Abstract
Background
A growing body of evidence suggests that repetitive transcranial magnetic stimulation (rTMS) can alleviate negative and positive symptoms of refractory schizophrenia. However, trials to date have been small and results are mixed.
Methods
We performed meta-analyses of all prospective studies of the therapeutic application of rTMS in refractory schizophrenia assessing the effects of high-frequency rTMS to the left dorsolateral prefrontal cortex (DLPFC) to treat negative symptoms, and low-frequency rTMS to the left temporo-parietal cortex (TPC) to treat auditory hallucinations (AH) and overall positive symptoms.
Results
When analyzing controlled (active arms) and uncontrolled studies together, the effect sizes showed significant and moderate effects of rTMS on negative and positive symptoms (based on PANSS-N or SANS, and PANSS-P or SAPS, respectively). However, the analysis for the sham-controlled studies revealed a small non-significant effect size for negative (0.27, p=0.417) and for positive symptoms (0.17, p=0.129). When specifically analyzing AH (based on AHRS, HCS or SAH), the effect size for the sham-controlled studies was large and significant (1.04; p=0.002).
Conclusions
These meta-analyses support the need for further controlled, larger trials to assess the clinical efficacy of rTMS on negative and positive symptoms of schizophrenia, while suggesting the need for exploration for alternative stimulation protocols.
Keywords: Schizophrenia, rTMS, meta-analysis, negative symptoms, positive symptoms, auditory hallucinations
1. Introduction
Treatment for schizophrenia remains unsatisfactory. Current available antipsychotic drugs leave many symptoms of the illness untreated and cause unacceptable side-effects (Stone and Pilowsky, 2007). Therefore, the search for new antipsychotic drugs and the development of novel treatments for schizophrenia is critical. A growing body of evidence suggests that repetitive transcranial magnetic stimulation (rTMS) can provide alleviation of both positive and negative symptoms of schizophrenia. However, trials to date have been limited to small number of patients and overall results have been mixed. Previous review articles on this topic have elegantly described major findings of rTMS trials and identified the most extensively used and promising stimulation protocols (Cordes et al., 2006; Saba et al., 2006; Haraldsson et al., 2004). Recently, Stanford et al. (2008) contrasted the effects of different rTMS parameters and proposed methods to optimize dosage. Nevertheless, careful meta-analysis of the findings is sparse. Meta-analytic evidence suggests that severity of auditory hallucinations (AH) can be successfully reduced by rTMS (Aleman et al., 2007). However, this meta-analysis computed mean gain effect sizes of sham-controlled studies applying 1Hz rTMS to the left hemisphere, but the specific sites of stimulation varied across the included studies.
Thus, we conducted meta-analyses of all published prospective studies of the therapeutic application of rTMS in refractory schizophrenic patients. We specifically assessed both protocols most extensively used on the distinctive constellations of symptoms: high-frequency rTMS to the left dorsolateral prefrontal cortex (DLPFC) to treat negative symptoms, and low-frequency rTMS to the left temporoparietal cortex (TPC) for the specific treatment of AH. We also further explored the effects of rTMS to the left TPC on overall positive symptoms.
The original rationale supporting these protocols accommodates several lines of evidence from anatomical and functional neuroimaging studies. Indeed, activation of the prefrontal cortex seems to modulate the release of dopamine (Strafella et al., 2001), which may underlie improvement of negative symptoms (Heimer et al., 1997), whereas positive correlations between increased temporal cortical activity and the hallucinating state in schizophrenics have been reported (e.g., Silbersweig et al., 1995; Dierks et al., 1999; Shergill et al., 2000). Furthermore, there is a selective effect of stimulation frequency related to the different neurophysiological mechanisms triggered by low-frequency (≤1Hz) TMS, producing a decrease in cortical excitability, and high-frequency (>1Hz) TMS, generating the opposite effect (Pascual-Leone et al., 1998). Therefore, high-frequency TMS-triggered activation of prefrontal brain regions aims at reducing the severity of negative symptoms in schizophrenics, while low-frequency TMS is intended to relieve AH by decreasing temporal lobe activation. Moreover, further extension of the latter protocol for the treatment of other positive symptoms (delusions) has also been trialed.
2. Methods
2.1. Selection of studies
A systematic search of the literature was conducted using the Web of Science database (until July 2008). The identification of English language articles was based on the following keywords: schizo* and transcranial magnetic stimulation or TMS or rTMS. In addition, reference lists in systematic reviews and retrieved reports were also examined, but no other papers were included.
2.2. Selection criteria for the meta-analyses
Three different analyses were planned: two analyses designed to evaluate rTMS effects on the negative and overall positive symptoms of schizophrenia, and a third analysis to assess treatment efficacy on AH, architectured according to the symptom-dependent clinical ratings used. Initially, we adopted the following selection criteria: repetitive TMS was performed; study design was open, crossover or parallel; TMS was applied for more than a single session; psychotropic dosages were unchanged for at least 4 weeks before rTMS treatment (and maintained throughout the trial); and when published studies reported overlapping data sets, only the largest sample was included. Studies were excluded when they met at least one of the following criteria: single- or paired-pulse TMS was delivered; case reports; TMS effects were assessed after a single session; patients were on stable medication regimen for less than 4 weeks prior to rTMS. Furthermore, all studies had to report the mean and standard deviation (SD) of the outcome measures before and after treatment or provide other statistical parameters that could be used to deduce these values. For studies that met inclusion criteria but did not report these scores, the authors were contacted to provide these data.
2.2.1. Analyses for negative and overall positive symptoms
For the analysis of rTMS effects in the treatment of negative symptoms, we included papers in which: high-frequency TMS was used; rTMS was applied to the left DLPFC; outcome measures included the Negative symptom subscale of the Positive and Negative Syndrome Scale (PANSS-N) or the Scale for the Assessment of Negative Symptoms (SANS). We excluded studies when low-frequency TMS was used; TMS was applied bilaterally or to the right DLPFC; outcome measures were non-specific for the assessment of negative symptoms.
For the analysis of rTMS efficacy in the treatment of overall positive symptoms, we applied the following inclusion criteria: low-frequency TMS was used; TMS was applied to the left TPC with coil placed halfway between left temporal (T3) and left parietal (P3), according to the International 10/20 EEG electrode position system; outcome measures included the Positive symptom subscale of the PANSS (PANSS-P) or the Scale for the Assessment of Positive Symptoms (SAPS). We excluded reports when high-frequency rTMS to the temporal cortex was used; TMS was applied above other brain sites (not TPC) or over the left TPC concomitantly to other sites without wash-out periods; outcome measures were non-specific for the assessment of positive symptoms.
2.2.2. Analysis for auditory hallucinations
Within the identification of specific potential TMS-triggered benefits on AH, an analysis was carried out in order to estimate the magnitude of the TMS effects in the reduction of AH severity, measured with a composite score of the following measures: Auditory Hallucination Rating Scale (AHRS); Hallucination Change Scale (HCS); or Scale for Auditory Hallucinations (SAH).
2.3. Extraction of the outcome measures
The data were collected using a semi-structured form for each study by one of the authors and checked by another. The following variables were extracted: (1) mean and SD of the elected outcome measure for baseline and after treatment for the active (uncontrolled studies) and sham groups (controlled studies); (2) study design; (3) demographic and clinical characteristics (number of patients, gender, mean age, mean duration of illness, and medication approach during trial); (4) mean and SD of the baseline clinical status; and (5) TMS protocol [number of patients submitted to active/sham stimulation, frequency, intensity (% of motor threshold), number of sessions, total stimuli, type of coil, sham coil position].
2.4. Effect size calculation
All our analyses were performed using Stata statistical software, version 8.0 (StataCorp, College Station, TX). Effect sizes were computed as the standardized mean difference (Cohen d) based either on pre- and post-treatment values of one group (active group) within each study or comparison of the mean changes in pre- to post-treatment ratings of the two independent groups (sham and active rTMS) in the controlled trials, using the means and SDs. For the post-treatment value, we used the evaluation completed immediately after the end of rTMS treatment, since not all studies reported follow-up assessments. An individual effect size for each study was calculated, and a combined (pooled weighted) effect size was obtained through the implementation of random and fixed effect models. The random effect model gives relatively more weight to smaller studies and wider confidence intervals than the fixed effect model. Since some small studies were included in the meta-analyses, and these studies usually have large effect sizes, we evaluated the influence of individual studies by computing the meta-analysis’ estimates and omitting one study at a time. Furthermore, we assessed publication bias using the Begg-modified funnel plot (Egger et al., 1997), in which the standardized mean difference from each plot was plotted against the standard error.
3. Results
3.1. Selected studies
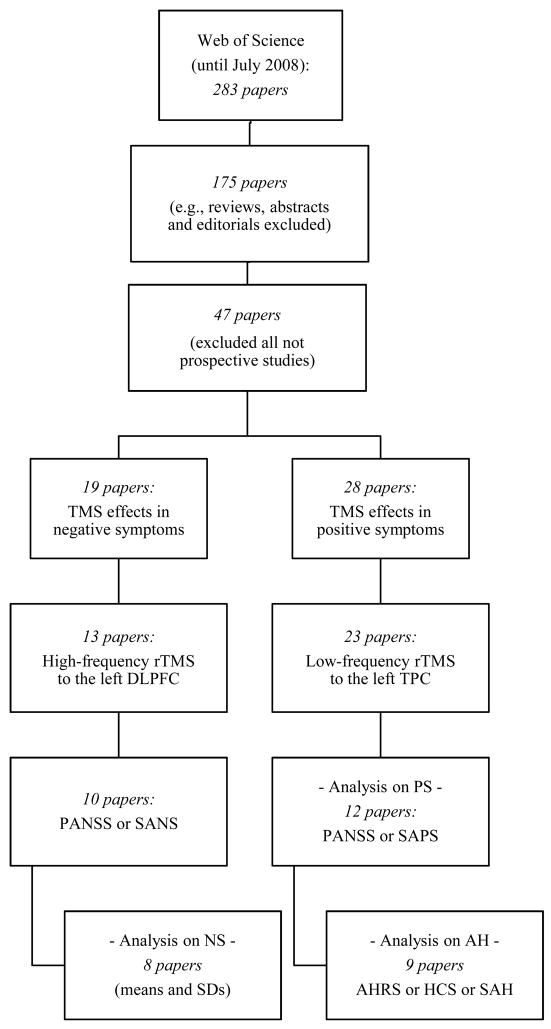
Figure 1 provides a flow diagram reflecting the process of selection of studies for the final analyses (figure 1). The initial strategy yielded 283 peer-reviewed papers. During the initial review, we excluded nearly 55% of the articles as they represented review or opinion articles. During the subsequent more detailed screening, we excluded about 38% of reports as TMS was not used as a therapeutic tool. Ultimately our search identified 47 published papers, of which 19 were devoted to rTMS treatment of negative symptoms and 28 to rTMS effects on positive symptoms.
Figure 1.
Flow diagram of the selection process of peer-reviewed articles for main analyses (negative and positive symptoms) and additional analysis (auditory hallucinations).
Within the subset of clinical trials aiming at treating negative symptoms, we searched for those that applied high-frequency rTMS to the left DLPFC, thus excluding trials in which bilateral (Geller et al., 1997; also single-pulse TMS), right (Feinsod et al., 1998; Grisaru et al., 1998; Klein et al., 1998), or the “dominant” (Rollnik et al., 2000) DLPFC was probed. We also excluded the study by Huber et al. (2003), since it was a reevaluation of previous data (Rollnik et al., 2000). This last strategy yielded 13 published reports, of which 8 were sham-controlled (6 parallel and 2 crossover) and 5 were open-label (2 case reports) studies. We then excluded case reports (Saba et al., 2002; Prikryl et al., 2007b) and 1 crossover study (Nahas et al., 1999) in which a single rTMS session was performed. Finally, due to lack of data on means and SDs, 2 additional studies (Hajak et al., 2004; Jin et al., 2006) had to be excluded, and thus 8 final trials were entered in the meta-analysis for the negative symptoms (table 1).
Table 1.
Selected peer-reviewed articles for meta-analysis of rTMS effects on negative symptoms of schizophrenia.
| Study | Demographic and clinical characteristics |
TMS parameters | Baseline psychopathology | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Design | N | Gender (% M) |
Mean age (y) |
Mean duration SCZ (y) |
Active | Sham | Frequency | % MT | No. of sessions |
Total stimuli |
Sham coil position |
Type of coil |
Rating scale |
Active | Sham | ||
| N | N | Mean | SD | Mean | SD | |||||||||||||
| Cohen et al., 1999 | Open-label | 6 | 33.3% | 39.00 | . | 6 | . | 20 Hz | 80% | 10 | 8000 | . | Fig. 8 | PANSS-G | 37.67 | 11.15 | . | . |
| Holi et al., 2004 | Parallel | 22 | 86.4% | 36.70 | 13.20 | 11 | 11 | 10 Hz | 100% | 10 | 4000 | 90° | Fig. 8 | PANSS-T | 105.20 | 41.20 | 110.30 | 20.20 |
| Sachdev et al., 2005 | Open-label | 4 | 100% | 34.50 | 13.75 | 4 | . | 15 Hz | 90% | 20 | 36000 | . | Fig. 8 | . | . | . | . | . |
| Jandl et al., 2005 | Open-label | 10 | 50.0% | 42.70 | 18.10 | 10 | . | 10 Hz | 100% | 5 | 3500 | . | Circul | BPRS | 36.90 | 8.10 | . | . |
| Novak et al., 2006 | Parallel | 16 | 75.0% | 34.10 | 11.50 | 8 | 8 | 20 Hz | 90% | 10 | 20000 | 90° | Fig. 8 | PANSS-T | 65.50 | 19.70 | 61.10 | 16.90 |
| Mogg et al., 2007 | Parallel | 17 | 94.1% | 41.70 | 16.53 | 8 | 9 | 10 Hz | 110% | 10 | 20000 | Placebo coil | Fig. 8 | PANSS-T | 86.00 | 9.75 | 86.00 | 10.09 |
| Prikryl et al., 2007a | Parallel | 22 | 100% | 33.90 | 6.73 | 11 | 11 | 10 Hz | 110% | 15 | 22500 | 90° | Fig. 8 | PANSS-T | 57.73 | 8.40 | 64.00 | 13.10 |
| Goyal et al., 2007 | Parallel | 10 | 100% | 28.00 | . | 5 | 5 | 10 Hz | 110% | 10 | 9800 | 45° | Fig. 8 | PANSS-T | 104.20 | 8.16 | 103.20 | 12.40 |
|
| ||||||||||||||||||
| Total | . | 107 | . | . | . | 63 | 44 | . | . | . | . | . | . | . | . | . | . | . |
TMS: Transcranial magnetic stimulation; M = Male; SCZ = Schizophrenia; MT = Motor threshold; Fig 8 = Figure-of-eight coil; Circul = Circular coil; PANSS-G = General psychopathology subscale of the Positive and Negative Syndrome Scale; PANSS-T = Total score of PANSS; BPRS = Brief Psychiatric Rating Scale; . = data not available or not applicable.
Likewise, within the subset of clinical trials addressing treatment effects on positive symptoms, we searched for those that delivered low-frequency rTMS to the left TPC, and excluded all trials probing the primary auditory cortex (D’ Alfonso et al., 2002; Langguth et al., 2006; Jardri et al., 2007), Broca’s area (Schönfeldt-Lecuona et al., 2004), or the right DLPFC (Schreiber et al., 2002). Regarding Sommer et al.’s (2007) study, in which the coil was placed either by anatomical landmarks or according to functional activation maps, we only used data from the group stimulated according to the former strategy (n=6). Thus, we retrieved 23 papers, of which 14 were sham-controlled (8 parallel and 6 crossover) and 9 were uncontrolled studies. We then excluded 6 case reports (Franck et al., 2003; Fitzgerald et al., 2006; Poulet et al., 2006; Chung et al., 2007; Favalli et al., 2007; Poulet et al., 2008), 2 studies in which samples overlapped with other reports (Hoffman et al., 1999, 2003), 1 study reporting only 1 week of pre-treatment antipsychotic stabilization (Jandl et al., 2006), 1 study in which several sites were probed with no wash-out periods (Hoffman et al., 2007), and 1 study exploring activity in language regions after successful AH treatment with rTMS (Fitzgerald et al., 2007). Therefore, we were able to enter 12 trials into the meta-analysis for the positive symptoms (table 2).
Table 2.
Selected peer-reviewed articles for meta-analysis of rTMS effects on auditory hallucinations (*) and for meta-analysis of rTMS effects on overall positive symptoms of schizophrenia.
| Study | Demographic and clinical characteristics |
TMS parameters | Baseline psychopathology | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Design | N | Gender (% M) |
Mean age (y) |
Mean duration SCZ (y) |
Active | Sham | Frequency | % MT |
No. of sessions |
Total stimuli |
Sham coil position |
Type of coil |
Rating scale |
Active | Sham | ||
| N | N | Mean | SD | Mean | SD | |||||||||||||
| * Hoffman et al., 2000 | Crossover | 12 | 83.3% | 41.80 | . | 12 | 12 | 1 Hz | 80% | 4 | 2400 | 45° | Fig. 8 | PANSS-G | 38.33 | 7.69 | 38.75 | 11.85 |
| * McIntosh et al., 2004 | Crossover | 16 | 43.8% | 35.90 | 11.60 | 16 | 16 | 1 Hz | 80% | 4 | 2400 | 45° | Fig. 8 | PANSS-G | 36.00 | 6.80 | 33.90 | 8.40 |
| * Fitzgerald et al., 2005 | Parallel | 32 | 53.1% | 36.03 | 14.31 | 17 | 15 | 1 Hz | 90% | 10 | 9000 | 45° | Fig. 8 | PANSS-G | 38.12 | 6.33 | 41.27 | 6.63 |
| * Chibbaro et al., 2005 | Parallel | 16 | 68.8% | 40.05 | 8.15 | 8 | 8 | 1 Hz | 90% | 4 | 3600 | 45° | Fig. 8 | . | . | . | . | . |
| Lee et al., 2005 | Parallel | 27 | 59.0% | 40.57 | . | 13 | 14 | 1 Hz | 100% | 10 | 12000 | 90° | Fig. 8 | PANSS-G | 42.46 | 14.04 | 43.93 | 11.06 |
| * Hoffman et al., 2005 | Parallel | 50 | 66.0% | 35.28 | 24.07 | 27 | 23 | 1 Hz | 90% | 9 | 7920 | 45° | Fig. 8 | PANSS-G | 31.06 | 8.66 | 31.89 | 11.19 |
| * Poulet et al., 2005 | Crossover | 10 | 70.0% | 34.90 | 10.60 | 10 | 10 | 1 Hz | 90% | 10 | 10000 | Placebo coil | Fig. 8 | . | . | . | . | . |
| * Brunelin et al., 2006 | Parallel | 24 | 66.7% | 34.53 | 9.33 | 14 | 10 | 1 Hz | 90% | 10 | 10000 | Placebo coil | Fig. 8 | PANSS-T | 81.40 | 11.40 | 79.60 | 11.50 |
| Saba et al., 2006 | Parallel | 16 | 81.3% | 30.65 | 7.95 | 8 | 8 | 1 Hz | 80% | 10 | 3000 | Placebo coil | Fig. 8 | PANSS-G | 42.25 | 5.73 | 48.38 | 7.47 |
| * Sommer et al., 2007 | Open-label | 6 | 100% | 38.83 | 14.00 | 6 | . | 1 Hz | 90% | 15 | 18000 | . | Fig. 8 | PANSS-G | 38.00 | 7.32 | . | . |
| * Horacek et al., 2007 | Open-label | 12 | 58.3% | 34.40 | 6.36 | 12 | . | 0.9 Hz | 100% | 10 | 10800 | . | Fig. 8 | PANSS-T | 72.83 | 17.89 | . | . |
| Rosa et al., 2007 | Parallel | 11 | 54.6% | 31.27 | 7.03 | 6 | 5 | 1 Hz | 90% | 10 | 9600 | Placebo coil | Fig. 8 | PANSS-G | 52.17 | 9.37 | 52.20 | 5.89 |
|
| ||||||||||||||||||
| Total | . | 232 | . | . | . | 149 | 121 | . | . | . | . | . | . | . | . | . | . | . |
: Selected peer-reviewed articles for analysis of rTMS effects on AH; TMS: Transcranial magnetic stimulation; M = Male; SCZ = Schizophrenia; MT = Motor threshold; Fig 8 = Figure-of-eight coil; Circul = Circular coil; PANSS-G = General psychopathology subscale of the Positive and Negative Syndrome Scale; PANSS-T = Total score of PANSS; . = data not available or not applicable.
For the analysis on the therapeutic use of rTMS on AH, exclusively, we extracted a subset of 9 studies, from the previously selected 12 reports, excluding 2 trials for not reporting a composite score for the AHRS (Lee et al., 2005; Rosa et al., 2007), and 1 concerning the treatment of delusions (Saba et al., 2006) (table 2).
3.2. Treatment of negative symptoms
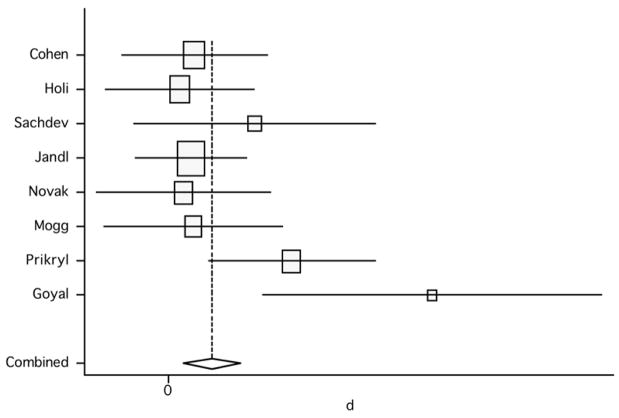
We initially pooled the results of the 8 studies assessing negative symptoms, and compared the results of post-rTMS treatment vs. baseline, using the active arms only for the controlled studies. The random effect model showed a pooled effect size of 0.58 [95% confidence interval (CI): 0.11, 1.04; p=0.014] and the fixed effect model revealed an effect size of 0.49 (95% CI: 0.17, 0.82; p=0.003) (figure 2). The test for heterogeneity failed to show significant heterogeneity between studies (Q7, χ2=12.64; p=0.081). These results indicate that high-frequency rTMS above the DLPFC induced a significant, although modest-to-moderate, reduction of negative symptoms in patients receiving active treatment.
Figure 2.
Pooled effect size (before versus after treatment) for studies of rTMS effects on negative symptoms (random effect model).
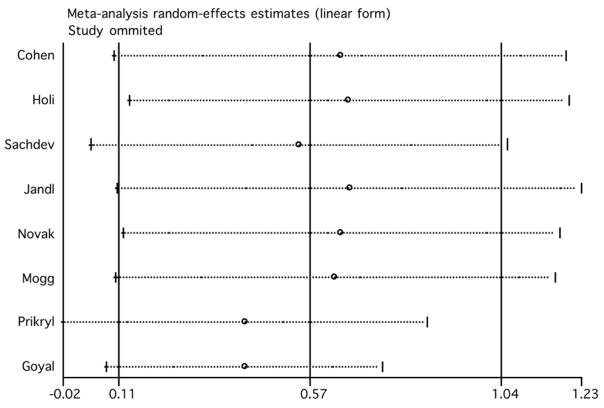
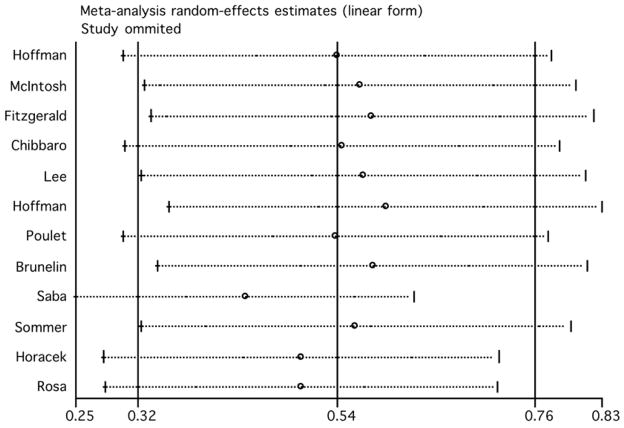
We evaluated the influence of individual studies by computing the meta-analysis’ random effect model estimates and omitting one study at a time (figure 3). The two studies that induced the largest individual difference when removed were those by Prikryl et al. (2007) and Goyal et al. (2007). With their exclusion, the overall estimate decreased to 0.418 (95% CI: −0.02, 0.86) and 0.417 (95% CI: 0.09, 0.75), respectively. The exclusion of Jandl et al.’s (2005) study produced the highest increase of the overall estimate (0.67, 95% CI: 0.11, 1.23). The overall finding of a beneficial effect of rTMS on negative symptoms in schizophrenia remained significant after the exclusion of any single study, except for the exclusion of the study of Prikryl et al. (2007), in which the results are only marginally significant (95% CI: −0.02 to 0.86).
Figure 3.
Estimates of the random effect model omitting one study at a time (rTMS effects on negative symptoms).
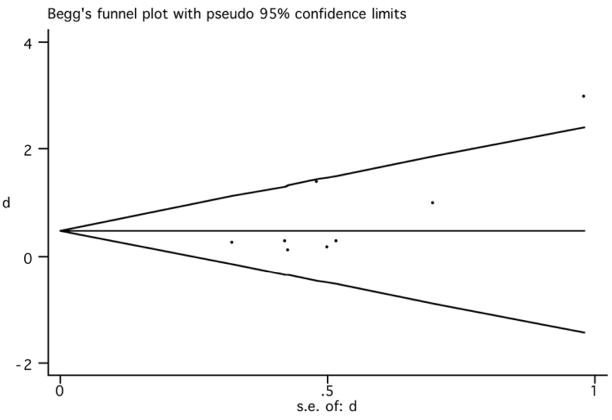
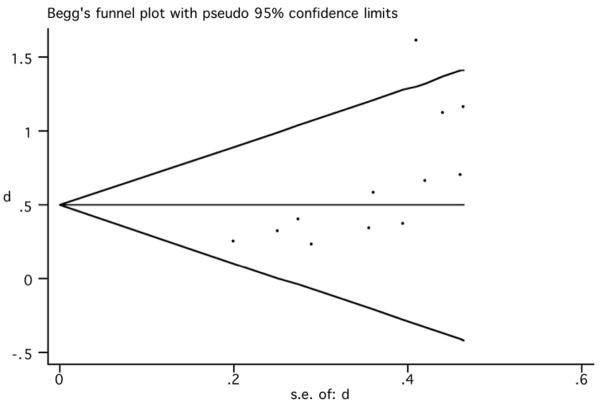
The funnel plot was used to identify whether the results were biased due to exclusion of unpublished, negative studies, which would render an asymmetrical funnel plot. The obtained plot (figure 4) showed that large studies had effect sizes that were near the pooled effects and showed a smaller effect according to our results. In addition, two studies with large standard error (indicative of small sample sizes) (Prikryl et al., 2007; Goyal et al., 2007) showed remarkably positive results, one of them being outside the 95% CI (Goyal et al., 2007). Although the distribution of the funnel plot might be considered somewhat asymmetrical, and the Egger test was significant (p=0.046), the sensitivity analysis showed that the exclusion of these two positive studies did not change our overall conclusions remarkably.
Figure 4.
Begg’s funnel plot (95% CI) (rTMS effects on negative symptoms).
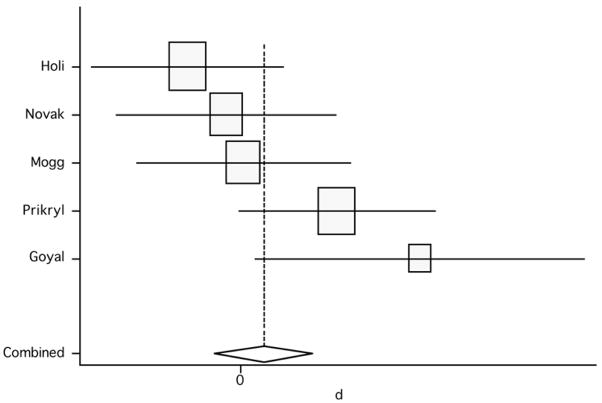
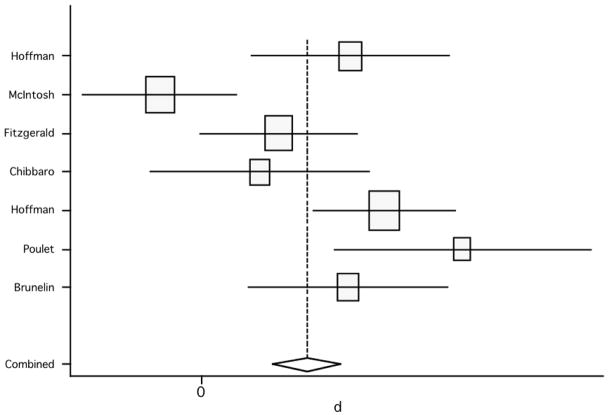
Lastly, we compared the scores between placebo versus active group in the double-blind studies (only 5 studies met this inclusion criterion). The analysis showed a pooled weighted effect size from the random effect model of 0.27 (95% CI: −0.38, 0.92; p=0.417) and from the fixed effect model of 0.21 (95% CI: −0.23, 0.64; p=0.351) (figure 5; table 3). Since in controlled studies the effect is defined as a difference from the control group, any positive effect in the control conditions would be expected to lower the net effect. In addition, effects of chance due to small number of studies are likely and, finally, the non-significant results here might be due the differences in the relative weighting of studies – i.e., because effect sizes are inversely proportional to the variance, small studies have larger variances and therefore smaller effects sizes. The test for heterogeneity failed to show significant heterogeneity across studies (Q4, χ2=8.65; p=0.07). However, due to low power of this analysis and, in fact, given the limited number of studies included, a p-value of 0.07 might represent indeed a considerable heterogeneity. For this reason, we also calculated the between-studies analysis of variance. This analysis yielded a relatively large value of 0.293, therefore suggesting a significant heterogeneity across these studies..
Figure 5.
Pooled effect size (placebo versus active treatment) for studies of rTMS effects on negative symptoms (random effect model).
Table 3.
Pooled weighted effect sizes for negative symptoms, overall positive symptoms, and auditory hallucinations.
| Negative symptoms | Random effect model | 95% CI | p value | Fixed effect model | 95% CI | p value | Q statistics | p value |
|---|---|---|---|---|---|---|---|---|
| Pooled weighted effect size* (all studies) | 0.58 | 0.11, 1.04 | 0.014 | 0.49 | 0.17, 0.82 | 0.003 | 12.64 | 0.081 |
| Pooled weighted effect size* (controlled studies) | 0.27 | −0.38, 0.92 | 0.417 | 0.21 | −0.23, 0.64 | 0.351 | 8.65 | 0.07 |
|
| ||||||||
| Positive symptoms | Random effect model | 95% CI | p value | Fixed effect model | 95% CI | p value | Q statistics | p value |
|
| ||||||||
| Pooled weighted effect size* (all studies) | 0.54 | 0.32, 0.76 | <0.001 | 0.50 | 0.31, 0.68 | <0.001 | 14.92 | 0.186 |
| Pooled weighted effect size* (controlled studies) | 0.17 | −0.05, 0.39 | 0.129 | 0.17 | −0.05, 0.39 | 0.129 | 2.96 | 0.966 |
|
| ||||||||
| Auditory hallucinations | Random effect model | 95% CI | p value | Fixed effect model | 95% CI | p value | Q statistics | p value |
|
| ||||||||
| Pooled weighted effect size* (all studies) | 1.28 | 0.89, | <0.001 | 1.35 | 1.11, | <0.001 | 19.50 | 0.012 |
| Pooled weighted effect size* (controlled studies) | 1.04 | 0.38, 1.71 | 0.002 | 0.96 | 0.65, 1.27 | <0.001 | 26.85 | <0.001 |
Effect size: standardized mean difference. The mean difference was calculated using the change from pre- to post-treatment for the active group. CI: confidence interval.
3.3. Treatment of positive symptoms
We first pooled the results of the 12 studies assessing positive symptoms, and compared the results of post-rTMS treatment vs. baseline, using the active arms solely for the controlled studies. The random effect model showed a pooled effect size of 0.54 (95% CI: 0.32, 0.76; p<0.001) and the fixed effect model a pooled effect size of 0.50 (95% CI: 0.31, 0.68; p<0.001) (figure 6). The test for heterogeneity failed to show significant heterogeneity (Q11, χ2=14.92; p=0.186). Thus, these results suggest that low-frequency rTMS delivered to the TPC induced a significant, but modest-to-moderate, reduction of overall positive symptoms in patients receiving active treatment.
Figure 6.
Pooled effect size (before versus after treatment) for studies of rRMS effects on positive symptoms (random effect model).
Next, we assessed the influence of individual studies (figure 7). The two studies that induced the largest individual difference in the pooled effects when excluded were the ones by Hoffman et al. (2005) and Saba et al. (2006). Interestingly, each study had the opposite influence: exclusion of Hoffman et al.’s study increased the overall estimate (0.59, 95% CI: 0.35, 0.84), whereas exclusion of Saba et al.’s trial decreased the overall estimate (0.44, 95% CI: 0.25, 0.62). Yet, the overall finding of a positive effect of TMS on positive symptoms in schizophrenia remained basically unaffected after the exclusion of any single study.
Figure 7.
Estimates of the random effect model omitting one study at a time (rTMS effects on positive symptoms).
Begg’s funnel plot (figure 8) showed that large studies had effect sizes that were near the pooled effects. However, three studies (Saba et al., 2006; Horacek et al., 2007; Rosa et al., 2007) with relatively large standard error showed very positive results, one of them being outside the 95% CI (Saba et al., 2006). Although the distribution of the funnel plot was asymmetrical, and the Egger test was significant (p=0.008), the sensitivity analysis showed that the exclusion of these positive studies did not change our overall conclusions.
Figure 8.
Begg’s funnel plot (95% CI) (rTMS effects on overall positive symptoms).
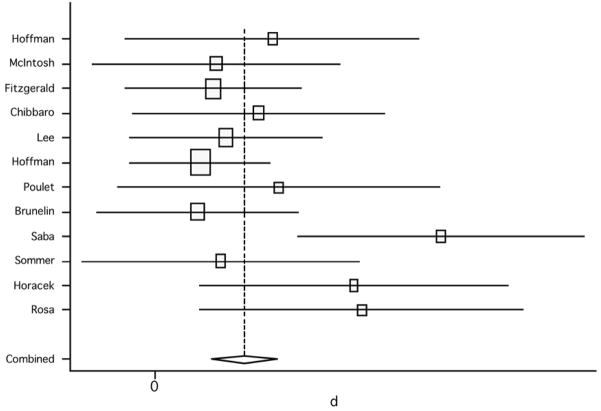
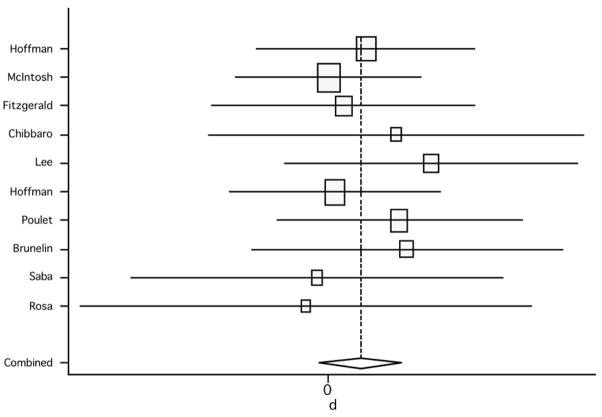
When assessing only sham-controlled studies and comparing scores between active and sham groups, the pooled weighted effect size from both the random effect model and the fixed effects model was 0.17 (95% CI: −0.05, 0.39; p=0.129) (figure 9); no significant heterogeneity was found (Q7, χ2=2.96; p=0.966) (table 3).
Figure 9.
Pooled effect size (placebo versus active treatment) for studies of rTMS effect on overall positive symptoms (random effect model).
3.4. Treatment of auditory hallucinations
Finally, for positive symptoms, we analyzed a subset of studies assessing the effect of rTMS on AH. The pooled effect size, again defined as the pre- versus post-treatment effect within the active arms only for controlled studies, using both the random effect model and the fixed effect model, was of 1.28 (95% CI: 0.89, 1.66; p<0.001) and of 1.35 (95% CI: 1.11, 1.58; p<0.001), respectively (figure 10). The test for heterogeneity showed that there was a significant heterogeneity across studies (Q8, χ2=19.5; p=0.012). Nevertheless, these results reveal a significant and robust effect of left TPC stimulation on AH in patients receiving active rTMS.
Figure 10.
Pooled effect size (before versus after treatment) for studies of rTMS effects on auditory hallucinations (random effect model).
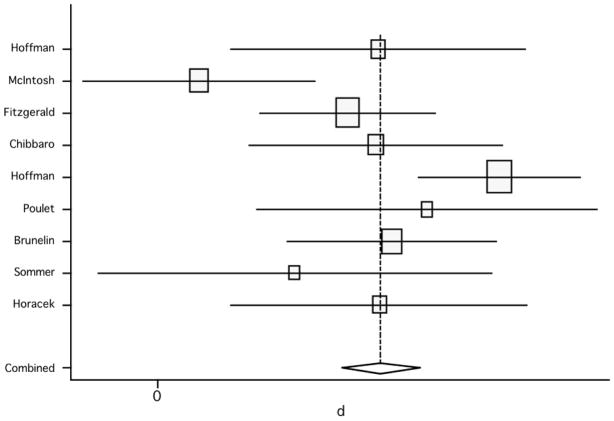
When assessing only sham-controlled studies, the comparison of scores between active versus sham groups showed an effect size from the random effect model of 1.04 (95% CI: 0.38, 1.71; p=0.002) and from the fixed effect model of 0.96 (95% CI: 0.65, 1.27; p<0.001) (figure 11); significant heterogeneity was found (Q6, χ2=26.85; p<0.001). Thus, the inclusion of controlled studies showed that a significant improvement of severity of AH after treatment was obtained, and the effect was robust (table 3).
Figure 11.
Pooled effect size (placebo versus active treatment) for studies of rTMS effects on auditory hallucinations (random effect model).
4. Discussion
4.1. Summary of findings
Our analyses yielded several clear findings. First, negative symptoms show significant post-treatment improvement across uncontrolled trials but not across controlled studies. When open trials are included, the effect is modest. The main finding, though, is that there is no statistically significant improvement of this symptom cluster in patients receiving active as compared to sham stimulation. Hence, the treatment of negative symptoms with rTMS as currently performed and measured (PANSS-N or SANS) does not seem to be efficacious. However, the number of sham-controlled studies is small, and two trials with positive results (Hajak et al., 2004; Jin et al., 2006) had to be excluded because of insufficient data. It is possible that inclusion of these studies might have resulted in significant and larger effect sizes. In particular, the study by Jin et al. (2006) clearly showed that the impact of rTMS on negative symptoms, compared to sham stimulation, was statistically significant and relatively impressive. Moreover, their approach suggested that rTMS to the left DLPFC can be beneficial if, in a synergistic fashion, concomitantly tuned with individualized frontal alpha frequency.
Second, positive symptoms, as globally assessed by PANSS-P or SAPS, do not show a statistically significant improvement after rTMS when only sham-controlled studies are analyzed. They do follow the same trend seen for negative symptoms, in the sense that the effect is significant, but modest, when all trials are included. This suggests, however, that overall positive symptoms do not benefit from stimulation of the TPC region, or there was not a sufficient number of studies.
Third, a marked and significant improvement of AH severity is obtained whether controlled or uncontrolled studies are analyzed. Indeed, not only the effect is significant, but it is also large. Thus, these results pinpoint a strong efficacy for the low-frequency rTMS protocol when probing left TPC on AH.
4.2. rTMS treatment of negative symptoms
If all trials are considered, this meta-analysis showed that rTMS to the left DLPFC results in statistically significant effects on negative symptoms in schizophrenia. The effect size was relatively discrete and this raises questions about the clinical relevance of the findings. When a potential placebo effect was considered, the effect of rTMS on negative symptoms was small and non-significant, and, accordingly, this had no clinical impact. Although we did not assess long-lasting effects in our analysis (as only few studies report this assessment), survivorship of effects seems to follow the same trend of poor results. Despite the fact that patients were followed-up in only 3 studies, no improvement was obtained at 2 (Mogg et al., 2007) or 8 weeks post-treatment (Novak et al., 2006). Sachdev et al. (2005) showed, however, that effects can last at least 30 days.
Several possible explanations can be offered for the weak results and divergence across studies. One explanation might be the severity of psychopathology. Indeed, the degree of illness severity (baseline psychopathology, table 1) varied substantially across studies and probably influenced treatment response. For instance, patients in Holi et al. (2004) study, who found no benefit of rTMS, had almost doubled baseline total scores of PANSS as compared to the patients of Prykril et al. (2007) who clearly benefited from rTMS. However, patients in the study by Goyal et al. (2007) were as severely affected as those of Holi et al. but significantly improved after treatment. This differential outcome might be related to the fact that Goyal et al. applied more than twice the number of rTMS stimuli that Holi and colleagues did.
Indeed rTMS parameters appear to be important, though no clear picture emerges. Jin et al. (2006) compared alpha-range (8–13Hz) and 20Hz rTMS, with patients submitted to the former showing significant post-treatment improvement while the latter being no better than sham. This might also justify the negative results of Novak et al. (2006) who used 20Hz rTMS. However, Cohen et al. (1999) found significant improvement after 20Hz rTMS, even though the number of stimuli delivered in their trial was less than half (8,000) of Novak et al.’s trial (20,000). On the other hand, it seems that trials in which a low number of stimuli were delivered produced negative (4,000; Holi et al., 2004) or worse results (3,500; Jandl et al., 2005) than when total number of stimuli were 10,000 or higher (e.g. 22,500; Prikryl et al., 2007). The highest number of stimuli (36,000) was delivered at 15Hz rTMS in the longest trial (Sachdev et al., 2005), and improvement in negative symptoms was observed, although the sample size was the smallest of all group studies. Therefore, it seems clear that more work is needed to identify optimal rTMS parameters. Ultimately, a multivariate regression analysis including patient characteristics (e.g., age, gender, or baseline psychopathology) as well as TMS parameters (e.g. number of stimuli, stimulation intensity, or number of session) is necessary to assess the contribution of clinical characteristics versus parameters of stimulation.
Another potential explanation regards the outcome measures that are currently being used as main assessments to target changes, and even the underlying definition of negative symptoms. In most studies, cognitive and functional outcome measures (assessing the impact of potential benefits in key outcome areas such as social behavior, work performance, and activities of daily living) were scarcely used, but retrieved interesting results. For example, Mogg et al. (2007)’s study found significant improvement of verbal learning (delayed recall) at 2-week follow-up, although none of the patients met criterion for response (20% reduction from baseline in PANSS-N). In Cohen et al. (1999)’s study, where positive results were achieved, there was a trend for general cognitive improvement, and significance was achieved for delayed visual memory. These two domains of cognition are among those identified by the NIMH-MATRICS project as important for schizophrenia (Green and Nuechterlein, 2004; Green et al., 2004). Sachdev et al. (2005) showed significant improvement of functional level (around 33%), while Fitzgerald et al. (2005)’s negative study found improvement in global function but no difference between groups. Thus, consideration of specific cognitive deficits might be valuable, though Novak et al. (2006) did not find any improvement of cognitive deficits.
Also important to consider is the site of stimulation, the left DLPFC. Evidence from anatomical and functional neuroimaging studies has pointed out several sources of dysfunction in schizophrenia patients. With respect to negative symptoms, structural and functional deficits have been shown in medial frontal areas (Koutsouleris et al., 2008; Siegel et al., 1993) and anterior cingulate (Fujimoto et al., 2007; Haznedar et al., 1997), as well as in posterior cortical parietal cortex (Zetzsche et al., 2008), including the inferior parietal lobule (for review, Torrey, 2007), and occipital regions (Onitsuka et al., 2007). The cerebellum has also been strongly related to schizophrenia. Indeed, Andreasen and Pierson (2008) extensively reviewed several lines of evidence for cerebellar abnormalities in schizophrenia and argued its role in the modulation of higher cognitive processes, largely impaired in schizophrenics. Moreover, the DLPFC is a region of the “task-related network” and, at least theoretically, more prone to cognitive enhancement than to core negative symptom improvement. Thus, it is possible that other brain sites would respond better to rTMS and should thus be carefully judged as potential stimulation targets for the treatment of negative symptoms of schizophrenia.
Finally, the total number of patients included in this meta-analysis is limited. Indeed, our sample was of 107 patients and only 63 received active treatment. Of these, almost half (n=27) were included in three studies (Holi et al., 2004; Novak et al., 2006; Mogg et al., 2007) showing negative results. Although we might have been underpowered in our analysis, the effect size of sham-controlled studies was small. Nevertheless, further controlled studies with larger sample sizes using designs shown to induce positive results [as, for instance, the design used by Jin et al. (2006)] seem to be of major importance at this point.
4.3. rTMS treatment of overall positive symptoms
As shown by this meta-analysis, low-frequency rTMS to the left TPC does not seem to be a suitable protocol for the treatment of positive symptoms other than AH. When placebo was concomitantly used, the effect size was small and non-significant. Moreover, these results are in agreement with previously reported meta-analytic findings of absence of significant improvement in overall positive symptoms (Aleman et al., 2007).
Probably, a major reason for such poor outcome is the targeted site. Positive psychotic symptoms, other than hallucinations, have been associated with dysfunctions in the orbitofrontal cortex (OFC) (Premkumar et al., 2008; Baas et al., 2008). Furthermore, altered distribution of OFC sulco-gyral pattern in schizophrenics and a smaller left middle orbital gyrus, strongly associated with worse positive formal thought disorder, were recently described (Nakamura et al., 2007; 2008). A role for the medial temporal lobe in positive psychotic symptoms was also suggested, while the lateral temporal cortex is involved in hallucinations (Whalley et al., 2007). White matter (WM) changes detected in diffusion tensor imaging studies have also been seen as one source of the illness and seem to be detectable in the early phases (Karlsgodt et al., 2008). Specifically, their functional impact on psychopathology and cognition is unraveling: for instance, frontal WM reduction is correlated with prefrontal alterations in working memory (Schlösser et al., 2007), whereas parietal and cerebellar WM abnormalities may contribute to the emergence of psychotic symptoms in early-onset schizophrenia (Kyriakopoulos et al., 2008). Moreover, cerebellar activation has been associated with delusions and suspiciousness/persecution (Whalley et al., 2007). This suggests that positive psychotic symptoms, such as delusions, might be better addressed if brain regions other than the TPC are targeted.
4.4. rTMS treatment of auditory hallucinations
Confirming prior studies, our meta-analysis demonstrates that rTMS to the left TPC results in robust therapeutic effects on AH. Indeed, even when only considering sham-controlled studies, the obtained effect size remained large and significant. In fact, this effect size was higher than the one obtained by Aleman et al. (2007)’s meta-analytic approach on this topic (d=0.76), in which different stimulation sites were analyzed together. Thus, the fact that we narrowed down our analysis to a single brain location and observed a larger effect size seems to indicate that the temporal association cortex plays a crucial role in the pathophysiology of AHs and offers a promising target for neuromodulatory therapeutic approaches. In this regard, the study by Hoffman and colleagues (2007) was particularly important, since it clearly showed an elevated response rate for rTMS to this region, as compared with rTMS to anterior temporal, frontal or right temporal areas.
Nevertheless, the treatment of AH deserves a few comments directed at possible ways for enhancing outcome. A critical finding of Hoffman et al. (2007)’s study concerned the discrepancy between the fMRI-guided TPC sites used in their trial and the standard TP3 site, which had little to no overlap. Moreover, in Sommer et al. (2007)’s study, 5 of the 7 patients undergoing functional-guided rTMS had predominant right-sided hallucinatory activity, and were therefore stimulated over the right TPC. Hence, this strongly suggests the need for individual assessment of the functional anatomy of hallucinations, so that the use of hallucination-activation maps, obtained either by PET or fMRI, might enhance TMS efficacy. Furthermore, it was recently showed that highest precision is achieved with individual, or even probabilistic, fMRI-guided stimulation (Sparing et al., 2008), as compared to other, less sophisticated approaches including coil position using the International 10/20 EEG electrode system.
Although global results are convincing, not all features of AHS seem to equally and adequately respond to rTMS. For instance, Hoffman et al. (2005) and Lee et al. (2005) found that frequency of AH was subject to significant treatment effects, while Fitzgerald et al. (2005) found a significant effect for loudness of voices. In contrast, Rosa et al. (2007) showed significant improvement after active rTMS in six of the seven AHRS items (except loudness), and maintenance of significant changes in four AH features at one-month follow-up. Yet, results are still mixed and further work focusing on the impact of rTMS on specific characteristics of AH is needed.
Another relevant finding by Hoffman et al. (2007) relates to the optimal number of sessions: only after the fourth site was probed (about 12 sessions) was there a significant improvement, regardless of the region being stimulated. Thus, the number of sessions might also impact on rTMS efficacy and outcome, and by far the majority of studies included in our analysis included a protocol with a maximum of 10 sessions. In other words, greater efficacy might be obtained if the number of sessions is expanded.
Finally, it is worth noting that only 5 out of the 12 studies entered in this meta-analysis included follow-up analysis. Hoffman et al. (2005) found that mean duration of survivorship was 13 weeks and close to 20 weeks among patients achieving responder status. In the study by Poulet et al. (2005), 50% of patients were still responders when they were followed up to 8 weeks. Chibbaro et al. (2005) found a delayed effect of rTMS on reduction of AH distinguishable from that of the sham group only at 3 weeks post-treatment and thereafter until week 8. In Sommer et al.’s study (2007) severity of AH was still significantly lower than baseline 10 weeks after the last treatment. Lastly, in Rosa et al.’s trial (2007) some AH features were still significantly improved at 6 weeks follow-up. However, further work on the duration of the effects of rTMS on AH is critically needed to assess the practical significance of this treatment. In this respect, modified parameters of rTMS that lead to longer-lasting cortical modulation, such as theta-burst stimulation (Huang and Rothwell, 2004), would seem worth testing.
Acknowledgments
We respectfully thank Drs. Alagona, Brunelin, Chae, D’Amato, Desarkar, Fitzgerald, George, Hoffman, Horacek, McLoughlin, Mogg, Pennisi, Rosa, and Sommer for their kind support and prompt contribution of data. Work on this study was supported in part by the Berenson-Allen Foundation, the Nancy Lurie Marks Family Foundation, the Harvard Clinical and Translational Science Center (Harvard Catalyst; NCRR – NIH UL1 RR025758) and a K24-RR018875 award from the National Institutes of Health to APL.
Footnotes
Conflicts of interest
We disclose no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Sommer IE, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68 (3):416–21. doi: 10.4088/jcp.v68n0310. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64 (2):81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, van’t Wout M, Aleman A, Kahn RS. Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychol Med. 2008;38 (5):747–54. doi: 10.1017/S0033291707001729. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Poulet E, Bediou B, Kallel L, Dalery J, D’amato T, Saoud M. Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res. 2006;81 (1):41–5. doi: 10.1016/j.schres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Chibbaro G, Daniele M, Alagona G, Di Pasquale C, Cannavò M, Rapisarda V, Bella R, Pennisi G. Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett. 2005;383 (1–2):54–7. doi: 10.1016/j.neulet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Chung YC, Im ES, Cho GH, Ko MH. Second run of transcranial magnetic stimulation has no effects on persistent auditory hallucinations. World J Biol Psychiatry. 2007;8 (1):48–50. doi: 10.1080/15622970600954044. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bernardo M, Masana J, Arrufat FJ, Navarro V, Valls-Solé, Boget T, Barrantes N, Catarineu S, Font M, Lomeña FJ. Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67 (1):129–30. doi: 10.1136/jnnp.67.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes J, Arends M, Mobascher A, Brinkmeyer J, Kornischka J, Eichhammer P, Klimke A, Winterer G, Agelink MW. Potential clinical targets of repetitive transcranial magnetic stimulation treatment in schizophrenia. Neuropsychobiology. 2006;54 (2):87–99. doi: 10.1159/000096990. [DOI] [PubMed] [Google Scholar]
- d’Alfonso AA, Aleman A, Kessels RP, Schouten EA, Postma A, van Der Linden JA, Cahn W, Greene Y, de Haan EH, Kahn RS. Transcranial magnetic stimulation of left auditory cortex in patients with schizophrenia: effects on hallucinations and neurocognition. J Neuropsychiatry Clin Neurosci. 2002;14 (1):77–9. doi: 10.1176/jnp.14.1.77. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22 (3):615–21. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli G, Gil A, Lobato MI, Marcolin MA, Belmonte-de-Abreu P. Repeated transcranial magnetic stimulation effect over treatment-resistant auditory hallucinations in a female patient with schizophrenia. Rev Bras Psiquiatr. 2007;29 (3):291–2. doi: 10.1590/s1516-44462007000300018. [DOI] [PubMed] [Google Scholar]
- Feinsod M, Kreinin B, Chistyakov A, Klein E. Preliminary evidence for a beneficial effect of low-frequency, repetitive transcranial magnetic stimulation in patients with major depression and schizophrenia. Depress Anxiety. 1998;7 (2):65–8. [PubMed] [Google Scholar]
- Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NA, de Castella A, Kulkarni J. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25 (4):358–62. doi: 10.1097/01.jcp.0000168487.22140.7f. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Benitez J, Daskalakis JZ, De Castella A, Kulkarni J. The treatment of recurring auditory hallucinations in schizophrenia with rTMS. World J Biol Psychiatry. 2006;7 (2):119–22. doi: 10.1080/15622970500474705. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Sritharan A, Benitez J, Daskalakis ZJ, Jackson G, Kulkarni J, Egan GF. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res. 2007;155 (1):83–8. doi: 10.1016/j.pscychresns.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Franck N, Poulet E, Terra JL, Daléry J, d’Amato T. Left temporoparietal transcranial magnetic stimulation in treatment-resistant schizophrenia with verbal hallucinations. Psychiatry Res. 2003;120 (1):107–9. doi: 10.1016/s0165-1781(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Takeuch K, Matsumoto T, Kamimura K, Hamada R, Nakamura K, Kato N. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psychiatry Res. 2007;154 (1):49–58. doi: 10.1016/j.pscychresns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Geller V, Grisaru N, Abarbanel JM, Lemberg T, Belmaker RH. Slow magnetic stimulation of prefrontal cortex in depression and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21 (1):105–10. doi: 10.1016/s0278-5846(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Goyal N, Nizamie SH, Desarkar P. Efficacy of adjuvant high frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: preliminary results of a double-blind sham-controlled study. J Neuropsychiatry Clin Neurosci. 2007;19 (4):464–7. doi: 10.1176/jnp.2007.19.4.464. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72 (1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56 (5):301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Grisaru N, Chudakov B, Yaroslavsky Y, Belmaker RH. Catatonia treated with transcranial magnetic stimulation. Am J Psychiatry. 1998;155 (11):1630. [PubMed] [Google Scholar]
- Hajak G, Marienhagen J, Langguth B, Werner S, Binder H, Eichhammer P. High-frequency repetitive transcranial magnetic stimulation in schizophrenia: a combined treatment and neuroimaging study. Psychol Med. 2004;34 (7):1157–63. doi: 10.1017/s0033291704002338. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ferrarelli F, Kalin NH, Tononi G. Transcranial Magnetic Stimulation in the investigation and treatment of schizophrenia: a review. Schizophr Res. 2004;71 (1):1–16. doi: 10.1016/j.schres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV, Jr, Lohr J, Wu J, Haier RJ, Bunney WE., Jr Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry. 1997;154 (5):682–4. doi: 10.1176/ajp.154.5.682. [DOI] [PubMed] [Google Scholar]
- Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76 (4):957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, Charney DS. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol Psychiatry. 1999;46 (1):130–2. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355 (9209):1073–5. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu YT, Carroll K, Krystal JH. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58 (2):97–104. doi: 10.1016/j.biopsych.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hampson M, Wu K, Anderson AW, Gore JC, Buchanan RJ, Constable RT, Hawkins KA, Sahay N, Krystal JH. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17 (11):2733–43. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60 (1):49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- Holi MM, Eronen M, Toivonen K, Toivonen P, Marttunen M, Naukkarinen H. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr Bull. 2004;30 (2):429–34. doi: 10.1093/oxfordjournals.schbul.a007089. [DOI] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, Novak T, Skrdlantova L, Klirova M, Bubenikova-Valesova V, Krajca V, Tislerova B, Kopecek M, Spaniel F, Mohr P, Höschl C. Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology. 2007;55 (3–4):132–42. doi: 10.1159/000106055. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Schneider U, Rollnik J. Gender differences in the effect of repetitive transcranial magnetic stimulation in schizophrenia. Psychiatry Res. 2003;120 (1):103–5. doi: 10.1016/s0165-1781(03)00170-7. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol. 2004;115 (5):1069–75. doi: 10.1016/j.clinph.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Jandl M, Bittner R, Sack A, Weber B, Günther T, Pieschl D, Kaschka WP, Maurer K. Changes in negative symptoms and EEG in schizophrenic patients after repetitive transcranial magnetic stimulation (rTMS): an open-label pilot study. J Neural Transm. 2005;112 (7):955–67. doi: 10.1007/s00702-004-0229-5. [DOI] [PubMed] [Google Scholar]
- Jandl M, Steyer J, Weber M, Linden DE, Rothmeier J, Maurer K, Kaschka WP. Treating auditory hallucinations by transcranial magnetic stimulation: a randomized controlled cross-over trial. Neuropsychobiology. 2006;53 (2):63–9. doi: 10.1159/000091721. [DOI] [PubMed] [Google Scholar]
- Jardri R, Lucas B, Delevoye-Turrell Y, Delmaire C, Delion P, Thomas P, Goeb JL. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry. 2007;12 (4):320. doi: 10.1038/sj.mp.4001968. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Kemp AS, Huerta ST, Alva G, Thai TM, Carreon D, Bunney WE., Jr Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (alphaTMS) on the negative symptoms of schizophrenia. Schizophr Bull. 2006;32 (3):556–61. doi: 10.1093/schbul/sbj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63 (5):512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Klein E, Kolsky Y, Puyerovsky M, Koren D, Chistyakov A, Feinsod M. Right prefrontal slow repetitive magnetic stimulation in schizophrenia: a double-blind sham-con- trolled pilot study. Biol Psychiatry. 1999;46:1451–1454. doi: 10.1016/s0006-3223(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Jäger M, Bottlender R, Frodl T, Holzinger S, Schmitt GJ, Zetzsche T, Burgermeister B, Scheuerecker J, Born C, Reiser M, Möller HJ, Meisenzahl EM. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39 (4):1600–12. doi: 10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23 (4):255–73. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Langguth B, Eichhammer P, Zowe M, Marienhagen J, Spiessl H, Hajak G. Neuronavigated transcranial magnetic stimulation and auditory hallucinations in a schizophrenic patient: monitoring of neurobiological effects. Schizophr Res. 2006;84 (1):185–6. doi: 10.1016/j.schres.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim W, Chung YC, Jung KH, Bahk WM, Jun TY, Kim KS, George MS, Chae JH. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376 (3):177–81. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DG, Johnstone EC, Ebmeier KP. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2004;127 (1–2):9–17. doi: 10.1016/j.psychres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mogg A, Purvis R, Eranti S, Contell F, Taylor JP, Nicholson T, Brown RG, McLoughlin DM. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93 (1–3):221–8. doi: 10.1016/j.schres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Nahas Z, McConnell KCS, Molloy M, Oliver NC, Risch SC, Christie S, Arana GW, George MS. Could left prefrontal rTMS modify negative symptoms and attention in schizophrenia? Biol. Psychiatry. 1999;45 (8 Suppl 1):37S. [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, McCarley RW. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131 (Pt 1):180–95. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Niznikiewicz M, Shenton ME. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130 (Pt 3):693–707. doi: 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák T, Horácek J, Mohr P, Kopecek M, Skrdlantová L, Klirova M, Rodriguez M, Spaniel F, Dockery C, Höschl C. The double-blind sham-controlled study of high-frequency rTMS (20 Hz) for negative symptoms in schizophrenia: negative results. Neuro Endocrinol Lett. 2006;27 (1–2):209–13. [PubMed] [Google Scholar]
- Onitsuka T, McCarley RW, Kuroki N, Dickey CC, Kubicki M, Demeo SS, Frumin M, Kikinis R, Jolesz FA, Shenton ME. Occipital lobe gray matter volume in male patients with chronic schizophrenia: A quantitative MRI study. Schizophr Res. 2007;92 (1–3):197–206. doi: 10.1016/j.schres.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15 (4):333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, d’Amato T, Saoud M. Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry. 2005;57 (2):188–91. doi: 10.1016/j.biopsych.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Kallel L, Bediou B, Dalery J, D’amato T, Saoud M. Is rTMS efficient as a maintenance treatment for auditory verbal hallucinations? A case report. Schizophr Res. 2006;84 (1):183–4. doi: 10.1016/j.schres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Kallel L, D’Amato T, Saoud M. Maintenance treatment with transcranial magnetic stimulation in a patient with late-onset schizophrenia. Am J Psychiatry. 2008;165 (4):537–8. doi: 10.1176/appi.ajp.2007.07060868. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Kumari V, Corr PJ, Fannon D, Sharma T. Neuropsychological function-brain structure relationships and stage of illness: an investigation into chronic and first-episode schizophrenia. Psychiatry Res. 2008;162 (3):195–204. doi: 10.1016/j.pscychresns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007a;95 (1–3):151–7. doi: 10.1016/j.schres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Skotakova S, Kasparek T, Ceskova E, Kucerova H, Ustohal L. Influencing negative symptoms of schizophrenia with repetitive transcranial magnetic stimulation: a case study. Acta Neuropsychiatrica. 2007b;19:53–55. doi: 10.1111/j.1601-5215.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Rollnik JD, Huber TJ, Mogk H, Siggelkow S, Kropp S, Dengler R, Emrich HM, Schneider U. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport. 2000;11 (18):4013–5. doi: 10.1097/00001756-200012180-00022. [DOI] [PubMed] [Google Scholar]
- Rosa MO, Gattaz WF, Rosa MA, Rumi DO, Tavares H, Myczkowski M, Sartorelli MC, Rigonatti SP, Elkis H, Cabral SB, Teixeira MJ, Marcolin MA. Effects of repetitive transcranial magnetic stimulation on auditory hallucinations refractory to clozapine. J Clin Psychiatry. 2007;68 (10):1528–32. doi: 10.4088/jcp.v68n1009. [DOI] [PubMed] [Google Scholar]
- Saba G, Rocamora JF, Kalalou K, Benadhira R, Plaze M, Aubriot-Delmas B, Januel D. Catatonia and transcranial magnetic stimulation. Am J Psychiatry. 2002;159 (10):1794. doi: 10.1176/appi.ajp.159.10.1794. [DOI] [PubMed] [Google Scholar]
- Saba G, Rocamora JF, Kalalou K, Benadhira R, Plaze M, Lipski H, Januel D. Repetitive transcranial magnetic stimulation as an add-on therapy in the treatment of mania: a case series of eight patients. Psychiatry Res. 2004;128 (2):199–202. doi: 10.1016/j.psychres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Saba G, Schurhoff F, Leboyer M. Therapeutic and neurophysiologic aspects of transcranial magnetic stimulation in schizophrenia. Neurophysiol Clin. 2006;36 (3):185–94. doi: 10.1016/j.neucli.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Loo C, Mitchell P, Malhi G. Transcranial magnetic stimulation for the deficit syndrome of schizophrenia: a pilot investigation. Psychiatry Clin Neurosci. 2005;59 (3):354–7. doi: 10.1111/j.1440-1819.2005.01382.x. [DOI] [PubMed] [Google Scholar]
- Schlösser RG, Nenadic I, Wagner G, Güllmar D, von Consbruch K, Köhler S, Schultz CC, Koch K, Fitzek C, Matthews PM, Reichenbach JR, Sauer H. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89 (1–3):1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Schönfeldt-Lecuona C, Grön G, Walter H, Büchler N, Wunderlich A, Spitzer M, Herwig U. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15 (10):1669–73. doi: 10.1097/01.wnr.0000126504.89983.ec. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Dannon PN, Goshen E, Amiaz R, Zwas TS, Grunhaus L. Right prefrontal rTMS treatment for refractory auditory command hallucinations - a neuroSPECT assisted case study. Psychiatry Res. 2002;116(1–2):113–7. doi: 10.1016/s0925-4927(02)00065-3. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57 (11):1033–8. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Jr, Buchsbaum MS, Bunney WE, Jr, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG, et al. Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am J Psychiatry. 1993;150 (9):1325–36. doi: 10.1176/ajp.150.9.1325. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RST. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378 (6553):176–9. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Sommer IE, de Weijer AD, Daalman K, Neggers SF, Somers M, Kahn RS, Slotema CW, Blom JD, Hoek HW, Aleman A. Can fMRI-guidance improve the efficacy of rTMS treatment for auditory verbal hallucinations? Schizophr Res. 2007;93 (1–3):406–8. doi: 10.1016/j.schres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29 (1):82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford AD, Sharif Z, Corcoran C, Urban N, Malaspina D, Lisanby SH. rTMS strategies for the study and treatment of schizophrenia: a review. Int J Neuropsychopharmacol. 2008;11 (4):563–76. doi: 10.1017/S1461145707008309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Pilowsky LS. Novel targets for drugs in schizophrenia. CNS Neurol Disord Drug Targets. 2007;6 (4):265–72. doi: 10.2174/187152707781387323. [DOI] [PubMed] [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97 (1–3):215–25. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Gountouna VE, Hall J, McIntosh A, Whyte MC, Simonotto E, Job DE, Owens DG, Johnstone EC, Lawrie SM. Correlations between fMRI activation and individual psychotic symptoms in un-medicated subjects at high genetic risk of schizophrenia. BMC Psychiatry. 2007;7:61. doi: 10.1186/1471-244X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Frodl T, Leinsinger G, Born C, Reiser M, Hegerl U, Möller HJ, Meisenzahl EM. White matter alterations in schizophrenic patients with pronounced negative symptomatology and with positive family history for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258(5):278–84. doi: 10.1007/s00406-007-0793-4. [DOI] [PubMed] [Google Scholar]