Abstract
Opsonization by antibodies represents a critical component of the host immune response against many pathogens. The mechanisms by which virulent microbes evade this protective response are not completely understood. In disease mediated by Francisella tularensis, antibody can effectively protect against infections with attenuated strains, e.g. LVS, but not virulent strains such as SchuS4. Thus, it is likely that SchuS4 has mechanisms, which are not present in LVS, that allow evasion of opsonization by antibody, dampening the protective effects of these host molecules. Here we demonstrate that evasion of antibody mediated opsonization and phagocytosis by the highly virulent SchuS4 is associated with its ability to bind the host serine protease plasmin. SchuS4, but not the closely related LVS, bound active plasmin. Plasmin bound SchuS4 degraded exogenous and opsonizing antibodies, whereas LVS failed to do so. Further, plasmin mediated inhibition of antibody opsonization by SchuS4 also inhibited antibody mediated uptake of this bacterium by macrophages. Antibody mediated uptake of uncoated and opsonized, SchuS4 elicited a strong pro-inflammatory response in infected macrophages. However, plasmin coated, opsonized SchuS4 poorly elicited production of these protective pro-inflammatory cytokines. This unique host-pathogen interplay is a novel immune evasion strategy utilized by virulent F. tularensis, and provides one explanation for the ability of antibody to protect against attenuated, but not virulent, strains of F. tularensis. This mechanism may also represent a more common hereto unrecognized strategy by which virulent bacteria evade detection and clearance by immunoglobulin.
Keywords: opsonization, plasmin, antibody, bacteria
Introduction
Antibody is recognized as an essential host factor for control of multiple microbial pathogens. Thus, it is not surprising that virulent microbes, unlike their more attenuated counterparts, have developed a number of strategies to evade the protective effects of antibodies in the host. One example of this dichotomy in the protective capabilities of antibody among attenuated and virulent bacterial infections is found in infections mediated by F. tularensis. Recent studies have demonstrated that passive transfer of either immune serum or monoclonal antibodies can protect against a lethal challenge with the attenuated F. tularensis strain LVS (1, 2). However, experiments testing protective efficacy of immune serum and monoclonal antibodies against challenge with virulent F. tularensis strain SchuS4 failed to reciprocate this protection, and resulted in only a modest extension of the mean time to death among treated animals (1, 2).
There are a number of explanations for the difference in antibody mediated protection between attenuated and virulent bacteria, including variation in surface antigens and affinity or avidity of the antibodies to the targeted bacterial antigen. However, an additional explanation is direct interference with the opsonic activity of antibodies by the bacteria themselves. This interference could be mediated by proteases generated directly by the bacteria or by the microbe co-opting host proteases, such as the serine protease plasmin.
Plasmin is normally generated in the host following conversion of the precursor plasminogen (Plg) to plasmin via the enzyme urokinase plasminogen activator (uPA) (as reviewed, (3). Generation of plasmin is a tightly regulated process and has been primarily associated with tissue remodeling in the host. However, plasmin has also been demonstrated to have proteolytic activity on host immunoglobulin (Ig) (4). Several species of bacteria have been shown to either bind or participate in the generation of plasmin (5). However, the contribution of plasmin and its effect on antibody mediated protection in bacterial pathogenesis has remained largely unexplored.
In this report, we provide evidence that, in contrast to attenuated LVS, virulent SchuS4 directly interferes with the activity of opsonizing antibodies in a process requiring plasmin. Together, our data describes one mechanism by which virulent bacteria evade antibody mediated protection and also serves as evidence for a novel strategy by which plasmin may be used as a virulence factor for other bacterial pathogens.
Materials and Methods
Bacteria
F. tularensis SchuS4 was kindly provided by Jeannine Peterson, Ph.D. and Marty Schriefer, Ph.D. (Centers for Disease Control, Fort Collins, CO). F. tularensis strain LVS was provided by Dr. Jean Celli (Rocky Mountain Laboratories, NIH, Hamilton, MT). LVS and SchuS4 were cultured as previously described (6, 7). Briefly, LVS and SchuS4 were cultured in modified Mueller-Hinton broth (Mueller-Hinton broth supplemented with CaCl2, MgCl, 0.1% glucose, 0.025% ferric pyrophosphate and 2% Isovitalex [BD Biosciences]) at 37°C with constant shaking overnight, aliquoted into 1 ml samples, frozen at −80°C and thawed just prior to use. Frozen stocks were titered by enumerating viable bacteria from serial dilutions plated on modified Mueller-Hinton agar as previously described. The number of viable bacteria in frozen stock vials varied less than 5% over 10 month period.
Detection of plasminogen bound to bacteria
The ability of LVS and SchuS4 to bind Plg was tested using a modified ELISA. SchuS4 and LVS were coated onto Immulon 2HB (Thermo, Milford, MA) plates as previously described (8). Plates were then washed with TBST, blocked with 1% bovine serum albumin (BSA) (Pierce, Rockford, IL) and washed again. Then, Plg (R&D Systems, Minneapolis, MN) or BSA (Sigma, St. Louis, MO; negative control) was added at the indicated concentrations, in triplicate, and plates were incubated for 1 hour at room temperature. Wells without bacteria served as negative controls to account for non-specific binding of Plg and antibodies (Ab). Bound Plg was detected using anti-human plasminogen Ab (clone 270409, R&D Systems) and HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). Bound Ab were detected using 1-step TMB ELISA substrate (Pierce) and analysis at 450 nm using a MRX Revelation and Revelation Software (Dynex Technologies, Chantilly, Virginia).
Detection of active plasmin bound to bacteria
Detection of active plasmin bound to the surface of LVS and SchuS4 was performed as previously described (9). Briefly, 4×108 CFU SchuS4 were centrifuged and resuspended in 100 µl PBS (Invitrogen, Carlsbad, CA) followed by addition of 40 µg Plg and 30 IU of human urokinase plasminogen activator (American Diagnostica, Inc, Stamford, CT). Additional tubes of bacteria received either PBS (control), 40 µg Plg, or 30 IU of urokinase uPA. To account for any carryover of active plasmin adhering to the tube, a final tube which initially contained only uPA and Plg (no bacteria) was washed in an identical fashion to that done with Francisella containing tubes. Each sample was incubated at 37°C/5%CO2 for 90 minutes. Bacteria were then washed 3 times and each tube was resuspended in 400 µl PBS. Three-hundred microliters from each tube was divided into 100 µl aliquots into fresh microcentrifuge tubes resulting in triplicate samples for each test condition. One hundred microliters (0.5 mg/ml) of the chromagenic substrate S-2251 (Chromagenix/Diapharma, West Chester, Ohio) was added to each new tube and samples were incubated for 90 minutes at 37°C/5%CO2. Samples were then centrifuged and the supernatants were transferred to an Immulon 2 HB plate for analysis at 405 nm using a MRX Revelation plate reader and Revelation software (Dynex Technologies).
Degradation of immunoglobulin
The ability of plasmin bound LVS or SchuS4 to degrade IgG was assessed using a modified ELISA. Briefly, Immulon 2 HB plates were coated with purified human IgG (Sigma) in 0.1M sodium bicarbonate. Plates were incubated at 4°C overnight, washed with PBS-0.05% Tween 20 (PBST) and blocked with 1% BSA-PBS overnight at 4°C. Plasmin coated bacteria and the indicated controls were prepared as described above. Bacteria were added to wells and incubated for 2 hr at 37°C. The last wash from tubes containing bacteria served as negative controls. Supernatants from tubes that contained Plg and uPA alone (plasmin) served as positive controls. Degradation of IgG was detected as an inability of HRP rabbit anti-human Fcγ specific antibodies (Jackson ImmunoResearch) to bind IgG in each well. Bound anti-IgG antibodies were detected following addition of HRP goat anti-rabbit IgG (Sigma) and 1-step TMB ELISA substrate (Pierce) and analysis as described above. Total percent degradation of IgG was calculated using the following formula:
Assessment of antibody mediated opsonization
Plasmin coated F. tularensis was generated as described above. Bacteria incubated in the presence of PBS served as negative controls. Bacteria were diluted to 4 × 107/100 µl and aliquoted into duplicate tubes. Alexa Fluor 488 conjugated anti-Francisella LPS antibody (anti-FT; US Biologicals) or FITC conjugated mouse IgG2a (BD Biosciences) was added to the indicated tubes at 4.4 µg/ml. Bacteria were incubated for 2 hours at 37°C/7%CO2. Bacteria were centrifuged, the resulting pellets resuspended in 1 ml 3% paraformaldehyde (PFA), and incubated for 30 minutes at 37°C/7%CO2. Then, bacteria were centrifuged again and pellets were resuspended in 100 µl PBS prior to transfer to a 96-well plate (Corning). Fluorescence was assessed and reported as relative fluorescence units (RFU) using a Tecan Safire2 microplate reader and XFluor software (Tecan, San Jose, CA).
Culture and infection of bone marrow derived macrophages
Bone marrow derived macrophages were generated as previously described (10) with the exception that cells were differentiated into macrophages in the presence of 10 ng/ml recombinant murine M-CSF (Peprotech, Rocky Hill, NJ). Macrophages were infected with a MOI = 25 of F. tularensis as previously described (10), with the following modifications. F. tularensis was co-incubated with macrophages at 37°C in 7% CO2 for 1.5 hours followed by treatment with 50 µg/ml gentamicin (Invitrogen) to eliminate extracellular bacteria. Cultures were incubated for an additional 45 min, washed with PBS, and fixed with 3% paraformaldehyde in PBS for 30 min at 37°C/7%CO2 prior to analysis for bacteria as described below. The infection inoculum was confirmed by plating serial dilutions of stock F. tularensis on modified Mueller-Hinton agar plates immediately prior to addition to cell cultures.
Fluorescence Microscopy
Macrophages were fixed and stained for F. tularensis and LAMP-1 as previously described (11). LAMP-1 and bacteria were visualized following incubation with rat anti-mouse LAMP-1 antibodies followed by Alexa Fluor 568 conjugated anti-rat antibodies (Invitrogen) and Alexa Fluor 488 mouse conjugated anti-F. tularensis (US Biological, Swampscot, MA), respectively. Samples were observed on a Carl Zeiss (Thornwood, NY) Axio Imager.M1 epifluoresence microscope for quantitative analysis. Approximately 75–100 cells/field and a minimum of three fields per coverslip were analyzed for presence of intracellular bacteria. The percent of infected cells was calculated as follows:
Detection of Secreted Cytokines
Culture supernatants were assayed for the presence of TNF-α and IL-6 by ELISA using commercially available kits according to the manufacturer’s instructions (R&D Systems).
Statistical analysis
Statistical differences between 2 groups were determined using unpaired t-test, with significance set at p < 0.05. For comparisons between 3 or more groups, analysis was done by one-way ANOVA, followed by Tukey's multiple comparisons test, with significance determined for p < 0.05.
Results
F. tularensis binds plasminogen
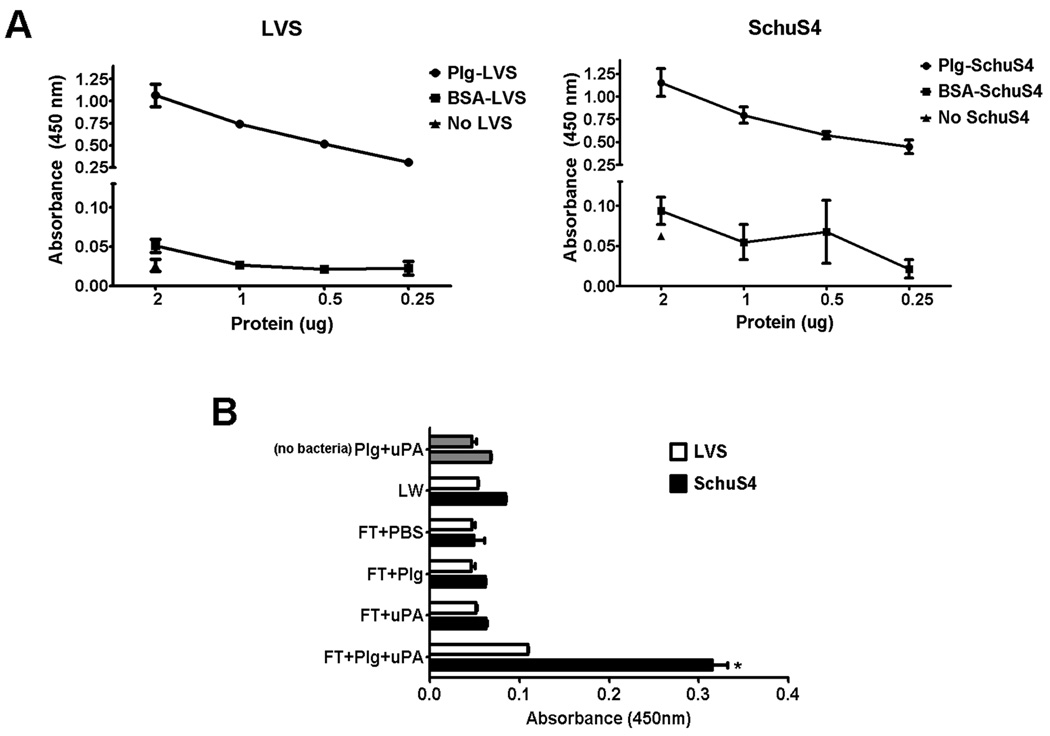
Active plasmin is generated following its transformation from Plg by the enzyme urokinase plasminogen activator (uPA) (as reviewed, (5)). Therefore, the typical pre-requisite for binding of plasmin to bacteria is the ability of the organism to bind Plg. Thus, we first determined if attenuated LVS and virulent SchuS4 could directly bind Plg. Both LVS and SchuS4 bound Plg in a dose dependent manner (Fig. 1A). Samples that did not include bacteria (no LVS or no SchuS4, respectively), failed to bind anti-Plg antibodies, indicating that antibody binding was specific for Plg bound to F. tularensis (Fig. 1A). Further evidence of a specific interaction of the bacteria with Plg was indicated by the failure of bacteria incubated with BSA to bind anti-Plg antibodies. These controls confirmed that detection of Plg bound to LVS and SchuS4 was not a result of non-specific interaction of the antibodies used for detection with exogenous protein (Fig. 1A).
Figure 1. Francisella binds Plg and plasmin.
(A) Binding of Plg to LVS and SchuS4 was assessed using a modified ELISA. Both LVS and SchuS4 bound human Plg in a dose dependent manner (closed circles). Bacteria incubated with the same concentrations of bovine serum albumin (BSA) (closed squares) served as negative controls. Additional wells incubated with the highest concentration of Plg tested in the absence of bacteria (closed triangles) also served as negative controls. (B) SchuS4 (black bars), but not LVS (white bars), binds active plasmin. Bacteria were incubated in Plg and uPA and then assessed for association of active plasmin. In contrast to LVS, SchuS4 was associated with significantly more active plasmin compared to bacteria treated with PBS, Plg or uPA alone (* = p<0.001). The last wash (LW) from Plg and uPA treated LVS and SchuS4 and tubes incubated with uPA+Plg without bacteria (no bacteria; gray bars) that were washed in the same manner as other samples served as negative controls for unbound or carry-over plasmin, respectively. Error bars represent SEM. All data is representative of three experiments of similar design.
Virulent, but not attenuated, F. tularensis binds active plasmin
Many pathogens have been noted to bind Plg (12–15); however, the ability of bacteria to interact with this protein may not correlate with their ability to bind the active protease plasmin. Thus, we next assessed the ability of Plg coated LVS and SchuS4 to bind active plasmin. Because F. tularensis species do not encode any known Plg activator enzymes, we supplied exogenous, host-derived, uPA as the catalytic enzyme. Supernatants from washed tubes in which Plg was incubated with uPA in the absence of bacteria ([nobacteria] Plg+uPA), accounted for any carryover of plasmin activity and served as a negative control. Additional controls included supernatants from the last wash (LW) from tubes which contained bacteria, Plg and uPA to account for residual plasmin that was not bound to the bacteria. Despite the ability of LVS to bind Plg, in the presence of uPA, LVS failed to bind significant quantities of active plasmin. In contrast, Plg coated SchuS4 readily bound active plasmin when uPA was present (Fig. 1B). Plasmin activity in samples containing LVS or SchuS4 incubated with either Plg or uPA alone was similar to that observed for the negative control, indicating that SchuS4 could not independently convert Plg to plasmin (Fig. 1B). SchuS4 and LVS incubated in the presence of PBS, but not Plg or uPA, also failed to have detectable levels of active plasmin (Fig. 1B). Furthermore, the plasmin activity observed in samples which contained all three components (SchuS4, Plg and uPA) was significantly greater than that observed in supernatant from the last wash of SchuS4 incubated in the presence of Plg and uPA (LW) (p<0.001) (Fig. 1B). Therefore, the significant plasmin activity observed in SchuS4 samples incubated with Plg and uPA was not due to carryover of free plasmin in the supernatant. Given the proteolytic capabilities of plasmin, it was possible that this enzyme might negatively affect the viability of LVS and SchuS4. Thus, we compared viability of LVS and SchuS4 incubated with Plg and uPA to bacteria incubated in PBS. There were no differences in the number of viable bacteria present in PBS or uPA+Plg samples suggesting that presence of plasmin did not affect viability of bacteria (data not shown). Together, these data demonstrate that, despite the apparent equivalent ability of attenuated LVS and virulent SchuS4 to bind Plg, only virulent SchuS4 was capable of binding significant concentrations of the active protease plasmin.
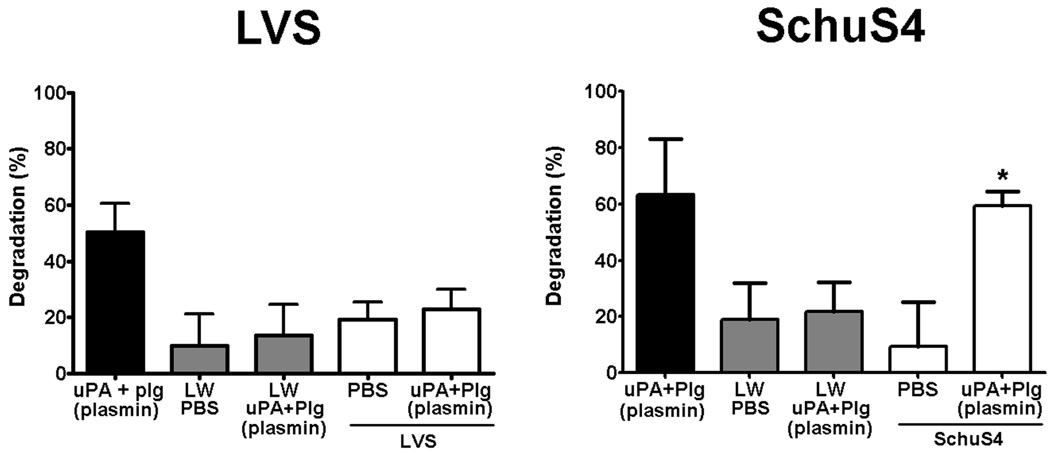
Plasmin coated SchuS4 degrades immunoglobulin
Clearance of bacteria has been tightly associated with opsonization of the microbe with Ig. However, the strategies by which pathogens evade this opsonization have not been completely elucidated. Early studies examining the stability of Ig demonstrated that these molecules were susceptible to degradation by plasmin (4). Thus, plasmin associated with LVS or SchuS4 may endow the bacteria with the ability to directly degrade Ig and evade opsonization. To test this hypothesis, we next assessed the ability of plasmin coated LVS and SchuS4 to degrade non-specific IgG. Consistent with the poor association of plasmin with LVS, LVS incubated in the presence of uPA and Plg failed to degrade IgG. In contrast, plasmin coated SchuS4 degraded significantly more IgG than SchuS4 incubated in PBS, supernatant from the last wash of bacteria incubated in the presence of Plg and uPA, and all LVS samples (p<0.01) (Fig. 2). Although there was some carryover of IgG degradation in the supernatant from the last wash of plasmin coated bacteria, this was significantly less than the degradation observed with plasmin coated bacteria themselves (Fig. 2). Thus, plasmin associated with SchuS4 resulted in degradation of host IgG, whereas attenuated LVS incubated under similar conditions was unable to mediate this proteolytic function.
Figure 2. Plasmin coated bacteria degrade IgG.
LVS and SchuS4 incubated with Plg and uPA to generate surface bound plasmin were added to plates coated with human IgG. Wells incubated with PBS or SchuS4 alone served as negative controls, whereas supernatant from Plg treated with uPA served as positive controls. Plasmin coated SchuS4 degraded significantly more human IgG compared to SchuS4 incubated in PBS, the last wash (LW) from SchuS4 incubated with Plg and uPA, or LVS incubated under all conditions (* = p<0.01). Error bars represent SEM. Data is representative of four experiments of similar design.
Plasmin coated SchuS4 interferes with opsonization and antibody mediated phagocytosis
The primary function of host antibody directed against invading pathogens is opsonization. Binding of pathogen specific antibody to microbes enables the host to more efficiently kill the pathogen via Fc receptor (FcR) mediated uptake by phagocytic effector cells or formation of a complement driven membrane attack complex on the surface of the bacterium. Since plasmin coated SchuS4, but not LVS, readily degraded IgG (Fig.2), we predicted that these bacteria would also be more resistant to opsonization by Francisella specific antibody. To determine if our hypothesis was correct, we compared the ability of SchuS4 and LVS incubated in PBS or Plg+uPA (plasmin) to bind Francisella specific IgG (anti-FT). Bacteria exposed to isotype control antibody served as negative controls for non-specific binding. There were no differences between bacteria incubated with PBS or uPA+Plg (plasmin) and their interaction with isotype control antibody (Fig.3). Similarly, regardless of pre-incubation conditions (PBS or uPA+Plg), LVS bound similar concentrations of anti-FT antibodies. Compared to PBS treated SchuS4, LVS (regardless of treatment) bound slightly less, but not significantly different amounts, of anti-FT antibodies. This suggests that opsonization with anti-FT antibodies may be slightly more efficient for SchuS4 bacteria. In marked contrast to plasmin treated LVS, plasmin coated SchuS4 bound significantly less (p = 0.0047) anti-FT antibody compared to PBS treated SchuS4 (Fig.3). Furthermore, plasmin coated SchuS4 bound less anti-FT antibodies than LVS pre-incubated with either PBS or uPA+Plg. Together, this data suggests that surface bound plasmin aids in the interference of opsonization of virulent, but not attenuated, F. tularensis by Francisella specific antibodies.
Figure 3. Plasmin inhibits opsonization of SchuS4 by Francisella specific antibodies.
LVS and SchuS4 were coated with plasmin following incubation with uPA+Plg as described above. Bacteria incubated in PBS served as negative controls (PBS). Bacteria were incubated with Alexa Fluor 488 anti-FT antibodies or matched FITC isotype control, washed, fixed and assessed for binding of antibody using a fluorimeter. PBS treated and plasmin coated bacteria bound small amounts of isotype control antibody equally. However, plasmin coated SchuS4 bound significantly less anti-FT antibodies compared to PBS treated SchuS4 controls and all LVS samples (* = p = 0.0047).
Opsonization of bacteria by antibody enables more efficient uptake by host effector cells. As described for other bacteria, antibody mediated uptake of F. tularensis results in compromised replication of the bacteria within macrophages ((16–18) and J. Celli, personal communication). Given that plasmin coated SchuS4 interfered with the binding of anti-FT antibody, we next examined the impact plasmin present on the bacterial surface had on antibody mediated uptake of LVS and SchuS4 by macrophages. Bacteria were coated with plasmin and exposed to anti-FT antibodies or isotype controls, as described above. Murine bone marrow derived macrophages were infected with the prepared SchuS4 or LVS and uptake of bacteria was monitored by microscopy. As expected, LVS and SchuS4 treated with PBS and opsonized with anti-FT antibodies were phagocytosed with greater efficiency than bacteria incubated with isotype control antibodies. This enhanced uptake of opsonized LVS and SchuS4 was evident as both an increase in both the number of infected macrophages and the number of bacteria per cell (Fig. 4 A and B). Interestingly, PBS treated, antibody opsonized SchuS4 were phagocytosed by more macrophages than PBS treated opsonized LVS. Incubation of LVS and SchuS4 in uPA+Plg (to generate plasmin) had no effect on the uptake of either LVS or SchuS4 in the presence of isotype control antibody (Fig. 4 A and B), thus presence of plasmin alone did not affect phagocytosis of bacteria by macrophages. Similarly, pre-incubation of LVS with uPA+Plg had no significant effect on the number of macrophages infected with opsonized LVS, nor the number of bacteria per cell (Fig.4 A and B). In contrast, and in correlation with approximately 50% inhibition of opsonization by anti-FT antibodies by plasmin coated SchuS4 (Fig. 3), significantly fewer macrophages (approximately 50% fewer) were infected with plasmin coated SchuS4 opsonized with anti-FT antibodies compared to PBS treated controls (p = 0.0081) (Fig. 4A). Furthermore, significantly fewer macrophages had 1–2 and 3–10 plasmin coated, anti-FT opsonized SchuS4 compared to PBS, anti-FT opsonized SchuS4 controls (p = 0.0210 and 0.0100, respectively) (Fig. 4B). Thus, unlike more attenuated strains of Francisella, plasmin associated with virulent SchuS4 inhibited antibody mediated opsonization and sub-sequentially, antibody mediated phagocytosis by macrophages.
Figure 4. Plasmin inhibits antibody mediated uptake of SchuS4.
LVS and SchuS4 were coated with plasmin following incubation with uPA+Plg as described above. Bacteria then were incubated with anti-FT or matched isotype control antibodies prior to addition to macrophages. Phagocytosis of SchuS4 and LVS by macrophages was monitored by microscopy and was assessed as the total number of cells infected (A) and the number of bacteria per cell (B). (A) Significantly fewer macrophages were infected with plasmin coated SchuS4 opsonized with anti-FT compared to PBS incubated, anti-FT opsonized controls (* = p = 0.0081). Pre-incubation with uPA and Plg did not significantly affect the ability of macrophages to take up anti-FT opsonized LVS. (B) Significantly fewer macrophages had 1–2 and 3–10 plasmin coated, anti-FT opsonized SchuS4 compared to PBS treated, anti-FT opsonized controls (* = p = 0.0210 and 0.0100, respectively).
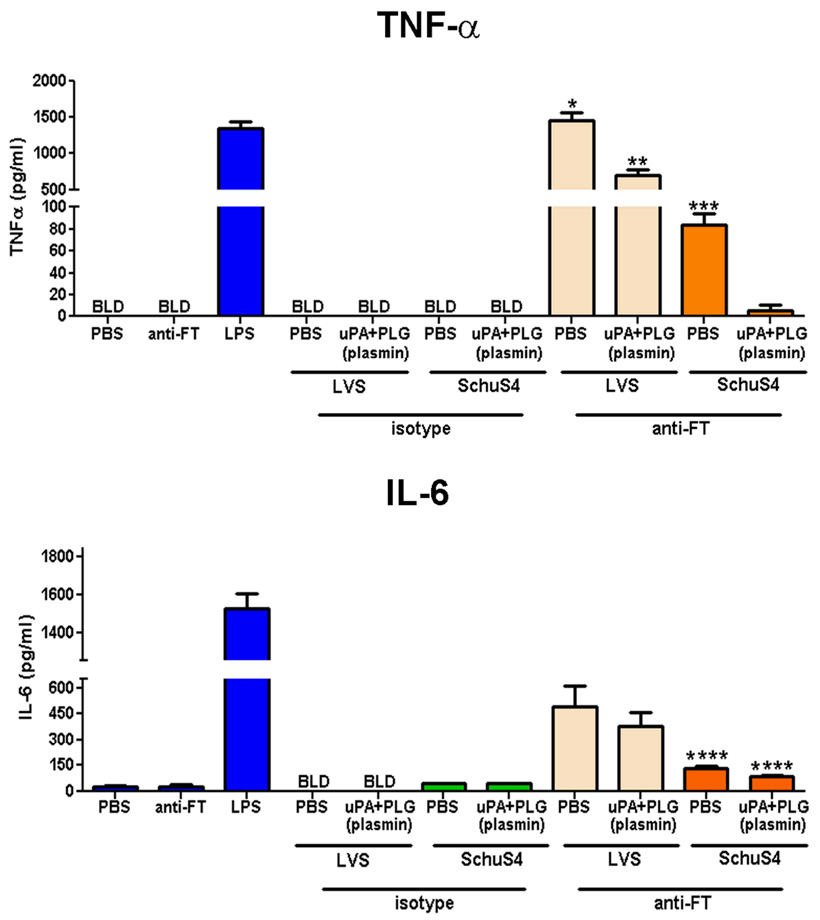
Plasmin coated SchuS4 inhibit induction of pro-inflammatory cytokines by opsonized bacteria
One mechanism by which antibody mediated phagocytosis aids in clearance and killing of invading pathogens is by enhancing the production of inflammatory cytokines, e.g. TNF-α, that trigger killing mechanisms in effector cells (19–21). Thus, in addition to plasmin coated SchuS4 interfering with antibody mediated phagocytosis, these bacteria may also limit the production of cytokines that are produced following uptake of antibody opsonized bacteria. To test this hypothesis we measured cytokines secreted by macrophages exposed to opsonized, plasmin coated or PBS control treated, LVS and SchuS4. As expected, all LVS and SchuS4 incubated in the presence of isotype control antibodies failed to stimulate secretion of TNF-α or IL-6 from macrophages in quantities that were significantly different from uninfected controls. Antibody opsonized LVS and SchuS4 readily induced significantly greater amounts of TNF-α and IL-6 from macrophages compared to all other treatment groups (p<0.01) (Fig. 5). Surprisingly, despite lack of evidence suggesting active plasmin bound to LVS (Fig 2–Fig 4), plasmin treated, antibody opsonized, LVS elicited significantly less TNF-α and IL-6 secretion from macrophages compared to PBS treated, anti-FT opsonized LVS (Fig. 5). However, even in the presence of plasmin opsonized LVS elicited significantly more TNF-α and IL-6 compared to unopsonized LVS controls and all SchuS4 infected cultures (p>0.01) (Fig. 5). Thus, although presence of plasmin effected the production of cytokines following phagocytosis of LVS, this effect was modest. In contrast, SchuS4 coated in plasmin and then exposed to opsonizing antibody induced significantly less TNF-α from macrophages compared to opsonized, PBS treated controls (p<0.05) (Fig. 5). Plasmin treated, antibody opsonized SchuS4 also induced less IL-6 compared to PBS treated opsonized SchUS4, but these values were not significantly different. Furthermore, in the presence of plasmin, antibody opsonized SchuS4 failed to induce TNF-α or IL-6 at concentrations significantly different from uninfected controls (Fig. 5). Thus, phagocytosis of antibody opsonized LVS and SchuS4 induced cytokine production from macrophages. However, the impact of plasmin on the interference of cytokine production following uptake of opsonized SchuS4 was significantly greater than that observed in cultures containing opsonized LVS (p<0.05). Together, this suggests that plasmin bound to the surface of SchuS4 modulates both the effect of antibody mediated phagocytosis and induction of two key pro-inflammatory cytokines from macrophages.
Figure 5. Plasmin coated SchuS4 interferes with antibody mediated induction of pro-inflammatory cytokines.
LVS and SchuS4 were prepared as described above. Bacteria incubated in PBS served as negative controls (PBS). LPS treated cells served as positive controls. Bacteria were incubated with anti-FT or matched isotype control antibodies prior to addition to macrophages. Eighteen hours after infection culture supernatants were assessed for cytokines by ELISA. Opsonized, uncoated, LVS induced secretion of significantly higher concentrations of TNF-α, and IL-6 (* = p<0.01) by macrophages compared to all other samples. Plasmin coated, antibody opsonized LVS induced significantly less TNF-α than unopsonized controls, but significantly more than all SchuS4 samples (** = p<0.05). Opsonized, uncoated SchuS4 induced secretion of significantly higher concentrations of TNF-α than plasmin coated, opsonized SchuS4 (*** = p<0.05). Opsonized SchuS4 induced significantly less IL-6 than LVS (**** = p<0.05). BLD = below level of detection. Error bars represent SEM. Data is representative of two experiments of similar design.
Discussion
Successful colonization and replication of pathogens in the host requires evasion and/or manipulation of the host immune response. The most infectious pathogens have developed a number of mechanisms to avoid detection or destruction by both innate and adaptive host effector mechanisms. One such effector mechanism is antibody mediated clearance of pathogens. Evasion of antibody mediated immunity is an important hurdle for both extra- and intracellular pathogens to overcome. For example, opsonization of Mycobacteria by antibody enhances uptake of the bacterium by phagocytic cells, activates the cells, and ultimately results in destruction of the microbe (22).
Antibody has also recently been shown to be an important component for optimal protective responses against F. tularensis. Transfer of immune serum or monoclonal antibodies uniformly protects animals against challenge with the attenuated strains of F. tularensis such as LVS (1, 2). In contrast, although transfer of immune serum or purified antibodies extends the mean time to death, these treatments fail to increase overall survival following challenge with virulent F. tularensis (1, 2). There are a number of explanations for the failure of antibody to protect against infections with virulent F. tularensis. These include poor affinity or avidity of antibody directed against the bacterium, low abundance of the antigen targeted by the antibody associated with virulent organisms, direct suppression of pro-inflammatory responses typically elicited following antibody mediated uptake of bacteria by phagocytes, or direct interference with antibody opsonization.
Data presented herein suggests the inability of antibody to efficiently protect against virulent F. tularensis infection is not due to the failure of antibody to bind bacteria under normal tissue culture conditions (Fig 3). Nor is the ineffectiveness of antibody mediated protection due to the lack of production of pro-inflammatory cytokines following uptake of opsonized F. tularensis (Fig 4). Rather, data presented herein suggest that the inability of antibody to adequately protect against virulent F. tularensis infections is directly related to the ability of virulent, but not attenuated, F. tularensis to more efficiently harness the proteolytic capability of host plasmin for degradation of Francisella specific antibody (Fig. 2–5).
The interaction of SchuS4 and LVS with plasmin and antibody revealed a previously unappreciated difference in the ability of these bacteria to modulate the host immune response. For example, antibody opsonization of SchuS4 resulted in both greater numbers of infected macrophages and greater numbers of cells infected with 3–10 and >10 bacteria per cell compared to LVS (Fig. 4A and B). This increase in phagocytosis may be a result of SchuS4 binding the anti-FT antibody with greater affinity than LVS. Alternatively, SchuS4 may have slightly greater amounts of the antigen recognized by the antibody used in these studies and, thus, bound more antibody on their surfaces. Experiments depicted in Figure 3 suggest that SchuS4 was capable of binding modestly larger amounts of anti-FT than LVS. Regardless of the reason for increased phagocytosis of antibody opsonized SchuS4 versus LVS, this data inferred that antibody mediated clearance would be more efficient for SchuS4 infections than LVS. However, previous reports demonstrate that passive transfer of immune serum or monoclonal antibodies fails to protect infection with SchuS4 (1, 2). Thus, it is likely that functional differences in antibody mediated uptake (outside of phagocytosis) may contribute to control of LVS, but not SchuS4, infections.
In this report we demonstrate that one such functional difference is the induction of pro-inflammatory cytokines following uptake of antibody opsonized Francisella. Although more macrophages were infected with antibody opsonized SchuS4 than opsonized LVS, production of TNF-α and IL-6 from cells infected with opsonized LVS was significantly greater than that observed in cultures infected with opsonized SchuS4 (Fig. 5). This disparity in cytokine production between infections with either opsonized SchuS4 and LVS was even more dramatic in cultures infected with plasmin coated, antibody opsonized, bacteria. uPA+Plg treated, antibody opsonized LVS induced less TNF-α and IL-6 than PBS treated, opsonized LVS. However, the concentration of cytokines detected in plasmin treated, opsonized LVS cultures was significantly higher than that detected in any SchuS4 culture. In contrast, the presence of plasmin on the surface of SchuS4 resulted in nearly complete reversal of the antibody mediated cytokine production observed in PBS treated, opsonized SchuS4 infections. Thus, although the number of opsonized SchuS4 engulfed by macrophages was either greater or equivalent to that observed with opsonized LVS, virulent SchuS4 was less efficient at inducing cytokine responses. Furthermore, the presence of plasmin had a more profound effect on the induction of cytokines by antibody opsonized SchuS4 compared to opsonized LVS.
We have previously demonstrated that SchuS4, in the absence of antibody, is capable of actively suppressing cytokine responses in various host target cells and tissues (6, 23). It has also been reported that LVS interferes with cytokine production in host cells (24, 25). This suggested that SchuS4 and LVS are similar in their disruption of innate immune responses However, data presented herein clearly demonstrates that (unlike LVS) in addition to disrupting innate immunity, SchuS4 is capable of profoundly modulating cytokine responses that are associated with adaptive immune responses as well, i.e. production of cytokines following uptake of antibody opsonized SchuS4. These data reveal an important advance in our understanding of the interplay between host immune responses and virulent Francisella. Furthermore, they describe an important, fundamental difference in LVS and SchuS4 mediated infections of host cells. Together this data confirms that antibody mediated uptake is likely an important component of protective immunity against virulent Francisella infections, and that the bacterium has specific mechanisms in place to resist this host response. Moreover, our data also suggests that antibody mediated protection against virulent F. tularensis may be improved following inhibition of specific components of the host plasminogen activating system (PAS).
Typically, bacteria interacting with the PAS via binding of Plg also efficiently bind plasmin (as reviewed, (5)). Thus, our observation that both LVS and SchuS4 bound Plg, but only SchuS4 bound active plasmin was unexpected. To our knowledge, this is the first demonstration of a direct comparison of an attenuated and virulent strain of bacteria, with nearly equivalent ability to interact with Plg, to interact with plasmin in such a disparate way. The most likely explanation of the dissimilar nature in which LVS and SchuS4 interact with plasmin is that the molecule responsible for binding plasmin to the surface of the bacterium is either absent or present in low abundance on LVS compared to SchuS4. In a recent study, the only protein present in outer membrane fractions of SchuS4 that was absent in LVS was a homologue to the yersinia autotransporter protein (Yap) designated as YapH-N and YapH-C (26, 27). In Yersinia, these proteins function as adhesins similarly to the plasminogen activator protein (Pla) that is also found on the surface of Yersinia (28–30). Pla is well known for its ability to interact with Plg and plasmin (31). However, the potential interaction of Yap with Plg and plasmin has not been examined. Thus, it is tempting to speculate that YapH-N and YapH-C proteins present on the surface of SchuS4 may play an important role in securing host plasmin to the bacterial surface.
In addition to differences in the species of outer membrane proteins present on LVS and SchuS4, it is also possible that small changes in the overall structure of LPS on these two related bacteria contributes to conversion of bound Plg to plasmin on SchuS4, but not LVS. Previous studies conducted with Yersinia and Salmonella have shown that the structure of LPS associated with these organisms, specifically the ability of LPS to interact with arginine residues located in Yersinia and Salmonella proteins interacting with Plg (Pla and PgtE, respectively), was critical for the optimal conversion of Plg to plasmin on the surface of these bacteria (32). Although, LPS associated with LVS and SchuS4 are both poorly inflammatory, a direct comparison of their molecular structure and their ability to bind arginine residues located in outer membrane proteins has not been performed (6, 33). Therefore, in addition to specific differences in plasmin binding proteins, small variations in the structure of SchuS4 LPS may contribute to the ability of outer membrane proteins present in this virulent bacterium to bind plasmin.
In conclusion, our data demonstrate a novel role for plasmin associated with virulent, but not attenuated, bacterial pathogens. With regard to Francisella specific immunity, we demonstrate that antibody mediated opsonization and phagocytosis of virulent F. tularensis results in the elicitation of pro-inflammatory cytokines by infected macrophages. This supports the hypothesis that Francisella specific antibody plays an important role in protective immunity against this bacterium. Further, our data provide a potential mechanism by which antibody may aid in the control of Francisella infections and how virulent strains utilize host systems to modulate this response. Our data also have important implications for many other bacterial diseases. Specifically, the majority of studies examining the role of Plg and plasmin in bacterial infections have focused on the contribution these compounds make toward dissemination of the pathogens via degradation of extracellular matrix proteins. A direct demonstration of plasmin mediated degradation of pathogen specific antibody and the consequence of this activity for control of infection has not been addressed. Furthermore, a direct comparison of the ability of attenuated and virulent strains of a bacterial pathogen to harness the proteolytic potential of plasmin has not been reported. Considering the number of pathogens known to interact with plasminogen and plasmin, e.g. Neisseria, Haemophilus and Borrelia, it is tempting to speculate that this strategy of plasmin mediated degradation of immunoglobulin for interference of antibody mediated opsonization and uptake by macrophages may occur for a number of pathogenic microbes (9, 34–36). Recognition and identification of the specific function of plasmin during bacterial infections will undoubtedly be a critical step in the development of effective therapeutics and vaccines directed against many infectious diseases, including those mediated by F. tularensis, where antibody is important.
Acknowledgements
We would also like to thank Drs. Jean Celli, Robert Heinzen and Henriette Geier for their helpful comments and suggestions regarding this work.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
References
- 1.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 2.Savitt AG, Mena-Taboada P, Monsalve G, Benach JL. Francisella tularensis infection-derived monoclonal antibodies provide detection, protection, and therapy. Clin Vaccine Immunol. 2009;16:414–422. doi: 10.1128/CVI.00362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholl SM, Roztocil E, Davies MG. Plasminogen activator system and vascular disease. Curr Vasc Pharmacol. 2006;4:101–116. doi: 10.2174/157016106776359880. [DOI] [PubMed] [Google Scholar]
- 4.Harpel PC, Sullivan R, Chang TS. Binding and activation of plasminogen on immobilized immunoglobulin G. Identification of the plasmin-derived Fab as the plasminogen-binding fragment. J Biol Chem. 1989;264:616–624. [PubMed] [Google Scholar]
- 5.Lahteenmaki K, Kuusela P, Korhonen TK. Bacterial plasminogen activators and receptors. FEMS Microbiol Rev. 2001;25:531–552. doi: 10.1111/j.1574-6976.2001.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 6.Chase JC, Celli J, Bosio CM. Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4. Infect Immun. 2009;77:180–195. doi: 10.1128/IAI.00879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman JL, Benach JL. The generation of enzymatically active plasmin on the surface of spirochetes. Methods. 2000;21:133–141. doi: 10.1006/meth.2000.0984. [DOI] [PubMed] [Google Scholar]
- 10.Bosio CM, Elkins KL. Susceptibility to secondary Francisella tularensis live vaccine strain infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect Immun. 2001;69:194–203. doi: 10.1128/IAI.69.1.194-203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, Benach JL. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe JD, Sievwright IK, Auld GC, Moore NR, Gow NA, Booth NA. Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol Microbiol. 2003;47:1637–1651. doi: 10.1046/j.1365-2958.2003.03390.x. [DOI] [PubMed] [Google Scholar]
- 14.Ehinger S, Schubert WD, Bergmann S, Hammerschmidt S, Heinz DW. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: crystal structure and evaluation of plasmin(ogen)-binding sites. J Mol Biol. 2004;343:997–1005. doi: 10.1016/j.jmb.2004.08.088. [DOI] [PubMed] [Google Scholar]
- 15.Kuusela P, Saksela O. Binding and activation of plasminogen at the surface of Staphylococcus aureus. Increase in affinity after conversion to the Lys form of the ligand. Eur J Biochem. 1990;193:759–765. doi: 10.1111/j.1432-1033.1990.tb19397.x. [DOI] [PubMed] [Google Scholar]
- 16.Janoff EN, Fasching C, Orenstein JM, Rubins JB, Opstad NL, Dalmasso AP. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Invest. 1999;104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modolell M, Schaible UE, Rittig M, Simon MM. Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol Lett. 1994;40:139–146. doi: 10.1016/0165-2478(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 18.Moore T, Ananaba GA, Bolier J, Bowers S, Belay T, Eko FO, Igietseme JU. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105:213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parcina M, Wendt C, Goetz F, Zawatzky R, Zahringer U, Heeg K, Bekeredjian-Ding I. Staphylococcus aureus-induced plasmacytoid dendritic cell activation is based on an IgG-mediated memory response. J Immunol. 2008;181:3823–3833. doi: 10.4049/jimmunol.181.6.3823. [DOI] [PubMed] [Google Scholar]
- 20.Shannon JG, Cockrell DC, Takahashi K, Stahl GL, Heinzen RA. Antibody-mediated immunity to the obligate intracellular bacterial pathogen Coxiella burnetii is Fc receptor- and complement-independent. BMC Immunol. 2009;10:26. doi: 10.1186/1471-2172-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein M, Gordon S. Regulation of tumor necrosis factor (TNF) release by murine peritoneal macrophages: role of cell stimulation and specific phagocytic plasma membrane receptors. Eur J Immunol. 1991;21:431–437. doi: 10.1002/eji.1830210227. [DOI] [PubMed] [Google Scholar]
- 22.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2007;178:4538–4547. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 24.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 25.Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNF-alpha and IL-1 from murine macrophages. Cell Microbiol. 2003;5:41–51. doi: 10.1046/j.1462-5822.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 26.Huntley JF, Conley PG, Hagman KE, Norgard MV. Characterization of Francisella tularensis outer membrane proteins. J Bacteriol. 2007;189:561–574. doi: 10.1128/JB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect Immun. 2008;76:3664–3671. doi: 10.1128/IAI.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felek S, Lawrenz MB, Krukonis ES. The Yersinia pestis autotransporter YapC mediates host cell binding, autoaggregation and biofilm formation. Microbiology. 2008;154:1802–1812. doi: 10.1099/mic.0.2007/010918-0. [DOI] [PubMed] [Google Scholar]
- 29.Kienle Z, Emody L, Svanborg C, O'Toole PW. Adhesive properties conferred by the plasminogen activator of Yersinia pestis. J Gen Microbiol. 1992;138(Pt 8):1679–1687. doi: 10.1099/00221287-138-8-1679. [DOI] [PubMed] [Google Scholar]
- 30.Lahteenmaki K, Virkola R, Saren A, Emody L, Korhonen TK. Expression of plasminogen activator pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect Immun. 1998;66:5755–5762. doi: 10.1128/iai.66.12.5755-5762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 32.Kukkonen M, Suomalainen M, Kyllonen P, Lahteenmaki K, Lang H, Virkola R, Helander IM, Holst O, Korhonen TK. Lack of O-antigen is essential for plasminogen activation by Yersinia pestis and Salmonella enterica. Mol Microbiol. 2004;51:215–225. doi: 10.1046/j.1365-2958.2003.03817.x. [DOI] [PubMed] [Google Scholar]
- 33.Ancuta P, Pedron T, Girard R, Sandstrom G, Chaby R. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect Immun. 1996;64:2041–2046. doi: 10.1128/iai.64.6.2041-2046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knaust A, Weber MV, Hammerschmidt S, Bergmann S, Frosch M, Kurzai O. Cytosolic proteins contribute to surface plasminogen recruitment of Neisseria meningitidis. J Bacteriol. 2007;189:3246–3255. doi: 10.1128/JB.01966-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullberg M, Kronvall G, Karlsson I, Wiman B. Receptors for human plasminogen on gram-negative bacteria. Infect Immun. 1990;58:21–25. doi: 10.1128/iai.58.1.21-25.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahteenmaki K, Kukkonen M, Jaatinen S, Suomalainen M, Soranummi H, Virkola R, Lang H, Korhonen TK. Yersinia pestis Pla has multiple virulence-associated functions. Adv Exp Med Biol. 2003;529:141–145. doi: 10.1007/0-306-48416-1_28. [DOI] [PubMed] [Google Scholar]