Abstract
Curcumin has potential as a chemopreventative and chemotherapeutic agent however its interactions with clinically relevant cytokines are poorly characterized. Since cytokine immunotherapy is a mainstay of treatment for malignant melanoma, we hypothesized that curcumin could modulate the cellular responsiveness to interferons and interleukins. As a single agent, curcumin induced a dose-dependent increase in apoptosis of human melanoma cell lines, which was most prominent at doses >10 µM. Immunoblot analysis confirmed that curcumin induced apoptosis and revealed caspase-3 processing, PARP cleavage, reduced Bcl-2 and decreased basal phosphorylated STAT3. Despite its pro-apoptotic effects, curcumin pre-treatment of human melanoma cell lines inhibited the phosphorylation of STAT1 protein and downstream gene transcription following IFN-α and IFN-γ as determined by immunoblot analysis and Real Time PCR, respectively. Pre-treatment of peripheral blood mononuclear cells (PBMCs) from healthy donors with curcumin also inhibited the ability of IFN-α, IFN-γ and IL-2 to phosphorylate STAT proteins critical for their anti-tumor activity (STAT1 and STAT5, respectively) and their respective downstream gene expression as measured by Real Time PCR. Finally, stimulation of natural killer (NK) cells with curcumin reduced the level of IL-12-induced IFN-γ secretion, and production of granzyme b or IFN-γ upon co-culture with A375 melanoma cells or NK sensitive K562 cells as targets. These data demonstrate that although curcumin can induce apoptosis of melanoma cells, it can also adversely affect the responsiveness of immune effector cells to clinically relevant cytokines that possess anti-tumor properties.
Keywords: Curcumin, Melanoma, Interferon, Interleukin, Apoptosis
Introduction
Curcumin, 1,7-bis(4-hydroxy-3methoxyphenyl)-1,6-heptadien-3,5-dione, is the primary bioactive component isolated from turmeric, a dietary spice made from the rhizome of Curcuma longa (1). Curcumin has chemopreventative and chemotherapeutic properties in numerous experimental models and has been shown to inhibit a variety of cellular targets that promote survival and metastasis including nuclear factor kappa-B (NF-κB) and signal transducer and activator of transcription-3 (STAT3), among others (1–3). These data suggest that curcumin may be an effective means to modulate pathways that contribute to chronic inflammation leading to cancer. Despite its broad target specificity, curcumin has a favorable safety profile in humans (no dose-limiting toxicity up to 12/g/day) (4, 5), although dietary curcumin has poor bioavailability (6). Nonetheless, curcumin has demonstrated anti-tumor effects in vivo when administered via dietary supplement, oral gavage or injection, even at the low concentrations achieved in these physiologic systems (7–14). Although our understanding of how curcumin can elicit anti-tumor activity is incomplete, this agent is being investigated in human oncology trials and its use by cancer patients as a form of alternative medicine will likely continue or even increase as anecdotal reports of its anti-cancer potential become widespread.
One clinical area in which effective chemopreventative agents could make a tremendous impact is in the adjuvant setting of melanoma. Here patients have been rendered disease-free following surgical excision of a high-risk lesion (>4 mm in depth, or with lymph node involvement). These patients are at a high-risk for local recurrence or even metastatic disease. The only FDA-approved adjuvant therapy is the cytokine, interferon-alpha (IFN-α). The use of this agent is somewhat controversial due to its toxicity profile, however a recent meta-analysis demonstrated a significant benefit of IFN-α in relation to both relapse-free and overall survival in the adjuvant setting of melanoma (15, 16). Cytokine immunotherapy with single agent, high-dose IFN-α and interleukin-2 (IL-2) are also mainstays of treatment in the setting of metastatic disease where they can mediate complete clinical responses in approximately 10% of patients (17, 18). The mechanisms by which IFN-α or IL-2 mediate anti-tumor activity involves stimulation of host immune effector cells (18–21), although IFN-α can also exert direct anti-proliferative, pro-apoptotic and anti-angiogenic effects on melanoma cells (22–26).
In addition to the clinical use of cytokines, endogenously produced type I interferons (IFN-α, IFN-β) and type II interferons (IFN-γ) are critical components of tumor immunosurveillance (27–29). In a similar manner, IL-2 has been shown to play an important role in survival, proliferation and effector function of NK and T cells (30–32). The adjuvant setting of melanoma and the presence of micrometastatic disease represent scenarios in which effective tumor immunosurveillance could be of great clinical benefit. Based on these data, identifying chemopreventative agents that might compliment the cellular actions of interferons and interleukins could have the greatest potential for clinical success.
In the present report, we have demonstrated that curcumin can induce apoptosis and down-regulate proteins involved in the survival of human melanoma cells. However, pre-treatment with curcumin inhibited IFN-α and IFN-γ induced signal transduction and downstream gene expression in human melanoma cell lines. Curcumin also significantly inhibited the response of peripheral blood mononuclear cells from healthy donors to key cytokines involved in anti-tumor immunity (IFN-α, IFN-γ, IL-2) at the level of signal transduction and gene expression. Finally, curcumin adversely modulated the function of NK cells to secrete IFN-γ in response to IL-12, and inhibited cytotoxicity of A375 human melanoma cells and NK sensitive K562 target cells in vitro. Although the ability of curcumin to inhibit the response to cytokines is desirable for prevention of cancers associated with chronic inflammation, the present data caution that it may negate the cellular response to immunotherapeutic cytokines that mediate cancer immunosurveillance.
Materials and Methods
Cell Culture and Reagents
A375 and Hs294T human metastatic melanoma cell lines and K562 chronic myelogenous leukemia cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The 1106 MEL and F01 human metastatic melanoma cell lines were a kind gift from Dr. Soldano Ferrone (University of Pittsburgh, Pittsburgh, PA) and were cultured as previously described (23). The radial growth phase WM 1552c and vertical growth phase WM 793b human melanoma cell lines were provided by Dr. M. Herlyn (Wistar Institute, Philadelphia, PA) and cultured as previously described (33). Recombinant human IFN-α2b was purchased from Schering-Plough, Inc. (Kenilworth, NJ). Recombinant human IFN-γ and IL-2 were purchased from R & D Systems, Inc. (Minneapolis, MN). Recombinant human IL-12 was provided by Genetics Institute (Cambridge, MA).
Peripheral Blood Mononuclear Cell Isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from source leukocytes of healthy donors (American Red Cross, Columbus, OH) via density gradient centrifugation using Ficoll-Paque (Amersham, Pharmacia Biotech, Bjorkgatan, Sweeden) as previously described (34). Where indicated, NK cells were enriched from source leukocytes by negative selection with Rosette Sep reagents (Stem Cell Technologies, Inc., Vancouver, British Columbia, Canada).
Curcumin
Curcumin was prepared via condensation of pentane-2,4-dione and vanillin according to the procedure of Venkateswarlu (35). Recrystallization of the crude precipitate from ethanol provided curcumin as orange needles, mp: 181–183 °C [182–184 °C (36)]. The spectral data for the synthetic curcumin (1H NMR and 13C NMR) was also identical to that reported for the natural product (36). This approach routinely resulted in curcumin that was of >95% purity as determined by nuclear magnetic resonance (NMR) spectroscopy, which is equal or superior to most commercially available samples (data not shown).
Immunoblot Analysis
Lysates were prepared from melanoma cell lines or PBMCs in Laemelli buffer and assayed for protein expression by immunoblot analysis as previously described with antibodies to STAT1 (BD Biosciences), pSTAT1, STAT3, pSTAT3, PARP, Bcl-2, Cyclin D1, Caspase-3 (Cell Signaling Technology), or β-actin (Sigma) (23). Following incubation with the appropriate horseradish-peroxidase-conjugated secondary Ab, immune complexes were detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL).
Flow cytometric analysis of phosphatidyl serine exposure
Phosphatidyl serine exposure was assessed in tumor cells by flow cytometry using allophycocyanin (APC)-labeled Annexin V (BD Pharmingen, San Diego, CA) as previously described (37). Propidium iodide exclusion was not used concurrently in these experiments due to the observation that curcumin produces considerable spectral overlap at wavelengths used to detect this viability exclusion dye. Each analysis was performed utilizing at least 10,000 events.
Real Time PCR Analysis
Real-time polymerase chain reaction was used to evaluate the expression of the IFN-α stimulated genes (IFIT2, ISG-15), IFN-γ stimulated gene (IRF1) and IL-2 stimulated gene (cytokine-induced SH2-containing protein; CIS) in melanoma cell cultures or PBMCs as previously described (38, 39). Following cytokine treatment, total RNA was isolated using via TRIzol extraction (Invitrogen), quantitated, and converted to cDNA using random hexamers (Invitrogen) as primers for first-strand synthesis. Two microliters of the resulting cDNA was used as a template to measure mRNA transcripts by Real-time RT-PCR with pre-designed primer/probe sets (Assays On Demand; Applied Biosystems, Foster City, CA) and 2X TaqMan Universal PCR Master Mix (Applied Biosystems) per the manufacturer’s recommendations. Pre-designed primer/probe sets for 18s rRNA (Applied Biosystems) were used to normalize expression values (housekeeping gene) and data was acquired and analyzed using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Enzyme Linked Immunosorbent Assay for Quantitation of IFN-γ
Culture supernatant from normal human NK cells were assayed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) using commercially available monoclonal antibody (Ab) pairs as previously described (40). Standard curves were prepared for each plate, and all plasma samples from a single donor were run in duplicate and in parallel to minimize interassay variability.
ELISPOT Assays for Granzyme B and IFN-γ
To measure IFN-γ and granzyme B (GrB) secretion, ELISPOT experiments were conducted using MultiScreen 96-well plates (Millipore, Bedford, MA) as described previously (41). The plates were coated overnight at 4°C with 50 µl/well of anti-human GrB (Mabtech, Cincinnati, OH) or anti-human IFN-γ capture antibody (Mabtech, Cincinnati, OH) diluted to 10 µg/ml in PBS. After incubation, coated plates were blocked with 1% fetal bovine serum in PBS for an hour at room temperature, then washed four times with media. Freshly isolated NK cells (effectors) were incubated overnight in media with either 20 µM curcumin or DMSO (vehicle). Effector cells were then co-incubated in triplicate with A375 melanoma cells as targets, or K562 (positive control target, ATCC) at an effector:target rato of 20:1 for four hours. Targets and effectors cultured alone were used as negative controls. After incubation, the plates were washed four times with 100 µl/well of PBS/0.05% Tween-20 and 100 µl of biotinylated monoclonal anti-human GrB or IFN-γ detecting antibody (Mabtech, Cincinnati, OH) diluted to 1 µg/ml was added. After a three hour incubation at room temperature, the plates were washed four times with PBS-Tween and 100 µl/well of Strepavidin-Alkaline Phosphatase (Mabtech, Cincinnati, OH) diluted to 1:1000 with PBS was added for two hours at room temperature. After the incubation, the plates were washed again with PBS-Tween and 150 µl of BCIP/NBT phosphatase substrate was added to each well. Plates were developed for five minutes at room temperature in the dark and the reaction stopped by rinsing plates with distilled water. The membranes were air dried and spots were visualized and counted using the ImmunoSpot Imaging Analyzer system (Cellular Technology Ltd, Cleveland, OH).
Statistical Analysis
Mixed effects regression models were used to analyze the results of the PBMC and ELISPOT experiments, including a random effect for donor and treatment. For the PBMC experiments, fold changes in gene expression were log-transformed to meet the model assumptions of normality. For ELISPOT experiments, data were obtained in at least 3 independent experiments with different NK cell donors. P-values less than or equal to 0.05 were considered to be statistically significant.
Results
Curcumin induces apoptosis in human melanoma cell lines
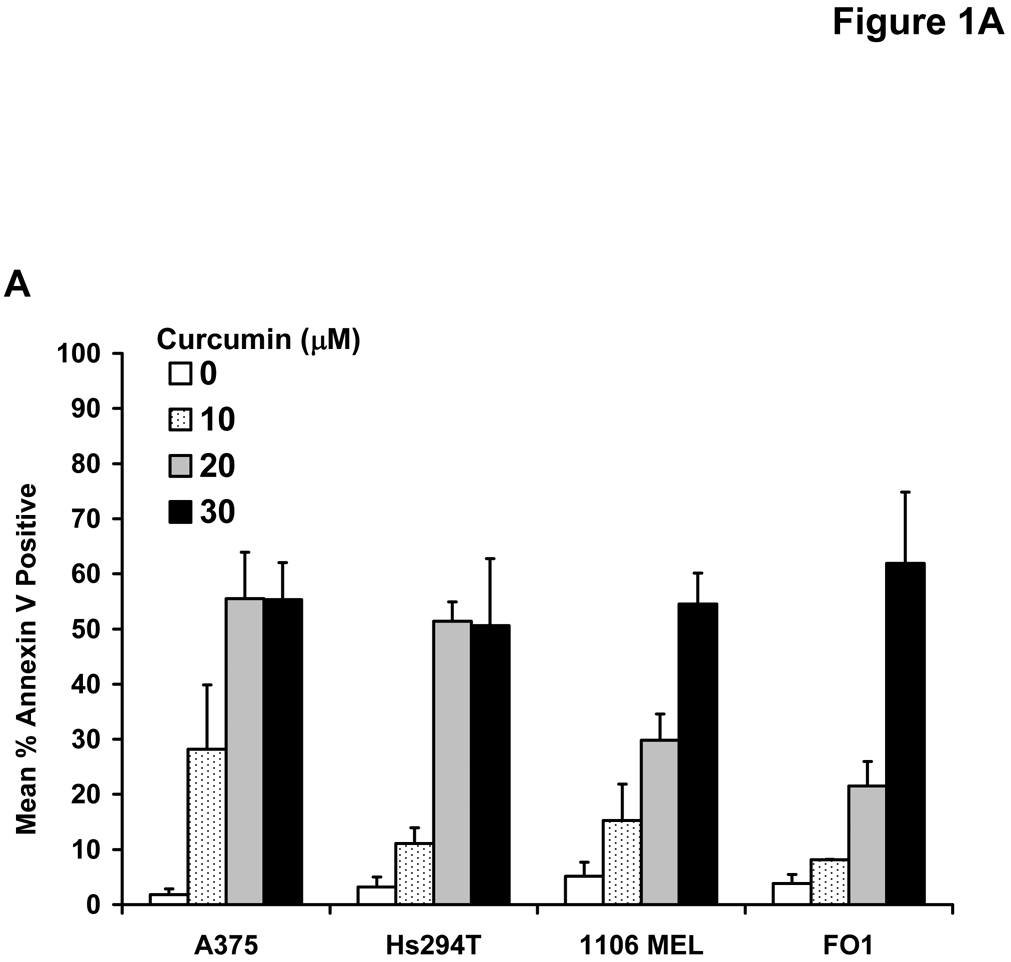
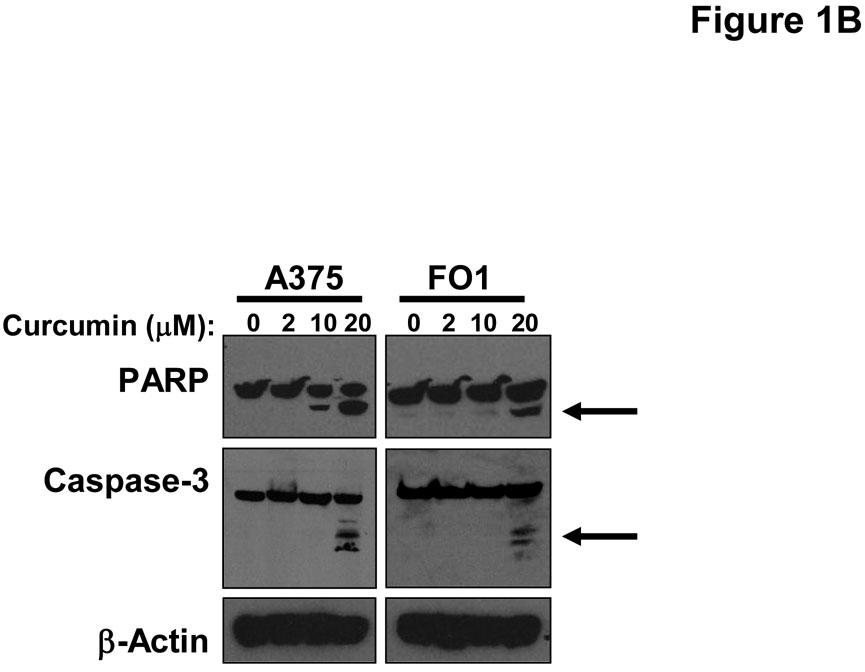
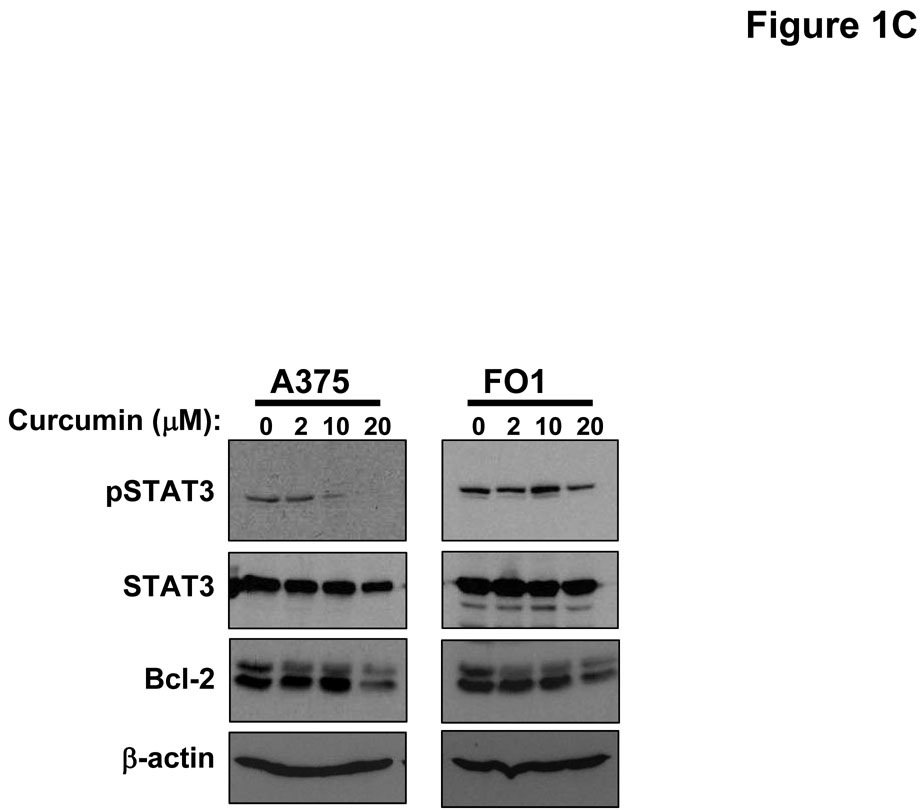
Treatment of multiple human metastatic melanoma cell lines (A375, FO1, Hs294T, 1106 MEL) with curcumin for 48 hours induced a dose-dependent increase in Annexin V-positive cells which was most prominent at doses >10 µM (Figure 1A). Immunoblot analysis was used to confirm curcumin-induced apoptosis in representative lines by measuring the processing of caspase-3 and cleavage of PARP (Figure 1B). Consistent with the documented ability of curcumin to inhibit the STAT3 and NF-κB signal transduction pathways (1, 42, 43), a reduced level of basal phosphorylated STAT3 and Bcl-2 proteins were observed in melanoma cells following curcumin treatment (Figure 1C). Individual melanoma cell lines displayed differential curcumin sensitivity at the lower 10 µM dose, however apoptosis was relatively uniform across all cell lines (~50%) after a 48 hour treatment with higher doses of curcumin (30 µM; Figure 1A). Curcumin also induced apoptosis at comparable concentrations in both the WM 1552c radial growth phase (41% apoptosis at 20 µM curcumin) and WM 793b vertical growth phase (36% apoptosis at 20 µM curcumin) human melanoma cell lines.
Figure 1. Curcumin induces apoptosis in melanoma cells.
(A) Annexin V staining of apoptosis in human metastatic melanoma cell lines by flow cytometry following a 48 hour treatment with various doses of curcumin. DMSO (vehicle)-treated cells served as negative controls. Error bars represent the standard deviation from three separate experiments. (B) The pro-apoptotic effects of curcumin were confirmed by immunoblot analysis of PARP cleavage and caspase-3 processing (arrows) in representative metastatic melanoma cell lines following a 24 hour treatment. Membranes were probed with antibodies against β-actin to control for loading. (C) Treatment of the representative A375 and FO1 metastatic melanoma cell lines with curcumin (0 – 20 µM) resulted in decreased levels of basal STAT3 phosphorylation and Bcl-2 protein. Membranes were probed with antibodies against total STAT3 and β-actin protein to control for loading.
Curcumin inhibits interferon-induced signal transduction in melanoma cells
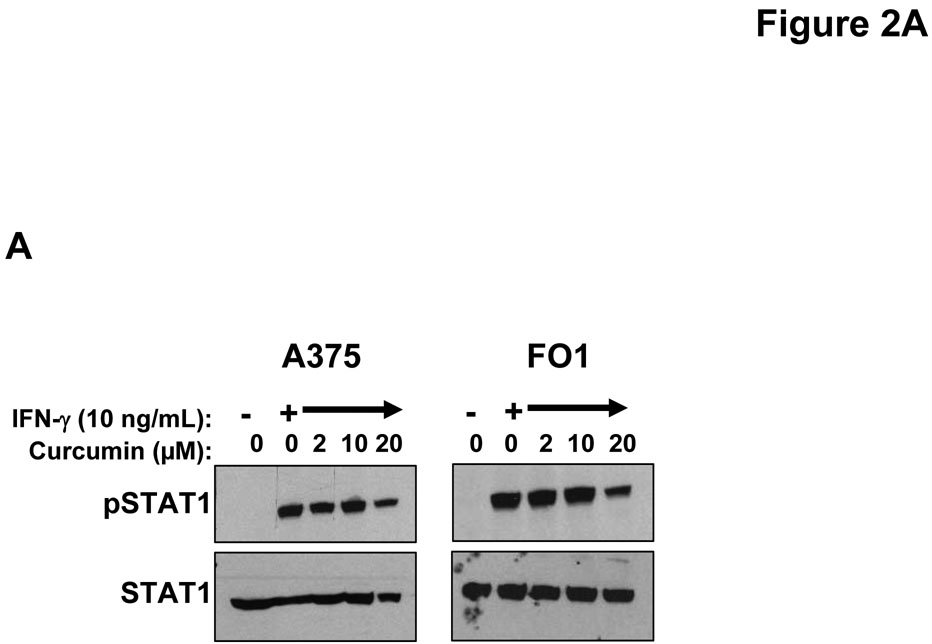
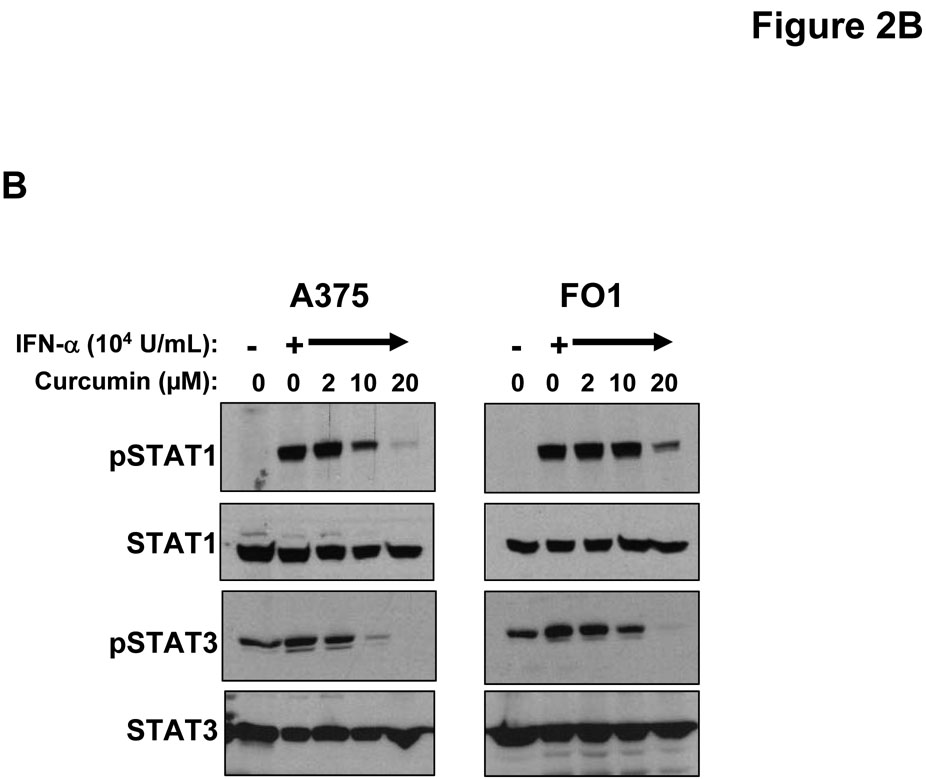
IFN-α and IFN-γ are clinically relevant cytokines that can induce direct pro-apoptotic effects against melanoma cells (26, 44). To determine whether curcumin could modulate the cellular response to these cytokines, representative human melanoma cell lines (A375 and FO1) were pre-treated with curcumin (20 µM) or vehicle (DMSO; negative control) for 6 hours (prior to the induction of apoptosis) and subsequently cultured for 15 minutes with PBS (vehicle), IFN-α (104 U/mL) or IFN-γ (10 ng/mL) to stimulate signal transduction. These doses were chosen as they induce a maximal degree of STAT1 phosphorylation at this time point (23). Cell lysates were evaluated by immunoblot analysis. Reduced levels of IFN-α and IFN-γ-induced STAT1 phosphorylation and IFN-α-induced STAT3 phosphorylation were observed in these melanoma cell lines following pre-treatment with curcumin (Figure 2A–B). These data indicated that curcumin could modulate signal transduction events involved in promoting tumor progression (e.g. STAT3-mediated) or apoptosis (e.g. STAT1-mediated) (45).
Figure 2. Curcumin inhibits interferon-induced signal transduction and gene expression in melanoma cells.
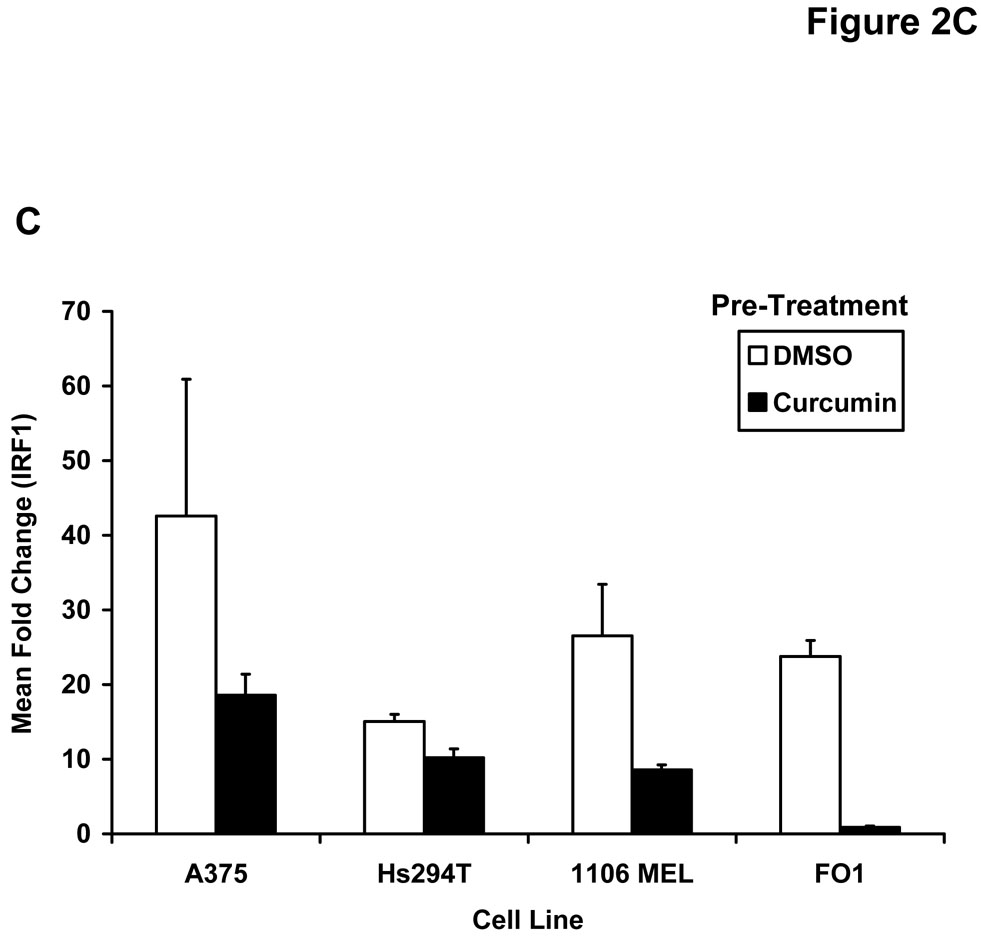
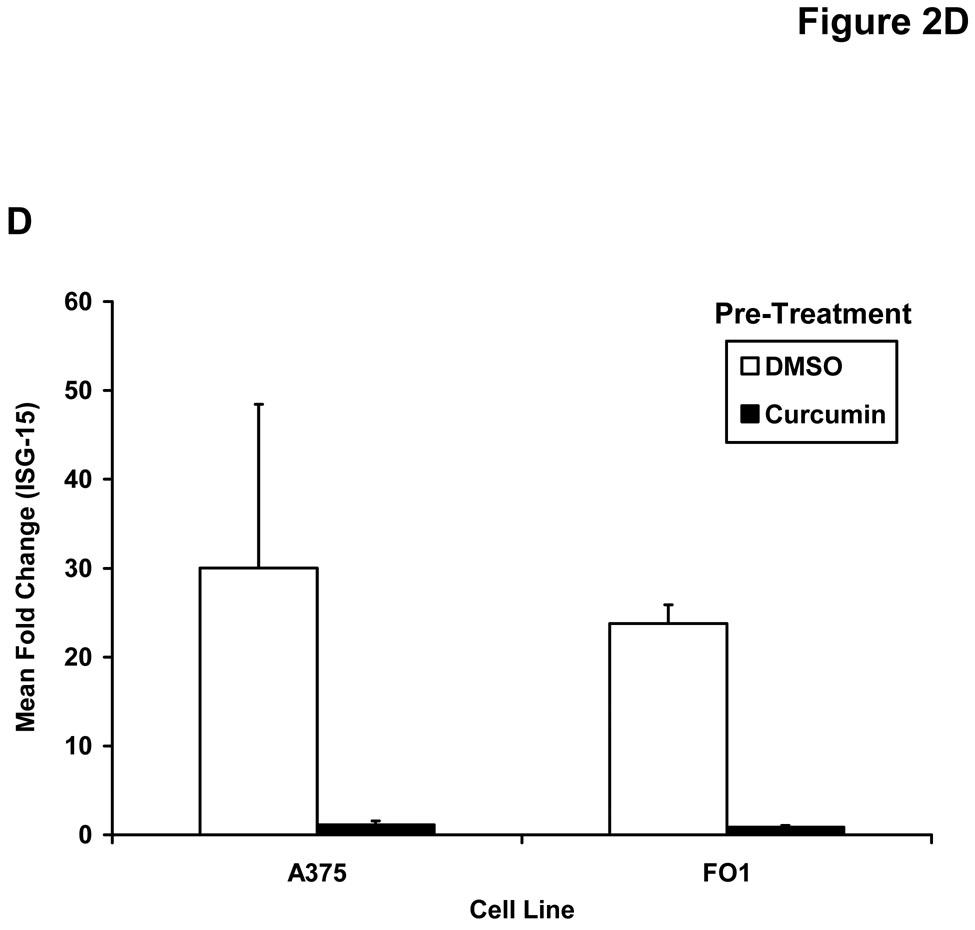
Pre-treatment of human melanoma cells for 6 hours with curcumin inhibited the phosphorylation of STAT1 (at Tyr701) in response to a subsequent 15 minute stimulation with (A) IFN-γ (10 ng/mL) or (B) IFN-α (104 U/mL). Similar data were obtained in both the A375 and FO1 melanoma cell lines. Total STAT1 and STAT3 proteins were measured to control for loading. Curcumin pre-treatment (20 µM) inhibits the (C) IFN-γ-induced transcription of IRF1 and the IFN-α-induced transcription of (D) ISG-15 as determined by Real Time PCR. All PCR data were normalized to expression values for18s rRNA (housekeeping gene) and expressed as the fold change in gene expression relative to DMSO-treated cells. PCR data shown are representative of at least three independent experiments with similar results.
Curcumin inhibits interferon-induced gene expression in melanoma cells
The effect of curcumin on downstream IFN-induced gene expression was next evaluated by Real Time PCR. In these experiments, melanoma cell lines were pre-treated for one hour with curcumin (20 µM) or DMSO (vehicle) as a control and cultured in the presence of PBS, IFN-α (104 U/mL) or IFN-γ (10 ng/mL) for an additional four hours. Curcumin pre-treatment of the multiple human melanoma cell lines led to consistent reductions in expression of the pro-apoptotic interferon-regulatory factor-1 (IRF1) gene expression following IFN-γ stimulation as compared to DMSO-pre-treated cells (Figure 2C). This gene has been previously shown to be transcribed via a STAT1-STAT1 homodimer binding to a gamma-activated sequence (GAS) element and is well-characterized as an IFN-γ-responsive gene (46). Similar reductions in transcription of IFN-α stimulated genes regulated by the ISGF3 complex (e.g. ISG-15) were also noted following curcumin pre-treatment in representative melanoma cell lines (Figure 2D and not shown).
Curcumin inhibits interferon-responsiveness of PBMCs
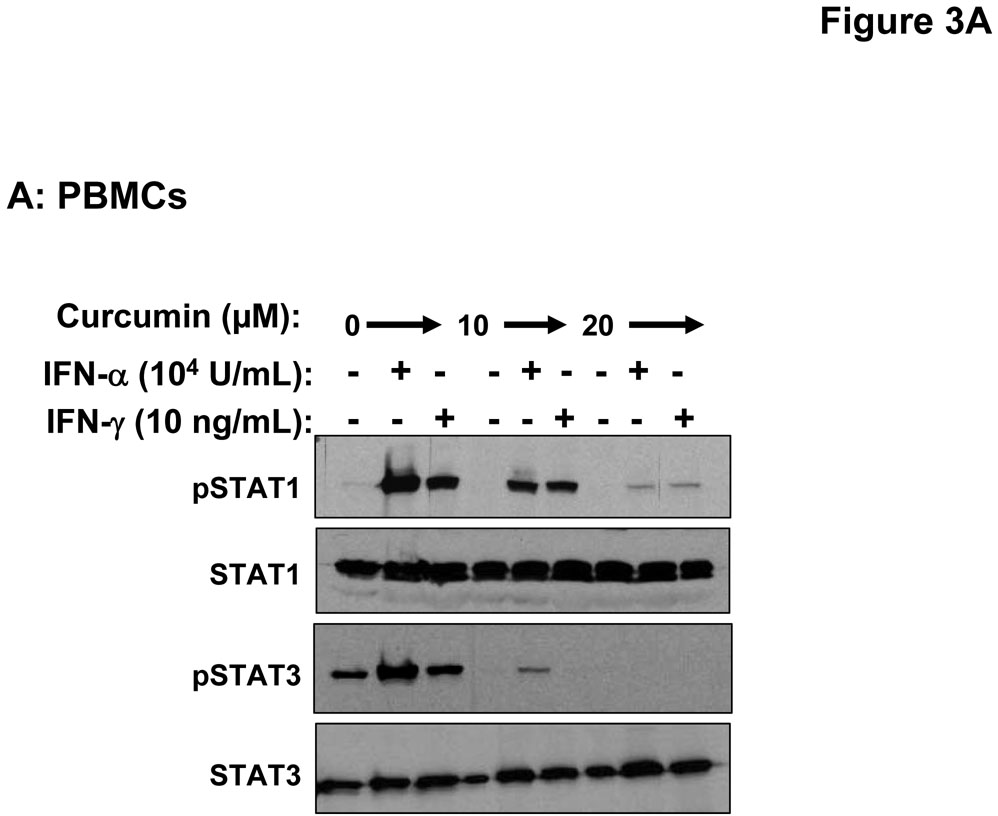
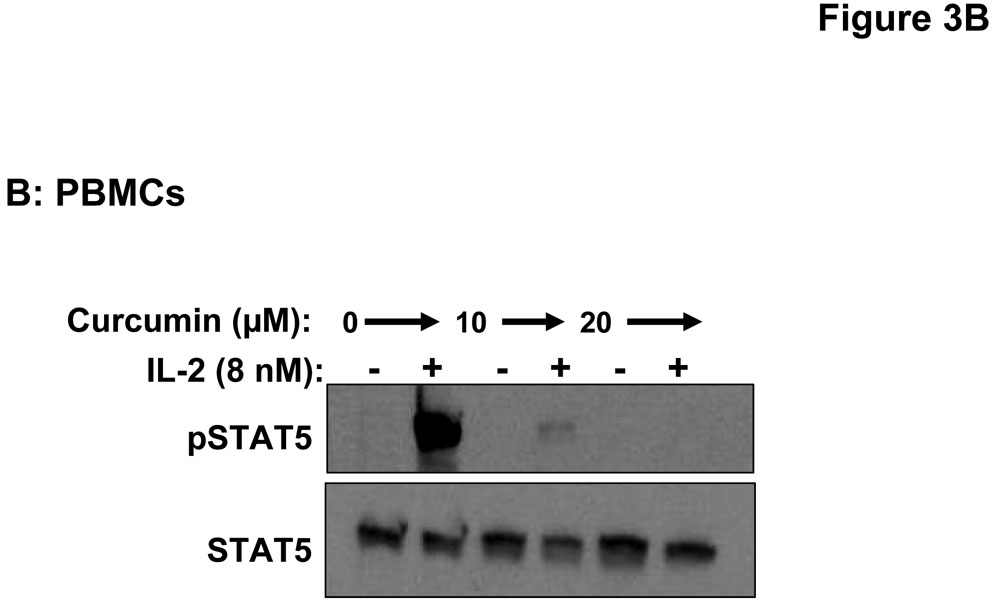
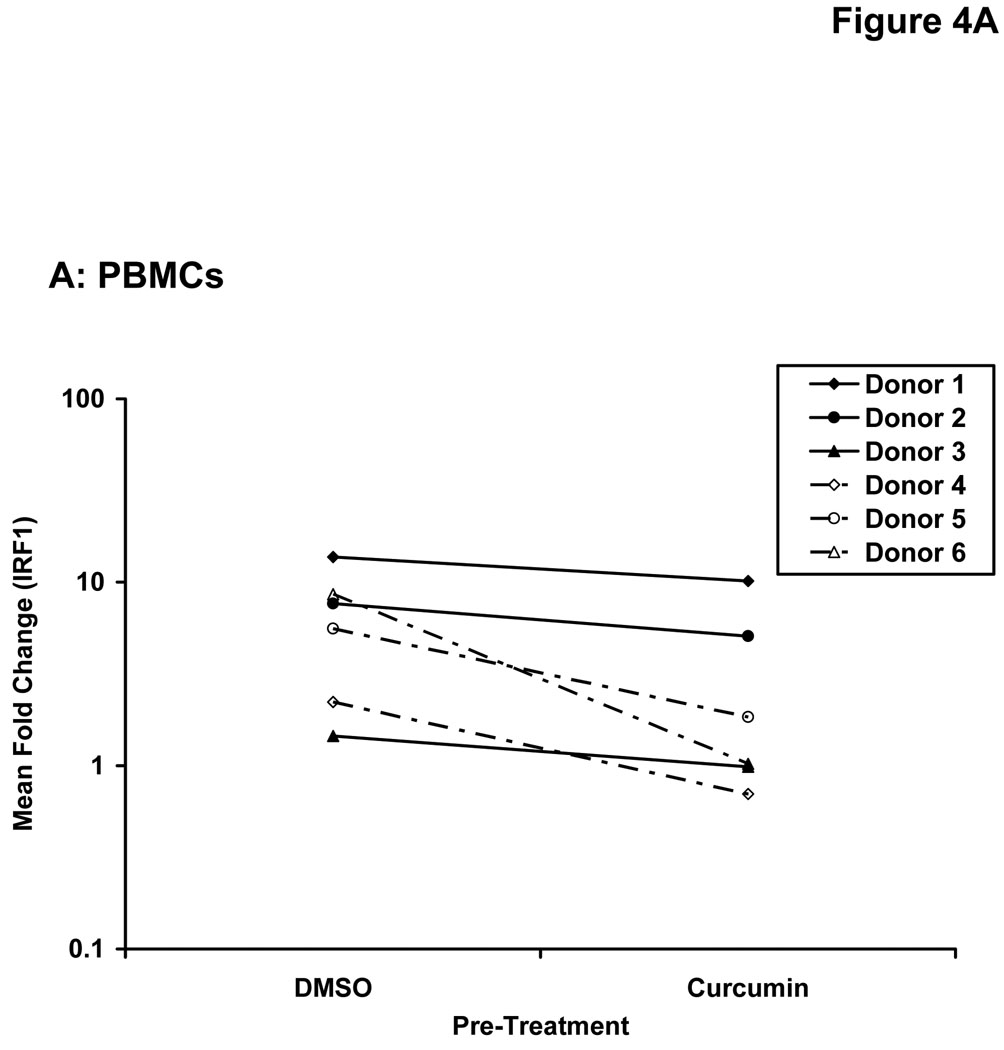
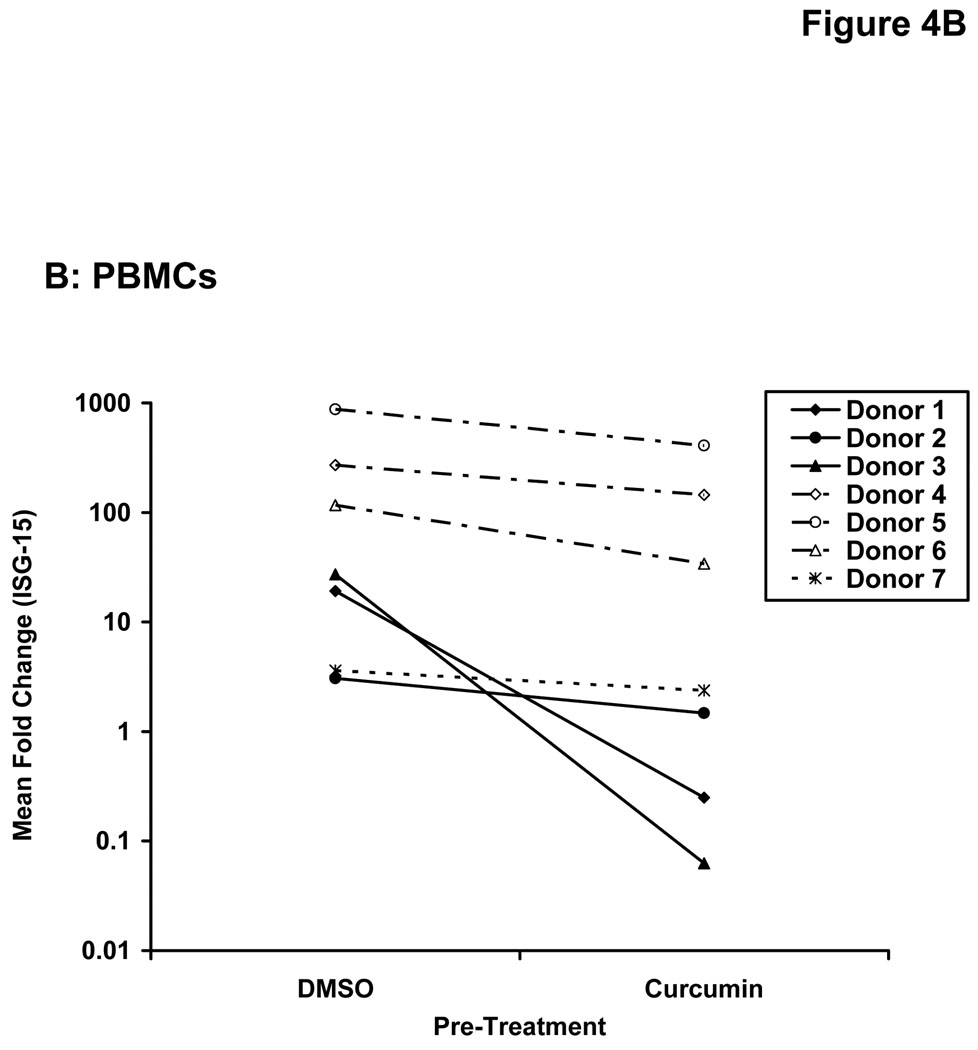
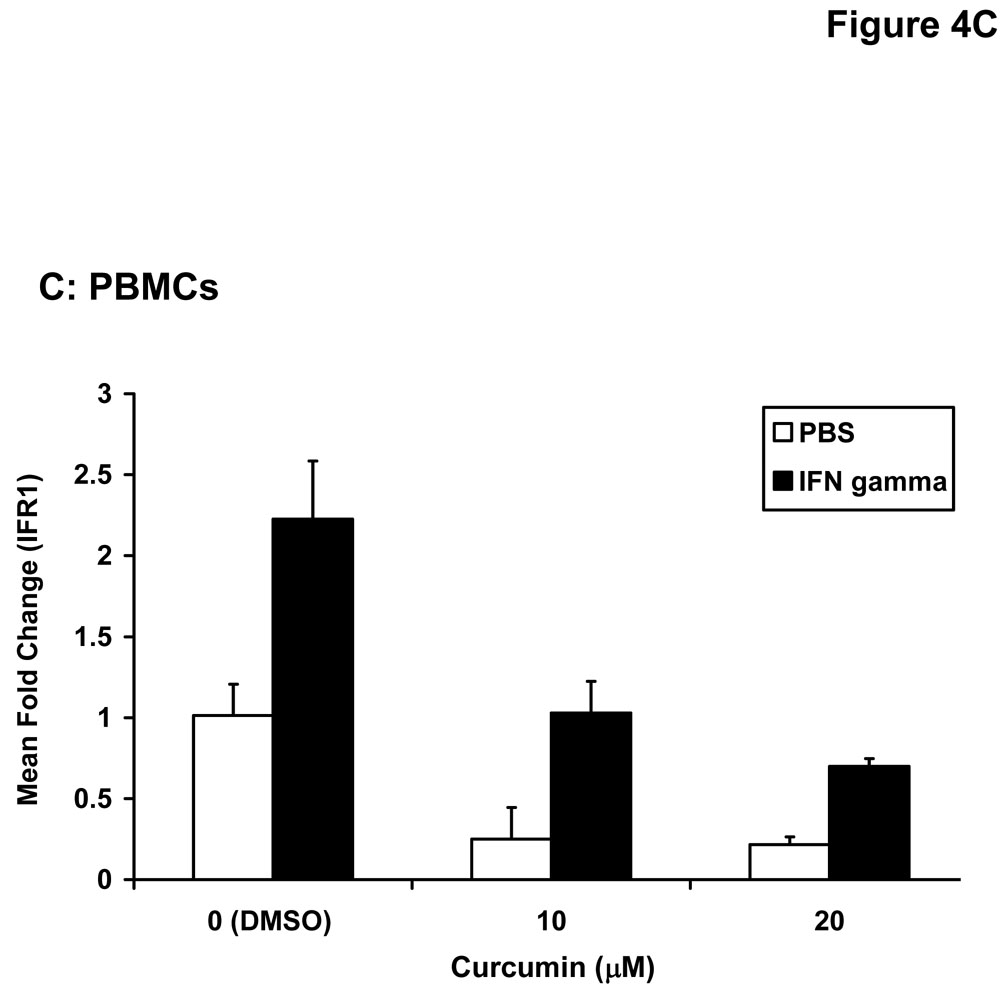
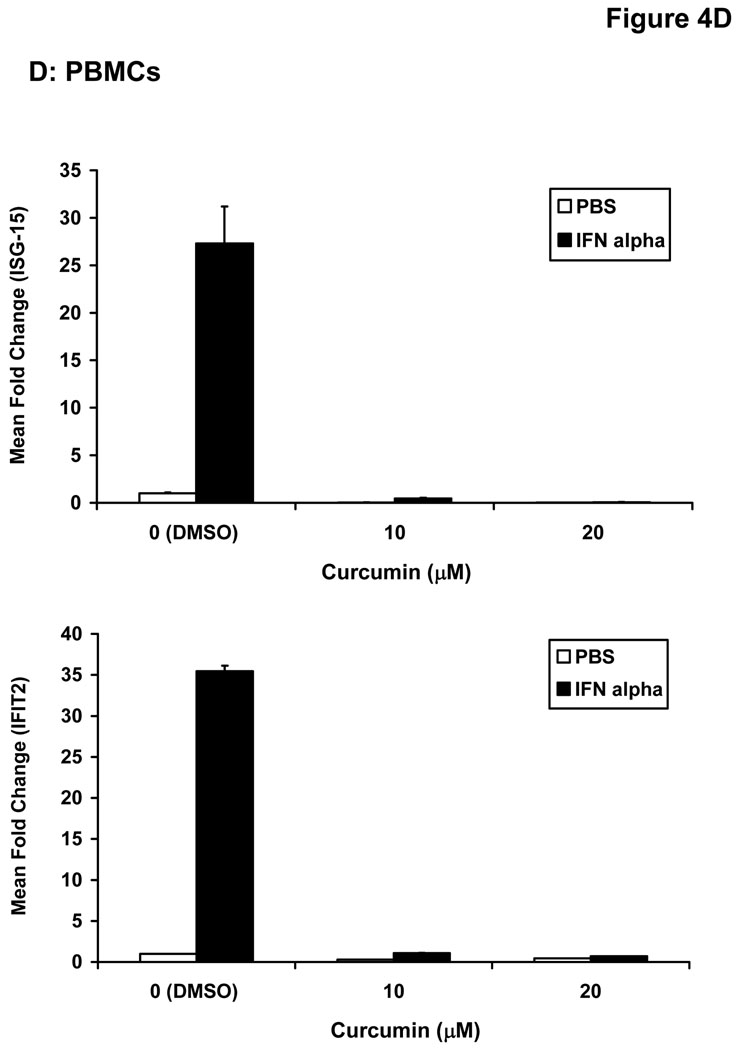
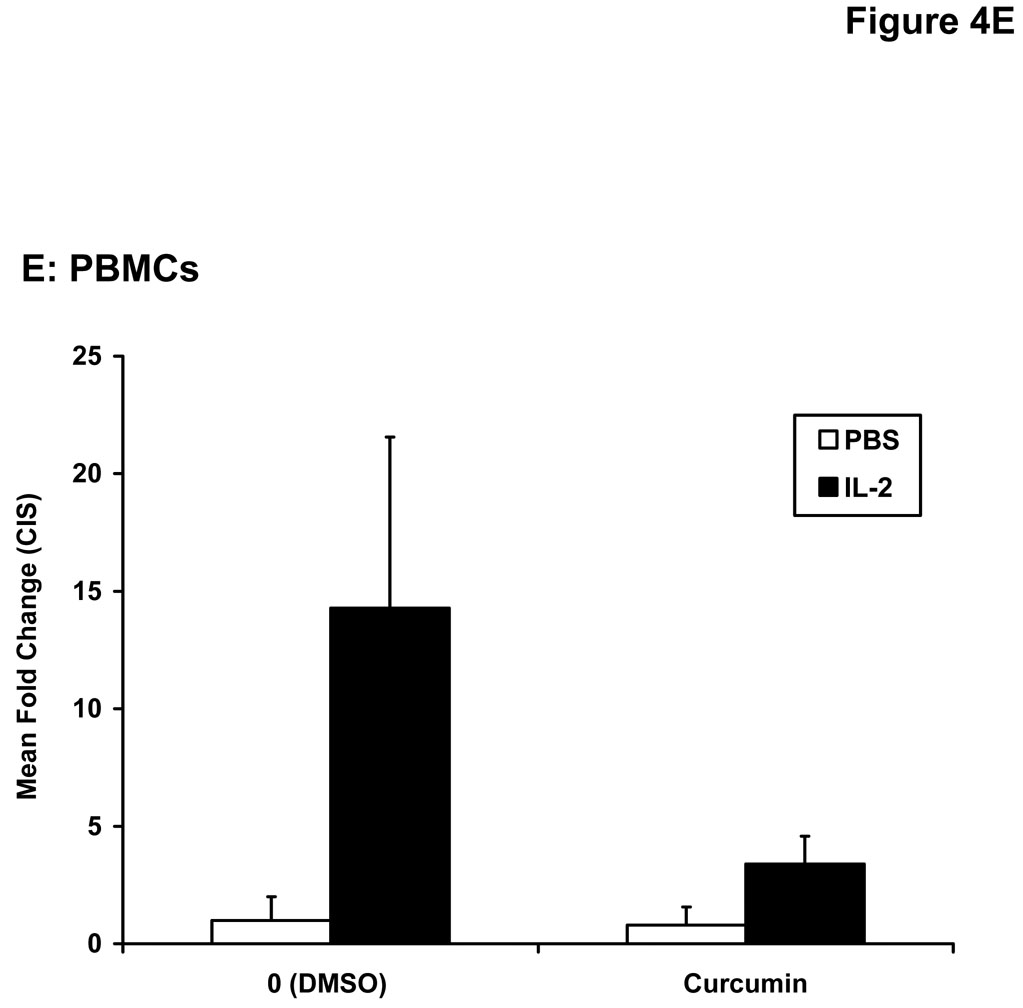
Stimulation of host immune effector cells is thought to contribute to the anti-tumor potential of interferons or interleukins (20, 21, 47). Similar to data obtained in melanoma cell lines, a 5 hour pre-treatment of PBMCs isolated from healthy donors with curcumin (10 µM or 20 µM) also inhibited the ability of IFN-α and IFN-γ to phosphorylate STAT1 or STAT3 (Figure 3A). Curcumin pre-treatments also led to a striking inhibition of IL-2-induced STAT5 phosphorylation in PBMCs (Figure 3B). These data suggested the inhibitory effects of curcumin were broad and not specific to the interferons. Subsequent Real Time PCR analysis in multiple donors further indicated that curcumin pre-treatment significantly inhibited the IFN-γ-induced transcription of IRF1, and the IFN-α-induced transcription of ISG-15 in PBMC from all donors examined (Figure 4A–B; p = 0.02 for IRF1; p = 0.05 for ISG-15). In some donors, the ability of curcumin to inhibit IFN-responsiveness was dramatic. However, as demonstrated in prior published studies, there was a high degree of donor variability in the individual responsiveness to IFN-stimulation (38, 48). The curcumin-mediated inhibition of gene expression appeared to be dose-dependent, and was more pronounced for IFN-α-induced genes as compared to IFN-γ-induced genes (Figure 4C–D). Similar data were obtained in PBMCs following curcumin pre-treatment for the IL-2 responsive gene, CIS (Figure 4E), and were reproduced following a 4 hour co-stimulation of PBMCs with curcumin and these cytokines (not shown).
Figure 3. Curcumin inhibits cytokine-induced signal transduction in immune cells.
PBMCs were pre-treated for 5 hours with curcumin (10 or 20 µM) and subsequently stimulated with (A) IFN-α, IFN-γ or (B) IL-2 for 15 minutes. Phosphorylation of STAT1, STAT3 and STAT5 proteins were evaluated by immunoblot analysis. Total STAT1, STAT3 or STAT5 proteins were measured to control for loading.
Figure 4. Curcumin inhibits cytokine induced gene expression in immune cells.
PBMCs from normal donors were pre-treated with curcumin (20 µM) or DMSO (negative control) prior to stimulation with (A) IFN-γ (10 ng/mL) to induce IRF1 transcription or (B) IFN-α (104 U/mL) to induce ISG-15 transcription. (C) IFN-γ-induced IRF1 expression and (D) IFN-α-induced ISG-15 and IFIT2 expression and (E) IL-2-induced CIS expression were evaluated by Real Time PCR in a representative normal donor following pre-treatment with multiple doses of curcumin (10 or 20 µM). All PCR data were normalized to expression values for 18s rRNA (housekeeping gene) and expressed as the fold change in gene expression relative to DMSO-treated cells. Error bars in C – E are derived from triplicate wells in a single experiment with a representative donor.
Curcumin inhibits innate natural killer cell function
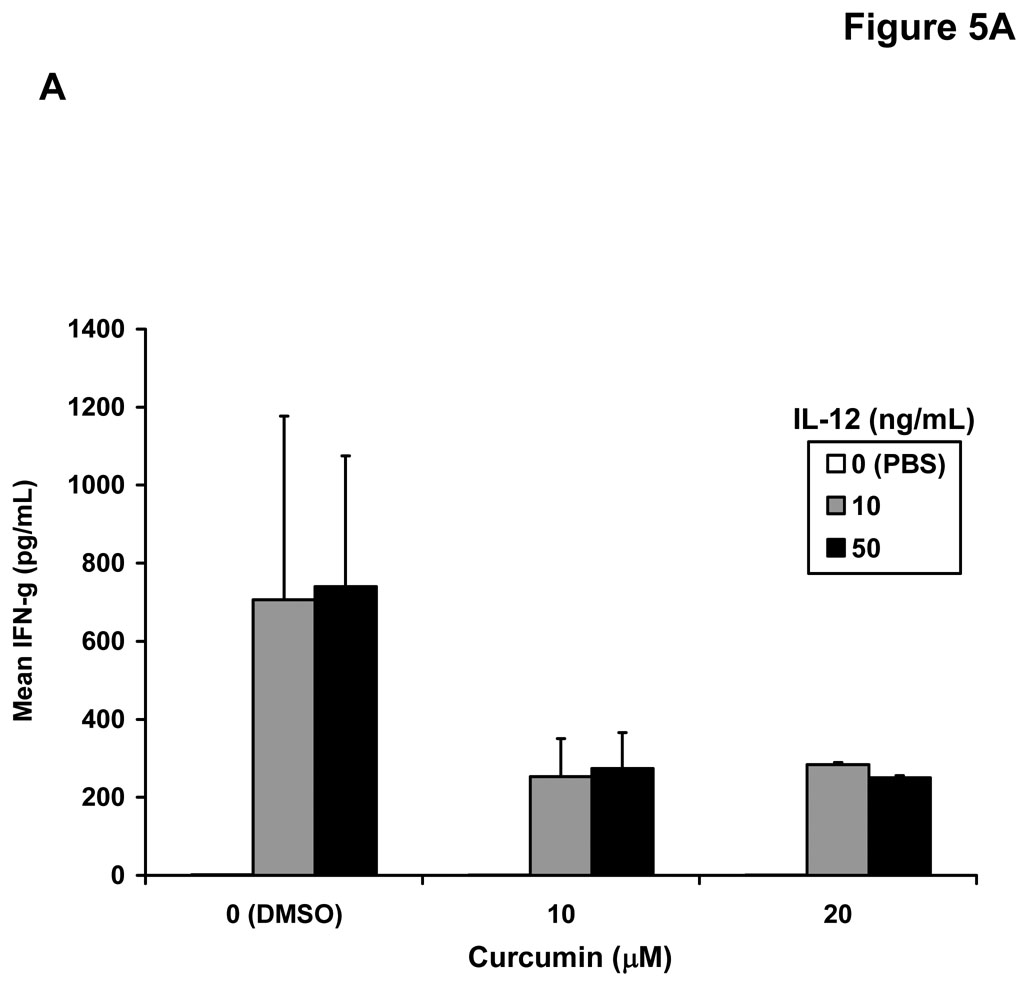
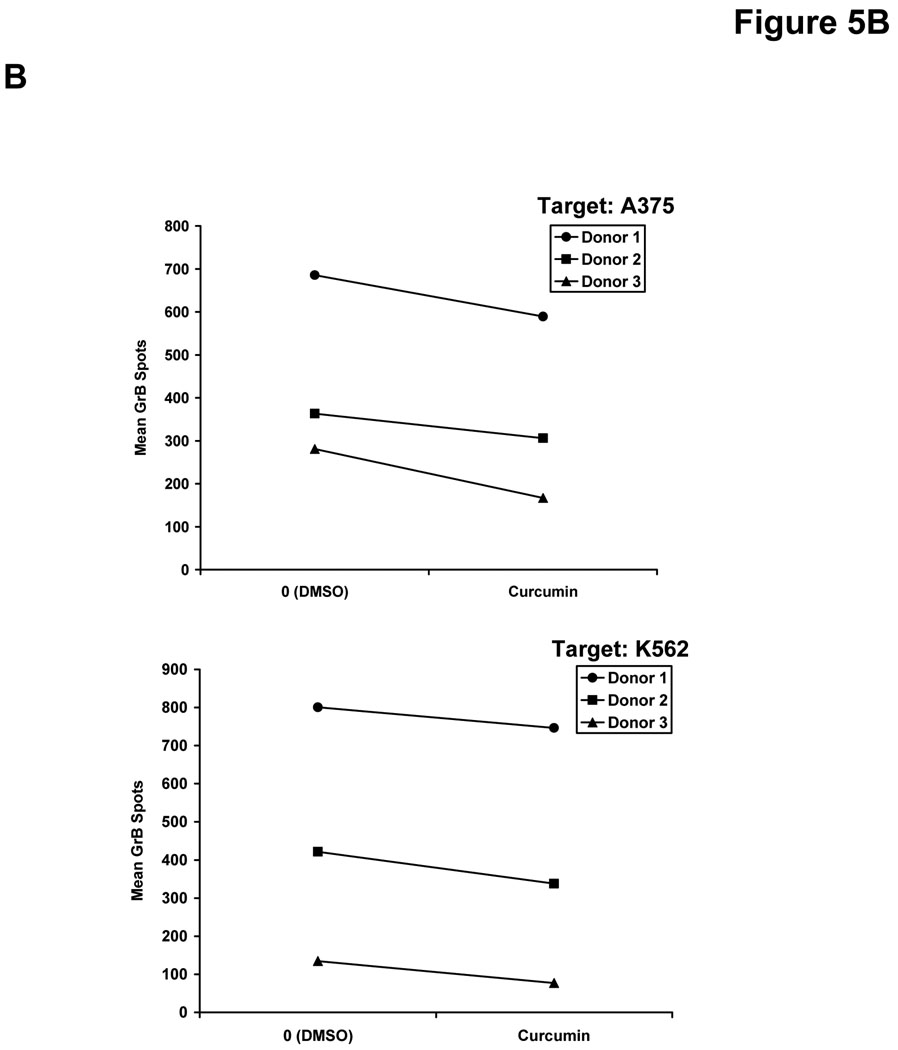
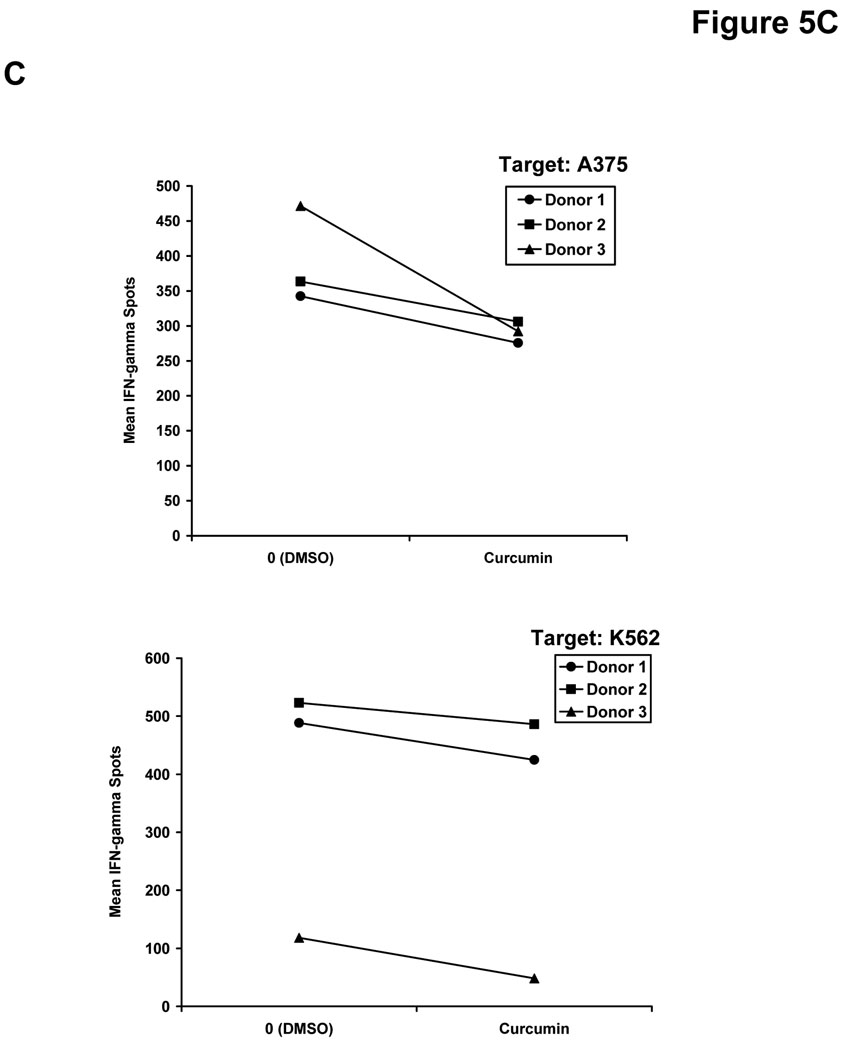
We hypothesized that curcumin-mediated inhibition of signal transduction might also lead to a similar decrease in the function of NK cells. This cell subset can directly eliminate malignant cells and is activated in response to stimulation with a variety of immunoregulatory cytokines (49). Co-treatment of NK cells from healthy donors with curcumin and IL-12 (10 or 50 ng/mL) led to reduced secretion of IFN-γ as compared to NK cells co-treated with DMSO (vehicle) and IL-12 for 24 hours (Figure 5A). Importantly, overnight treatment of healthy donor NK cells with curcumin (20 µM) also impaired GrB release from NK cells against the A375 melanoma cell line, and the NK sensitive K562 cell line, serving as a positive control target (Figure 5B). Targets or effector NK cells cultured alone showed no GrB release (data not shown). Although an inherent degree of inter-individual variability was observed in the NK cell response, the inhibitory effect of curcumin on NK cell function was consistently observed in three of three individual donors. Curcumin-treated NK cells exposed to A375 targets exhibited on average a 89 GrB spots/well decrease (± 29) over vehicle-treated NK cells from the same donor (p = 0.03). A similar trend was seen against the K562 line where curcumin-treated NK cells yielded on average 65 fewer GrB spots/well (± 16) than vehicle-treated NK cells (p = 0.02).
Figure 5. Curcumin inhibits functional properties of human NK cells.
(A) Levels of IFN-γ were assayed in the culture supernatants of NK cells from normal human donors following a 24 hour co-culture with curcumin (10 or 20 µM) or recombinant human IL-12 (10 or 50 ng/mL). Cells stimulated with DMSO and PBS (vehicles) served as negative controls. Error bars represent data combined data derived from two separate donors. Detection of (B) granzyme B (GrB) and (C) IFN-γ release from NK cells by ELISPOT. Following overnight culture with DMSO (vehicle) or curcumin (20 µM), NK cells from normal donors were cultured with A375 human melanoma cells or K562 cells as targets at a 20:1 effector:target ratio. Data shown are derived from independent experiments with NK cells from n = 3 normal donors.
Consistent with our ELISA data obtained following IL-12 stimulation (Figure 5A), curcumin also led to a slightly reduced production of IFN-γ by NK cells in the presence of A375 melanoma and K562 target cell lines (Figure 5C). Curcumin-treated NK cells exposed to A375 targets exhibited on average a 101 IFN-γ spots/well (± 68) decrease compared to vehicle-treated NK cells from the same donor (p = 0.12). A similar effect was seen against the K562 line where curcumin-treated NK cells produced on average 57 fewer IFN-γ spots/well (± 18) than untreated NK cells (p = 0.03).
Discussion
The use of medicinal natural products is widespread in patients with various types of cancer. Recent estimates indicate that one third of Americans use medicinal natural products daily, and that possibly more than 50% of cancer patients use them as well (1). Curcumin represents a compound used in dietary supplements and its anti-tumor potential is currently being evaluated in human clinical trials for various cancers including multiple myeloma, pancreatic cancer and colon cancer (50). Although the precise mechanisms by which many medicinal natural products elicit anti-tumor activity are unknown, these compounds do possess bioactive components that inhibit growth and promote apoptosis of malignant cells. However, few studies have explored in detail the off-target effects of these compounds on human immune cells, or their ability to modulate the cellular response to immunotherapeutic cytokines (51–53)
Due to the ability of the immune system to regulate tumor outgrowth, the interaction between bioactive compounds and host immune function is an important area of research. Data from the present study demonstrated that curcumin induced apoptosis of human melanoma cells in vitro. Curcumin also inhibited the IFN-responsiveness of melanoma cells and abrogated signal transduction, gene expression and functional activity of PBMCs in response to numerous cytokines including IFN-α, IFN-γ, IL-2 and IL-12. These data indicate that despite its beneficial, direct anti-tumor actions, curcumin (and potentially other natural products) may adversely modulate the cellular response to clinically-relevant cytokines.
Curcumin has demonstrated cytotoxic activity against a variety of tumor targets suggesting that it has broad potential as an effective chemopreventative or chemotherapeutic agent (1). The present data further confirm that single agent curcumin is active against human melanoma cells. Previous reports have also shown that curcumin induces apoptosis against the B16F10 murine melanoma cell line, and that this cell death was mediated by endoplasmic reticulum stress and downregulation of the anti-apoptotic protein, Mcl-1 (54). In human melanoma cells, curcumin has also been shown to induce apoptosis through a Fas receptor/caspase-8 pathway, to suppress NF-κB activity, and induce G2/M cell cycle arrest (55–57). Consistent with these observations, our data demonstrated that curcumin induced apoptosis in radial growth phase, vertical growth phase and metastatic human melanoma cell lines and down-regulated basal pSTAT3 and Bcl-2. Since curcumin can inhibit multiple pro-survival pathways, there is considerable interest in studying whether this compound could augment existing therapies for melanoma or other malignancies (42, 43).
The ability of curcumin to inhibit various aspects of innate immune function and Jak-STAT signal transduction could be considered controversial with respect to its clear anti-tumor activity (52, 53, 58–60). Indeed, these inhibitory properties of curcumin are likely beneficial for preventing cancers that arise from chronic inflammation and reducing certain mediators that contribute to immune suppression. For example, curcumin suppressed STAT1 activation in murine dendritic cells and inhibited indoleamine 2,3-dioxygenase (IDO)-mediated suppression of T cell responses (53). Oral administration of curcumin to mice bearing ascites carcinoma also inhibited tumor-induced T cell death (51). Conversely, curcumin has been shown to inhibit basic functional properties of immune effector cells that could facilitate immune-mediated recognition of tumors including IL-2 induced proliferation of cytotoxic T lymphocytes and expression of the CD80 and CD86 co-stimulatory molecules (60). Data from the present report suggests that curcumin may adversely affect the responsiveness of immune cells to clinically relevant cytokines that are often administered to patients with advanced melanoma. Of note, previous studies have demonstrated that STAT1 signal transduction in immune cells was required for the anti-tumor actions of exogenously administered IFN-α in murine models of melanoma (61). Similarly, the STAT5 transcription factor plays a crucial role in normal immune function by promoting IL-2 induced cell cycle progression in T cells (62–64) and regulates NK cell-mediated proliferation and cytolytic activity (65–67). These data caution that despite its anti-inflammatory, chemopreventative properties, curcumin may potentially negate the anti-tumor properties of therapeutically administered cytokines.
It has been difficult to truly characterize the immunomodulatory effects of curcumin because many prior studies performed in murine models have not documented plasma or tissue concentrations of curcumin. This information would have allowed for a more accurate interpretation as to whether the concentrations achieved in vivo were relevant for inducing tumor cell apoptosis. Efforts to improve the in vivo bioavailability and absorbtion of curcumin are being rigorously investigated and have produced promising initial results. For example simply heating curcumin has been shown to increase its water solubility by 12-fold (68). In a similar manner, co-administration of curcumin together with piperine, an extract from black pepper has been shown to increase the bioavailability of curcumin by 1000% in normal human donors (5). This effect is primarily due to the ability of piperine to inhibit hepatic and intestinal glucuronidation, a major mechanism by which curcumin is rapidly metabolized (5). The present data suggest careful attention should be given to the immunomodulatory effects of curcumin once the hurdle of achieving systemic levels that induce apoptosis is overcome.
Although our understanding of melanoma development is in its infancy, the presence of activated lymphocytes in regressing tumors, the clinical activity of exogenous cytokines such as IL-2 and IFN-α, and the high incidence of autoimmune vitiligo observed in patients who respond clinically to IFN-α suggest that the immune system can influence the development and metastatic progression of melanoma (20, 23, 69). Consequently, melanoma represents a form of cancer which may be amenable to immune-based therapy. In particular, melanoma patients rendered disease free following surgery who are at high risk for recurrence represent a population in which chemopreventative agents could have a positive impact. Ideally, these agents could compliment the actions of exogenous or endogenous immunostimulatory cytokines to provide a potentially greater clinical benefit.
In conclusion, data from the present study raise concern that utilizing medicinal natural products could negate the effects of immunotherapy or of the ability of the immune system to recognize tumors. Curcumin represents a single example of the numerous alternative medicine or nutritional supplements available over-the-counter which are used regularly in the absence of strong scientific data. In line with these concerns are recent reports that green tea polyphenols block the anti-tumor properties of bortezomib and other boron based proteasome inhibitors against multiple myeloma cells (70). These data suggest that the ability of bioactive natural products to modulate immune function and cytokine responsiveness deserves further investigation in a well-controlled manner before recommending their widespread use.
Acknowledgment
We thank Dr. William E. Carson, III for critical review of this manuscript. We thank the OSU CCC Analytical Cytometry, Nucleic Acid and Biostatistics Shared Resources. This work was supported by NIH Grant K22 CA134551 (G.B. Lesinski), The Valvano Foundation for Cancer Research (G.B. Lesinski) and Grant # IRG-67-003-44 from the American Cancer Society (D. Benson, Jr.). The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources, funded by the Office of the Director, National Institutes of Health (OD) and supported by the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 5.Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 6.Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 8.Bachmeier B, Nerlich AG, Iancu CM, et al. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol Biochem. 2007;19:137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 9.Busquets S, Carbo N, Almendro V, et al. Curcumin, a natural product present in turmeric, decreases tumor growth but does not behave as an anticachectic compound in a rat model. Cancer Lett. 2001;167:33–38. doi: 10.1016/s0304-3835(01)00456-6. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Baiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 11.LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 12.Menon LG, Kuttan R, Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–225. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- 13.Odot J, Albert P, Carlier A, et al. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer. 2004;111:381–387. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- 14.Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109–115. [PubMed] [Google Scholar]
- 15.Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. J Transl Med. 2008;6:62. doi: 10.1186/1479-5876-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheatley K, Ives N, Eggermont A, et al. Interferon-alpha as adjuvant therapy for melanoma: An individual patient data meta-analysis of randomised trials. Proc Am Soc Clin Oncol. 2007:478S. (abstr 8526) [Google Scholar]
- 17.Agarwala S. Improving survival in patients with high-risk and metastatic melanoma: immunotherapy leads the way. Am J Clin Dermatol. 2003;4:333–346. doi: 10.2165/00128071-200304050-00004. [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 20.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 21.Waithman J, Gebhardt T, Davey GM, Heath WR, Carbone FR. Cutting edge: Enhanced IL-2 signaling can convert self-specific T cell response from tolerance to autoimmunity. J Immunol. 2008;180:5789–5793. doi: 10.4049/jimmunol.180.9.5789. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene. 2002;21:2504–2512. doi: 10.1038/sj.onc.1205341. [DOI] [PubMed] [Google Scholar]
- 23.Lesinski GB, Trefry J, Brasdovich M, et al. Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13:5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- 24.Levy DE, Gilliland DG. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene. 2000;19:2505–2510. doi: 10.1038/sj.onc.1203480. [DOI] [PubMed] [Google Scholar]
- 25.Pansky A, Hildebrand P, Fasler-Kan E, et al. Defective Jak-STAT signal transduction pathway in melanoma cells resistant to growth inhibition by interferon-alpha. International Journal of Cancer. 2000;85:720–725. doi: 10.1002/(sici)1097-0215(20000301)85:5<720::aid-ijc20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Thyrell L, Erickson S, Zhivotovsky B, et al. Mechanisms of Interferon-alpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–1262. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 28.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 29.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 30.Baker PE, Gillis S, Smith KA. Monoclonal cytolytic T-cell lines. J Exp Med. 1979;149:273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm EA, Mazumder A, Zhangs HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41:4420–4425. [PubMed] [Google Scholar]
- 33.Satyamoorthy K, DeJesus E, Linnenbach AJ, et al. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7 Suppl 2:S35–S42. [PubMed] [Google Scholar]
- 34.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkateswarlu S, Ramachandra MS, Subbaraju GV. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg Med Chem. 2005;13:6374–6380. doi: 10.1016/j.bmc.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 36.Park BS, Kim JG, Kim MR, et al. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. J Agric Food Chem. 2005;53:9005–9009. doi: 10.1021/jf051765z. [DOI] [PubMed] [Google Scholar]
- 37.Lesinski GB, Raig T, Guenterberg K, et al. IFN-alpha and bortezomib overcome Bcl-2 and Mcl-1 overexpression in melanoma cells by stimulating the extrinsic pathway of apopotsis. Cancer Res. 2008;68:8351–8360. doi: 10.1158/0008-5472.CAN-08-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesinski GB, Kondadasula SV, Crespin T, et al. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon alfa immunotherapy. J Natl Cancer Inst. 2004;96:1331–1342. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 39.Varker KA, Kondadasula SV, Go MR, et al. Multiparametric flow cytometric analysis of signal transducer and activator of transcription 5 phosphorylation in immune cell subsets in vitro and following interleukin-2 immunotherapy. Clin Cancer Res. 2006;12:5850–5858. doi: 10.1158/1078-0432.CCR-06-1159. [DOI] [PubMed] [Google Scholar]
- 40.Parihar R, Nadella P, Lewis A, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- 41.Shafer-Weaver KA, Sayers T, Kuhns DB, et al. Evaluating the cytotoxicity of innate immune effector cells using the GrB ELISPOT assay. J Transl Med. 2004;2:31. doi: 10.1186/1479-5876-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 43.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 46.Ramsauer K, Sadzak I, Porras A, et al. p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc Natl Acad Sci U S A. 2002;99:12859–12864. doi: 10.1073/pnas.192264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malek TR, Yu A, Zhu L, et al. IL-2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28:635–639. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 48.Whitney AR, Diehn M, Popper SJ, et al. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci U S A. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 2008;31:685–692. doi: 10.1097/CJI.0b013e318182de23. [DOI] [PubMed] [Google Scholar]
- 50.Milacic V, Banerjee S, Landis-Piwowar KR, et al. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharyya S, Mandal D, Saha B, et al. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282:15954–15964. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- 52.Jagetia GC, Aggarwal BB. "Spicing up" of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 53.Jeong YI, Kim SW, Jung ID, et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem. 2009;284:3700–3708. doi: 10.1074/jbc.M807328200. [DOI] [PubMed] [Google Scholar]
- 54.Bakhshi J, Weinstein L, Poksay KS, et al. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis. 2008;13:904–914. doi: 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 56.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 57.Zheng M, Ekmekcioglu S, Walch ET, Tang CH, Grimm EA. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;14:165–171. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]
- 58.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 59.Blasius R, Reuter S, Henry E, Dicato M, Diederich M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem Pharmacol. 2006;72:1547–1554. doi: 10.1016/j.bcp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 60.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 61.Lesinski GB, Anghelina M, Zimmerer J, et al. The anti-tumor effects of interferon-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112:170–180. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behbod F, Nagy ZS, Stepkowski SM, et al. Specific inhibition of Stat5a/b promotes apoptosis of IL-2-responsive primary and tumor-derived lymphoid cells. J Immunol. 2003;171:3919–3927. doi: 10.4049/jimmunol.171.8.3919. [DOI] [PubMed] [Google Scholar]
- 63.Fung MM, Rohwer F, McGuire KL. IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells. Cell Signal. 2003;15:625–636. doi: 10.1016/s0898-6568(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 64.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164:6244–6251. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 65.Imada K, Bloom ET, Nakajima H, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11:225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 67.Moriggl R, Topham DJ, Teglund S, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 68.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 69.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 70.Golden EB, Lam PY, Kardosh A, et al. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood. 2009 doi: 10.1182/blood-2008-07-171389. [DOI] [PubMed] [Google Scholar]