Abstract
Objective
To determine the factors associated with increased levels of fatigue over the course of the disease in systemic lupus erythematosus (SLE) patients from LUMINA (Lupus in Minorities: Nature versus Nurture), a longitudinal multiethnic cohort.
Methods
Patients with SLE (American College of Rheumatology revised and updated criteria), age ≥16 years, disease duration ≤ 5 years at entry into the cohort (T0), of Hispanic (Texan or Puerto Rican), African America or Caucasian ethnicity, were studied. The association between socioeconomic-demographic, health behaviors, behavioral and psychological, functional and clinical characteristics and fatigue was examined using generalized estimating equations to account for the longitudinal nature of the data.
Results
Five-hundred and fifteen patients (~91% female) contributed 2,609 visits to these analyses; there were: 93 (18.1%) Texan Hispanics, 101 (19.6%) Puerto Rican Hispanics, 169 (32.8%) African Americans, and 152 (29.5%) Caucasians; the patients mean (SD) age and follow up time were 37.2 (12.0) and 4.7 (3.2) years, respectively. Variables associated with increased levels of fatigue in the multivariable analyses were Caucasian ethnicity, the presence of constitutional symptoms(fever, weight loss), higher levels of pain, of abnormal illness-related behaviors and of helplessness (p’s between 0.0018 and <0.0001).
Conclusions
The presence of pain, abnormal illness-related behaviors, helplessness and constitutional manifestations were associated with increased levels of fatigue; however, lupus specific measures, such as disease activity and damage were not. Interventions aimed at decreasing fatigue need to take into account these findings.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease which is quite heterogeneous in its presentation, course and outcome. Fatigue is a very common complaint among SLE patients and it is characterized by tiredness that can, if extreme, prevent patients from accomplishing their daily activities (1–12). It should be noted, however, that fatigue is not a well-understood symptom as it is oftentimes reported in the absence of observable disease activity (9;13). Furthermore, fatigue is not a manifestation that can be documented by physical examination or measured with a laboratory test; rather, it is assessed using self-reported instruments (14–17). The American College of Rheumatology (ACR) Ad Hoc Committee on response criteria for fatigue has recommended the use of the Fatigue Severity Scale (FSS), an instrument developed and validated by Krupp et al. for the purpose of assessing this construct in patients with SLE and multiple sclerosis (14); the Committee has also recommended the inclusion of possible confounding factors when fatigue is ascertained (18).
We have previously examined the factors associated with fatigue in SLE patients from LUMINA (LUpus in MInorities: NAture vs Nurture), a multiethnic US cohort using a cross-sectional approach (8). We found fatigue to be a pervasive symptom among our SLE patients; factors associated with fatigue included older age, high levels of self-reported pain, lack of health insurance, abnormal illness-related behaviors, a greater degree of helplessness and increased levels of disease activity. With more than double the number of patients in the cohort and several years more of follow up of its members, we have now examined the factors associated with fatigue over the duration of the disease. We hypothesized that both disease and non-disease features may explain the presence of fatigue among patients with SLE.
Patients and Methods
Studied patients were from the LUMINA cohort which is a joint effort between the University of Alabama at Birmingham (UAB), the University of Texas Health Science Center at Houston (UTH), and the University of Puerto Rico Medical Sciences Campus (UPR). The cohort (n=635 patients) is comprised of SLE patients [as defined by the American Rheumatism Association/ACR revised and updated classification criteria (19;20), with ≤ 5 years of disease duration] from four ethnic groups: Texan Hispanics, Puerto Rican Hispanics, African Americans, and Caucasians; particulars of this cohort have been previously reported (21–23). An intake, enrollment or baseline visit (T0), semi-annual visits for the first year, and annual visits are conducted. All available medical records from the time of diagnosis (TD) when ACR criteria were met until T0 were used to gather additional patients’ clinical information. LUMINA patients’ visits include self-report questionnaires, a medical interview, a physical examination and laboratory tests. Only patients in whom three study visits (including T0) were available were included in these analyses.
Variables
As previously reported (21–23), the LUMINA database is comprised of variables from the following domains: socioeconomic-demographic, health behaviors, behavioral and psychological, functional, clinical and immunologic. Variables are obtained during each study visit; only those variables included in this study will be described.
Fatigue
The short version of the FSS was used in this study; this version includes nine items (Appendix 1). Each of these nine-items is scored from 1 (indicating no fatigue) to 7 (indicating extreme fatigue); a FSS score of ≥3 indicates the presence of fatigue according to Krupp (14); however, for the purpose of these analyses, the FSS score was examined as a continuous variable.
Socioeconomic-demographic features included are: age, gender, ethnicity, education, poverty (according to the US Federal government regulations and adjusted for the number of members in the household) (24), marital status, occupation and health insurance.
Health behavior features included are: smoking, exercise, use of alcoholic beverages, and the intake of recreational drugs. Exercise was defined as regular participation in physical activities including aerobic exercise (e.g., walking, jogging, swimming, cycling) and /or muscle strength training (e.g., weights, tubing, dynamic bands).
Behavioral and psychological features included are: depression, social support, abnormal illness-related behaviors and learned helplessness. The use of anti-depressants was considered a proxy for the presence of depression. Social support was measured using the 40-item Interpersonal Support Evaluation List (ISEL) instrument which determines the patients’ perception of available social support (25). The ISEL comprises four subscales: appraisal, tangible support, self-esteem support and belonging support; a score ranging from 0 to 10 is computed for each subscale and the mean total score was used. Higher ISEL scores indicate higher perceived social support. Learned helplessness was measured with a validated 15-item instrument, the Arthritis Helplessness Index (AHI). This self-reported instrument examines the patients’ perception that their symptoms cannot be modified or managed; higher scores indicate higher degrees of helplessness (26;27). Abnormal illness-related behaviors were evaluated using a validated 62-item instrument, the Illness Behavior Questionnaire (IBQ), which measures patients’ attitudes, responses and ideas related to illness; the IBQ has seven scales, two factors and a total score (range 0–35); for the purpose of these analyses, we have used the total score. The higher the score the less adequate the individual is facing illness. thus higher levels of abnormal illness behaviors (28).
Functional features
Self-reported physical and mental functioning were evaluated using the SF-36 instrument which comprises 8 scales (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) and two component summary measurements, physical and mental (PCS and MCS, respectively) (15;29). This validated 36-item instrument measures patients’ perceptions about their ability to function both physically and mentally.
Clinical features included are: disease duration, disease activity, disease damage, disease manifestations, weight, fibromyalgia, pain, and medication usage. Disease duration was recorded from T0 until TL. Disease activity was ascertained using the Systemic Lupus Activity Measure-Revised (SLAM-R) (30;31) which includes features of twenty-five clinical manifestations and seven laboratory tests; for the purpose of this study fatigue was excluded from the SLAM-R. The SLAM-R also includes a 10 cm VAS that measures disease activity from both the patient’s (a subjective estimate) and physician’s perspective. Disease damage was evaluated using the Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (SDI) (32); the SDI examines irreversible damage in 12 organ systems present at for least 6 months. Disease manifestations were examined according with the ACR criteria and as previously described (21) and are presented by organ system; of note, fatigue was not included among the constitutional group of manifestations. The presence of fibromyalgia was determined by ≥11/18 pre-specified ACR tender points (33). Since this data element was not obtained when the cohort was first started in 1994, the first available measurement was included in these analyses. Weight was obtained at every study visit. Pain (attributed to lupus) was estimated on a 10 cm visual analog scale (VAS) where 0 indicates no pain and 10 indicates the most pain possible. Autoantibodies obtained at T0 included antinuclear antibodies (ANA by immunofluorescence using HEp-2 cell line), anti-double-stranded DNA (anti-dsDNA, by immunofluorescence against Crithidia luciliae), anti Smith and RNP (by immunodiffusion) and antiphospholipid antibodies (APL by enzyme-liked immunoabsorbent assay and /or lupus anticoagulant by the Staclot assay). Past and present medication usage was also recorded.
Statistical analyses
The association between variables from the aforementioned domains and fatigue scores was examined using generalized estimating equations (GEE) to account for the correlated nature of the data. For the purpose of these analyses all continuous independent variables were dichotomized into above and below the median. Variables from the preceding study visit or the last observation carried forward (if a variable was not available in the preceding visit) were used in these analyses. This provides the opportunity to temporally separate the dependent and independent variables thus allowing us to identify characteristics that are prospectively associated with fatigue. Variables with a p value ≤0.10 in the univariable analyses and/or considered to be clinically relevant were entered into the multivariable models; for highly correlated variables the most significant variable was included and the other omitted (e.g. pain and fibromyalgia; neurological manifestations and headache). Medications were not included in the final multivariable models as they were felt not to be truly explanatory of the outcome. Each variable was systematically entered (or omitted) from the model contingent upon it being associated with the end point at p≤0.10 until a final parsimonious model was obtained. Given that some of the scales from which the SF-36’s summary measurements (PCS and MCS) are derived include highly overlapping constructs with the end point being examined (vitality, for example) and that some are also highly correlated with other independent variables included in our analyses (bodily pain, for example), the PCS and MCS of the SF-36 were omitted from the models.
Results
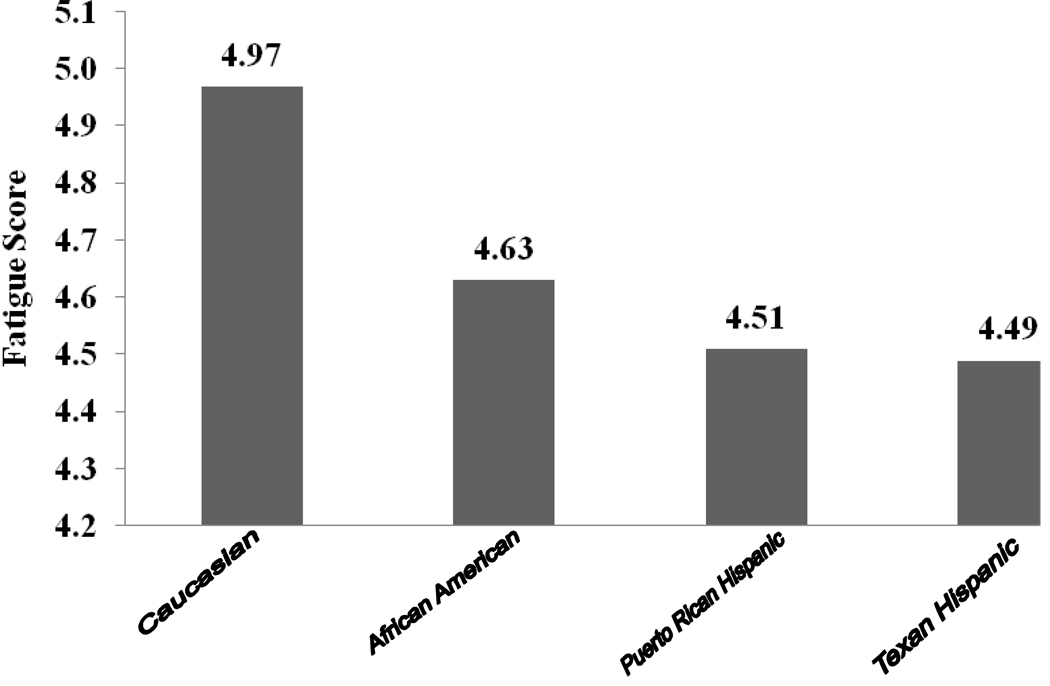
A total of 515 patients provided 2,609 study visits and were included in these analyses. This subset of the LUMINA cohort included 93 (18.1%) Texan Hispanics, 101 (19.6%) Puerto Rican Hispanics, 169 (32.8%) African Americans, and 152 (29.5%) Caucasians. The majority of the patients (90.5%) were women and their mean (Standard deviation, SD) age was 37.2 (12.6) years whereas their mean (SD) disease duration was 4.7 (3.2) years. Fatigue was reported in 91.7% of the patients ranging from as high of 94.7% in the Caucasians to a low of 89.1% in the Puerto Rican Hispanics. The mean (SD) FSS score was 4.7 (1.1) whereas the median score was 4.5. These scores were higher in the Caucasians than in patients from the other ethnic groups (p=0.0022). The mean FSS scores as a function of ethnic group are depicted in Figure 1. Fibromyalgia at some point over the disease course occurred in about 14% of the patients whereas antidepressant use occurred in nearly 40% of them; however, both fibromyalgia and antidepressant use were more frequent among the Caucasians than among patients from the other ethnic groups (22.4% vs. 11.1% and 58.2 % vs. 32.6%, respectively, x2 = 9.5, p = 0.0021 and x2 = 26.4, p = <0.0001).
Figure 1.
Distribution of the mean Fatigue Severity Scale scores as a function of ethnic group.
Univariable analyses
Within the socioeconomic-demographic features, age, gender, education, occupation, poverty, drinking or using recreational drugs and health insurance were not found to be significantly associated with fatigue. On the other hand, ethnicity (Caucasian), smoking and marital status (being married) were associated with higher levels of fatigue whereas exercise was associated with lower levels of fatigue. These data are depicted in Table 1.
Table 1.
Socioeconomic-demographic Variables Associated with Persistent Fatigue in LUMINA Patients by Univariable Analyses
| Variable* | Estimate | Z value | p value |
|---|---|---|---|
| Age* | 0.0073 | 0.72 | 0.4721 |
| Gender | −0.1570 | −0.77 | 0.4400 |
| Ethnicity | |||
| Texan Hispanic | −0.0027 | −0.02 | 0.9880 |
| Puerto Rican Hispanic | Reference Group | ||
| African American | 0.1443 | 0.91 | 0.3618 |
| Caucasian | 0.4560 | 2.94 | 0.0033 |
| Marital status (married) | 0.2329 | 2.16 | 0.0309 |
| Education* | −0.1246 | −0.10 | 0.2976 |
| Occupation (employed) | −0.1587 | −1.40 | 0.1617 |
| Poverty (below)† | 0.0022 | 0.02 | 0.9942 |
| Having health insurance | 0.0736 | 0.58 | 0.5617 |
| Drinking | −0.0430 | −0.24 | 0.8117 |
| Smoking | 0.3519 | 3.00 | 0.0027 |
| Recreational drug use | 0.7143 | 1.43 | 0.1521 |
| Exercising | −0.2710 | −2.46 | 0.0140 |
These variables have been dichotomized using their median values as the reference point (age=37 years; education=12 years).
As per the US Federal government guidelines adjusted for the number of persons in the household.
The association of self-reported quality of life (MCS and PCS) along with the behavioral and psychological variables and levels of fatigue is depicted in Table 2. Lower PCS and MCS scores as well as higher levels of abnormal illness-related behaviors and helplessness were associated with higher levels of fatigue whereas higher levels of social support were associated with lower levels of fatigue.
Table 2.
Behavioral, Psychological and Functional Variables Associated with Persistent Fatigue in LUMINA Patients by Univariable Analyses
| Variable* | Estimate | 95% CI | Z value | p value |
|---|---|---|---|---|
| Helplessness | 0.7477 | 0.61 – 0.99 | 9.93 | <0.0001 |
| Abnormal illness–related behaviors† | 0.8147 | 0.65 – 0.98 | 9.75 | <0.0001 |
| Social Support | −0.1447 | −0.58 – −0.25 | −4.97 | <0.0001 |
| SF-36‡ | ||||
| PCS‡ | −0.9139 | −1.06 – −0.77 | −12.23 | <0.0001 |
| MCS‡ | −0.7515 | −0.90 – −0.60 | −9.73 | <0.0001 |
All variables have been dichotomized for the purpose of these analyses using their median values as the reference point (helplessness=39; IBQ score=18; social support=8.5; PCS=34.6, MCS=41.1).
As per the illness Behavior Questionnaire (IBQ) and the Interpersonal Support Evaluation List (ISEL), respectively
Physical Component Summary and Mental Component Summary measurements of the Short Form-36.
The relationship between clinical manifestations and levels of fatigue is depicted in Table 3. Constitutional, integument, cardiorespiratory, headache and neurological manifestations were associated with higher levels of fatigue. The same was the case for higher levels of pain, disease activity and the presence of fibromyalgia. On the other hand, premature gonadal failure was associated with lower levels of fatigue. Peripheral vascular disease, increased weight, and disease damage were associated with higher levels of fatigue albeit not significantly. Digestive, renal, hematological and ocular manifestations as well as hypothyroidism, anemia, leukopenia/lymphopenia, thrombocytopenia, increased erythrocyte sedimentation rate and increased creatinine were not associated with either higher or lower levels of fatigue. The same was the case for all immunological features. In terms of medications, the use of anti-depressants was associated with higher levels of fatigue while non steroidal anti-inflammatory drugs (NSAIDs) did not quite reach statistical significance. Other medications were not associated with either higher or lower levels of fatigue. These data are also depicted in Table 3.
Table 3.
Clinical Variables Associated with Persistent Fatigue in LUMINA Patients by Univariable Analyses
| Variable* | Estimate | Z value | p value |
|---|---|---|---|
| Organ system manifestations (present)† | |||
| Constitutional‡ | 0.7770 | 10.82 | <0.0001 |
| Integument | 0.4070 | 4.90 | <0.0001 |
| Cardiorespiratory | 0.0166 | 2.49 | 0.0127 |
| Digestive | 0.2371 | 1.51 | 0.1316 |
| Renal | 0.0097 | 0.11 | 0.9093 |
| Peripheral vascular | 0.1599 | 1.94 | 0.0523 |
| Hematological | 0.0014 | 0.02 | 0.9868 |
| Neurological | 0.4520 | 5.27 | <0.0001 |
| Ocular | 0.1704 | 1.81 | 0.0699 |
| Other clinical manifestations (present) | |||
| Headache | 0.5000 | 4.46 | <0.0001 |
| Hypothyroidism | −0.2170 | −1.36 | 0.1741 |
| Premature gonadal failure | −0.3379 | −2.58 | 0.0099 |
| Increased weight* | 0.2136 | 1.95 | 0.0512 |
| Fibromyalgia | 0.8332 | 8.10 | <0.0001 |
| Pain* | 0.6637 | 8.61 | <0.0001 |
| Laboratory features | |||
| Anemia | 0.0479 | 0.56 | 0.5742 |
| Leukopenia | −0.1001 | −0.78 | 0.4356 |
| Lymphopenia | −0.0566 | −0.71 | 0.4754 |
| Thrombocytopenia | 0.2257 | 1.76 | 0.0777 |
| Increased erythrocyte sedimentation rate | −0.0636 | −0.75 | 0.4560 |
| Increased creatinine | 0.0132 | 0.08 | 0.9346 |
| Disease activity (SLAM-R) *§ | 0.3574 | 4.96 | <0.0001 |
| Disease damage (SDI)*¶ | 0.1904 | 1.94 | 0.0518 |
| Autoantibodies (present)** | |||
| Anti-dsDNA | −0.1759 | −1.74 | 0.0817 |
| Anti-Sm | −0.1466 | −1.04 | 0.2965 |
| APL | 0.1099 | 0.81 | 0.4194 |
| Medications used | |||
| Hydroxychloroquine | 0.1179 | 1.20 | 0.2302 |
| Glucocorticoids | 0.2066 | 2.03 | 0.2242 |
| Cyclophosphamide | −0.1645 | −1.22 | 0.2242 |
| Azathioprine | 0.1513 | 1.32 | 0.1860 |
| Non steroidal anti-inflammatory drugs | 0.1512 | 1.93 | 0.0532 |
| ACE inhibitors†† | 0.0895 | 0.92 | 0.3551 |
| Anti-depressants | 0.7772 | 8.99 | <0.0001 |
| Statins | 0.0535 | 0.37 | 0.7126 |
Continuous variables have been dichotomized using their median values as the reference point (weight=71.7 kg, Pain=2.8, SLAM-R=6, SDI >0)
Attributable to lupus, as previously described (21)
Fever and/or weigh loss without fatigue
Systemic Lupus Activity Measure-Revised
Systemic Lupus International Collaborating Clinics Damage Index
Anti-double stranded DNA, anti-Smith and antiphospholipid antibodies, respectively
Angiotensin converting enzyme
Multivariable analyses
The parsimonious and full GEE regresion models are depicted in Table 4. Variables found to be associated with higher levels of fatigue in the final or parsimonious model were Caucasian ethnicity, the presence of constitutional symptoms, higher levels of pain, of abnormal illness-related behaviors and of helplessness.
Table 4.
Variables Independently Associated with Persistent Fatigue in LUMINA Patients
| Variable* | Full Model | Reduced Model | ||||
|---|---|---|---|---|---|---|
| Estimate | Z value | p value | Estimate | Z value | p value | |
| Age* | 0.0413 | 0.44 | 0.6624 | |||
| Gender (male) | −0.2121 | −1.47 | 0.1407 | |||
| Ethnicity | ||||||
| Texan Hispanic | −0.1570 | −1.01 | 0.3145 | −0.1806 | −1.25 | 0.2121 |
| Puerto Rican Hispanic | Reference group | |||||
| African American | 0.0611 | 0.40 | 0.6906 | 0.0167 | 0.12 | 0.9055 |
| Caucasian | 0.4780 | 3.40 | 0.0007 | 0.4146 | 3.11 | 0.0018 |
| Exercising | −0.1031 | −1.12 | 0.2621 | |||
| Smoking | 0.0182 | 0.17 | 0.8630 | |||
| Marital status (married) | −0.0039 | −0.04 | 0.9678 | |||
| Clinical Manifestations | ||||||
| Constitutional† | 0.5000 | 7.04 | <0.0001 | 0.4925 | 7.21 | <0.0001 |
| Integument | 0.0821 | 1.08 | 0.2821 | 0.1189 | 1.61 | 0.1070 |
| Cardiorespiratory | 0.0194 | 0.23 | 0.8209 | |||
| Peripheral vascular | −0.0405 | −0.58 | 0.5631 | |||
| Neurological | 0.1592 | 2.12 | 0.0337 | 0.1342 | 1.86 | 0.0625 |
| Ocular | −0.0511 | −0.67 | 0.4997 | |||
| Hypothyroidism | −0.0525 | −0.40 | 0.6896 | |||
| Increased weight* | 0.0867 | 0.96 | 0.3346 | |||
| Pain* | 0.1518 | 2.33 | 0.0197 | 0.2089 | 3.26 | 0.0011 |
| Disease activity (SLAM-R)*‡ | 0.0595 | 0.89 | 0.3757 | |||
| Disease damage (SDI)*§ | 0.1391 | 1.71 | 0.0865 | 0.1443 | 1.81 | 0.0698 |
| Thrombocytopenia | 0.0052 | 0.05 | 0.9628 | |||
| Anti-dsDNA antibodies¶ | −0.1788 | −2.07 | 0.0381 | −0.1394 | −1.70 | 0.0889 |
| Helplessness* | 0.3148 | 4.28 | <0.0001 | 0.3332 | 4.92 | <0.0001 |
| Abnormal illness–related behaviors* | 0.6583 | 8.09 | <0.0001 | 0.6607 | 8.42 | <0.0001 |
| Social Support* | 0.0486 | 0.66 | 0.5095 | |||
Continuous variables have been dichotomized using the median values as the reference point (age=37 years; weight=71.7 kg; pain=2.8; SLAM-R=6; SDI >0)
Attributable to lupus, as previously described (21)
Fever and/or weigh loss without fatigue; ‡ Systemic Lupus Activity Measure-Revised
Systemic Lupus International Collaborating Clinics Damage Index
Anti double stranded DNA, anti-Smith and antiphospholipid antibodies
Discussion
In addition to corroborate the fact that fatigue is commonly present in patients with SLE (1;6;8;10–12;34;35), we have now expanded our previous cross-sectional observations by examining the factors associated with the levels of fatigue occurring over the course of the disease utilizing our longitudinal database. In fact 90% of the patients experienced fatigue at the baseline visit, and in nearly three quarter of the patients visits the scores neither improve nor worsened; in addition, for proximate visits the scores were quite high correlations (r = 0.8–0.9); however, as the time passes there was a high degree of correlation of the fatigue scores for each individual patient; correlations weaken some (r = 0.5–0.7). These data, taken together inform us about the pervasiveness and persistence of this clinical symptom. Consistent with our previously published baseline cross-sectional analyses (8), we have now found the presence of pain, abnormal illness-related behaviors and helplessness to be associated with higher levels of fatigue over the course of disease in these patients with SLE. We have also found Caucasian ethnicity and constitutional manifestations to be associated with higher levels of fatigue. Although a number of other variables were significant or borderline significant in the univariable analyses (marital status, smoking, exercising, integument, cardiorespiratory, neurological manifestations, premature gonadal failure, disease activity and damage, the use of NSADs and anti-depressants), they were not retained in the multivariable models examined. Nevertheless, it is important to point out that some of the disease manifestations significantly associated with higher levels of fatigue in the univariable analyses may reflect a less severe disease. As expected, the SF-36 component summary measurements (PCS and MCS) were found to be highly associated with higher levels of fatigue. However, given that some of the scales from which the SF-36’s summary measurements (PCS and MCS), particularly vitality are derived, and that some of the scales are highly correlated with some of the independent variables examined (bodily pain) we chose not to include these variables in the multivariable models. Although depression and fibromyalgia are very likely to be associated with higher levels of fatigue, the ascertainment of depression in our LUMINA patients was not done using a defined instrument, rather the use of antidepressants was considered a proxy for its presence; however, we omitted this variable from the multivariable models because of a possible reduction in the sample size due to missing values; furthermore, medications in general are unlikely to be in the causal pathway of fatigue and all were omitted. In terms of fibromyalgia, we included the highly correlated construct of pain which was significantly associated with increased levels of fatigue. We chose this variable rather than the first to be able to include a larger number of data points in our analyses given that fibromyalgia per se was not ascertained during the first years of the LUMINA cohort. The omission of these two variables from the multivariable analyses, certainly constitutes limitation to our study.
Fatigue in SLE, as in other chronic diseases, is a very difficult clinical manifestation to assess, yet it has a tremendous impact on the lives of patients with lupus; in fact, in some patients it is the primary and the most disabling complaint (2;4;6). Physicians, in general, are not well-suited to assess a manifestation which cannot possibly be ascertained objectively, which lacks a laboratory correlate and which is not amenable or responsive to specific pharmacological therapies (14–16). Searching for a biological explanation for the presence of fatigue such as anemia, hypothyroidism and other clinical manifestations indicative of active disease is commonly done with oftentimes discouraging results (1;9;13). In fact, neither hypothyroidism nor anemia were found to be associated with fatigue in our analyses. Nevertheless, fatigue in SLE is, with all likelihood, multifactorial and is probably mediated to a certain extent by disease activity (pro-inflammatory cytokines) but also by other disease factors such as the coexistence of depression, pain and fibromyalgia as well as of purely psychological and behavioral constructs such as inadequate social support, the presence of helplessness or the patients perception that nothing can be done to modify their symptoms, or of inadequate coping mechanisms in dealing with the disease. In fact, most of these variables were significant in the multivariable analyses performed; of interest, however, neither disease activity nor damage accrual were retained in the multivariable models examined (3;11–13;36;37). Finally, the association of Caucasian ethnicity with higher levels of fatigue has not been previously reported. However, fatigue (and fibromyalgia) occurred more frequently among these patients than in patients from the other ethnic groups; this has not been previously reported in the literature given that the work related to fatigue in lupus has mainly emanated from more ethnically homogeneous and predominantly Caucasian populations (10–13;34). In only one relatively small study in which Caucasians and African Americans were studied, ethnicity was not found to be associated with its occurrence (38).
Our results are consistent, for the most part, with our previous observations (8) and with studies performed by other investigators in terms of the variables found to be associated with fatigue (10;11;13;34). Of importance, we found no association with disease activity in these analyses which contrast with our initial observations (8) but supports the assertions made by Wang et al. (13) and Petri et al. (39) over a decade ago, that is fatigue in lupus is not a function of disease activity. We nevertheless have now examined the factors associated with higher levels of fatigue over the course of the disease, rather than at one time point, suggesting that the association with disease activity may be operative only early in the course of the disease but not subsequently.
It should be noted that despite the fact that we have conducted longitudinal analyses, given the fact that once fatigue occurs it is very likely to remain present, some of the predictor variables (helplessness, abnormal-illness behaviors) could be considered outcomes rather than predictors. Given that these variables could not have possibly been measured before lupus ensued, the intricate relationship between fatigue and these constructs cannot be further disentangled for now.
Nevertheless, the information gathered in this study is particularly valuable for the design of clinical intervention studies aimed at ameliorating this pervasive clinical manifestation; for example we have now shown that exercise may be associated with decreased levels of fatigue given further evidence to the relatively small studies conducted in which exercise has been shown to have a positive impact on this symptom in patients with SLE (40–43). We were surprised, however, that this variable was not retained in the multivariable models; it is conceivable that exercise was negatively and highly correlated with other variables significant in the final model including pain, helplessness and abnormal illness-related behaviors. In short, while managing disease activity in the lupus patients is undoubtedly of critical importance, comprehensive intervention programs aimed at modifying the comorbid conditions of fibromyalgia and depression, the presence of pain, sedentarism, helplessness and of negative coping styles are also important. Certainly physicians alone cannot possibly tackle such a program while also managing other important aspects of the disease (such as unexpected flares and premature atherosclerosis). Nevertheless, such a comprehensive approach is needed if the quality of life of our lupus patients is going to be substantially improved.
Acknowledgments
Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Disease P01 AR49084, General Clinical Research Centers M01-RR02558 (UTH) and M01-RR00032 (UAB) and from the National Center for Research Resources (NCRR/HIH) RCMI Clinical Research Infrastructure Initiative (RCRII) 1P20RR11126 (UPR) and by the STELLAR (Supporting Training Efforts in Lupus for Latin American Rheumatologist) Program funded by Rheuminations, Inc (UAB).
Appendix 1
Fatigue Severity Scale Instrument Nine-Item:
My motivation is lower when I am fatigued
Exercise brings on my fatigue
I am easily fatigued
Fatigue interferes with my physical functioning
Fatigue causes frequent problems for me
My fatigue prevents sustained physical functioning
Fatigue interferes with carrying out certain duties and responsibilities
Fatigue is among my three most disabling symptoms
Fatigue interferes with my work, family or social life
Reference List
- 1.Wysenbeek AJ, Leibovici L, Weinberger A, Guedj D. Fatigue in systemic lupus erythematosus. Prevalence and relation to disease expression. Br J Rheumatol. 1993;32:633–635. doi: 10.1093/rheumatology/32.7.633. [DOI] [PubMed] [Google Scholar]
- 2.Krupp LB, LaRocca NG, Muir J, Steinberg AD. A study of fatigue in systemic lupus erythematosus. J Rheumatol. 1990;17:1450–1452. [PubMed] [Google Scholar]
- 3.McKinley PS, Ouellette SC, Winkel GH. The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. A proposed model. Arthritis Rheum. 1995;38:826–834. doi: 10.1002/art.1780380617. [DOI] [PubMed] [Google Scholar]
- 4.Hastings C, Joyce K, Yarboro CH, Berkebile C, Yocum D. Factors affecting fatigue in systemic lupus erythematosus. Arthritis Rheum. 1986;29:S176. doi: 10.1002/anr.1790020208. [DOI] [PubMed] [Google Scholar]
- 5.Petri M. Clinical features of systemic lupus erythematosus. Curr Opin Rheumatol. 1995;7:395–401. doi: 10.1097/00002281-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Joyce K, Berkebile C, Hastings C, Yarboro C, Yocum D. Health status and disease activity in systemic lupus erythematosus. Arthritis Care Res. 1989;2:65–69. doi: 10.1002/anr.1790020208. [DOI] [PubMed] [Google Scholar]
- 7.Tench CM, McCurdie I, McCarthy J, White PD, D'Cruz D. The assessment of aerobic capacity in a group of patients with systemic lupus erythematosus and its association with fatigue quality and disease activity. Arthritis Rheum. 1998;41:S332. [Google Scholar]
- 8.Zonana-Nacach A, Roseman JM, McGwin G, Jr, Friedman AW, Baethge BA, Reveille JD, et al. Systemic lupus erythematosus in three ethnic groups, VI: Factors associated with fatigue within 5 years of criteria diagnosis. Lupus. 2000;9:101–109. doi: 10.1191/096120300678828046. [DOI] [PubMed] [Google Scholar]
- 9.Omidal R, Husby G, Koldingsnes W. Intravenous and oral cyclophosphamide pulse therapy in rheumatic diseases: side effects and complications. Clin Exp Rheumatol. 1993;11:283–288. [PubMed] [Google Scholar]
- 10.Da Costa D, Dritsa M, Bernatsky S, Pineau C, Menard HA, Dasgupta K, et al. Dimensions of fatigue in systemic lupus erythematosus: relationship to disease status and behavioral and psychosocial factors. J Rheumatol. 2006;33(7):1282–1288. [PubMed] [Google Scholar]
- 11.Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. J Rheumatol. 2005;32(9):1699–1705. [PubMed] [Google Scholar]
- 12.Tench CM, McCurdie I, White PD, D'Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatol. 2000;39:1249–1254. doi: 10.1093/rheumatology/39.11.1249. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Gladman DD, Urowitz MB. Fatigue in lupus is not correlated with disease activity. J Rheumatol. 1998;25:892–895. [PubMed] [Google Scholar]
- 14.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res. 1993;37:753–762. doi: 10.1016/0022-3999(93)90104-n. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Luft BJ, Hapern JJ. Comparison of neurologic and psychologic findings in patient with Lyme disease and chronic fatigue syndrome. Neurology. 1989;39:S144. [Google Scholar]
- 17.Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 18.Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis Rheum. 2007;57(8):1348–1357. doi: 10.1002/art.23113. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Alarcon GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 22.Alarcon GS, McGwin G, Jr, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–2806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Reveille JD, Moulds JM, Ahn C, Friedman AW, Baethge B, Roseman J, et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, Nature versus Nurture. Arthritis Rheum. 1998;41:1161–1172. doi: 10.1002/1529-0131(199807)41:7<1161::AID-ART4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.U.S.Department of Commerce, Bureau of the Census. Current population reports, Series P-23, No. 28 and Series P-60, No. 68 and subsequent years. Washington, DC: Housing and Household Economic Statistics Division; 1995. [Google Scholar]
- 25.Cohen S, Mermelstein R, Kamarck T, Hoberman HN. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social Support: Theory, Research and Applications. Boston: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- 26.Engle EW, Callahan LF, Pincus T, Hochberg MC. Learned helplessness in systemic lupus erythematosus: Analysis using the Rheumatology Attitude Index. Arthritis Rheum. 1990;33:281–286. doi: 10.1002/art.1780330220. [DOI] [PubMed] [Google Scholar]
- 27.Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the Arthritis Helplessness Index. J Rheumatol. 1985;12:462–467. [PubMed] [Google Scholar]
- 28.Pilowsky I. Dimensions of illness behavior as measured by the Illness Behavior Questionnaire: A replication study. J Psychosom Res. 1993;37:53–62. doi: 10.1016/0022-3999(93)90123-w. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 30.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 31.Liang MH, Fortin PR, Isenberg DA, Snaith L. Quantitative clinical assessment of disease activity in systemic lupus erythematosus: progress report and research agenda. Rheumatol Int. 1991;11:133–136. doi: 10.1007/BF00304502. [DOI] [PubMed] [Google Scholar]
- 32.Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27:373–376. [PubMed] [Google Scholar]
- 33.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 34.Bruce IN, Mak VC, Hallett DC, Gladman DD, Urowitz MB. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis. 1999;58:379–381. doi: 10.1136/ard.58.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozora E, Ellison MC, West S. Depression, fatigue, and pain in systemic lupus erythematosus (SLE): relationship to the American College of Rheumatology SLE neuropsychological battery. Arthritis Rheum. 2006;55(4):628–635. doi: 10.1002/art.22101. [DOI] [PubMed] [Google Scholar]
- 36.Omdal R, Waterloo K, Koldingsnes W, Husby G, Mellgren SI. Fatigue in patients with systemic lupus erythematosus: the psychosocial aspects. J Rheumatol. 2003;30(2):283–287. [PubMed] [Google Scholar]
- 37.Branden HSU, Udden MM, Lynch EC. Autoimmune hemolytic anemia, primary adrenal insufficiency and the antiphospholipid syndrome. Am J Med Sci. 1997 July;314:41–44. doi: 10.1097/00000441-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Doninger NA, Fink JW, Utset TO. Neuropsychologic functioning and health status in systemic lupus erythematosus: does ethnicity matter? J Clin Rheumatol. 2005;11(5):250–256. doi: 10.1097/01.rhu.0000182149.67967.cc. [DOI] [PubMed] [Google Scholar]
- 39.Petri M, Steckroth J. Fibromyalgia, not active SLE, explains fatigue, HAQ scores, disability and depression scores. Arthritis Rheum. 1996;39(9):S68. [Google Scholar]
- 40.Robb-Nicholson LC, Daltroy L, Eaton H, Gall V, Wright E, Hartley LH, et al. Effects of aerobic conditioning in lupus fatigue: a pilot study. Br J Rheumatol. 1989;28:500–505. doi: 10.1093/rheumatology/28.6.500. [DOI] [PubMed] [Google Scholar]
- 41.Daltroy LH, Robb-Nicholson LC, Iversen MD, Wright EA, Liang MH. Effectiveness of minimally spupervised home aerobic training in patients with systemic rheumatic disease. Br J Rheumatol. 1995;34:1064–1069. doi: 10.1093/rheumatology/34.11.1064. [DOI] [PubMed] [Google Scholar]
- 42.Tench CM, McCarthy J, McCurdie I, White PD, D'Cruz DP. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology. 2003;42:1050–1054. doi: 10.1093/rheumatology/keg289. [DOI] [PubMed] [Google Scholar]
- 43.Ramsey-Goldman R, Schilling EM, Dunlop D, Langman C, Greenland P, Thomas RJ, et al. A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthitis Care Res. 2000;13:262–269. doi: 10.1002/1529-0131(200010)13:5<262::aid-anr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]