Abstract
Previous work has shown ICOS can function independently of CD28, but whether either molecule can compensate for the other in vivo is not known. Since ICOS is a potent inducer of Th2 cytokines and linked to allergy and elevated serum IgE in humans, we hypothesized that augmenting ICOS costimulation in murine allergic airway disease may overcome CD28 deficiency. While ICOS was expressed on T cells from CD28−/− mice, Th2-mediated airway inflammation was not induced in CD28−/− mice by increased ICOS costimulation. Further, we determined if augmenting CD28 costimulation could compensate for ICOS deficiency. ICOS−/− mice had a defect in airway eosinophilia that was not overcome by augmenting CD28 costimulation. CD28 costimulation also did not fully compensate for ICOS for antibody responses, germinal center formation or the development of follicular B helper T cells. CD28 and ICOS play complementary non-overlapping roles in the development of Th2 immunity in vivo.
Keywords: Costimulation, CD28, ICOS, follicular B helper T cells, Rodent, Th2 Cells, Antibodies, Allergy, Asthma
INTRODUCTION
CD28 was arguably the most well studied costimulatory molecule until the discovery of ICOS in 1999 expanded the CD28 family [1, 2]. Similar to CD28, ICOS has been shown to augment T cell proliferation, Th2 differentiation and production of IL-4 but unlike CD28, ICOS costimulation does not induce high levels of IL-2 production [2, 3]. We have previously found an association of ICOS single nucleotide polymorphisms with increased serum IgE levels and allergen sensitivity in a human population, suggesting ICOS plays a role in augmenting allergy in humans [4]. In mouse models of Th2-mediated allergic airway disease, several groups including ours have found that blockade of CD28 completely inhibits Th2-mediated airway inflammation and IgE production [5–8], while ICOS blockade decreases Th2-mediated airway inflammation [9, 10]. Further, ICOS−/− mice have defects in Th2 differentiation similar to CD28−/− mice [11–15]. These data support a model defined by the requirement of CD28 for Th2 development while ICOS is an important mediator of the magnitude of Th2 responses.
Individuals who have a total defect in ICOS expression due to a deletion within the ICOS gene present clinically with common variable immunodeficiency syndrome (CVID), characterized by hypogammalobulinemia and recurrent infections of the respiratory and gastrointestinal tract.[16–18]. ICOS-null individuals have been found to have profound defects in B cell maturation and immunoglobulin isotype switching. These individuals like ICOS−/− mice have a defect in the production of specialized follicular B helper T cells (TFH). TFH are a novel sub-population of CD4+ T cells characterized by expression of the chemokine receptor CXCR5, high ICOS expression and the production of IL-21 [19–21]. Thus similar to CD28, a major role for the ICOS-B7RP-1 pathway in T helper cell-dependent B cell responses has emerged.
Previous work using mice deficient for both ICOS and CD28 has shown that ICOS can play a role independent of CD28 in viral immunity and immunoglobulin isotype switching [22, 23]. However, these reports have not investigated whether augmenting CD28 or ICOS costimulation can compensate for the function of the other molecule. Since costimulatory molecules provide a signal that is additive to the TCR it is possible that increasing activation of ICOS or CD28 may overcome the deficiency of the other for Th2 immunity. We found that while ICOS can be expressed in vivo in CD28−/− mice, augmenting ICOS costimulation did not surmount the requirement for CD28 to induce Th2 mediated airway inflammation or antibody responses. In ICOS−/− mice Th2-mediated airway eosinophilia was significantly less than wild-type mice and augmenting CD28 costimulation had no significant effect. Further, augmenting CD28 costimulation did not restore to wild-type levels antigen-specific antibody responses, germinal center size or the expansion of CD4+CXCR5+ TFH in the absence of ICOS. Thus CD28 and ICOS play complementary non-overlapping roles in the development of Th2 immunity in vivo.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice, ages 4–6 wk, were purchased from National Cancer Institute (Frederick, MD) or Charles River Laboratories (Wilmington, MA). C57Bl/6. CD28−/− mice, 4–6 wk old, were purchased from Jackson Laboratory (Bar Harbor, ME). C57Bl/6. ICOS−/− mice were the gift of Dr. Richard Flavell and were bred in our facilities. Wild-type littermate controls from in-house breeding were used in the experiments with ICOS−/− mice. Animals were housed in a specific pathogen-free barrier facility, maintained by the University of Chicago Animal Resources Center (Chicago, IL) and were used under the guidelines of our Institutional Animal Care and Use Committee.
Animal models
S. mansoni eggs and antigen were prepared as previously described [24]. The protocol used for animal sensitization and challenge was modified from that Padrid et al. [7]. Mice, immunized on day 0 with 5,000 inactivated S. mansoni eggs delivered by intraperitoneal (i.p.) injection, were challenged on day 7 via intratracheal (i.t.) delivery of 5μg of soluble egg antigen. Mice were treated i.p. every other day throughout sensitization and challenge with either 50μg B7RP-1-Fc or 25 μg human Fc (molar equivalent, Amgen); or mice received one dose i.p. of either 100 or 150 μg anti-CD28 (PV-1) [25], hamster isotype control (BioXCell), or PBS at the time of sensitization unless noted in the text. In some experiments mice were sensitized i.p. with 10 μg ovalbumin (OVA) in alum. For restimulation ex vivo, lungs or mediastinal lymph nodes were disassociated as previously described [10].
Bronchoalveolar Lavage (BAL)
Mice were sacrificed on day 11. BAL was performed as previously described [10]. Differential cell counts were determined by counting a total of 200 cells. Alternatively, differential cell counts were determined by flow cytometry.
Serum Antibody Levels
Total serum IgE was measured by ELISA (BD Biosciences). Antigen-specific IgG1, IgG2a, IgG2b were measured by coating plates with either SEA (10 μg/ml) or OVA (100 μg/ml) and using secondary istoype specific antibodies (BD Biosciences). OVA specific IgE was measured by coating with an IgE specific antibody (BD Biosciences) and using a biotinylated OVA secondary reagent. OVA-specific IgE was determined by comparison with a standard curve constructed with the use of purified, mouse, OVA-specific IgE secreted by the hybridoma TOε, a kind gift of Dr. Paul Bryce [26].
Flow Cytometry Analysis
BAL cells were stained with anti-CD3, anti-CD4, anti-CD8 and anti-CCR3 (eosinophils). All antibodies were purchased from BD Pharmingen with the exceptions of anti-CD4-allophycocyanin (eBioscience) and anti-CCR3-FITC (R&D Systems). Flow cytometry analysis was performed using either a FACSCalibur or LSR II (BD Pharmingen). Data was analyzed using CellQuest or Flow Jo software.
Histology
Lungs were fixed by immersion into 4% paraformaldehyde. Spleens were freshly frozen and embedded in Tissue-Tek II OCT embedding compound (Baxter Scientific) and sliced into 6 μm sections. Biotinylated peanut agglutinin (PNA; Vector, B-1075) was used for staining of germinal center B cells. Entire spleen sections were imaged at 4x magnification using an Axioskop microscope and digital camera. Germinal center area was measured using Image J version 1.36b software. An arbitary standard was set for calibrating distance for all samples at 11.67 pixels per cm. The size of each PNA positive area within the B cell follicles in the entire section was analyzed and 1 to 2 slides per spleen were analyzed.
Statistical analysis
Statistics were done using an unpaired Student two-tailed t test, Mann Whitney or two way ANOVA (*p<0.05, **p<0.01, ***p<0.001). Error bars represent SEM.
RESULTS
ICOS costimulation enhances serum IgE production but does not affect the severity of allergic airway disease
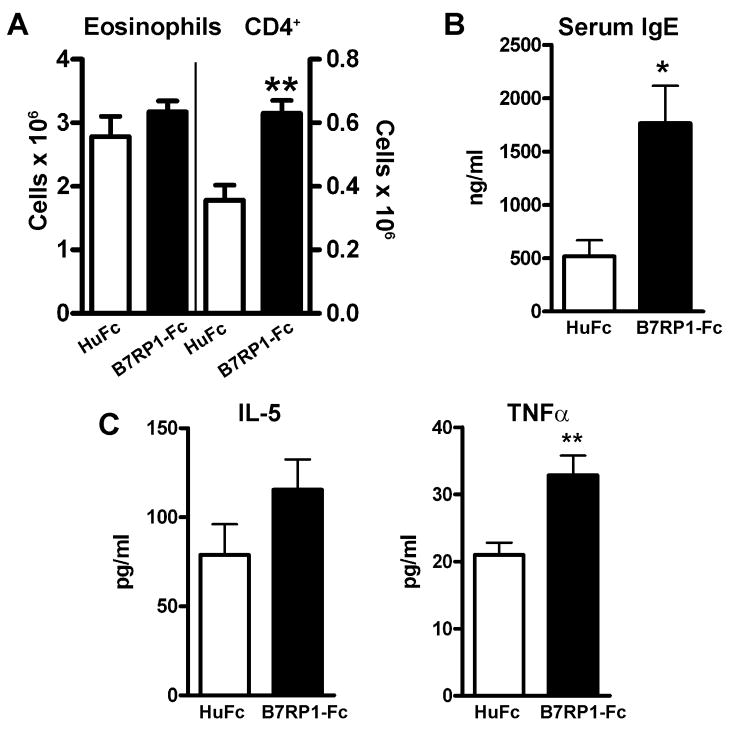
To investigate the effect of augmented ICOS costimulation on Th2-mediated inflammation, we used a model of allergic airway disease that we have previously shown induces a robust Th2 type response in the lung [7, 8, 10]. A soluble form of the ICOS ligand, B7RP-1-Fc, that has previously been shown to expand the cellular and humoral responses to different antigens was used to augment ICOS costimulation [27, 28]. C57Bl/6 mice were sensitized i.p. on day 0 with inactivated S. mansoni eggs and challenged in the lung on day 7 with S. mansoni soluble egg antigen (SEA). Evaluation of the bronchoalveolar lavage (BAL) on day 11 revealed a significant increase in lymphocytes, predominantly CD4+ T cells, in the airways of mice treated every other day throughout sensitization and challenge with B7RP-1-Fc by i.p. injection compared to human Fc (HuFc) treated control mice (Fig. 1A and data not shown). No significant difference was found in the total number of eosinophils or in the cellular infiltration in the lungs by histology (Fig. 1A and data not shown). In addition, the levels of IL-5 and IL-4 in the BAL were not significantly different. The level of IFNγ in the BAL in both groups was undetectable (<5 pg/ml). However, the level of TNFα in the BAL was significantly higher in the B7RP-1-Fc treated mice suggesting a pro-inflammatory effect of B7RP-1-Fc (Fig. 1C and data not shown). The most striking difference found was an increase in serum IgE in B7RP-1-Fc treated mice consistent with the role of ICOS in augmenting humoral immunity (Fig. 1B). To determine if an increase in Th2 differentiation had occurred, lung and mediastinal lymph node (MLN) cells were restimulated ex vivo. Increased IL-5 (lung) and IL-13 (lung and MLN) levels were found in cultures from mice treated with B7RP-1-Fc compared to HuFc treated controls (Supp. Fig. S1). The findings suggest that B7RP-1-Fc has a modest effect on Th2 inflammation but significantly augments Th2 differentiation and serum IgE.
Figure 1. ICOS costimulation enhances serum IgE production.
C57Bl/6 mice sensitized and challenged with S. mansoni were injected i.p. every other day with either B7RP-1-Fc (black bars) or HuFc (open bars). (A) The total numbers of BAL eosinophils and CD4+ T cells, (B) levels of serum IgE and (C) the levels of BAL IL-5 and TNFα were measured. The data are representative of 3 independent experiments with five mice per group.
ICOS can be expressed in the absence of CD28, but cannot induce Th2-airway inflammation independent of CD28
Previous work has shown that in the absence of CD28, S. mansoni cannot induce Th2-mediated airway inflammation or systemic IgE responses [8]. Since ICOS is a potent stimulator of humoral immunity we hypothesized that B7RP-1-Fc may compensate for the defect in CD28−/− mice. Given that ICOS expression has been shown to be enhanced by CD28 costimulation in vitro, it was possible that ICOS would not be expressed in vivo in the absence of CD28 [3]. CD28−/− and CD28+/+ mice were sensitized i.p. with S. mansoni eggs and ICOS expression was examined 7 days later on T cells harvested from the spleen. We found that a population of both CD4+ and CD8+ T cells from CD28−/− mice did express ICOS although to a lesser extent than CD28+/+ T cells (Suppl. Fig. S2).
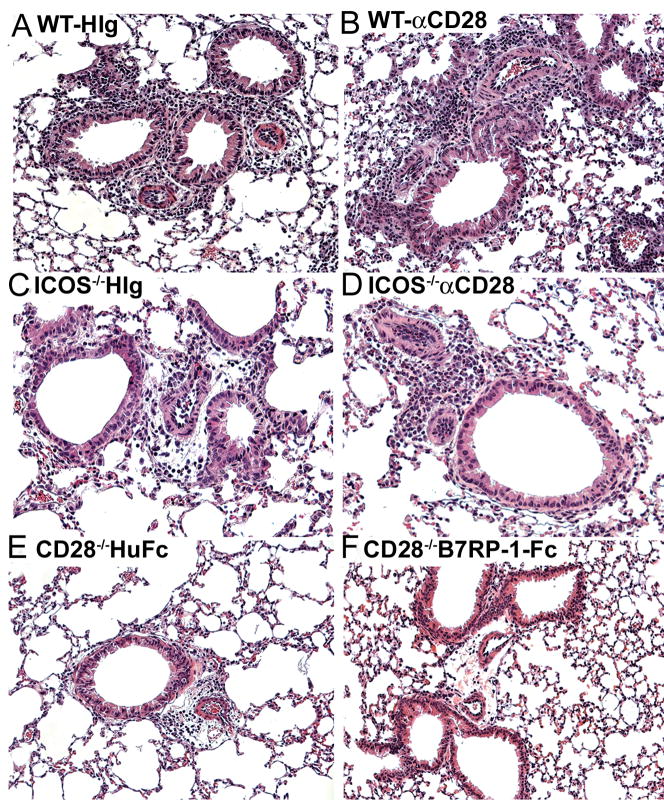
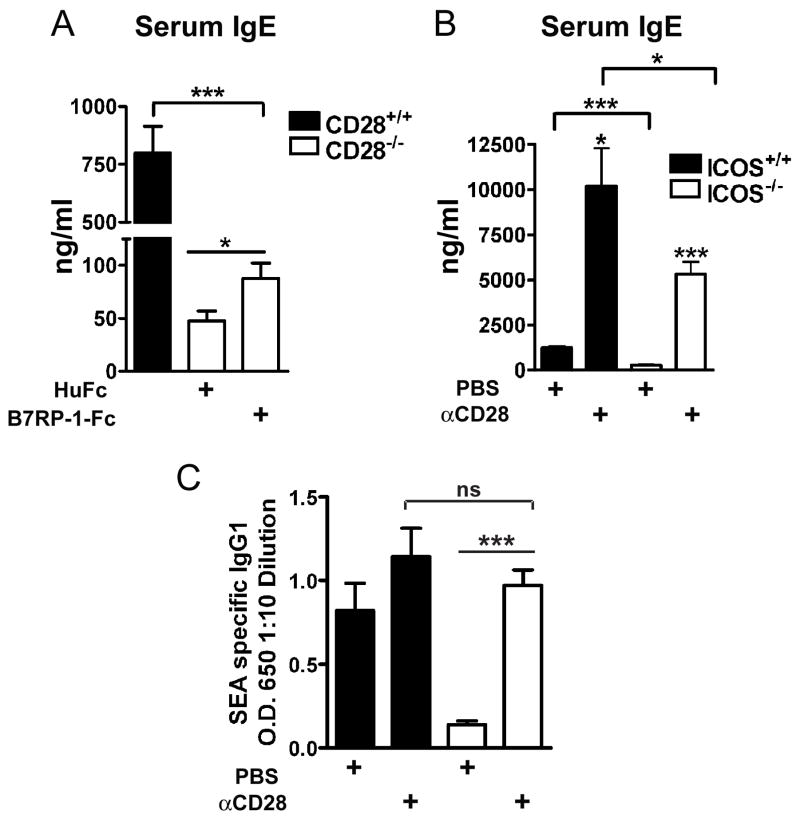
To determine if ICOS costimulation augmented Th2-mediated inflammation independent of CD28, we administered either B7RP-1-Fc or HuFc i.p. to CD28−/− mice every other day throughout sensitization and challenge, as in Figure 1, compared to sensitization and challenge of CD28+/+ mice without treatment. We have previously established that eosinophilia in our model is totally dependent on both sensitization and challenge [7, 8]. B7RP-1-Fc did not affect total BAL cell counts, eosinophils or lymphocytes in the CD28−/− mice compared to controls (Fig. 2A and data not shown), and did not induce an appreciable increase in peribronchial or perivascular inflammation (Fig. 3E, F). CD28−/− control mice did not have significant airway inflammation as previously shown (Fig. 2A) [8]. In the absence of CD28, ICOS costimulation can only induce a small, although significant, increase in serum IgE (Fig. 4A). We were unable to detect a significant difference in other isotypes measured, IgG1 or IgG2a (data not shown). Our data show that CD28 is necessary for Th2-mediated airway inflammation and increased ICOS costimulation with B7RP-1-Fc cannot overcome the defects in CD28−/− mice.
Figure 2. Responses of CD28−/− and ICOS−/− mice to ICOS or CD28 co-stimulation in S. mansoni model of Th2-mediated airway inflammation.
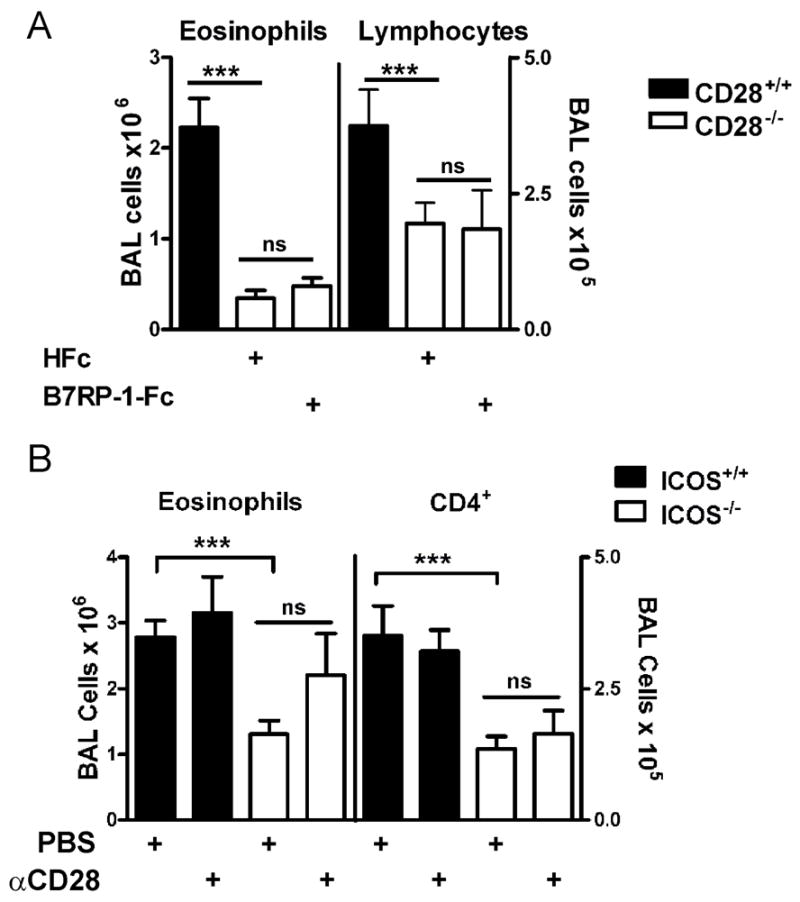
Wild-type, CD28−/− or ICOS−/− mice were sensitized and challenged with S. mansoni. A, BAL cell counts from CD28+/+ mice (black bars) or CD28−/− mice (open bars) treated every other day i.p. with either control 25μg HuFc or 50μg B7RP-1-Fc. Data are from two combined experiments that are representative of four independent experiments. B, BAL cell counts from ICOS+/+ (black bars) or ICOS−/− mice (open bars) injected i.p. on day 0 with either 100μg anti-CD28 or PBS. The data were combined from three independent experiments.
Figure 3. Augmenting ICOS costimulation does not affect airway inflammation in the absence of CD28.
Representative H&E staining from lungs of mice sensitized and challenged with S. mansoni. A, B, Wild-type (WT) mice treated with control HIg (A) or anti-CD28 (B). C, D, ICOS−/− mice treated with HIg (C) or anti-CD28 (D). E, F, CD28−/− mice treated with HuFc (E) or B7RP-1-Fc (F).
Figure 4. After sensitization and challenge, anti-CD28 significantly increased serum IgE and antigen-specific IgG1 in ICOS−/− mice.
Serum IgE or SEA specific IgG1 was measured on day 11 after sensitization and challenge with S. mansoni. A, Serum IgE from CD28+/+ mice (n=12) or CD28−/− mice injected i.p. every other day with HuFc (n=15) or B7RP-1-Fc (n=13). B, C, Serum IgE (B) and SEA specific IgG1 (C) from ICOS+/+ (n=3 each condition) or ICOS−/− mice (n=9 each condition) treated i.p. with 100μg anti-CD28 or PBS once at the time of sensitization.
CD28 cannot augment Th2 airway eosinophilia in the absence of ICOS
We have previously shown that ICOS blockade decreases airway inflammation in our model [10]. We found that ICOS−/− mice have significantly less airway eosinophilia and CD4+ T cells in the BAL after sensitization and challenge (Fig. 2B), confirming that ICOS affects the severity of the response in our model. We hypothesized that augmenting CD28 costimulation may provide an additive signal for T cell activation that would overcome the need for ICOS. We augmented CD28 costimulation by injecting one dose i.p. of anti-CD28 (PV-1) at the time of sensitization in ICOS−/− and ICOS+/+ mice compared to controls treated with PBS [29]. Anti-CD28 treatment of ICOS−/− mice did not significantly increase eosinophils or CD4+ T cells in the BAL compared to control ICOS−/− mice (Fig. 2B). This was not due to the inability of ICOS−/− T cells to express CD28 (Suppl. Fig. S2). In addition, no differences were detected in peribronchial and perivascular inflammation with anti-CD28 treated ICOS−/− mice compared to control ICOS−/− mice (Fig. 3C, D). Our data suggest that for Th2-mediated airway inflammation ICOS deficiency cannot be overcome by augmenting CD28 costimulation.
CD28 cannot fully compensate for ICOS for germinal center formation or Ig production
Since ICOS has been found to play an essential role in humoral immunity, we also investigated if increased CD28 costimulation would compensate for ICOS for Th2-mediated isotype switching and germinal center formation. After sensitization and challenge with S. mansoni, anti-CD28 significantly increased total serum IgE and antigen-specific IgG1 in ICOS−/− mice (Fig. 4B, C). However the levels of serum IgE in ICOS−/− mice were still significantly decreased compared to ICOS+/+ mice. To further investigate the ability of anti-CD28 to overcome the defects in the ICOS−/− mice the immunoglobulin response after sensitization with S. mansoni only (without challenge) was tested. We found that anti-CD28 significantly augmented total IgE and SEA-specific IgG1in ICOS−/− mice compared to isotype control antibody or PBS treated ICOS−/− mice (Suppl. Fig. S3). To more fully examine antigen-specific responses, we sensitized mice with ovalbumin in alum (Fig. 5). Anti-CD28 significantly augmented OVA-specific IgG1, IgG2a, IgG2b and IgE (Fig. 5). The OVA-specific IgG2a and IgG2b responses were relatively small consistent with a Th2 type response. However, we found that the increased OVA-specific IgE and IgG1 levels in ICOS−/− mice treated with anti-CD28 were significantly less than wild-type mice given anti-CD28 (Fig. 5). These differences could not be overcome by increasing the dose of anti-CD28 (Suppl. Fig. S3).
Figure 5. CD28 cannot fully recover primary antigen-specific IgE and IgG1 responses in ICOS−/− mice.
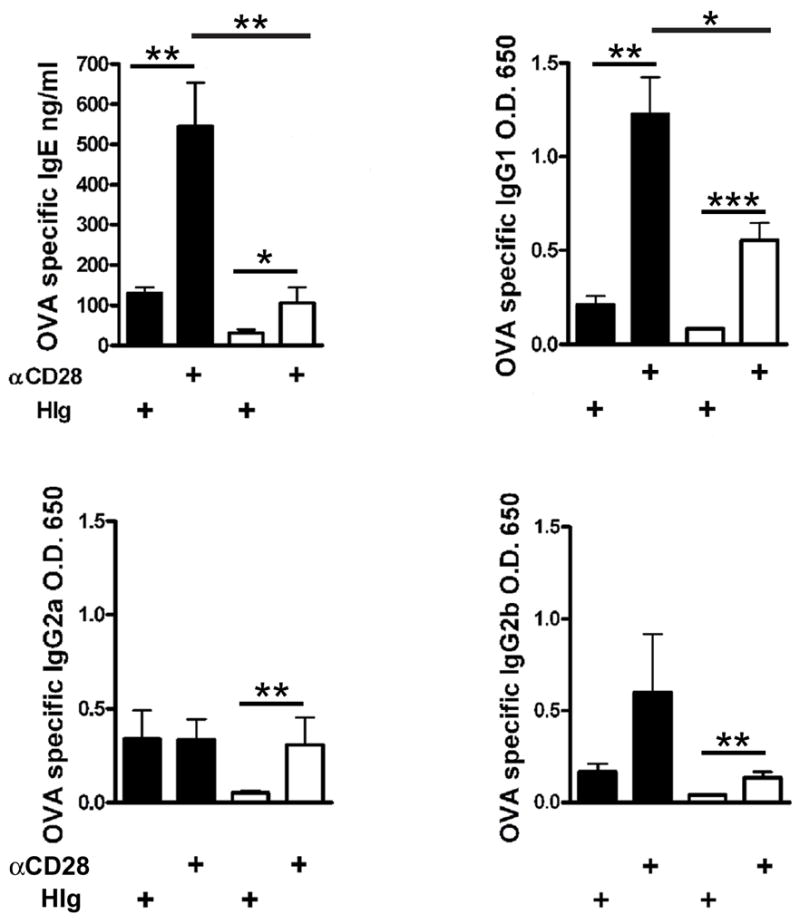
ICOS+/+ (black bars) or ICOS−/− (white bars) mice were sensitized i.p. with ovalbumin in alum and treated i.p. on day 0 with either anti-CD28 or HIg. OVA specific IgE, IgG1, IgG2a and IgG2b were measured on day 14 after sensitization with ova in alum. IgG1 and IgG2b levels were measured at 1:1000 dilution and IgG2a at 1:20.
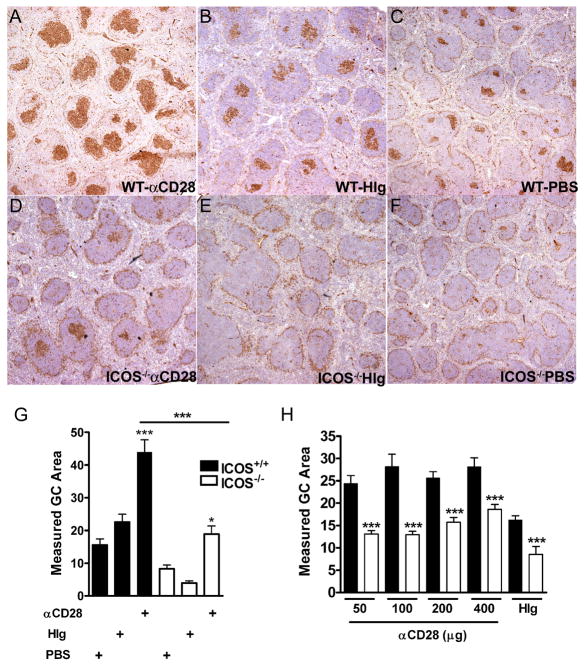
To determine if augmenting either ICOS or CD28 costimulation in the absence of the other was affecting germinal center formation, spleens were cryosectioned and stained for germinal center B cells using peanut agglutinin (PNA). As previously found, CD28−/− mice had almost no discernible germinal centers by PNA staining and treatment with B7RP-1-Fc had no detectable effect (data not shown) [14]. While ICOS−/− control mice had few germinal centers (Fig. 6), anti-CD28 did induce an increase in the size of germinal centers in ICOS−/− mice (Fig. 6, C, D, and quantified in G). As with serum IgE, the level of response with anti-CD28 in ICOS−/− mice was not as large as in ICOS+/+ mice (Fig. 6A, D, G). Increasing the dose of anti-CD28 also did not abrogate the differences in germinal center size found between ICOS+/+ or ICOS−/− mice (Fig. 6H).
Figure 6. Augmenting CD28 costimulation cannot fully recover germinal center formation in the absence of ICOS.
A–F, Spleens from ICOS+/+ (WT) (A–C) or ICOS−/− mice (D–F) after sensitization with S. mansoni were stained with PNA for germinal center B cells. Mice received either anti-CD28 (A, D), HIg (B, E) or PBS (C, F). All images are at 4x magnification. G, Germinal center area was measured using Image J software on pictures of 4x magnification (3–4 mice per group were analyzed). Data are representative of two independent experiments. H, Germinal center area was measured after treatment with 50, 100, 200, 400 μg of anti-CD28 or 400 μg of HIg.
CD28 cannot compensate for ICOS in the development of TFH
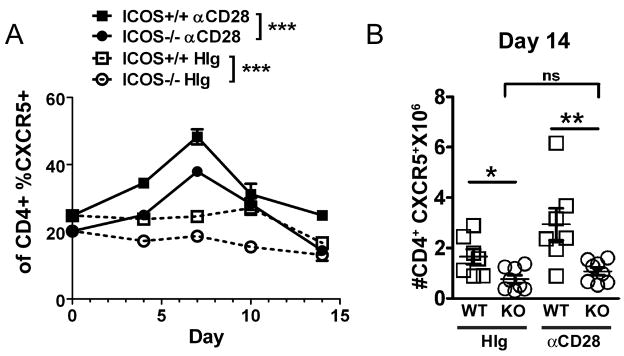
One possible mechanism by which T cell costimulation may regulate antibody responses is through differentiation of CD4+CXCR5+ follicular B helper T cells (TFH). ICOS has previously been associated with the generation and function of these specialized T cells [19–21, 30]. Since ICOS−/− mice have been found to be deficient in the number of TFH in vivo, we investigated whether CD28 costimulation could augment the number of TFH in the absence of ICOS [21, 30]. Mice were sensitized i.p. with S. mansoni and treated one time i.p. with either anti-CD28 or control hamster Ig. Anti-CD28 treatment significantly increased the percentage of CD4+ T cells expressing CXCR5 in the spleens of both ICOS−/− and ICOS+/+ mice peaking at day 7 (Fig. 7A and Suppl. Fig. S4). However, at day 14 post-sensitization the number of CD4+CXCR5+ T cells in ICOS−/− mice treated with anti-CD28 was significantly less than anti-CD28 treated ICOS+/+ mice and was not significantly different than control ICOS−/− mice (Fig. 7B). Consistent with previous work ICOS−/− control treated mice had significantly less CD4+CXCR5+ T cells than control treated ICOS+/+ mice [31]. Anti-CD28 also significantly increased the numbers of CD4+ T cells in ICOS+/+ mice compared to ICOS−/− mice but had no effect on CD19+ B cells (Suppl. Fig. S4 and data not shown). A significant increase in the percentage of CD4+ T cells that had up-regulated CD44 was also found in both ICOS−/− and ICOS+/+ mice (Suppl. Fig. S4). Thus, although we found a transient increase in the percentage of CD4+CXCR5+ T cells in ICOS−/− mice after anti-CD28 treatment, the response was not sustained and by day 14 there is no significant difference between anti-CD28 and control treated ICOS−/− mice. Our findings demonstrate CD28 cannot compensate for ICOS in the development of TFH cells.
Figure 7. Anti-CD28 cannot sustain an increase in CD4+CXCR5+ T cells (TFH) in the absence of ICOS.
After sensitization with S. mansoni, ICOS+/+ and ICOS−/− mice injected i.p. on day 0 with anti-CD28 or HIg were sacrificed on the days indicated. A, Percentage of spleen CD4+CXCR5+ T cells in αCD28 or HIg treated ICOS+/+ mice or ICOS−/− mice. Significance determined by two way ANOVA as indicated in figure. B, Number of CD4+CXCR5+ T cells found in the spleen on Day 14.
DISCUSSION
In evaluation of the ability of CD28 and ICOS costimulation to compensate for each other in Th2-mediated immunity, we have found that CD28 is the dominant Th2 costimulatory molecule. Augmenting ICOS costimulation cannot overcome the requirement for CD28 to initiate a Th2 immune response. In contrast, CD28 can partially compensate for lack of ICOS in vivo. While, increased CD28 costimulation did not affect airway eosinophilia in ICOS−/− mice, anti-CD28 significantly increased antigen-specific IgG1 and IgE and germinal center formation in the absence of ICOS. However, the effects of anti-CD28 in the absence of ICOS were not as large as in wild-type mice. Further, anti-CD28 treatment failed to sustain an increase in TFH. Our findings demonstrate that augmenting CD28 costimulation can only partially compensate for ICOS for Th2 mediated immunity and suggest that CD28 and ICOS have complementary non-overlapping roles.
Our prior data with ICOS blockade suggested augmenting ICOS costimulation with B7RP-1-Fc would increase Th2-mediated airway inflammation, but we did not find a significant increase in BAL eosinophilia with B7RP-1-Fc treatment or changes in lung histology in wild-type mice [10]. We did find a small but consistent increase in BAL lymphocytes predominantly CD4+ T cells, as well as augmented Th2 cytokine production upon restimulation of lung and draining lymph node cells after B7RP-1-Fc treatment. Further, the most significant effect was on serum IgE. These data suggest that ICOS costimulation expands Th2 development. Our data are consistent with previous studies showing a role for ICOS in expanding Th2 responses in vivo [32, 33]. Although we found an effect of B7RP-1-Fc on Th2 development in wild-type mice, B7RP-1-Fc did not affect airway inflammation in the absence of CD28. The lack of response was not due to an absence of ICOS expression in CD28−/− mice. It is perhaps not totally surprising that ICOS cannot compensate for loss of CD28, as CD28 is known to be important for T cell derived IL-2 and IL-4, as well as for survival of T cells [34, 35].
While the development of Th2-mediated airway inflammation was dependent on CD28, we also found that ICOS−/− mice had a defect in Th2-mediated airway inflammation with a significant reduction in BAL eosinophils and T cells. Previous reports in the literature on allergic airway inflammation in ICOS−/− and B7RP-1−/− mice have been conflicting [11, 36, 37]. The differences may be due to the different models used and the variation in backcrossing the mice. In our study, we have used mice backcrossed more than nine generations that are compared to littermate controls. In addition, our model induces a very robust response in the BAL and lung compared to other models using ovalbumin and it is possible that our system is more sensitive to detect differences between ICOS−/− mice and controls. Nevertheless the differences we found in the ICOS−/− mice were only in the BAL and did not translate into significant differences in histology suggesting ICOS modifies the response but is not necessary for a Th2-mediated inflammatory response in the lung.
The most striking defects in Th2-mediated immunity in ICOS−/− mice are in humoral immunity. Interestingly, we found that increased CD28 costimulation in vivo did increase immunoglobulin responses and germinal center formation in the absence of ICOS. The effect of anti-CD28 on ICOS−/− mice was not as significant as in wild-type mice suggesting CD28 costimulation did not fully compensate for ICOS costimulation. While anti-CD28 initially increased the frequency of CD4+CXCR5+ T cells in ICOS−/− mice there was also an effect on T cell activation signified by increased CD44 expression. CXCR5 is a marker for TFH that can be upregulated on all T cells after activation [38]. By day 14 the effect of anti-CD28 on CD4+CXCR5+ T cells in ICOS−/− mice was abrogated. Our findings suggest that ICOS plays a distinct role from CD28 in modulating the expansion of B cell responses which may be due to its effect on the persistence of CD4+CXCR5+ TFH cells. It is interesting that CD28 cannot fully compensate for ICOS in vivo since ICOS does not bind any unique signaling molecules from CD28. ICOS like CD28 is known to upregulate phosphoinositide-3 kinase (PI-3K) upon T cell activation but unlike CD28, PI-3K is the only known binding partner for ICOS [9, 39]. However, ICOS has been shown to upregulate PI-3K to a greater extent than CD28 [40]. Thus ICOS costimulation may provide a qualitatively different signal through PI-3K than CD28 or perhaps another binding partner has yet to be identified.
Our study demonstrates that the requirement for ICOS can at least in part be overcome by augmenting CD28 costimulation and suggest that some of the differences in the literature regarding ICOS may be due to the strength of the aggregate costimulation signal induced in vivo. These findings may have implications for immunotherapy for humans. Individuals who are deficient for ICOS suffer from humoral immunodeficiency [16, 18]. Our work suggests anti-CD28 may benefit these individuals, as well as be useful for boosting responses to vaccines. However, the recent trial of a humanized superagonist anti-CD28 antibody with the deleterious consequence of inducing the systemic inflammatory response syndrome and multi-system organ failure make the current formulation of anti-CD28 for humans unacceptable [41]. Further, the fact that anti-CD28 cannot sustain the TFH response supports the essential role for ICOS in humoral immunity. Future work to determine the downstream signals of ICOS costimulation in T cells may provide novel targets for therapies for autoimmune diseases and allergies.
Supplementary Material
Acknowledgments
We thank K. Blaine, D. Decker, and Dr. P. Mody, for helpful discussions and assistance during these studies and Dr. Paul Bryce for the gift of supernatant from the hybridoma TOε. This work was supported by NIH grants R01 AI50180, P01 AI56352, K08 AI 059105, the American Lung Association of Metropolitan Chicago and Louis Block Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen- specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 2.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 3.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4(+) T cells. J Immunol. 2000;165:5035–40. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 4.Shilling RA, Pinto JM, Decker DC, Schneider DH, Bandukwala HS, Schneider JR, Camoretti-Mercado B, Ober C, Sperling AI. Cutting edge: polymorphisms in the ICOS promoter region are associated with allergic sensitization and th2 cytokine production. J Immunol. 2005;175:2061–5. doi: 10.4049/jimmunol.175.4.2061. [DOI] [PubMed] [Google Scholar]
- 5.Krinzman SJ, De Sanctis GT, Cernadas M, Mark D, Wang Y, Listman J, Kobzik L, Donovan C, Nassr K, Katona I, Christiani DC, Perkins DL, Finn PW. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. Journal of Clinical Investigation. 1996;98:2693–2699. doi: 10.1172/JCI119093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keane-Myers A, Gause WC, Linsley PS, Chen SJ, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158:2042–9. [PubMed] [Google Scholar]
- 7.Padrid PA, Mathur M, Li X, Herrmann K, Qin Y, Cattamanchi A, Weinstock J, Elliott D, Sperling AI, Bluestone JA. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol. 1998;18:453–462. doi: 10.1165/ajrcmb.18.4.3055. [DOI] [PubMed] [Google Scholar]
- 8.Mathur M, Herrmann K, Qin Y, Gulmen F, Li X, Krimins R, Weinstock J, Elliott D, Bluestone JA, Padrid P. CD28 interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 1999;21:498–509. doi: 10.1165/ajrcmb.21.4.3714. [DOI] [PubMed] [Google Scholar]
- 9.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell- dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 10.Tesciuba AG, Subudhi S, Rother RP, Faas SJ, Frantz AM, Elliot D, Weinstock J, Matis LA, Bluestone JA, Sperling AI. Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J Immunol. 2001;167:1996–2003. doi: 10.4049/jimmunol.167.4.1996. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Juedes Amy E, Temann UllaAngela, Shresta Sujan, Allison James P, Ruddle Nancy H, Flavell Richard A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 12.McAdam AJ, Greenwald Rebecca J, Levin Michele A, Chernova Tatyana, Malenkovich Nelly, Ling Vincent, Freeman Gordon J, Sharpe Arlene H. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 13.Tafuri A, Shahinian Arda, Bladt Friedhelm, Yoshinaga SteveK, Jordana Manel, Wakeham Andrew, Boucher Louis-Martin, Bouchard Denis, Chan Vera SF, Duncan Gordon, Odermatt Bernhard, Ho Alexandra, Itie Annick, Horan Tom, Whoriskey John S, Pawson Tony, Penninger Josef M, Ohashi Pamela S, Mak Tak W. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–81. [PubMed] [Google Scholar]
- 15.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–11. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, Kroczek RA, Peter HH. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–8. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 17.Salzer U, Maul-Pavicic A, Cunningham-Rundles C, Urschel S, Belohradsky BH, Litzman J, Holm A, Franco JL, Plebani A, Hammarstrom L, Skrabl A, Schwinger W, Grimbacher B. ICOS deficiency in patients with common variable immunodeficiency. Clin Immunol. 2004;113:234–40. doi: 10.1016/j.clim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, van Dongen JJ, Orlowska-Volk M, Knoth R, Durandy A, Draeger R, Schlesier M, Peter HH, Grimbacher B. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107:3045–52. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- 19.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The Role of ICOS in the CXCR5+ Follicular B Helper T Cell Maintenance In Vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 22.Suh WK, Tafuri A, Berg-Brown NN, Shahinian A, Plyte S, Duncan GS, Okada H, Wakeham A, Odermatt B, Ohashi PS, Mak TW. The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J Immunol. 2004;172:5917–23. doi: 10.4049/jimmunol.172.10.5917. [DOI] [PubMed] [Google Scholar]
- 23.Vidric M, Suh WK, Dianzani U, Mak TW, Watts TH. Cooperation between 4–1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol. 2005;175:7288–96. doi: 10.4049/jimmunol.175.11.7288. [DOI] [PubMed] [Google Scholar]
- 24.Elliott D. Methods used to study immunoregulation of schistosome egg granulomas. Immunol Methods. 1996;9:255–257. doi: 10.1006/meth.1996.0032. [DOI] [PubMed] [Google Scholar]
- 25.Abe R, Vandenberghe P, Craighead N, Smoot DS, Lee KP, June CH. Distinct signal transduction in mouse CD4+ and CD8+ splenic T cells after CD28 receptor ligation. J Immunol. 1995;154:985–997. [PubMed] [Google Scholar]
- 26.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. J Allergy Clin Immunol. 2003;112:149–58. doi: 10.1067/mai.2003.1616. [DOI] [PubMed] [Google Scholar]
- 27.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–32. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Stolina M, Bready JV, Yin S, Horan T, Yoshinaga SK, Senaldi G. Stimulatory effects of b7-related protein-1 on cellular and humoral immune responses in mice. J Immunol. 2001;166:5578–84. doi: 10.4049/jimmunol.166.9.5578. [DOI] [PubMed] [Google Scholar]
- 29.Mandelbrot DA, Oosterwegel MA, Shimizu K, Yamada A, Freeman GJ, Mitchell RN, Sayegh MH, Sharpe AH. B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4. J Clin Invest. 2001;107:881–7. doi: 10.1172/JCI11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 31.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, Peter HH, Warnatz K. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177:4927–32. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 32.Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170:2310–5. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- 33.Wilson EH, Zaph C, Mohrs M, Welcher A, Siu J, Artis D, Hunter CA. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. J Immunol. 2006;177:2365–72. doi: 10.4049/jimmunol.177.4.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 35.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 36.Gajewska BU, Tafuri A, Swirski FK, Walker T, Johnson JR, Shea T, Shahinian A, Goncharova S, Mak TW, Stampfli MR, Jordana M. B7RP-1 Is Not Required for the Generation of Th2 Responses in a Model of Allergic Airway Inflammation but Is Essential for the Induction of Inhalation Tolerance. J Immunol. 2005;174:3000–5. doi: 10.4049/jimmunol.174.5.3000. [DOI] [PubMed] [Google Scholar]
- 37.Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, Odermatt B, Ho A, Bouchard D, Whorisky JS, Jordana M, Ohashi PS, Pawson T, Bladt F, Tafuri A. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4:765–72. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 38.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 39.Zang X, Loke P, Kim J, Wojnoonski K, Kusdra L, Allison JP. A genetic library screen for signaling proteins that interact with phosphorylated T cell costimulatory receptors. Genomics. 2006;88:841–5. doi: 10.1016/j.ygeno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–74. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 41.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.