Abstract
Alterations in mitochondrial biology have long been implicated in neurotoxin, and more recently, genetic models of parkinsonian neurodegeneration. In particular, kinase regulation of mitochondrial dynamics and turnover are emerging as central mechanisms at the convergence of neurotoxin, environmental and genetic approaches to studying Parkinson's disease (PD). Kinases that localize to mitochondria during neuronal injury include mitogen activated protein kinases (MAPK) such as extracellular signal regulated protein kinases (ERK) and c-Jun N-terminal kinases (JNK), protein kinase B/Akt, and PTEN-induced kinase 1 (PINK1). Although site(s) of action within mitochondria and specific kinase targets are still unclear, these signaling pathways regulate mitochondrial respiration, transport, fission-fusion, calcium buffering, reactive oxygen species (ROS) production, mitochondrial autophagy and apoptotic cell death. In this review, we summarize accelerating experimental evidence gathered over the last decade that implicate a central role for kinase signaling at the mitochondrion in Parkinson's and related neurodegenerative disorders. Interactions involving α-synuclein, leucine rich repeat kinase 2 (LRRK2), DJ-1 and parkin are discussed. Converging mechanisms from different model systems support the concept of common pathways in parkinsonian neurodegeneration that may be amenable to future therapeutic interventions.
Keywords: autophagy, kinases, mitochondria, neurodegeneration, oxidative stress, Parkinson's disease
1. Introduction
The mitochondrion plays a central role in most eukaryotic metabolic processes. In addition to serving as “powerhouses” to produce the majority of cellular ATP, mitochondria buffer intracellular calcium levels, regulate lipid metabolism, integrate metabolic and apoptotic signaling pathways and represent the major source of intracellular reactive oxygen species (ROS). Mitochondria are dynamic organelles that exhibit bidirectional motility within neurons and plasticity to undergo extensive shape changes mediated by GTPases of the mitochondrial fission/fusion machinery (MFF) (Karbowski & Youle 2003). In neurons, MFF-dependent transport of mitochondria to dendrites promotes synaptogenesis, while axonal transport of mitochondria to pre-synaptic sites regulates the refilling of neurotransmitter pools (Li et al. 2004, Verstreken et al. 2005). Given the key role of mitochondria in neuronal function (Mandemakerset al. 2007), it is not surprising that disturbances in mitochondrial function, transport, dynamics and turnover have emerged as central mechanisms at the convergence of neurotoxin, environmental and genetic approaches to Parkinson's disease.
2. Mitochondrial dysregulation in Parkinson's and related parkinsonian disorders (PD)
Parkinson's disease is a debilitating, progressive movement disorder that affects ∼1 million people in North America. The major motor symptoms can be attributed to degeneration of endogenously pigmented midbrain neurons of the nigrostriatal projection, while involvement of other neuronal populations result in olfactory, autonomic and cognitive dysfunction. While most cases have no known cause, oxidative stress, disordered protein handling/degradation, and mitochondrial dysfunction are mechanistically implicated in sporadic PD, in parkinsonism due to toxin/pesticide exposures, and in several models of familial PD (Giasson et al. 2000, Munch et al. 2000, Betarbet et al. 2002, Dawson & Dawson 2003).
Decreased mitochondrial complex I function has been observed in post-mortem PD midbrain tissues (Schapira et al. 1990) and in cybrid cells containing PD patient mitochondria (Swerdlow et al. 1996). These data suggest a role for mitochondrial DNA (mtDNA) alterations (Gu et al. 2002), although distinguishing potential causative changes remains elusive given the frequency of similar mutations in elderly controls (Simon et al. 2004). Cybrid PD lines exhibit rounded, swollen mitochondria with rarefied cristae (Trimmer et al. 2000) similar to autophagocytosed mitochondria observed in association with phosphorylated mitogen activated protein kinases (MAPK) in PD/Lewy body disease substantia nigra neurons (Zhu et al. 2003). Since substantia nigra DA neurons exhibit decreased basal mitochondrial content compared to other midbrain neurons, it has been proposed that diminished mitochondrial reserves may render them more susceptible to compromise of mitochondrial homeostasis during PD pathogenesis (Liang et al. 2007).
2.1 Mitochondria in toxin models of PD
Mitochondria are central to the actions of diverse neurotoxins that preferentially injure dopaminergic neurons. The heroin contaminant 1-methyl-4-phenyl-1,2,3,6-tetrahydroxypyridine (MPTP) causes acute parkinsonian intoxication, and represents one of the earlier models of parkinsonian neuronal injury (Przedborski & Jackson-Lewis 1998). The active metabolite MPP+ is a mitochondrial complex I inhibitor (Sherer et al. 2002, Brill & Bennett 2003), as is the pesticide rotenone used to model environmental contributions to PD (Betarbet et al. 2002). 6-OHDA is a redox-active dopamine analog used widely to lesion the DA nigrostriatal system (Zigmond & Keefe 1997). While there are differences in cell death mechanisms elicited by MPP+ and 6-OHDA (Choi et al. 1999, Chu et al. 2005), mitochondrial oxidative stress (Klivenyi et al. 1998, Callio et al. 2005), mitogen activated protein kinases (Kulich & Chu 2001, Kuan & Burke 2005, Zhu et al. 2007), endoplasmic reticulum stress (Ryu et al. 2002), and mitochondrial autophagy (Zhu et al. 2007, Dagda et al. 2008) have emerged as common factors.
2.2 Mitochondria in genetic models of PD
The discovery of α-synuclein mutations and later, gene multiplication (Polymeropoulos et al. 1997, Singleton et al. 2003), as causes of autosomal dominant forms of PD triggered a decade of additional gene discoveries and new efforts to model parkinsonian neurodegeneration. α-Synuclein aggregation and Lewy bodies are observed in both sporadic and dominant forms of PD. The leucine rich repeat kinase 2 (LRRK2) is the most commonly mutated gene in both familial and sporadic settings (Kachergus et al. 2005), accounting for up to a third of cases in some populations. Proteins involved in autosomal recessive parkinsonism include parkin, ATP13A2, DJ-1, and PTEN-induced kinase 1 (PINK1) (Kitada et al. 1999, Bonifati et al. 2003, Valente et al. 2004b, Ramirez et al. 2006). PINK1 is the first kinase discovered to be regulated by a canonical N-terminal mitochondrial targeting sequence. The discovery of PINK1 mutations in recessive PD (Valente et al. 2004a), combined with observations that DJ-1 localizes to mitochondria during oxidative stress (Dekker et al. 2003), presaged the current renaissance of interest in the role of mitochondria in PD. Growing evidence implicates PINK1 and parkin in the regulation of mitochondrial morphology (Exner et al. 2007, Yang et al. 2008, Dagda et al. 2009) and turnover (Narendra et al. 2008, Dagda et al. 2009). Both α-synuclein and LRRK2 show at least partial localization to mitochondria (Biskup et al. 2006, Devi et al. 2008). Thus, as discussed in more detail below, toxin and genetic studies converge on cytoplasmic–mitochondrial signaling and protein trafficking in PD pathogenesis.
3. Overview of mitochondrial transport, dynamics and turnover
Mitochondrial fission is mediated by cytosolic and outer membrane proteins including dynamin-related protein (Drp1) and hFis1, which induce mechanical constriction powered by GTP hydrolysis (Karbowski & Youle 2003). Mitochondrial fission or fragmentation is often associated with cell death, playing an important role in the execution of apoptosis (Youle & Karbowski 2005, Yuan et al. 2007), including that elicited by the PD-neurotoxin 6-hydroxydopamine (Gomez-Lazaro et al. 2008). Little is known about the posttranslational regulation of mitochondrial dynamics, although sumoylation and phosphorylation of Drp1 have been recently reported (Cribbs & Strack 2007, Wasiak et al. 2007). Mitochondrial fusion results in an interconnected network of elongated mitochondria. It is mediated by the inner membrane protein optic atrophy 1 (Opa1) and two outer membrane GTPases termed mitofusion1 and mitofusion2 (Mfn1/2), which facilitate tethering and fusion of outer and inner membranes (Gazaryan & Brown 2007). Enhanced mitochondrial fusion and connectivity is associated with resistance to many forms of cellular injury (Cheung et al. 2007, Cribbs & Strack 2007). On the other hand, Drp-1 dependent mitochondrial fission can limit neuronal injury associated with propagating calcium waves (Szabadkai et al. 2004) and in models of PINK1 deficiency (Dagda et al. 2009).
Changes in mitochondrial dynamics are integrally linked to trafficking of these organelles to the most distant reaches of neuritic processes where they function to provide critical energy and calcium buffering at synapses (Fig. 1, upper left). In addition to kinesin adapter proteins such as Milton and Miro (Wang & Schwarz 2009), an intact MFF machinery is required for successful trafficking of mitochondria along axons. Disruption of either fission (Li et al. 2004, Verstreken et al. 2005) or fusion (Baloh et al. 2007) proteins impair this important process. Alterations in mitochondrial movement are linked to physiologic processes, such as those mediating synaptic plasticity (Li et al. 2004), and to pathologic processes (Chang et al. 2006, Orret al. 2008). Interestingly, calcium itself regulates cessation of mitochondrial movement along axons (Wang & Schwarz 2009).
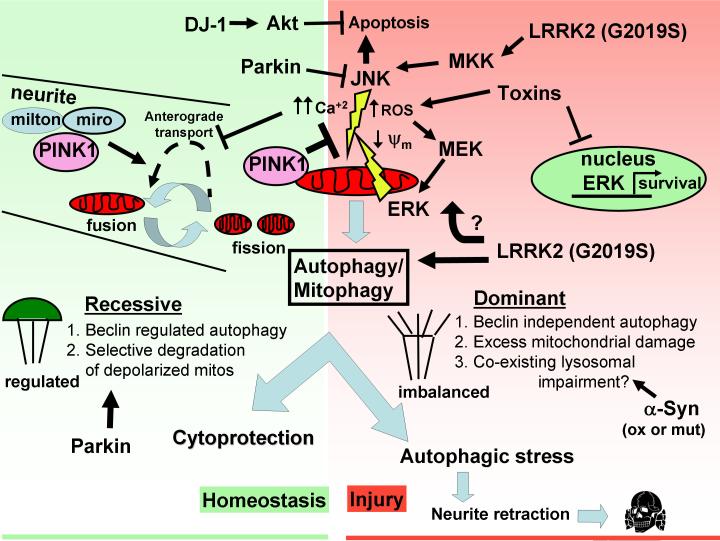
Figure 1. Hypothetical model integrating dominant (genetic or neurotoxin) and recessive (genetic or deficiency) influences on mitochondrial health and the autophagic/mitophagic injury response.
Neuronal homeostasis (green background) is mediated by Akt, PINK1 and nuclear ERK/CREB signals that promote transcription of genes that support neuronal survival, differentiation and function (BDNF, Elk1, cJun, Bcl-2). PINK1 promotes maintenance of healthy mitochondrial networks, potentially facilitating mitochondrial trafficking to synapses via its recently reported association with the kinesin adaptor proteins Miro and Milton. Parkin, DJ-1 and Akt oppose the pro-apoptotic effects of JNK, which is activated by neurotoxins and mutant LRRK2 expression. Loss of PINK1 function permits mitochondrial damage involving increased ROS, calcium dysregulation, and decreased respiratory function/membrane potential, which may signal fission and autophagic clearance of damaged mitochondria. Physiologic feedback mechanisms involving beclin 1 interactions and/or parkin-assisted selective clearance of damaged mitochondria serve as safety mechanisms (green parachute) to regulate and prevent overactivation of autophagy.
Dominant neuronal injuries (shaded in red) caused by parkinsonian neurotoxins promote large increases in cytosolic and mitochondrial ROS, activation/translocation of ERK and JNK to mitochondria and induction of beclin 1-independent autophagy, accompanied by decreased nuclear trafficking and neuroprotective transcription. Both JNK and ERK have been shown to contribute directly to mitochondrial dysfunction by suppressing oxidative respiration. An activating mutation in LRRK2 also results in activation of JNK pathways and ERK-dependent neurite retraction mediated by beclin1-independent autophagy. Factors that result in autophagic stress are still incompletely defined, but could hypothetically result from excessive loss of functioning mitochondria or reduced ability to complete lysosomal degradation, as oxidized or mutant forms of α-synuclein may interfere with certain forms of lysosomal degradation. We propose that excessive autophagy induction relative to the capacity of the neuron to undergo regenerative biosynthesis leads to a harmful state of imbalance that favors neurite retraction, neuronal atrophy and eventually cell death.
In addition to dynamic changes in mitochondrial fission/fusion and trafficking, autophagic degradation plays a major role in regulating mitochondrial quality and content (Kiselyov et al. 2007, Zhang et al. 2007). Macroautophagy involves the regulated, membranous engulfment of cytoplasmic cargo destined for lysosomal degradation (Mizushima et al. 2002, Cherra & Chu 2008), and represents the only major degradative pathway for organelles and insoluble proteins (Rubinsztein et al. 2005). Dysregulation of macroautophagy (Zhu et al. 2003, Alemi et al. 2007, Zhu et al. 2007, Dagda et al. 2008) and of chaperone-mediated autophagy (Cuervo et al. 2004, Martinez-Vicente et al. 2008, Yang et al. 2009) have been implicated in toxin and genetic models of PD. The autophagy machinery includes conjugating enzymes required for covalent attachment of ubiquitin-fold proteins Atg12 and Atg8/microtubule-associated protein light chain 3 (LC3) to nascent autophagic membranes (Mizushima et al. 2002). RNAi knockdown of Atg conjugation components are effective at inhibiting induction of autophagy and mitophagy (Chu et al. 2009). Depolarization, fission and mitochondrial ERK signaling have each been reported to trigger mitochondrial autophagy (Dagda et al. 2008, Gomes & Scorrano 2008, Narendra et al. 2008), with failure of depolarized fragments to re-fuse with the mitochondrial reticulum representing an alternative mechanism governing selective mitophagy (Twig et al. 2008). While well-regulated autophagic recycling of damaged mitochondria is beneficial, the outcome most likely also depends upon the degree of damage-induced autophagy and other factors that pre-dispose neurons to autophagic stress (Cherra & Chu 2008) (Fig. 1).
It is important to note that an increase in autophagosomes in degenerating neurons does not necessarily imply increased autophagic activity. The most robust accumulations of autophagosomes are observed experimentally when lysosomal fusion and degradation are inhibited. In human PD brain tissues, increased oxidative damage to mitochondria (Zhang et al. 1999) is correlated with a modest increase in autophagosomes containing ERK-labeled mitochondria (Zhu et al. 2002, Zhu et al. 2003). Experimental studies demonstrate intact autophagic flux and degradation in several PD models (Zhu et al. 2007, Dagda et al. 2008, Plowey et al. 2008, Dagda et al. 2009). In contrast, evidence of mitochondrial autophagy (Moreira et al. 2007) is readily identified in AD with robust accumulation of early and intermediate autophagic vacuoles (Nixon et al. 2005), potentially attributable to reduced autophagic clearance (Boland et al. 2008). Thus, both post-mortem and experimental studies suggest multiple mechanisms by which perturbations in mitochondrial dynamics and turnover could contribute to synaptic dysfunction and neurodegeneration.
4. Kinase signaling to the mitochondrion
Given that only a small fraction of mitochondrial proteins are encoded in the mitochondrial genome and mitochondria rely heavily on synthesis and import of nuclear encoded proteins, mitochondria have undoubtedly evolved complex mechanisms to communicate with the rest of the cell. Despite this central role in cellular metabolism, mitochondria were once though to be unlikely central sites for reversible protein phosphorylation due to compartmentalization from the rest of the cell by multiple membrane layers, and the absence of mitochondrial targeting leader sequences in most signaling proteins (reviewed by (Pagliarini & Dixon 2006)). In yeast, only about seven protein kinases out of 136 (5%) have been identified in mitochondria (Tomaska 2000). However, experimental evidence garnered over the past two decades have demonstrated a clear role for kinases in regulating electron transport chain function, and cytoplasmic kinases can reach not only the outer surface of mitochondria, but also distribute in intermembrane and matrix compartments (Reviewed in (Horbinski & Chu 2005). The discoveries of signaling scaffold proteins that function to target specific kinases to the mitochondrion and of a functional N-terminal mitochondrial targeting sequence in the serine/threonine kinase PINK1 (Feliciello et al. 2005) further confirm an important role for kinases in mitochondrial communication with the rest of the cell to include the nucleus (Butow & Avadhani 2004).
As general features of kinase/phosphatase signaling to the mitochondrion have been the subject of several recent reviews (Horbinski & Chu 2005, McBride et al. 2006, Pagliarini & Dixon 2006), the subsequent sections will focus upon the role of kinases implicated in PD. In particular, converging roles for specific mitochondrially targeted kinases derived from neurotoxin and genetic models will be emphasized. Regardless of etiology in this multifactorial disease, these data suggest common pathways of parkinsonian neurodegeneration that are potentially amenable to therapeutic intervention.
5. Extracellular signal regulated protein kinases (ERK1/2)
The extracellular signal-regulated kinases (ERK1/2) are conserved serine/threonine protein kinases that have emerged as important regulators of neuronal responses to both functional and pathologic stimuli (Chu et al. 2004). Although ERK1/2 typically translocates between the cytosol and nucleus to mediate well-characterized pro-survival and trophic functions (Yoon & Seger 2006), it is also found in mitochondria of neurons and non-neuronal cells such as in mouse heart (Baines et al. 2002); renal epithelial cells (Nowak et al. 2006, Zhuang et al. 2008); mitochondrial outer membrane and the intermembrane space of rat brain cells (Alonso et al. 2004); mouse hippocampus (Rumora et al. 2007), B65 cells (Kulich et al. 2007), SH-SY5Y cells (Dagda et al. 2008); Leydig cells (Poderoso et al. 2008), and human alveolar macrophages (Monick et al. 2008). The function of mitochondrial ERK1/2 is still not clear, but it appears to play a central role in regulating mitochondrial function (Nowak et al. 2006, Monick et al. 2008) and survival-death decisions (Kulich et al. 2007, Dagda et al. 2008, Lin et al. 2008, Zhuang et al. 2008). In human PD brain and diffuse Lewy body diseases, there are significant increases of phospho-ERK (p-ERK) in the cytoplasm and mitochondria of midbrain dopaminergic neurons (Zhu et al. 2002, Zhu et al. 2003). The punctate mitochondrial distribution of p-ERK in PD is distinct from the diffuse staining pattern observed after cerebral ischemia (Namura et al. 2001). Moreover, p-ERK is not elevated in substantia nigra degeneration due to progressive supranuclear palsy (author's unpublished data), indicating that this type of dysregulated ERK1/2 signaling may be relatively specific to PD.
5.1 ERK in parkinsonian neurotoxin models
Alterations in ERK signaling is observed during dopaminergic cell injury elicited by MPTP/MPP, 6-OHDA, rotenone, and toxic doses of dopamine (Kulich & Chu 2001, Gomez-Santos et al. 2002, Zhu et al. 2002, Chuenkova & Pereira 2003, Kulich et al. 2007, Zhu et al. 2007, Chen et al. 2008, Ren et al. 2009). ERK signaling is generally considered a pro-survival pathway (Baines et al. 2002), but increasing evidence suggests that activation of ERK also contributes to cell death (Chu et al. 2004, Zhuang & Schnellmann 2006, Ren et al. 2009). The level of ERK activation or its kinetics may play a role, as inhibiting basal ERK signaling has different effects than inhibiting toxin-induced ERK activation (Gomez-Santos et al. 2002). In the 6-OHDA model, we found that the time course of ERK activation is tightly correlated with mitochondrial ROS production; antioxidants inhibit ERK phosphorylation and rescue from neuronal injury (Kulich et al. 2007). Mitochondrially localized ERK induces autophagy/mitophagy even in the absence of toxin injury, suggesting that mitochondrially localized ERK could act as a sensor downstream of mitochondrial injury induced by toxins.
Factors that could determine the outcome of ERK activation include cell or organ type, nature of the treatments, and the temporal and/or spatial pattern of signaling within the cell (Colucci-D'Amato et al. 2003, Chu et al. 2004, Subramaniam & Unsicker 2006, Lin et al. 2008). A rapid and transient activation of ERK in mouse brain and MN9D cells promotes neuronal survival (Weng et al. 2007, Lin et al. 2008), while sustained or delayed ERK activation by 6-OHDA, MPP+ or dopamine promote cell death in neuronal cells (Kulich & Chu 2001, Gomez-Santos et al. 2002, Zhu et al. 2007). Detrimental effects of ERK activation are correlated with changes in the nuclear-cytoplasmic ratios of activated signaling phosphoproteins in the ERKRsk-CREB axis (Chen et al. 2004, Chalovich et al. 2006, Glotin et al. 2006, Poderoso et al. 2008). In both the 6-OHDA model and a dopamine toxicity model, only a small amount of p-ERK1/2 activated during injury translocates to the nucleus, with the majority located in the cytoplasm and mitochondria (Zhu et al. 2002, Chen et al. 2004, Dagda et al. 2008). This pattern of p-ERK trafficking causes a decline in neurotrophic transcription accompanied by an increase in pathologic mitophagy (Fig. 1, right side). While lower levels of mitophagy can confer neuroprotection, prolonged or excessive ERK2-driven mitophagy appears harmful in neuronal cells as MAPK inhibitors or expression of a dominant negative ERK2 reduces cell death (Zhu et al. 2007, Dagda et al. 2008). Thus, addressing the mechanisms underlying altered trafficking of p-ERK may offer insights into potential therapies.
5.2 ERK in parkinsonian genetic models
Mutations in the leucine-rich repeat kinase 2 gene (LRRK2) cause late-onset Parkinson's disease. The mechanisms by which missense alterations in the LRRK2 gene initiate neurodegeneration remain unknown. LRRK2 has putative Ras/GTPase-like, a protein kinase domain, leucine rich domain, and WD40 domains, all suggesting a major role in signaling (Ross & Farrer 2005, Greggio et al. 2006). Several mutations in the GTPase and kinase domains have been described. The kinase domain has a catalytic core common to tyrosine and serine/threonine kinases, and is homologous to mitogen activated protein kinase kinase kinases (MAPKKK) or mixed lineage kinases (West et al. 2005). The G2019S has been consistently shown to exhibit increased kinase activity. Interestingly, LRRK2 appears to activate ERK1/2 signaling in SH-SY5Y cells (Liou et al. 2008). However, in another study, ERK activity was not found to differ significantly in extracts of leukocytes from patients with PD carrying the G2019S mutation, healthy mutation carriers, patients with idiopathic PD, and healthy controls (White et al. 2007). We found that G2019S LRRK2, but not wild type LRRK2 or kinase-dead K1906M LRRK2, stimulated neuritic autophagy and neurite retraction by a pathway dependent upon ERK signaling in retinoic acid differentiated SH-SY5Y cells (Plowey et al. 2008). As with the toxin models, it is likely that the potential effects of ERK1/2 signaling will ultimately depend upon timing and compartmentalization of activation, which will determine which downstream pathways predominate in a given pathologic context.
6. c-Jun N-terminal kinases (JNK)
The c-Jun N-terminal kinases (JNK) represent another branch of the MAPK family that is activated by exposure of cells to environmental stress. Phospho-activation of JNK is mediated by MKK4 and MKK7. JNKs phosphorylate a variety of nuclear factors such as c-Jun, ATF2 and Elk1, and also cytoplasmic substrates such as cytoskeletal proteins and mitochondrial proteins including Bcl-2 and Bcl-xl. The spatial-temporal regulation of JNK is differently regulated in multiple intracellular compartments (Bonny et al. 2005, Borsello & Forloni 2007). Many studies indicate that JNK could be activated in or translocate to mitochondria, including work in ischemia-injured hippocampus, mouse cardiac mitochondria, H2O2-treated rat brain or primary cortical cultures, acetaminophen induced liver injury, HeLa cells treated with paclitaxel, and multiple myeloma cells treated with anti-cancer drugs (Baines et al. 2002, Chauhan et al. 2003, Zablocka et al. 2003, Brichese et al. 2004, Rumora et al. 2007, Hanawa et al. 2008, Zhou et al. 2008b). While most studies including those discussed below implicate JNK in death signaling, JNK shows neuroprotective effects in hypoxia-reoxygenation studies (Dougherty et al. 2004).
6.1 JNK in parkinsonian neurotoxin models
JNK represents one of the major signaling pathways implicated in PD pathogenesis. Increased JNK activity has been reported in MPTP animal models (Saporito et al. 1999, Xia et al. 2001, Hunot et al. 2004, Park et al. 2004), MPP+ cell culture model (Xia et al. 2001, Kim et al. 2007), rotenone neurotoxicity (Newhouse et al. 2004, Klintworth et al. 2007), and the 6-OHDA model (Hara et al. 2003, Eminel et al. 2004, Pan et al. 2007). Although the temporal and spatial patterns of JNK activation are different from model to model, activation of JNK almost exclusively leads to cell death. JNK2 and JNK3 mutant mice are more resistant to MPTP as compared with wide type littermates (Hunot et al. 2004). In mice, adenoviral gene transfer of the JNK binding domain of the scaffold protein JNK-interacting protein-1 inhibited MPTP-induced c-Jun and caspase activation and dopaminergic neuron cell death (Xia et al. 2001). Downstream targets of JNK implicated in MPTP toxicity include cyclooxygenase 2 and the p53 protein (Trimmer et al. 1996, Teismann et al. 2003, Hunot et al. 2004, Nair 2006). JNK inhibitors and transfection with dominant negative forms of JNK reduce 6-OHDA induced cell death in PC12 cells (Eminel et al. 2004) and rotenone toxicity in SH-SY5Y cells (Newhouse et al. 2004). Similar to pathological ERK activation, antioxidants reduce JNK activation and cell death in 6-OHDA injured neuronal cells (Tian et al. 2007). All these experiment suggest JNK plays an important role in mediating parkinsonian cell death.
Exposure of rat primary cortical neurons to H2O2 resulted in increased phosphorylated JNK associated with the outer mitochondrial membrane, where causes phosphorylation of pyruvate dehydrogenase (PDH), a key enzyme that links two major metabolic pathways: glycolysis and the tricarboxylic acid cycle. Given that PDH is a matrix-localized proteins, the mechanisms involved are unclear, but phosphorylation of PDH causes a decline in its activity and a shift to anaerobic metabolism and acidosis (Zhou et al. 2008b). Mitochondrial translocation of JNK also causes release of the Second Mitochondria-derived Activator of Caspase and cytochrome c from mitochondria (Chauhan et al. 2003, Eminel et al. 2004), promoting apoptosis and phospho-inactivating Bcl2 and Bcl-x (Kharbanda et al. 2000, Brichese et al. 2004). As with pathological ERK activation, the potential mechanisms by which JNK translocate to mitochondria to promote mitochondrial dysfunction and degeneration in PD remains to be fully determined.
6.2 JNK in parkinsonian genetic models
Parkin is an E3 ubiquitin ligase, encoded by parkin, the most common gene mutated in autosomal recessive familial parkinsonism. Parkin has been demonstrated to act as a protector of dopaminergic neurons against multiple PD-related toxicities. Overexpression of parkin in SHSY5Y cells significantly attenuated dopamine-induced activation of c-Jun N-terminal kinase (JNK) and caspase-3. It also decreased the level of reactive oxygen species (ROS) and protein carbonyls in the cell (Jiang et al. 2004). Conversely, JNK is highly activated in dopaminergic neurons of parkin mutants (Cha et al. 2005). While it was originally thought that deficits in Parkin biology stimulates aggregation of its substrates to cause cellular stress, Parkin has also been reported to directly inhibit JNK activation via ubiquitination of JNK pathway mediators (Cha et al. 2005) (Fig. 1, top). A recent report suggests that Parkin inactivation of JNK is mediated by multiple mono-ubiquitinations on Hsp70, although the mechanism by which Hsp70 mono-ubiquitination regulates this is not clear (Liu et al. 2008). Parkin has three independent microtubule binding domains in addition to its RING domains. Dopaminergic neurons in culture appear are sensitive to rotenone-induced depolymerization of microtubules with subsequent activation of ERK and JNK, and Parkin protects against these effects (Ren et al. 2009). These studies indicate a direct interaction between Parkin and MAPK signaling pathways. JNK has also been implicated in relation to mutations in LRRK2. Protein assays of cell extracts from patients with LRRK2 G2019S-associated PD showed significant reductions in phosphorylation of JNK, Src, and HSP27 compared to healthy controls (White et al. 2007). On the other hand, recent studies indicate that mutant LRRK2 activates pathologic JNK and p38 signaling through phosphorylation of MAPKKs (Gloeckner et al. 2009)(Fig. 1, top right). Given dual roles for ERK on survival in different experimental contexts, the role of mutant LRRK2 in modulating JNK signaling deserves further investigation.
7.0 Akt/Protein kinase B
Protein kinase B (PKB) or c-Akt is the downstream kinase that regulates class III phosphoinositide-3-kinase (PI3K) dependent signaling in neurons. Recruitment of cytosolic Akt to the cell membrane via a pleckstrin homology domain (PH) by phosphatidylinositol 1,3,5 triphosphate (PIP3) facilitates its phosphorylation and activation by protein dependent kinase-1 (PDK1), leading to enhanced survival of motor neurons, PC12 cells and in cerebellar granule cells (Namikawa et al. 2000, Alvarez-Tejado et al. 2001, Bijur & Jope 2003, Leeds et al. 2005, Zhong et al. 2005, Li et al. 2008). Akt also promotes sequestration of Bad and suppresses the pro-apoptotic activity of GSK-3β (Datta et al. 1997, del Peso et al. 1997). Although mostly cytosolic, a fraction of Akt is recruited to mitochondria upon stimulation of SH-SY5Y cells with growth factors such as insulin (Bijur & Jope 2003). Transient expression of mitochondrially targeted constitutively active Akt protects against staurosporine induced apoptosis (Mookherjee et al. 2007). A recent study describes a role for heat shock protein 90 in mediating neuroprotective mitochondrial translocation of Akt (Barksdale & Bijur 2009).
7.1 Akt in parkinsonian toxin models
Several studies demonstrate that upregulation of the Akt pathway is neuroprotective. 6-OHDA treatment of SH-SY5Y cells significantly promotes a decrease in Akt phosphorylation (Li et al. 2008). Likewise, stereotactic injection of adenovirus expressing constitutively active m-Akt into the substantia nigra and striatum is strongly neuroprotective against 6-OHDA in vivo (Ries et al. 2006) (Fig. 1, top). Akt also showed striking trophic effects with increased sprouting of dopaminergic projections and increased substantia nigra neuron sizes. While this study elegantly highlights a potential application of gene therapy for PD, the potential for long-term constitutive Akt activation to promote neoplasia would need to be investigated.
7.2 Akt in parkinsonian genetic models
Stable knockdown of DJ-1 in Drosophila is associated with mitochondrial dysfunction and decreased Akt signaling (Yang et al. 2005). Furthermore, oxidative stress induces aggregation of α-synuclein, which modulates Akt signaling in neurons (Hashimoto et al. 2001, Seo et al. 2002). Increased levels of β-synuclein, which seems to antagonize the toxic and aggregating effects of α-synuclein, protected against rotenone toxicity via upregulation of the Akt signaling pathway. (Hashimoto et al. 2001, Uversky et al. 2002, Hashimoto et al. 2004). Moreover, human genetic studies also support a role for Akt in protecting against PD-type degeneration, as a particular Akt1 haplotype is associated with a decreased risk of developing PD in a Greek cohort of PD cases (Xiromerisiou et al. 2008).
8. PTEN-induced kinase 1 (PINK1)
While mitochondrial kinases have been implicated in PD through human tissue studies and parkinsonian toxin models for nearly a decade, the seminal discovery that the PARK6 locus of autosomal recessive, early-onset PD encodes PTEN-induced kinase 1 (PINK1) launched an ongoing period of intensive interest in the regulation of mitochondrial pathobiology by kinases. PINK1 is a serine/threonine kinase with homology to calcium/calmodulin regulated kinases (Valente et al. 2004a). Notably, the primary sequence for PINK1 includes a canonical N-terminal mitochondrial leader sequence (Silvestri et al. 2005) and reviewed by (Mills et al. 2008), and has been shown to distribute to mitochondria in numerous cell types including human brain (Gandhi et al. 2006), where it is predicted to be cleaved by matrix proteases. PINK1 also appears to have cytoplasmic functions, and even cleaved forms can be found in the cytoplasm, suggesting mitochondrial export of the protein for signaling or clearance purposes. There is a putative transmembrane domain thought to arrest import of PINK1 in a manner that allows it to insert in the outer mitochondrial membrane (Zhou et al. 2008a). The C-terminal domain of PINK1 regulates its autophosphorylation activity [reviewed in (Mills et al. 2008)].
Multiple point mutations and truncations have been mapped throughout the transmembrane, kinase and C-terminal domains of PINK1. These mutations serve to reduce or impair kinase activity, promote accelerated degradation, or induce misfolding of PINK1 [reviewed by (Mills et al. 2008)]. The TNF receptor associated protein 1 (TRAP1) was identified as a potential substrate for PINK1, and the serine protease Omi/Htra2 and heat shock proteins, Hsp75 (TRAP1), Hsp90/Cdc37 are potential mitochondrial PINK1 binding partners (Plun-Favreau et al. 2007, Pridgeon et al. 2007, Moriwaki et al. 2008). Given that PINK1 loss of function leads to younger onset ages for parkinsonian neurodegeneration, a better understanding of the normal role(s) of PINK1 may offer important insight applicable to preventing or delaying onset of PD in general.
Although PINK1 is undoubtedly a mitochondrially targeted kinase, the subcellular localization of PINK1 in neurons has been controversial. Some studies suggest a mixed cytosolic/mitochondrial localization or localization in peroxisomes, while others indicate PINK1 is predominantly localized to the mitochondria (Beilina et al. 2005, Petit et al. 2005, Zhou et al. 2008a). Indeed, only some PINK1 functions appear dependent upon the mitochondrial localization signal, as N-terminal deletions of PINK1 that lead to cytoplasmic localization is sufficient to protect neurons from the classic mitochondrial toxin MPTP (Haque et al. 2008). However, a residual pool of mitochondrial leader peptide-truncated PINK1 can associate with mitochondria, possibly through association with the mitochondrial axonal transport proteins Miro and Milton (Weihofen et al. 2009). As observed with each of the kinases discussed above, localized activation and/or differential trafficking of different pools of PINK1 likely serve to mediate different physiological roles within neuronal cells.
8.1 PINK1 in parkinsonian toxin models
To date, all studies with PINK1 in toxin models have shown a prominent role for wild type PINK1 in neuroprotection. Transient or stable overexpression of PINK1 protects against a variety of toxic insults including staurosporine, rotenone, proteasome inhibition, MPP+ and 6-OHDA (Dagda & Chu, unpublished data), while RNA interference (RNAi) knockdown of PINK1 has the opposite effect (Deng et al. 2005, Petit et al. 2005, Pridgeon et al. 2007, Haque et al. 2008). Proposed prosurvival mechanisms include stabilizing the mitochondrial membrane potential, inhibiting superoxide generation and inhibiting the release of apoptogenic factors such as cytochrome c (Petit et al. 2005, Clark et al. 2006, Exner et al. 2007, Wang et al. 2007, Wood-Kaczmar et al. 2008) (Fig. 1, center).
On the other hand, the regulation of endogenous PINK1 responses by neurotoxic injuries has been less studied. Upon mitochondrial depolarization by oxidative stress, PINK1 in SHSY5Y cells rapidly translocates to mitochondria, is cleaved by matrix proteases and rapidly degraded by the proteasome pathway within minutes of toxin treatment (Lin & Kang 2008). We have observed alterations in PINK1 expression in the MPTP model in vivo and in a chronic MPP+ culture model (Zhu, Callio & Chu, unpublished data), which may play into either injury or compensatory mechanisms.
8.2 PINK1 in parkinsonian genetic models
Most PD-associated mutations in PINK1 result in loss of the ability of overexpressed PINK1 to confer neuroprotection against different forms of toxic insults (Wang et al. 2007). While some mutations are directly associated with loss of in vitro kinase activity, other mutations promote decreased protein stability or protein misfolding (Beilina et al. 2005). The possibility of an elevated risk for heterozygous PINK1 mutant carriers to develop PD (Valente et al. 2004b, Bonifati et al. 2005, Kumazawa et al. 2008), show accelerated disease progression (Marongiu et al. 2008), or develop neuropsychiatric disorders (Steinlechner et al. 2007, Reetz et al. 2008), remain controversial. In addition to a growing list of other proteins, PINK1 has been shown to be localized to Lewy bodies in human brain (Gandhi et al. 2006). While it is attractive to speculate that depletion of functional PINK1 through aggregation may be pathogenic, decreased PINK1 levels in sporadic PD patients has not yet been reported.
Multiple RNAi studies in cultured mammalian cells, Drosophila models, and studies of mutant PINK1 patient primary fibroblasts reveal strikingly aberrant mitochondrial morphology, with loss of membrane potential and increased oxidative stress, implicating PINK1 in the regulation of mitochondrial homeostasis (Clark et al. 2006, Park et al. 2006, Wang et al. 2006, Yang et al. 2006, Exner et al. 2007, Poole et al. 2008, Wood-Kaczmar et al. 2008). Interestingly, all these alterations are restored by transient or stable expression of Parkin, leading to the concept of Parkin as a downstream effector of PINK1 neuroprotection.
Recent data also suggest alternative mechanisms for Parkin-mediated complementation of PINK1 deficiency (Narendra et al. 2008, Dagda et al. 2009). We observed that PINK1 loss-of-function resulted in aberrations in mitochondrial morphology, increased mitochondrial ROS, Drp1-dependent mediated mitochondrial fission, and a protective macroautophagy/mitochondrial autophagy responses (Fig. 1, lower left). Interestingly, not only did the MFF machinery mediate mitochondrial fragmentation in stable loss of PINK1 but the autophagic machinery also cooperated in this process. Morever, instead of simply reversing each cellular effect of stable PINK1 knockdown, Parkin overexpression resulted in further amplification of the autophagic/mitophagic response (Dagda et al. 2009), consistent with a recently reported role for Parkin in autophagic clearance of depolarized mitochondria (Narendra et al. 2008).
In contrast to culture and Drosophila studies, the effects of PINK1 deficiency in mouse models has been much more subtle. While there is no frank degeneration in PINK1 shRNA mice or PINK1 knockout mice (Zhou et al. 2007, Gautier et al. 2008), PINK1 appears to regulate dopamine release, long-term potentiation (Kitada et al. 2007) and potentially metabotropic glutamate receptors in medium spiny neurons of the striatum supporting a role of PINK1 at the dendrites and synapse possibly by regulating mitochondrial bioenergetics and dynamics at those compartments (Martella et al. 2009). Ultimately, our understanding of mechanisms related to PINK1-associated PD will rely upon a better understanding of the many normal roles of PINK1 in the central nervous system.
9. Conclusions
Data from neurotoxin, environmental and genetic models of parkinsonian neurodegeneration have converged upon a key role for kinases in regulating mitochondrial pathobiology in which disturbances in mitochondrial function, transport, dynamics and turnover are central converging mechanisms (Fig. 1). Altered subcellular localization of signaling proteins and transcription factors is frequently observed in post-mortem tissues and models of several major neurodegenerative diseases [Reviewed in (Chu et al. 2007)], with a tendency towards nuclear depletion and cytoplasmic/mitochondrial accumulation. Mitochondrial kinase activity can be mediated by localized ROS-mediated activation at the mitochondria as well as by trafficking and recruitment of signaling proteins activated elsewhere in the cytoplasm. As mitochondria serve as central sensors of metabolic alterations, reverse mitochondrial to nuclear signaling (Dawson & Dawson 2004) may be just as important as traditional pathways mediating communication between extracellular and intracellular environments. With recent impetus and momentum offered by PD-related factors, the basic role of bi-directional kinase signaling involving mitochondrial, as well as nuclear, trafficking represents an important emerging field of study.
Interestingly, neuroprotective autophagy/mitophagy elicited in response to recessive deficiencies (e.g. loss of PINK1, amino acid starvation, insufficient trophic stimulation) are regulated by canonical beclin1-dependent signaling pathway, which serves to prevent overactivation of autophagy (Pattingre et al. 2005). In contrast, autophagy associated with dominant G2019S LRRK2 or MPP+ toxicity occur through beclin1-independent mechanisms (Zhu et al. 2007, Plowey et al. 2008), implying escape from this physiologic regulatory pathway. Hypothetically, either excessive mitochondrial damage or excessive mitophagy induced by neurotoxins, overactive LRRK2, or mitochondrial ERK1/2 (Dagda et al. 2008), could exceed the regenerative capacity of nigral neurons and prove detrimental (Fig. 1).
Although the mechanism(s) by which altered temporal and spatial dynamics of kinases that traffic between cytosolic, nuclear and mitochondrial compartments remain to be elucidated, over-activation and/or mitochondrial translocation of certain serine/threonine kinases (ERK2, JNK1/2, GSK3β, ?LRRK2), and impaired function of others (PINK1, Akt-1), promote PD-related pathogenic mechanisms including aberrations in mitochondrial cytoarchitecture, decreased mitochondrial function, and increased oxidative stress, contributing to protein aggregation. A compensatory mitochondrial autophagic response may represent a double-edged sword depending upon the degree of damage and ability of the neuron to successfully complete autophagic degradation and biogenesis of healthy mitochondria (Cherra & Chu 2008). This exciting new frontier of mitochondrial kinase regulation in PD raises the possibility that administration of known or yet-to-be discovered agents that inhibit kinase activity and/or mitochondrial translocation of ERK2, JNK1/2, LRRK2 or α-synuclein may rescue mitochondrial function, prevent activation of apoptotic or autophagic death pathways and/or prevent neurite degeneration. At the same time, potential therapies that increase kinase activity and/or mitochondrial functions of PINK1, Akt-1 or DJ-1 could aid in stabilizing mitochondrial networks, preventing activation of neurodegenerative and cell death pathways in PD.
ACKNOWLEDGMENTS
The authors'research is supported by the National Institutes of Health (CTC: AG026389, NS053777, DC009120) and the American Parkinson Disease Association (JZ). RKD is supported in part by F32 AG030821
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alemi M, Prigione A, Wong A, Schoenfeld R, DiMauro S, Hirano M, Taroni F, Cortopassi G. Mitochondrial DNA deletions inhibit proteasomal activity and stimulate an autophagic transcript. Free Radic Biol Med. 2007;42:32–43. doi: 10.1016/j.freeradbiomed.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Melani M, Converso D, Jaitovich A, Paz C, Carreras MC, Medina JH, Poderoso JJ. Mitochondrial extracellular signal-regulated kinases 1/2 (ERK1/2) are modulated during brain development. J Neurochem. 2004;89:248–256. doi: 10.1111/j.1471-4159.2004.02323.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, Carrera AC, Landazuri MO, del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale KA, Bijur GN. The basal flux of Akt in the mitochondria is mediated by heat shock protein 90. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson's disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. Journal of neurochemistry. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P. DJ-1( PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rohe CF, Breedveld GJ, Fabrizio E, De Mari M, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De Gaetano A, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Dalla Libera A, Tinazzi M, De Pandis F, Fabbrini G, Goldwurm S, de Klein A, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- Bonny C, Borsello T, Zine A. Targeting the JNK pathway as a therapeutic protective strategy for nervous system diseases. Rev Neurosci. 2005;16:57–67. doi: 10.1515/revneuro.2005.16.1.57. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Brichese L, Cazettes G, Valette A. JNK is associated with Bcl-2 and PP1 in mitochondria: paclitaxel induces its activation and its association with the phosphorylated form of Bcl-2. Cell Cycle. 2004;3:1312–1319. doi: 10.4161/cc.3.10.1166. [DOI] [PubMed] [Google Scholar]
- Brill LB, 2nd, Bennett JP,, Jr. Dependence on electron transport chain function and intracellular signaling of genomic responses in SH-SY5Y cells to the mitochondrial neurotoxin MPP(+). Exp Neurol. 2003;181:25–38. doi: 10.1016/s0014-4886(02)00045-6. [DOI] [PubMed] [Google Scholar]
- Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Molecular cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J. Biol. Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha GH, Kim S, Park J, Lee E, Kim M, Lee SB, Kim JM, Chung J, Cho KS. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc Natl Acad Sci U S A. 2005;102:10345–10350. doi: 10.1073/pnas.0500346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich EM, Zhu JH, Caltagarone J, Bowser R, Chu CT. Functional repression of cAMP response element in 6-hydroxydopamine-treated neuronal cells. J Biol Chem. 2006;281:17870–17881. doi: 10.1074/jbc.M602632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- Chen J, Rusnak M, Luedtke RR, Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J Biol Chem. 2004;279:39317–39330. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang X, Yang D, Du Y, Li L, Li X, Ming M, Le W. D2/D3 receptor agonist ropinirole protects dopaminergic cell line against rotenone-induced apoptosis through inhibition of caspase- and JNK-dependent pathways. FEBS Lett. 2008;582:603–610. doi: 10.1016/j.febslet.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, Chu CT. Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurol. 2008;3:309–323. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, McBride HM, Slack RS. Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis. 2007;12:979–992. doi: 10.1007/s10495-007-0745-5. [DOI] [PubMed] [Google Scholar]
- Choi WS, Yoon SY, Oh TH, Choi EJ, O'Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. Journal of neuroscience research. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Chu CT, Levinthal DJ, Kulich SM, Chalovich EM, DeFranco DB. Oxidative neuronal injury. The dark side of ERK1/2. Eur J Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Zhu JH, Cao G, Signore A, Wang S, Chen J. Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem. 2005;94:1685–1695. doi: 10.1111/j.1471-4159.2005.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Plowey ED, Wang Y, Patel V, Jordan-Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Plowey ED, Dagda RK, Hickey RW, III, S.J.C., Clark RS. Autophagy in neurite injury and neurodegeneration: in vitro and in vivo models. Methods in Enzymology. 2009;453:217–249. doi: 10.1016/S0076-6879(08)04011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuenkova MV, Pereira MA. PDNF, a human parasite-derived mimic of neurotrophic factors, prevents caspase activation, free radical formation, and death of dopaminergic cells exposed to the Parkinsonism-inducing neurotoxin MPP+. Brain Res Mol Brain Res. 2003;119:50–61. doi: 10.1016/j.molbrainres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Colucci-D'Amato L, Perrone-Capano C, di Porzio U. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–1095. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO reports. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress. Autophagy. 2008;4:770–782. doi: 10.4161/auto.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009 doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. Journal of bioenergetics and biomembranes. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- Dekker MC, Bonifati V, van Duijn CM. Parkinson's disease: piecing together a genetic jigsaw. Brain. 2003;126:1722–1733. doi: 10.1093/brain/awg172. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science (New York, N.Y. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. doi: 10.1016/j.bbrc.2005.09.178. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty CJ, Kubasiak LA, Frazier DP, Li H, Xiong WC, Bishopric NH, Webster KA. Mitochondrial signals initiate the activation of c-Jun N-terminal kinase (JNK) by hypoxia-reoxygenation. Faseb J. 2004;18:1060–1070. doi: 10.1096/fj.04-1505com. [DOI] [PubMed] [Google Scholar]
- Eminel S, Klettner A, Roemer L, Herdegen T, Waetzig V. JNK2 translocates to the mitochondria and mediates cytochrome c release in PC12 cells in response to 6-hydroxydopamine. J Biol Chem. 2004;279:55385–55392. doi: 10.1074/jbc.M405858200. [DOI] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–287. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, Holton JL, Wood NW, Revesz T. PINK1 protein in normal human brain and Parkinson's disease. Brain. 2006;129:1720–1731. doi: 10.1093/brain/awl114. [DOI] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazaryan IG, Brown AM. Intersection between mitochondrial permeability pores and mitochondrial fusion/fission. Neurochem Res. 2007;32:917–929. doi: 10.1007/s11064-006-9252-2. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha- synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Schumacher A, Boldt K, Ueffing M. The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2009.06024.x. [DOI] [PubMed] [Google Scholar]
- Glotin AL, Calipel A, Brossas JY, Faussat AM, Treton J, Mascarelli F. Sustained versus transient ERK1/2 signaling underlies the anti- and proapoptotic effects of oxidative stress in human RPE cells. Invest Ophthalmol Vis Sci. 2006;47:4614–4623. doi: 10.1167/iovs.06-0297. [DOI] [PubMed] [Google Scholar]
- Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochimica et biophysica acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med. 2008;44:1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Ferrer I, Reiriz J, Vinals F, Barrachina M, Ambrosio S. MPP+ increases alpha-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002;935:32–39. doi: 10.1016/s0006-8993(02)02422-8. [DOI] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J. Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol. 2002;61:634–639. doi: 10.1093/jnen/61.7.634. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, Thomas KJ, D'Souza C, Callaghan S, Kitada T, Slack RS, Fraser P, Cookson MR, Tandon A, Park DS. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Ohta M, Ohta K, Kuno S, Adachi T. Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res. 2003;119:125–131. doi: 10.1016/j.molbrainres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Bar-On P, Ho G, Takenouchi T, Rockenstein E, Crews L, Masliah E. Beta-synuclein regulates Akt activity in neuronal cells. A possible mechanism for neuroprotection in Parkinson's disease. J Biol Chem. 2004;279:23622–23629. doi: 10.1074/jbc.M313784200. [DOI] [PubMed] [Google Scholar]
- Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ren Y, Zhao J, Feng J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, Gibson JM, Ross OA, Lynch T, Wiley J, Payami H, Nutt J, Maraganore DM, Czyzewski K, Styczynska M, Wszolek ZK, Farrer MJ, Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell death and differentiation. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim MY, Mo JS, Park JW, Park HS. SAG protects human neuroblastoma SH-SY5Y cells against 1-methyl-4-phenylpyridinium ion (MPP+)-induced cytotoxicity via the downregulation of ROS generation and JNK signaling. Neurosci Lett. 2007;413:132–136. doi: 10.1016/j.neulet.2006.11.074. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Jennigs JJ, Jr., Rbaibi Y, Chu CT. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3:259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Matsumine H, Hattori N, Minoshima S, Shimizu N, Mizuno Y. Positional cloning of the autosomal recessive juvenile parkinsonism (AR-JP) gene and its diversity in deletion mutations. Parkinsonism Relat Disord. 1999;5:163–168. doi: 10.1016/s1353-8020(99)00032-2. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth H, Newhouse K, Li T, Choi WS, Faigle R, Xia Z. Activation of c-Jun N-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 2007;97:149–162. doi: 10.1093/toxsci/kfm029. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, St Clair D, Wermer M, Yen HC, Oberley T, Yang L, Flint Beal M. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Burke RE. Targeting the JNK signaling pathway for stroke and Parkinson's diseases therapy. Current drug targets. 2005;4:63–67. doi: 10.2174/1568007053005145. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–383. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa R, Tomiyama H, Li Y, Imamichi Y, Funayama M, Yoshino H, Yokochi F, Fukusako T, Takehisa Y, Kashihara K, Kondo T, Elibol B, Bostantjopoulou S, Toda T, Takahashi H, Yoshii F, Mizuno Y, Hattori N. Mutation analysis of the PINK1 gene in 391 patients with Parkinson disease. Arch Neurol. 2008;65:802–808. doi: 10.1001/archneur.65.6.802. [DOI] [PubMed] [Google Scholar]
- Leeds P, Leng Y, Chalecka-Franaszek E, Chuang DM. Neurotrophins protect against cytosine arabinoside-induced apoptosis of immature rat cerebellar neurons. Neurochemistry international. 2005;46:61–72. doi: 10.1016/j.neuint.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu Y, Zhu Q, Zhu J. Neurotrophin-3 reduces apoptosis induced by 6-OHDA in PC12 cells through Akt signaling pathway. Int J Dev Neurosci. 2008;26:635–640. doi: 10.1016/j.ijdevneu.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson's disease. Experimental neurology. 2007;203:370–380. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. Journal of neurochemistry. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AK, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Aneja R, Sun X, Xie S, Wang H, Wu X, Dong JT, Li M, Joshi HC, Zhou J. Parkin regulates Eg5 expression by Hsp70 ubiquitination-dependent inactivation of the c-Jun NH2-terminal kinase. J Biol Chem. 2008 doi: 10.1074/jbc.M806860200. [DOI] [PubMed] [Google Scholar]
- Mandemakers W, Morais VA, De Strooper B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J Cell Sci. 2007;120:1707–1716. doi: 10.1242/jcs.03443. [DOI] [PubMed] [Google Scholar]
- Marongiu R, Ferraris A, Ialongo T, Michiorri S, Soleti F, Ferrari F, Elia AE, Ghezzi D, Albanese A, Altavista MC, Antonini A, Barone P, Brusa L, Cortelli P, Martinelli P, Pellecchia MT, Pezzoli G, Scaglione C, Stanzione P, Tinazzi M, Zecchinelli A, Zeviani M, Cassetta E, Garavaglia B, Dallapiccola B, Bentivoglio AR, Valente EM. PINK1 heterozygous rare variants: prevalence, significance and phenotypic spectrum. Hum Mutat. 2008;29:565. doi: 10.1002/humu.20719. [DOI] [PubMed] [Google Scholar]
- Martella G, Platania P, Vita D, Sciamanna G, Cuomo D, Tassone A, Tscherter A, Kitada T, Bonsi P, Shen J, Pisani A. Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin. Experimental neurology. 2009;215:388–396. doi: 10.1016/j.expneurol.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Mills RD, Sim CH, Mok SS, Mulhern TD, Culvenor JG, Cheng HC. Biochemical aspects of the neuroprotective mechanism of PTEN-induced kinase-1 (PINK1). Journal of neurochemistry. 2008;105:18–33. doi: 10.1111/j.1471-4159.2008.05249.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A, Groskreutz DJ, Nyunoya T, Coleman M, Spitz DR, Hunninghake GW. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008;180:7485–7496. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee P, Quintanilla R, Roh MS, Zmijewska AA, Jope RS, Johnson GV. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. Journal of cellular biochemistry. 2007;102:196–210. doi: 10.1002/jcb.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- Moriwaki Y, Kim YJ, Ido Y, Misawa H, Kawashima K, Endo S, Takahashi R. L347P PINK1 mutant that fails to bind to Hsp90/Cdc37 chaperones is rapidly degraded in a proteasome-dependent manner. Neuroscience research. 2008;61:43–48. doi: 10.1016/j.neures.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P. Crosslinking of alpha-synuclein by advanced glycation endproducts--an early pathophysiological step in Lewy body formation? J Chem Neuroanat. 2000;20:253–257. doi: 10.1016/s0891-0618(00)00096-x. [DOI] [PubMed] [Google Scholar]
- Nair VD. Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson's disease. Apoptosis. 2006;11:955–966. doi: 10.1007/s10495-006-6316-3. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z. Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci. 2004;79:137–146. doi: 10.1093/toxsci/kfh089. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–855. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends in biochemical sciences. 2006;31:26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Pan J, Zhao YX, Wang ZQ, Jin L, Sun ZK, Chen SD. Expression of FasL and its interaction with Fas are mediated by c-Jun N-terminal kinase (JNK) pathway in 6-OHDA-induced rat model of Parkinson disease. Neurosci Lett. 2007;428:82–87. doi: 10.1016/j.neulet.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim SH, Park KH, Kim SD, Kim JY, Baek SY, Chung BS, Kang CD. Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease. Neurosci Lett. 2004;363:243–246. doi: 10.1016/j.neulet.2004.03.072. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nature cell biology. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S, Maciel FC, Paz C, Carreras MC, Poderoso JJ, Podesta EJ. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS ONE. 2008;3:e1443. doi: 10.1371/journal.pone.0001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS biology. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V. Mechanisms of MPTP toxicity. Mov Disord. 1998;13(Suppl 1):35–38. [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Reetz K, Lencer R, Steinlechner S, Gaser C, Hagenah J, Buchel C, Petersen D, Kock N, Djarmati A, Siebner HR, Klein C, Binkofski F. Limbic and frontal cortical degeneration is associated with psychiatric symptoms in PINK1 mutation carriers. Biol Psychiatry. 2008;64:241–247. doi: 10.1016/j.biopsych.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Ren Y, Jiang H, Yang F, Nakaso K, Feng J. Parkin Protects Dopaminergic Neurons against Microtubule-depolymerizing Toxins by Attenuating Microtubule-associated Protein Kinase Activation. J Biol Chem. 2009;284:4009–4017. doi: 10.1074/jbc.M806245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland R, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson's disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross OA, Farrer MJ. Pathophysiology, pleiotrophy and paradigm shifts: genetic lessons from Parkinson's disease. Biochem Soc Trans. 2005;33:586–590. doi: 10.1042/BST0330586. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin Z-H, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Rumora L, Lovric J, Sairam MR, Maysinger D. Impairments of heat shock protein expression and MAPK translocation in the central nervous system of follitropin receptor knockout mice. Exp Gerontol. 2007;42:619–628. doi: 10.1016/j.exger.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J Pharmacol Exp Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Seo JH, Rah JC, Choi SH, Shin JK, Min K, Kim HS, Park CH, Kim S, Kim EM, Lee SH, Lee S, Suh SW, Suh YH. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. Faseb J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;14:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- Simon DK, Lin MT, Zheng L, Liu GJ, Ahn CH, Kim LM, Mauck WM, Twu F, Beal MF, Johns DR. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson's disease. Neurobiol Aging. 2004;25:71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Stahlberg J, Volkel B, Djarmati A, Hagenah J, Hiller A, Hedrich K, Konig I, Klein C, Lencer R. Co-occurrence of affective and schizophrenia spectrum disorders with PINK1 mutations. Journal of neurology, neurosurgery, and psychiatry. 2007;78:532–535. doi: 10.1136/jnnp.2006.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. Extracellular signal-regulated kinase as an inducer of non-apoptotic neuronal death. Neuroscience. 2006;138:1055–1065. doi: 10.1016/j.neuroscience.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr., Davis RE, Parker WD., Jr. Origin and functional consequences of the complex I defect in Parkinson's disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian LL, Zhou Z, Zhang Q, Sun YN, Li CR, Cheng CH, Zhong ZY, Wang SQ. Protective effect of (+/−) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells. Cell Physiol Biochem. 2007;20:1019–1032. doi: 10.1159/000110682. [DOI] [PubMed] [Google Scholar]
- Tomaska L. Mitochondrial protein phosphorylation: lessons from yeasts. Gene. 2000;255:59–64. doi: 10.1016/s0378-1119(00)00315-2. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Smith TS, Jung AB, Bennett JP., Jr. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration. 1996;5:233–239. doi: 10.1006/neur.1996.0031. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr., Miller SW, Davis RE, Parker WD., Jr. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol. 2000;162:37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science (New York, N.Y. 2004a;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, Caputo V, Romito L, Albanese A, Dallapiccola B, Bentivoglio AR. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004b;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wang D, Qian L, Xiong H, Liu J, Neckameyer WS, Oldham S, Xia K, Wang J, Bodmer R, Zhang Z. Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proc Natl Acad Sci U S A. 2006;103:13520–13525. doi: 10.1073/pnas.0604661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chou AH, Yeh TH, Li AH, Chen YL, Kuo YL, Tsai SR, Yu ST. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiology of disease. 2007;28:216–226. doi: 10.1016/j.nbd.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Thomas KJ, Ostaszewski B, Cookson M, Selkoe DJ. Pink1 forms a multi-protein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009 doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–34491. doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LR, Toft M, Kvam SN, Farrer MJ, Aasly JO. MAPK-pathway activity, Lrrk2 G2019S, and Parkinson's disease. J Neurosci Res. 2007;85:1288–1294. doi: 10.1002/jnr.21240. [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AS, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS ONE. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiromerisiou G, Hadjigeorgiou GM, Papadimitriou A, Katsarogiannis E, Gourbali V, Singleton AB. Association between AKT1 gene and Parkinson's disease: a protective haplotype. Neuroscience letters. 2008;436:232–234. doi: 10.1016/j.neulet.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YX, Wood NW, Latchman DS. Molecular basis of Parkinson's disease. Neuroreport. 2009;20:150–156. doi: 10.1097/WNR.0b013e32831c50df. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Yuan H, Gerencser AA, Liot G, Lipton SA, Ellisman M, Perkins GA, Bossy-Wetzel E. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14:462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- Zablocka B, Dluzniewska J, Zajac H, Domanska-Janik K. Opposite reaction of ERK and JNK in ischemia vulnerable and resistant regions of hippocampus: involvement of mitochondria. Brain Res Mol Brain Res. 2003;110:245–252. doi: 10.1016/s0169-328x(02)00653-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–346. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- Zhong J, Deng J, Phan J, Dlouhy S, Wu H, Yao W, Ye P, D'Ercole AJ, Lee WH. Insulin-like growth factor-I protects granule neurons from apoptosis and improves ataxia in weaver mice. Journal of neuroscience research. 2005;80:481–490. doi: 10.1002/jnr.20490. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008a;105:12022–12027. doi: 10.1073/pnas.0802814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Falkenburger BH, Schulz JB, Tieu K, Xu Z, Xia XG. Silencing of the Pink1 gene expression by conditional RNAi does not induce dopaminergic neuron death in mice. International journal of biological sciences. 2007;3:242–250. doi: 10.7150/ijbs.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008b;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther. 2008;325:732–740. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Keefe KA. 6-Hydroxydopamine as a tool for studying catecholamines in adult animals. In: Kostrzewa RM, editor. Highly Selective Neurotoxins: Basic and Clinical Applications. Humana Press, Inc.; Totowa, NJ: 1997. pp. 75–107. [Google Scholar]