Abstract
Mechanical loading is known to alter tendon structure, but its cellular mechanisms are unclear. This study aimed to determine the effect of mechanical loading on tendon cells in vivo. C57BL/6J female mice were used in a treadmill running study. The treadmill running protocol consisted of treadmill training for one week, followed by sustained moderate running at 13 m/min for 50 min/day, 5 days/week, for 3 weeks. Immunohistochemical staining of tendon sections of mice after treadmill running revealed that numerous cells in the tendon section expressed α-SMA, whereas in the tendon sections of control mice, only a few cells exhibited weak α-SMA signals. Furthermore, mouse patellar tendon cells (MPTCs) derived from treadmill running mice were generally larger in culture, proliferated faster, expressed a higher level of α-SMA, and formed more abundant stress fibers compared to MPTCs from control mice. In addition, MPTCs from treadmill running mice generated larger traction forces (169 ± 66.1 Pa) than those from control mice (102 ± 34.2 Pa). Finally, cells from treadmill running mice produced higher levels of total collagen (516.4 ± 92.7 µg/10,000 cells) than their counterparts (303.9± 34.8 µg/10,000 cells). Thus, mechanical loading via treadmill running increased the presence of myofibroblasts in mouse patellar tendons. As myofibroblasts are activated fibroblasts, their presence in the tendon following treadmill running indicates that they actively repair and remodel tendon tissue under strenuous mechanical loading, leading to known changes in tendon structure.
Keywords: Treadmill running, tendon cell, myofibroblast, α-SMA, collagen, cell traction force
INTRODUCTION
Tendons, which consist of collagen, elastin, proteoglycans, and cells, transmit muscular forces to bones. Tendons alter their structure in response to mechanical loading 19. For example, exercise enhances both their structural and mechanical properties 22. Exercise also increases the collagen level, collagen fibril size, and fibril density 10, 22, 38. These ultra-structural morphological changes are manifested through improvement in mechanical properties, such as increased ultimate tensile strength of normal tendons following swimming and running 6, 32. However, the cellular mechanisms responsible for such effects are unclear. One possibility is that, as a result of chronic mechanical loading, a new type of cell becomes present in tendons and is responsible for structural changes. We suspected that the new cells might be myofibroblasts, since mechanical loading of tendon cells in vitro induces differentiation of tendon cells into myofibroblasts 36. Myofibroblasts are characterized by α-smooth muscle actin (α-SMA) expression, the formation of α-SMA-containing stress fibers, and the generation of large traction forces that are required for wound closure and extracellular matrix (ECM) remodeling 13.
The purpose of our study was to test the hypothesis that chronic mechanical loading induces the presence of myofibroblasts in tendons. We applied mechanical loading to mouse patellar tendons in vivo via treadmill running. Following loading, tendon samples were obtained to characterize the cells using immunohistochemistry, cell culture, Western blot, and cell traction force microscopy. We found that, as a result of mechanical loading, a large number of myofibroblasts were present in tendons.
MATERIALS AND METHODS
Mouse treadmill running
C57BL/6J female mice (2.5 months old) were used in a treadmill running study (Exer-6M Open Treadmill, Columbus Instruments). The protocol consisted of one week treadmill training, followed by a moderate running exercise at 13 m/min for 50 min/day, 5 days/week, for 3 weeks. Nine mice were used for treadmill running, whereas eight mice served as controls and were allowed to move freely in their cages. The study protocol (#0603480A) was approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Immunohistochemical staining of tendon sections for α-SMA
Immediately after treadmill running, mice were euthanized using CO2 asphyxiation. The patellar tendons of two exercised and two control mice were harvested for immunohistochemical analysis. Tendon samples were embedded in OCT 4583 and snap frozen in pre-cooled liquid isopentane. The samples were cut using a cryostat at 13 µm along the tendon’s long axis. Immunostaining protocols based on a previous study23 were used to stain the sections for α-SMA, which were then visualized using confocal microscopy.
Tendon cell culture and cell proliferation
Patellar tendons from seven exercised (14 tendons) and six control mice (12 tendons) were harvested in a sterile manner for cell culture. The tendon sheath and underlying paratenon were removed, and the tendon tissues were minced and placed in a standard tissue culture dish and grown in DMEM (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), penicillin (50U/ml), and streptomycin (50U/ml) at 5% CO2, 37°C as described in previous protocols 34. Mouse patellar tendon cells (MPTCs) grew out of the tendon explants in about 2 weeks. MPTCs at passages 5 to 8 were used. After these passages, no apparent changes in morphology or doubling time were noted. Cell proliferation was assessed by population doubling time (PDT), which is the total culture time divided by the number of generations. The number of generations was expressed as log2Nc/N0, where N0 is the population of the cells seeded initially, and Nc is the population at confluence.
Immunostaining α-SMA and actin filaments of cultured cells
Non-confluent MPTCs were fixed with 4% paraformaldehyde at room temperature for 15 min. The cells were then washed twice with wash buffer (0.05% Tween 20 in PBS) and permeabilized with 0.1% Triton X-100 for 3 min. After washing, blocking solution (1% BSA in PBS) was applied for 30 min. The primary antibody, mouse α-SMA monoclonal (Sigma-Aldrich, St. Louis, MO), was diluted to 1:500 in the blocking solution and then added. After 1 h incubation with the primary antibody, followed by three washes with washing buffer, the secondary antibody (goat anti-mouse, FITC-conjugated, 1:500 dilution) was added and incubated for 30 to 60 min before washing. For double labeling, TRITC-conjugated phalloidin was incubated simultaneously with the secondary antibody for 30 to 60 min. Confocal microscopy was used to image stained cells.
Western blot analysis
MPTCs at confluence were lysed using mammalian protein extraction reagent (Pierce, Rockford, IL) containing 1.5% protease inhibitors cocktail (Sigma-Aldrich, St. Louis, MO). After centrifugation at 12,000 rpm for 10 min, the protein concentrations of the supernatants were determined with a BCA Protein Assay (Pierce, Rockford, IL). Equal amounts of total protein (5 µg for α-SMA, 5 µg for β-actin, 10 µg for non-muscle myosin II) were run on 10% (4 to 15% for myosin II) SDS-polyacrylamide gels (Bio-Rad, Hercules, CA) at a constant voltage of 90 V. Proteins were blotted to a nitrocellulose membrane using a standard transfer module (Bio-Rad) for 90 min (overnight for myosin II). The membrane was blocked in a 5% dry milk/PBS-Tween 20 solution for 1 h at room temperature and then probed with a mouse monoclonal anti-α-SMA antibody (Sigma-Aldrich) at a dilution of 1:1500–2000 in a 1% dry milk/PBS-Tween 20 solution. Incubation with the primary antibody was followed by a peroxidase-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Lab, Inc., West Grove, PA) at a dilution of 1:2000 in a 1% dry milk/PBS-Tween 20 solution. The targeted protein bands were detected using an ECL detection kit (Amersham Biosciences, Piscataway, NJ), followed by exposure of the membrane to X-ray film. Membranes were also re-probed for GAPDH to verify equal protein loading in the gels. Finally, α-SMA protein bands were scanned and quantified by image analysis (Scion Imaging 4.0, Scion Corp., Frederick, MD). The density of each band was normalized to that of GAPDH.
Cell traction force microscopy (CTFM)
Two steps were involved in the application of CTFM to determine individual cell traction forces 35. First, polyacrylamide gel disks were prepared with a thickness of 120 µm and diameter of 10 mm. The Young’s modulus of the gel, which contained 5% acryalamide and 0.1% bis, was 3 kPa, and the Poisson’s ratio was taken to be 0.48. The gel was impregnated with 0.2 µm red fluorescent micro-beads (Molecular Probes, Eugene, OR) and attached to the bottom of a 35 mm glass dish (MatTek, Ashland, MA), which had a 14 mm circular inner glass area and had been consecutively treated with 0.1 M sodium hydroxide, 3-aminopropyltrimethoxysilane, and 0.5% glutaraldehyde. Following polymerization, the gel surface was treated with Sulfo-SANPAH (Pierce, Rockford, IL) and then coated with 200 µl of 100 µg/ml collagen type I solution overnight at 4 °C. Subsequently, cells were plated on the collagen-coated gel disks. The cell density was 3000 cells/disk; the cells were allowed to spread on the gel for 6 h in the same medium as the cell culture treatment. Phase contrast images of individual cells and fluorescent images of the embedded beads were obtained, after which the cells were trypsinized and images of the fluorescent beads in the unstrained gel were taken. Cell traction forces were determined by computation based on a published method 7. Note that the CTF is defined as the stress (or local force per unit area) imposed on the gel surface by an adherent cell7, 35.
Sircol assay for total collagen
The amount of total soluble collagen in cell culture supernatants was quantified using the Sircol collagen assay (Biodye Science, UK). For Sircol assay, confluent MPTCs were used. This assay uses a dye-binding method to quantify total collagen content. The Sircol dye reagent was mixed with cell-media samples for 30 minutes on an orbital shaker, followed by centrifugation to collect the collagen-dye complex pellet. The pellet was solubilized with an alkali reagent, and the absorbance of the samples was measured at a wavelength of 540 nm using a microplate reader (Spectra MAX 190, Molecular Devices, CA). A calibration standard of acid-soluble type I collagen was used to obtain the standard curve for calculating the amount of collagen present in samples.
Statistical Analyses
A student’s t-test was used for statistical analyses of α-SMA expression, traction forces, and total collagen production between treadmill running and control mice. The SPSS software package (version 12.0; SPSS, Chicago, Illinois) was used and a p-value<0.05 was considered significant.
RESULTS
Immunofluorescence microscopy revealed that numerous cells in tendon sections from treadmill running mice that expressed α-SMA. In contrast, only a few cells were present in the tendons of control mice that exhibited a weak α-SMA signal (Fig. 1).
Fig. 1.
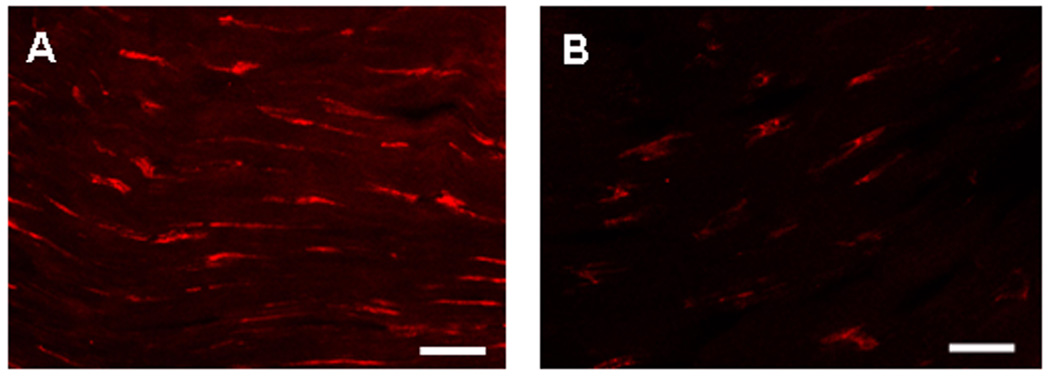
Confocal microscopy of representative tendon sections stained by immunohistochemistry for α-SMA expression of MPTCs from treadmill running mouse (A) and cage control mouse (B). (Scale bars: 20 µm).
The MPTCs obtained from exercised and control mice were markedly different in morphology. In general, cells from exercised mice exhibited greater surface area (Fig. 2), expressed a higher level of α-SMA, and presented more abundant stress fibers compared to cells from control mice (Fig. 3). These cells grew faster than control cells, with a shorter population doubling time (44.2 ± 3.9 hrs) compared to cells from control mice (93 ± 14.4 hrs; p < 0.05) (Fig. 4). In addition, MPTCs from treadmill running mice generated larger traction forces than those from control mice. The cell traction force generated by exercised mice was 102 ± 34.2 Pa compared to 169 ± 66.1 Pa (p < 0.05) for control mice (Fig. 5). Finally, MPTCs from exercised mice produced higher levels of total collagen (516.4 ± 92.7 µg/10,000 cells) than control mice (303.9± 34.8 µg/10,000 cells; p < 0.05) (Fig. 6).
Fig. 2.
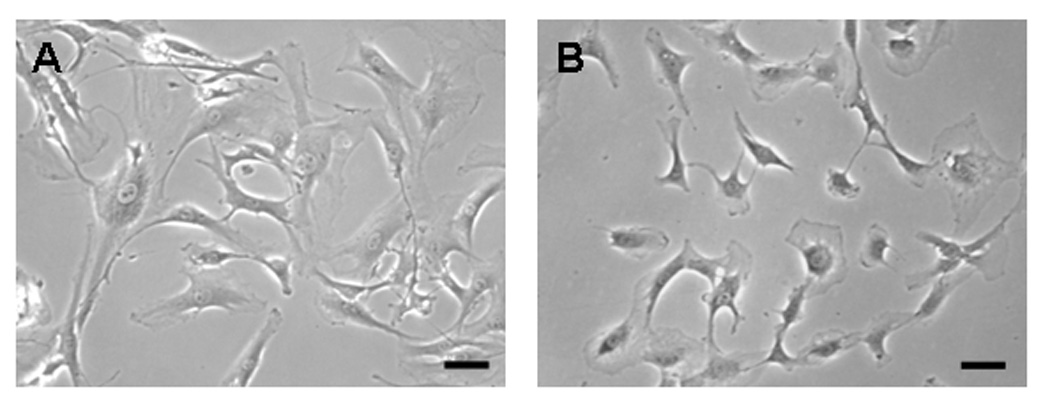
The morphology of MPTCs from treadmill running mouse (A) and cage control mouse (B). MPTCs from treadmill running group show a typical myofibroblast morphology with larger and more spread out cells compared to those from the cage control group. (Scale bars: 100 µm).
Fig. 3.
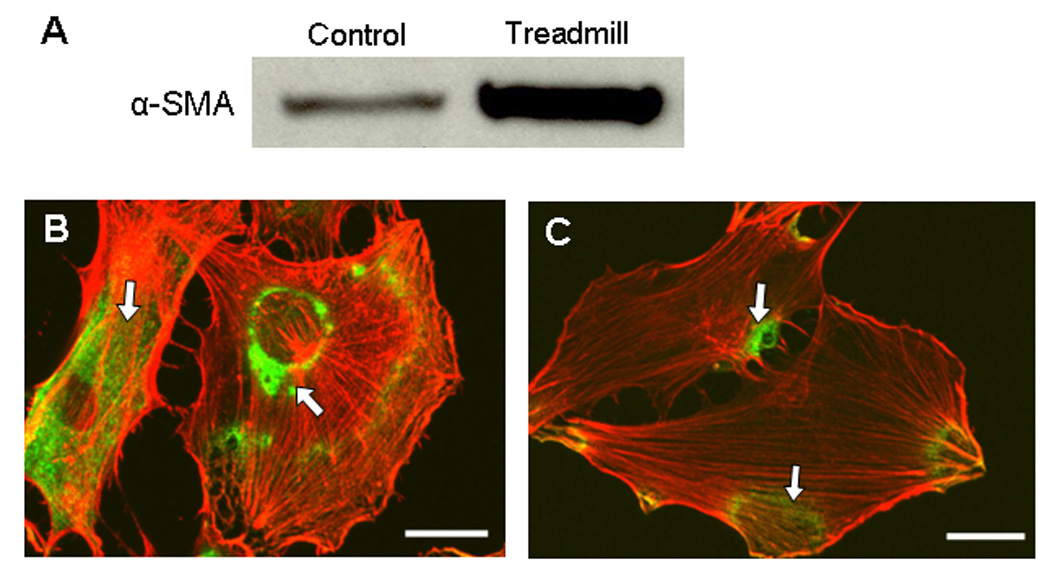
Western blot analysis of α-SMA expression in MPTCs from treadmill running and control mice (A). MPTCs from treadmill running mice had much higher levels of α-SMA expression compared to those cells from cage control mice. Immunocytochemistry for α-SMA (green) and actin filaments (red) of MPTCs from treadmill running mouse (B) and control mouse (C). More a-SMA green signals (arrows) were seen in MPTCs from treadmill running mice than in cells from cage control mice. (Scale bars: 20 µm.)
Fig. 4.
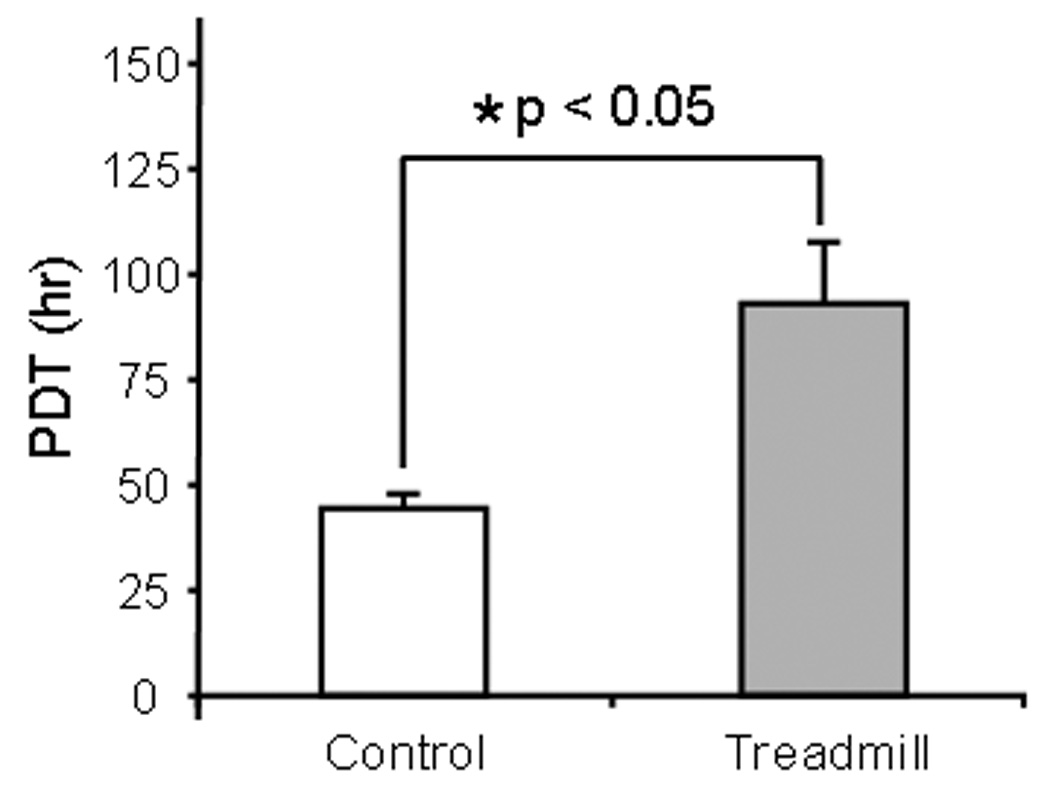
Population doubling time (PDT) of MPTCs. After treadmill running, myofibroblasts from exercised mice proliferated much faster than those cells from cage control mice.
Fig. 5.
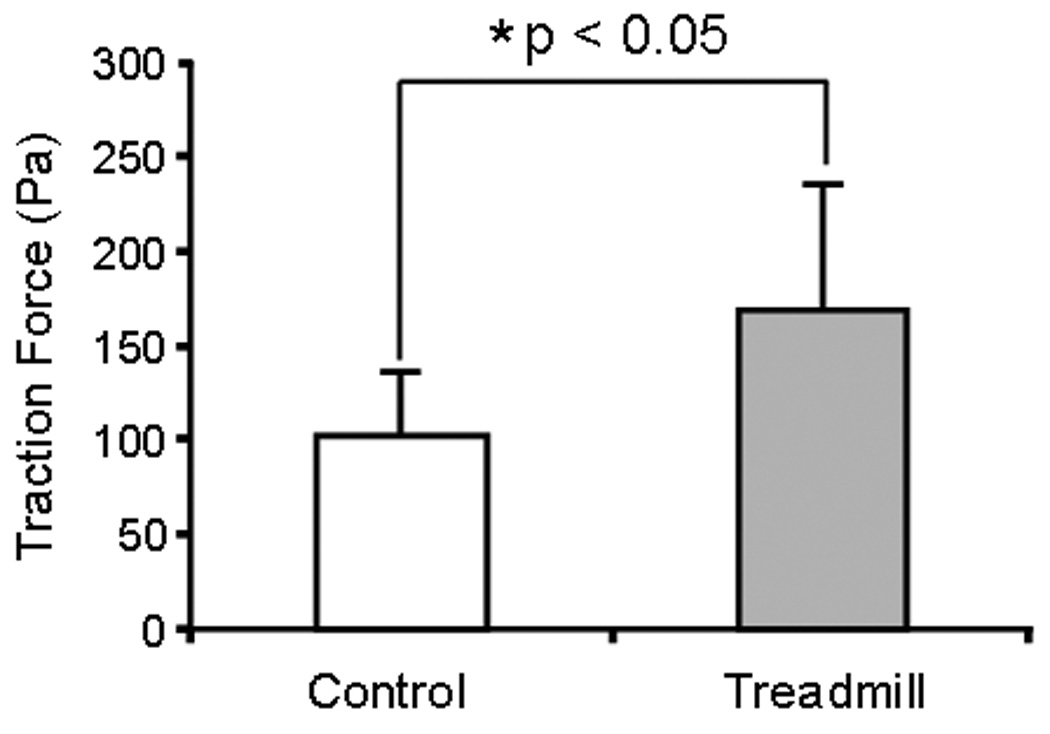
Traction forces of MPTCs from treadmill running and control mice. After treadmill running, MPTCs generated larger traction forces than cells from cage control mice.
Fig. 6.
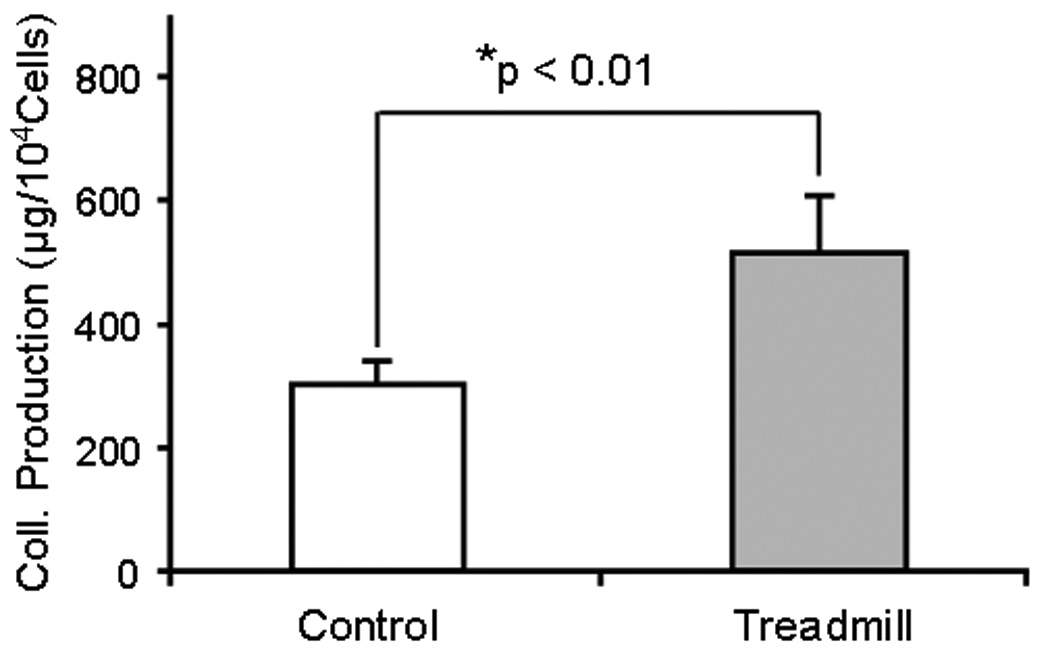
Total collagen production by MPTCs from treadmill running and control mice. Treadmill running significantly increased cellular collagen production compared to cage control mice.
DISCUSSION
Mechanical loading has been shown to enhance the structural and mechanical properties of tendons, but the cellular mechanisms responsible for this have not been elucidated. Using a treadmill running model, we showed that numerous cells expressed α-SMA in tendons from mice subjected to running compared to only a few cells that expressed weak α-SMA in control mice. This observation was supported by in vitro culture experiments; compared to tendon cells derived from control mice, MPTCs from treadmill running were larger, formed prominent stress fibers, and expressed larger amounts of α-SMA, all of which indicated that MPTCs from treadmill running are myofibroblasts 11, 17. To confirm this, we used CTFM to determine cell traction forces, as increased α-SMA expression relates directly to increased traction forces in myofibroblasts 8. We found that the MPTCs from exercised mice generated greater traction forces than those from control mice. Finally, we showed that the MPTCs of exercised mice produced more collagen than cells from control mice, consistent with previous studies, where running exercise induced collagen synthesis in the tendons of human subjects 15, 20, 21.
To our knowledge, this study provides the first evidence that myofibroblasts are present in tendons after treadmill running exercise. Myofibroblasts could be responsible for changes in tendon structure, because these cells are considered to be activated fibroblasts that produce abundant collagen 26 and generate large traction forces 8. Myofibroblasts also play a vital role in the repair and remodeling of wound tissues 18. A previous study showed that repetitive mechanical loading resulted in micro-tears in tendons 25. Therefore, the presence of myofibroblasts suggests that repetitive mechanical loading of tendons may cause micro-injuries, and thus elicit a healing response for repair of injured tendons.
Where the myofibroblasts in this study originated is unclear, but they may have a very heterogeneous origin. In normal conditions, fibroblastic cells exhibit few or no actin-associated cell-cell and cell-matrix contacts and little ECM production 33. After injury, these cells become activated due to the release of cytokines and growth factors such as TGF-β from inflammatory and resident cells 16. As a result, they migrate into the injured site to produce ECM components for tissue repair. Another possible cell source is circulating fibrocytes, which have been reported to migrate to the site of tissue injury and differentiate into myofibroblasts 27. Fibrocytes play an important role in the genesis of sub-epithelial fibrosis and airway remodeling in asthma 30. In addition, bone marrow stem cells (BMSCs) can circulate and give rise to lung fibroblasts 5, 14 as well as participate in wound healing 39. Nakama et al. showed that repetitive contraction of the flexor digitorum profundus in rabbits causes tendon microtears 25. Using high resolution ultrasound, Gibbon et al. showed an increase in Achilles tendon microtears in athletes with symptomatic tendonitis, while the same defects were seen only occasionally in asymptomatic volunteers 12. Therefore, BMSCs may migrate to injured tendon sites.
In recent years, tissue-specific adult stem cells have been identified in many tissues and organs 28. These stem cells contribute to tissue regeneration after injury by differentiating into specific cell types within the host tissue 24. In particular, Bi et al. isolated tendon stem cells that have multidifferentiation potential 4. Mechanical loading of stem cells in vitro promote selective differentiation of stem cells toward specific cell lineages. For example, concurrent application of tensile and rotational loading to human and bovine bone marrow stem cells (BMSCs) in collagen gels resulted in differentiation to ligament cells 2. Mechanical loading of bovine MSCs also enhances osteogenesis 9. Thus, residential tendon stem cells may proliferate and differentiate into myofibroblasts in response to treadmill running. Future studies should investigate whether BMSCs, circulating fibrocytes, or tendon stem cells are active in this response.
While short term, moderate mechanical loading via treadmill running showed an anabolic effect on tendons in our study, excessive mechanical loading is considered to be a major cause of tendinopathy. Using a rat treadmill running model, Archambault et al. found that treadmill running for four weeks induced a fibrocartilage phenotype in the supraspinatus tendons 3. In this regard, the anabolic effect on tendons seen in our study, namely increased cellular production of collagen, may reflect the early healing response of tendon cells to the development of tendinosis. In another study, tendinosis occurred in rat supraspinatus tendons following 12 to 16 wks of treadmill running 31. The difference between our study and the previous two studies may be attributed to species (mouse vs. rat), type of tendon (patellar vs supraspinatus tendon), and mechanical loading intensity such as loading duration (3 wks vs. 4 to 16 wks). Mechanical loading can be considered to have both beneficial and detrimental effects on tendon matrix. Under moderate loading conditions, it can stimulate collagen synthesis and protect the tendon from degeneration; however, it can cause collagen matrix damage and induce metalloproteinase release when mechanical loading exceeds the physiological limit of the tendon 29. Both these effects appear to be time and load-dependent 37. Therefore, tendon homeostasis depends on the balance between the intensity of mechanical loading and the ability of the tendon to adapt.
Like any tissue culture experiment, our study is limited by changes in cell phenotype that occurred after extraction of fibroblasts from tendon tissue and subsequent expansion in culture. The sub-culturing of cells is necessary to provide a sufficient number of cells assays such as Western blot for α-SMA expression. We found that cells from patellar tendons of exercised mice express a higher level of α-SMA than those from cage control mice. The results are consistent with the immunostaining results of α-SMA on tendon sections (Fig. 1). Moreover, our previous study showed that after multiple passages, the inherent differences in cell phenotype between healing and normal fibroblasts, at least in terms of α-SMA expression, collagen production, and contraction, were preserved 1. Thus, the limitation of using a cell culture model does not invalidate our current findings.
In summary, we showed the presence of numerous myofibroblasts in the tendons from treadmill running mice and demonstrated that cells from exercised mice grew faster, generated larger traction forces, and produced more collagen than cells from control mice. As myofibroblasts are known to be responsible for repair and remodeling of injured tissues, their presence in tendons after treadmill running suggests that the tendon is in a “healing state”, possibly due to repetitive loading-induced tendon micro-injuries. Further studies should investigate the origin of the myofibroblasts and in particular the possibility of the differentiation of tendon stem cells into myofibroblasts in response to chronic mechanical loading conditions.
Acknowledgements
This work was supported in part by NIH AR049921 and AR04992S1 (JHW).
REFERENCES
- 1.Agarwal C, Britton ZT, Alaseirlis DA, et al. Healing and normal fibroblasts exhibit differential proliferation, collagen production, alpha-SMA expression, and contraction. Ann Biomed Eng. 2006;34:653–659. doi: 10.1007/s10439-006-9090-z. [DOI] [PubMed] [Google Scholar]
- 2.Altman GH, Horan RL, Martin I, et al. Cell differentiation by mechanical stress. Faseb J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 3.Archambault JM, Jelinsky SA, Lake SP, et al. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 4.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 5.Brocker V, Langer F, Fellous TG, et al. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am J Respir Crit Care Med. 2006;173:1276–1282. doi: 10.1164/rccm.200509-1381OC. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90:164–171. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- 7.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol. 2002;282:C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64:248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 9.David V, Martin A, Lafage-Proust MH, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 10.Enwemeka CS. Inflammation, cellularity, and fibrillogenesis in regenerating tendon: implications for tendon rehabilitation. Phys Ther. 1989;69:816–825. doi: 10.1093/ptj/69.10.816. [DOI] [PubMed] [Google Scholar]
- 11.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 12.Gibbon WW, Cooper JR, Radcliffe GS. Sonographic incidence of tendon microtears in athletes with chronic Achilles tendinosis. Br J Sports Med. 1999;33:129–130. doi: 10.1136/bjsm.33.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto N, Jin H, Liu T, et al. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- 16.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 17.Hinz B, Celetta G, Tomasek JJ, et al. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B, Mastrangelo D, Iselin CE, et al. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaer M, Langberg H, Miller BF, et al. Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskelet Neuronal Interact. 2005;5:41–52. [PubMed] [Google Scholar]
- 20.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langberg H, Skovgaard D, Petersen LJ, et al. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports. 2003;13:211–223. doi: 10.1034/j.1600-0838.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 23.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189(Pt 3):593–600. [PMC free article] [PubMed] [Google Scholar]
- 24.Mimeault M, Batra SK. Recent Progress on Tissue-Resident Adult Stem Cell Biology and Their Therapeutic Implications. Stem Cell Rev. 2008 doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–263. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 27.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratajczak MZ, Machalinski B, Wojakowski W, et al. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 29.Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi: 10.1016/s0945-053x(01)00196-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Sun G, Stacey MA, et al. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 31.Scott A, Cook JL, Hart DA, et al. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen EB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. J Sports Sci. 1995;13:291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- 33.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 34.Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- 35.Wang JH, Lin JS. Cell traction force and measurement methods. Biomech Model Mechanobiol. 2007;6:361–371. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- 36.Wang JH, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J Biomech. 2004;37:573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Warden SJ. Animal models for the study of tendinopathy. Br J Sports Med. 2007;41:232–240. doi: 10.1136/bjsm.2006.032342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19:397–408. doi: 10.3233/bir-1982-19302. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(Suppl 1):S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]


