the prevalence of stress-related disorders, these findings may help identify new therapeutic targets for women.
Reversal of Dendritic Remodeling
Studies of the biology underlying stress’s effects in the brain are presumably done with the expectation that ultimately a way of preventing or reversing these effects will be found. Though lithium treatment has been shown to prevent stress-induced dendritic remodeling in hippocampal neurons (Wood et al., 2004), the ability of pharmacological agents to prevent or reverse this remodeling in the mPFC has not yet been tested. However, an extended period of rest following chronic stress exposure has been shown to induce potentially compensatory dendrite growth in mPFC neurons (Radley et al., 2005). As described above, male rats that are stressed for 21 days will show retraction in apical dendrites. But if these rats are allowed to remain undisturbed in their cages for another 21 days after the cessation of stress, these neurons will re-extend their branches, suggesting that rest alone may be sufficient to reverse the effects of stress in this region. Interestingly, this recovery period has been reported not to reverse stress-induced dendritic remodeling in the amygdala (Vyas et al., 2004). Whether this recovery is sufficient to restore the functional consequences of chronic stress is currently under investigation; preliminary work has found that while stress can reduce the dopamine D1 receptor population and suppress dopamine’s ability to enhance LTP in the mPFC, a rest period can restore both of these parameters to control levels (unpublished observations).
Functional and Clinical Implications
The studies described above reveal a remarkable degree of plasticity in the pyramidal neurons in the mPFC, capable of both expansion and retraction in response to stress, as well as re-expansion after a post-stress recovery period. The functional implications of these morphological changes remain unclear, however, especially with regards to whether retraction and expansion reflect detrimental or beneficial responses to stress. Common wisdom may dictate that dendritic loss be interpreted as maladaptive, while dendritic growth adaptive. Indeed, evidence in both hippocampal and mPFC neurons demonstrates that stress-induced dendritic retraction and spine loss are accompanied by cognitive impairments in tasks mediated by each respective structure (Conrad, 2006; Liston et al., 2006). Moreover, the function of brain structures that show stress-induced dendritc expansion or that are spared from the morphological effects of stress altogether appears not to be compromised after stress (Conrad et al., 1999).
However, the circuit specificity with which the mPFC responds to stress may suggest that the relationship between morphology changes and neuronal function is not so simple. Behaviorally, the projection pathway from the IL to the BLA is known to mediate the recall of extinction following fear conditioning (Lebron et al., 2004; Morgan et al., 1993) (Milad and Quirk, 2002), a task consistent with the mPFC’s regulation of amygdala activity and suppression of inappropriate responses. Extinction recall has been reported to be impaired after chronic stress (Miracle et al., 2006), but since BLA-projecting IL neurons appear to be resilient against the morphological effects of stress, this impairment is likely due to other stress-related changes. Additionally, the stress-induced expansion in BLA-projecting IL neurons of estrogen-treated females may indicate a potential for over-stimulation, and thus possible dysfunction. These hypotheses will likely need to be tested using careful physiology techniques in order for more confident causal claims to be made.
The cumulative findings here demonstrate that the morphological effects of stress in the mPFC are complex, varying with circuit, sex, hormonal status, and opportunity for recovery. These factors, then, must be taken into consideration in future studies, if progress in the treatment of stress-related disorders is to be made.
Future Directions
The degree to which the morphology of pyramidal neurons in mPFC can be altered by stress has been recognized for a fairly short period of time, and the heterogeneity of neuronal responses described above is only beginning to be revealed. Thus it is not surprising that the data summarized above raise numerous questions worthy of pursuit. For example, while we refer to the dendritic extension with rest as recovery, we know nothing about the degree to which the synaptic inputs and receptor profile of these spines are equivalent to controls (i.e., never stressed) or altered in a manner that changes the character of axospinous synapses on the affected dendrites. In addition, we do not know if the behavior altered by stress recovers as the neurons recover. In a related manner, we know little about the molecular mechanisms of dendritic retraction with stress and even less about the mechanisms for extension with rest. With increased knowledge of the mechanisms of retraction and expansion, we could presumably design interventions that could modulate both the response to stress and the potential for recovery. In addition, while it appears that the neuronal effects of stress are related to gender and the presence of estrogen, how do these neuronal effects impact behavior and are the behavioral effects gender-specific and estrogen-dependent? Finally, does the occurrence of stress and recovery alter the neurons’ future response to stress, capacity for recovery, or vulnerability to aging? All of these questions are addressable with current methodology and as the answers emerge, it will be critically important to develop therapeutic strategies that target and harness neuronal plasticity for protection against and recovery from affective disorders in humans.
Acknowledgments
The authors would like to thank Bill Janssen, Deena Goldwater and Carine Hamo for their contributions to the work described here.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int Rev Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacology, Biochemistry and Behavior. 2004;78:569–79. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–70. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Hovanesian S, Isakov I, Cervellione KL. Defense mechanisms and suicide risk in major depression. Arch Suicide Res. 2009;13:74–86. doi: 10.1080/13811110802572171. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–8. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–8. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Lechin F, Van der Dijs B, Benaim M. Stress versus depression. Progress in Neuropsychopharmacological and Biological Psychiatry. 1996;20:899–950. doi: 10.1016/0278-5846(96)00075-9. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–50. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–8. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–13. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–50. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Molecular Psychiatry. 2004;9:531–8. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–35. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Archives of General Psychiatry. 2004;61:481–8. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–9. [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–8. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–76. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]