Abstract
AIM: To examine the immunoreactivity of E-cadherin and four subtypes of catenin family in human hepatocellular carcinomas (HCCs) and to investigate the correlation between expression of E-cadherin/catenin complex and clinicopathologic parameters of HCC patients.
METHODS: An immunohistochemical study for E-cadherin and catenins was performed on 97 formalin-fixed, paraffin-embedded specimens of HCC.
RESULTS: Reduced expression of E-cadherin, α-, β-, γ-catenin and p120 was observed in 69%, 76%, 63%, 71% and 73%, respectively. Both expressions of E-cadherin and catenin components were significantly correlated with tumor grade (P = 0.000). It showed significant difference between expression of catenin members and tumor stage (P = 0.003, P = 0.017, P = 0.007 and P = 0.000, respectively). The reduced expression of E-cadherin in HCCs was significantly correlated with intrahepatic metastasis (IM) and capsular invasion (P = 0.008, P = 0.03, respectively). A close correlation was also observed between the expression of catenins and the tumor size (P = 0.002, P = 0.034, P = 0.016 and P = 0.000, respectively). In addition, the expression of each catenin was found correlated with IM (P = 0.012, P = 0.049, P = 0.026 and P = 0.014, respectively). No statistically significant difference was observed between the expression level of E-cadherin/catenin complex and lymph node permission, vascular invasion and satellite nodules. Interestingly, only expression of p120 showed correlation with AFP value (P = 0.035). The expression of E-cadherin was consistent with α-, β-, γ-catenin and p120 expression (P = 0.000). Finally, the abnormal expression of E-cadherin/catenin complex was significantly associated with patients’ survival (P = 0.0253, P = 0.0052, P = 0.003, P = 0.0105 and P = 0.0016, respectively). Nevertheless, no component of E-cadherin/catenin complex was the independent prognostic factor of HCC patients.
CONCLUSION: Down-regulated expressions of E-cadherin, catenins and p120 occur frequently in HCCs and contribute to the progression and development of tumor. It may be more exact and valuable to detect the co-expression of E-cadherin/catenin complex than to explore one of them in predicting tumor invasion, metastasis and patient’s survival.
Keywords: E-cadherin, Hepatocellular carcinomas, Histologic feature, Survival
INTRODUCTION
E-cadherin is a transmembrane glycoprotein and plays an important role in the contact between epithelial cells depending on calcium. Homophilic interaction of the extracellular part of E-cadherin mediates cell-cell adhesion[1]. The adhesion mediated by E-cadherin is significantly implicated in the establishment of cell polarity, the transformation of epithelial/interstitial cells and the classification of cell types in the development[2,3]. The cytoplasmic part of E-cadherin is linked to the actin cytoskeleton via the catenins, including α-catenin, β-catenin, γ-catenin and p120[4]. E-cadherin/catenin complex is widely acknowledged as both tumor and metastasis suppressor, and the search for strategies to repress metastasis has led to intense studies of the mechanisms and molecules regulating E-cadherin function[2,3]. In addition, several recent trials have displayed the bright future of E-cadherin/catenin complex as a targeted therapy for human cancers[5-7].
The expression of E-cadherin/catenin complex has been shown to associate with tumor histological features (tumor size, grade, stage, invasion, metastasis, prognosis, etc.) in several cancers[8-12]. For liver cancer, some studies have reported that E-cadherin gene methylation or β-catenin exon 3 mutation occurred frequently and there was significant correlations between these abnormal biologic behavior and tumor development and progression[13-15]. In addition, some studies have explored the relationship between the clinicopathologic parameters of hepatocellular carcinoma (HCC) and the expression of E-cadherin/catenins complex, but the conclusions are still controversial. Endo et al[16] revealed over-expression of α-, β-, and γ-catenins in most HCCs, which was inversely correlated with histological grade of HCC, whereas E-cadherin expression was down-regulated and displayed a significant positive correlation with HCC grade. However, Ihara et al[17] showed over-expression of E-cadherin in their 66 HCCs, which was inversely correlated with tumor histological grade.
At present, there are little reports to demonstrate the relationship between the co-expression of above-mentioned five cell adhesion molecules and the clinicopathologic parameters (including patients’ prognosis) in HCC. Especially, there was no report about expression of p120 catenin in HCC patients. In the present study, we used immunohistochemical staining of E-cadherin/catenin complex in the primary lesion of surgically resected HCC specimens to clarify the correlations between the expression of E-cadherin/cadherin complex and the development and progression of HCC.
MATERIALS AND METHODS
Patient selection and definition of clinicopathologic parameters
In this study, we selected 97 cases of HCC that had been collected and diagnosed at the Eastern Hepatobilliary Surgery Hospital, the Second Military Medical University between October 1998 and March 2004. The patients consisted of 67 men and 30 women and ranged in age from 34 to 72 years, with an average age of 54 years. In each case, HCC tissues and nontumorous liver tissues were obtained for pathological examination. The detailed pathologic results were gained from the department of pathology of our hospital. Background liver showed cirrhosis in 72 cases (74.2%) and chronic hepatitis in 69 cases (71.1%) which included hepatitis B virus in 60 cases and hepatitis C virus (HCV) in 5 cases, and cryptogenic in 4 cases.
Clinicopathologic parameters include histological grades, stages, size, capsular and vascular invasion (portal vein cancer thrombus), satellite nodules, IM, lymph node permission, AFP value and patients’ survival. HCC was classified into grade I, II, III and IV according to Edomndson and Steiner[18]. Histological types of HCC were adopted according to the system of World Health Organization. HCC staging was performed according to TNM staging system of the International Union against Cancer UICC[19]. IM was defined as recurrent tumors consisting of moderately or poorly differentiated HCC with the same or lower degree of differentiation compared with the differentiation of the primary tumors[20]. The satellite nodules of this HCC represent either intrahepatic spread of the tumor or multicentric origin of the tumor. The patients were followed up for 8-68 mo.
Immunohistochemistry
All samples were collected from 5-μm-thick histological sections. They were cut from the formalin-fixed paraffin-embedded material. Dako EnVisionTM Kit (Dakocytomation Company, Denmark) was used for the immunohistochemical staining of E-cadherin, α-, β- and γ-catenins and p120. Sections were dewaxed, and incubated with mathenol containing 30% H2O2 for 20 min to block endogenous peroxidase activity. To enhance antigen retrieval, sections were treated at this stage in a microwave oven. Briefly, sections were immersed in 0.01 mol/L citrate buffer (pH 6.0) and heated in a microwave oven at 100°C for 20 min. Subsequently, they were washed three times with distilled water, and then blocked with 1% BSA for 30 min. Sections were incubated overnight at 4°C with rabbit polyclonal IgG of E-cadherin, α-catenin and p120 (Santa Clauze Corporation, USA, dilution 1:200) and mouse monoclonal IgG of β-catenin and γ-catenin (Santa Clauze Corporation, USA, dilution 1:200). A subsequent reaction was carried out using second antibodies ((Dakocytomation Company, Denmark, dilution 1:200) for 30 min at 37°C. Sections were washed three times with PBS buffer and subsequently displayed color with DAB for about 5 min. Nuclei were lightly counterstained with hematoxylin. No staining was obtained when nonimmune serum or PBS was used instead of the primary antibodies, thus confirming the specificity of each primary antibody.
Evaluation of immunostaining
We used a scoring system[21] to evaluate semiquantitatively the immunoexpression. The expression of non-tumorous tissue served as an internal control. Briefly, immunoractivities were assessed by the extent (broadness) and intensity (color strength). Depending on the percentage of positive cells, the extent score was classified as follows: 0, no positive cell or less than 5%; +1, 5%-25%; +2, 26%-50%; +3, 51%-75% and +4, 76%-100% positive cells. The intensity score was also categorized into four groups: 0, no immunoreaction; +1, mild immunoreaction; +2, moderate immunoreaction; and +3, marked immunoreaction. Preserved E-cadherin or catenin expression was defined when the composite score was 6 or 7. In contrast, the expression of E-cadherin or catenin was also defined as “absent or loss” when the total score was 0.
Statistical analysis
Results from immunohistochemistry were analyzed by Yates’ correction or Fisher’s exact tests and statistical significance was accepted when < 0.05. For survival analysis, log-rank test was used with a significant level of P < 0.05. Survival curves were computed according to the method of Kaplan-Meier. The prognosis value of these five molecules was evaluated with univariate and multivariate analysis. The SPSS 10.1 software package for Windows (SPSS, Inc., Chicago, IL) was used.
RESULTS
Microscopic observations
In nontumorous liver tissues, both E-cadherin and catenins were expressed strongly at cell membrane, but the staining strength gradually weakened from nontumoral tissue to the tumor region. In addition, bile ducts, proliferated ductiles and intrahepatic vessels strongly expressed these two molecules at the cell membrane. There was no expression in other cell types in the liver.
In 97 specimens of HCCs, the abnormal expression of E-cadherin, α-, β-, γ-catenin and p120 was found in 57, 65, 55, 60 and 62 cases (completely absent in 16, 19, 13, 10 and 15 cases), respectively (Table 1). The immunostaining distribution of E-cadherin was only presented at cell membrane, whereas catenins were found at the membrane in the cytoplasm and/or nucleus (Table 2, Figures 1 and 2).
Table 1.
Expression of five cell adhesion molecules in HCCs n (%)
| E-cadherin | α-catenin | β-catenin | γ-catenin | p120 | |
| Normal | 40 (41.2) | 32 (35.0) | 42 (35.0) | 37 (37.5) | 35 (42.5) |
| Reduced | 41 (42.3) | 46 (65.0) | 42 (65.0) | 50 (62.5) | 47 (57.5) |
| Absent | 16 (16.5) | 19 | 13 | 10 | 15 |
Table 2.
Distribution of complex expression in HCCs
| Membrane | Cytoplasm | Nucleus | |
| E-cadherin | 97 | 0 | 0 |
| α-catenin | 70 | 27 | 0 |
| β-catenin | 55 | 36 | 6 |
| γ-catenin | 63 | 34 | 0 |
| p120 | 65 | 32 | 0 |
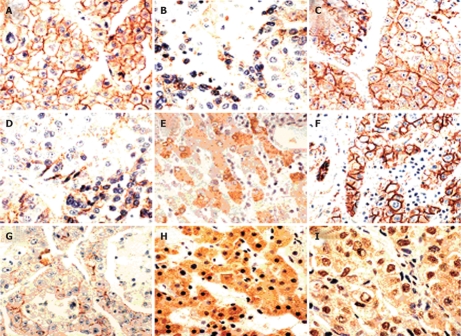
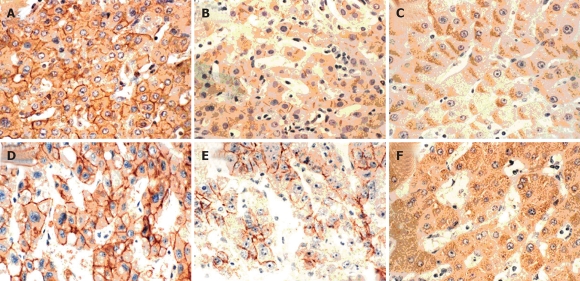
Figure 1.
Immunoreactivity of E-cadherin, α- and β-catenins in HCCs. A and B: Stained for E-cadherin: preserved type (+), reduced type (-); C-E: Stained for α-catenin: preserved type (+), reduced type (-) and staining in the cytoplasm; F-I: Stained for β-catenin: preserved type (+), reduced type (-) and staining in the cytoplasm and the nucleus (× 200).
Figure 2.
Immunoreactivity of γ-catenin and p120 in HCCs. A-C: Stained for γ-catenin: preserved type (+), reduced type (-) and staining in the cytoplasm; D-F: Stained for p120: preserved type (+), reduced type (-) and staining in the cytoplasm (× 200).
Relationship between expression of E-cadherin/catenin complex and histological features in HCCs
As shown in Table 3, expression of both E-cadherin and catenin components was significantly related with tumor grade (P = 0.000). There was a greater tendency for the expression of E-cadherin/catenin complex to reduce in poorly differentiated tumors than in well and moderately differentiated tumors. Except for E-cadherin, significant difference was found between expression of catenin members and tumor stage (P = 0.003, P = 0.017, P = 0.007 and P = 0.000, respectively).
Table 3.
Relationship between expression of E-cadherin/catenin complex and histological features and clinical parameters in HCCs
| E-cadherin | α-catenin | β-catenin | γ-catenin | p120ctn | ||
| n | +/- | +/- | +/- | +/- | +/- | |
| Histological grade | ||||||
| I-II | 23 | 20/3 | 19/4 | 22/1 | 20/3 | 19/4 |
| III | 58 | 17/41 | 11/47 | 20/38 | 11/47 | 15/43 |
| IV | 16 | 3/13 | 2/14 | 2/14 | 3/13 | 1/15 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Histological stage | ||||||
| I-II | 47 | 24/23 | 22/25 | 27/20 | 25/22 | 27/20 |
| III | 37 | 12/25 | 5/32 | 10/27 | 10/27 | 6/31 |
| IV | 14 | 4/10 | 3/11 | 5/9 | 2/12 | 2/12 |
| P | 0.136 | 0.003 | 0.017 | 0.007 | 0.000 | |
| Tumor size | ||||||
| < 3 cm | 29 | 15/14 | 17/12 | 18/11 | 17/12 | 19/10 |
| 3-10 cm | 50 | 18/32 | 10/40 | 16/34 | 13/37 | 12/38 |
| > 10 cm | 18 | 7/11 | 5/13 | 8/10 | 7/11 | 4/14 |
| P | 0.382 | 0.002 | 0.034 | 0.016 | 0.000 | |
| Capsular invasion | ||||||
| Present | 9 | 7/2 | 6/3 | 6/3 | 5/4 | 5/4 |
| Absent | 88 | 33/55 | 26/62 | 36/52 | 32/56 | 30/58 |
| P | 0.003 | 0.055 | 0.169 | 0.295 | 0.277 | |
| Satellite nodules | ||||||
| Present | 24 | 10/14 | 8/16 | 12/12 | 7/17 | 8/16 |
| Absent | 73 | 30/43 | 24/40 | 30/43 | 30/43 | 27/46 |
| P | 1.000 | 1.000 | 0.483 | 0.341 | 0.811 | |
| Vascular invasion | ||||||
| Present | 26 | 8/18 | 8/18 | 12/14 | 10/16 | 7/19 |
| Absent | 71 | 32/39 | 24/47 | 30/41 | 27/44 | 28/43 |
| P | 0.249 | 1.000 | 0.818 | 1.000 | 0.341 | |
| Lymph node permission | ||||||
| Present | 17 | 4/13 | 3/14 | 8/9 | 6/11 | 5/12 |
| Absent | 80 | 36/44 | 29/51 | 34/46 | 31/49 | 30/50 |
| P | 0.114 | 0.116 | 0.791 | 1.000 | 0.59 | |
| Intrahepatic metastasis | ||||||
| Present | 32 | 7/25 | 5/27 | 9/23 | 7/25 | 6/26 |
| Absent | 65 | 33/32 | 27/38 | 33/32 | 30/35 | 29/36 |
| P | 0.008 | 0.012 | 0.049 | 0.026 | 0.014 | |
| AFP-value | ||||||
| < 20 ng/dL | 28 | 6/12 | 13/15 | 16/12 | 14/14 | 15/13 |
| > 20 ng/dL | 69 | 24/45 | 19/50 | 26/43 | 23/46 | 20/49 |
| P | 0.068 | 0.096 | 0.113 | 0.167 | 0.035 |
Relationship between expression of E-cadherin/catenin complex and clinical parameters in HCCs
In Table 3, the reduced expression of E-cadherin in HCCs was significantly correlated with IM and capsular invasion (P = 0.008 and P = 0.03, respectively). A close correlation was also observed between expression of α-, β-, γ-catenin and p120 and the tumor size (P = 0.002, P = 0.034, P = 0.016 and P = 0.000, respectively). In addition, the expression of each catenin was found correlated with IM (P = 0.012, P = 0.049, P = 0.026 and P = 0.014, respectively). No statistically significant difference was observed between the expression level of E-cadherin/catenin complex and lymph node permission, vascular invasion and satellite nodules. Interestingly, only p120 expression was correlated with AFP value (P = 0.035).
Relationship between expression of E-cadherin and catenins in HCCs
As shown in Table 4, the expression of E-cadherin was significantly correlated to the expression of all kinds of catenins (P = 0.000). There was a significant concordance between the expression of E-cadherin and catenins.
Table 4.
Relationship between E-cadherin and catenin expressions in HCCs
| α-catenin |
β-catenin |
γ-catenin |
p120 |
||||||
| + | - | + | - | + | - | + | - | ||
| E-catenin | + | 28 | 12 | 33 | 7 | 29 | 11 | 31 | 9 |
| - | 4 | 53 | 9 | 48 | 8 | 49 | 4 | 53 | |
| P | 0.000 | 0.000 | 0.000 | 0.000 | |||||
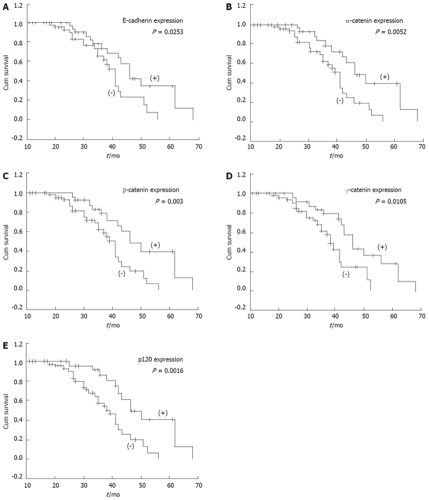
Relationship between expression of E-cadherin/catenin complex and patients’ survival
The overall patient survival according to the expression of E-cadherin and catenins in tumor is shown in Figure 3. Analysis of the survival for all patients showed that abnormal expression of E-cadherin and α-, β-, γ- and p120 catenins were correlated with poor survival (P = 0.0253, P = 0.0052, P = 0.003, P = 0.0105 and P = 0.0016, respectively). However, when E-cadherin/catenins complex status and other clinicopathological parameters were analyzed by the Cox regression model, abnormal expression of the E-cadherin/catenin complex was not found to be an independent prognostic factor (data not shown).
Figure 3.
Kaplan-Meier survival curves with normal (pink line) or abnormal (green line), A: Expression of E-cadherin (P = 0.0253); B: Expression of α-catenin (P = 0.0052); C: Expression of β-catenin (P = 0.003); D: Expression of γ-catenin (P = 0.0105); E: Expression of p120 (P = 0.0016). Abnormal expressions of E-cadherin/catenin complex significantly related with poor survival.
DISCUSSION
In accordance with previous reports[22,23], the expressions of all the members of E-cadherin/catenin complex were down-regulated in HCC, among of which the reduced expression of α-catenin was found most frequently. The expression of these five molecules was inversely correlated with tumor differentiation degree, thus all of them may be considered as good differentiation markers of HCC. Our result, however, is different from the reports of Endo and Ihara et al[16,17], which respectively showed over-expression of E-cadherin or α-, β- and γ-catenins in most HCCs. Therefore, together with CpG methylation of E-cadherin in HCC[13], it can be concluded that E-cadherin may act as a modulator of the maintenance of HCC histological architecture.
In addition, the reduced expression of all catenins, but E-cadherin, was significantly associated with tumor stage and tumor size in our study. The mechanism of these findings was unknown, but they were consistent with tumor clinical features, the small liver cancers have a relatively better clinical course. Interestingly, p120 expression was also correlated with AFP value. It is well known that AFP is only an indicator of malignant liver tumor, and its value is not always related to the classification of tumor cells, so it may be a coincidence and needs to be investigated in the future. However, the findings above may further demonstrate that E-cadherin/catenin complex, at least catenins, correlate with HCC’s biological behavior to some degree.
We found some distinct locations for different molecules of complex in HCC, especially for β-, γ-, and p120 catenin. In this study, hepatocytes, endothelial cells of bile ducts, proliferated ductiles and intrahepatic vessels of nontumorous liver tissue strongly expressed all of five molecules at cell membrane, but the staining strength gradually weakened from nontumorous tissue to tumor region. Besides expressing mostly at the membrane, these three members of armidillo protein family were also found in the cytoplasm or nucleus. It has been shown to be important in the development of several tumors that constitutionally activated the Wnt/Wingless signaling pathway by stabilization and accumulation of β-catenin in the nucleus and cytoplasm[24-27]. Like β-catenin, γ-catenin can also bind to APC protein and is able to translocate into the nucleus participating in the Wnt signaling pathway[28].
Several studies demonstrate that p120 can enter the nucleus and the Kaiso may be its receptor in nucleus[29]. Upon loss of E-cadherin, p120 translocates from the membrane to the cytoplasm. Alternatively, the cytoplasmic p120 pool may have increased access to the nucleus[30,31]. Therefore, these three molecules may play an important role in cell signal transduction and affect the development and progress of tumor. Unfortunately, there is no further investigation in relationship between p120 nucleus expression and clinicopathologic parameters in HCC due to the small number of cases with positive nucleus expression in our study.
As a focal point, we examined the relationships between expression of E-cadherin/catenin complex and some invasion and metastasis parameters of HCC. The reduced expression of E-cadherin in our experiment was significantly correlated with IM and capsular invasion. There was also a significant correlation between expression of catenins and IM in HCC. Nevertheless, no statistically significant difference was observed between the expression level of E-cadherin/catenin complex and lymph node permission, vascular invasion and satellite nodules. These results were different from those of the previous studies to some degree. Endo et al[16] showed significant positive correlations between γ-catenin high expression and capsular invasion or presence of satellite nodules, and between β-catenin high expression and vascular invasion. Kozyraki et al[23] found that E-cadherin and α-catenin expression in HCC was associated with occurrence of capsular and vascular invasion. Unlike most other types of tumors, very few HCC patients in our data had lymph node invasion due to their anatomic characters and complete liver capsule. Because of the small samples in our study (there were only 17 cases of lymph node, 9 cases of complete capsular and 26 cases of vascular invasion among 97 HCC patients), it is difficult to analyze the relationship between these parameters and expression of E-cadherin/catenin complex.
We were astonished that there was a significant correlation between expression of E-cadherin/catenin complex and IM in HCC. The mechanism and pathogenesis of the high frequency of IM in HCC have not yet been elucidated. Mitsunobu et al[32] demonstrated that tumor spread in HCC progresses from capsular invasion to intrahepatic invasion and that the portal vein may act as an efferent tumor vessel. Meada et al[33] pointed out that the histological features of the intrahepatic metastatic lesions are essentially the same as those of the main nodule. The proliferative activities in the intrahepatic metastatic lesions were generally higher than those in the main nodules. The fact that there are differences in the proliferative activity, despite the similarity in histology between the primary sites and the metastatic lesions, suggests that tumor cells of the metastatic lesions might acquire some characteristic advantages in forming metastasis. Preserved or recovered function of E-cadherin may be one of these advantages. Osada et al[34] found that E-cadherin is involved in the IM of HCC. Asayama et al[35] had a similar report in HCC-CC patients. These results indicated that E-cadherin/catenin complex might play an important role in the detachment of cancer cells.
To our knowledge, this is the first report of p120 catenin expression in human HCC. p120 was found to strangely correlate with differentiation, IM and patients’ survival, and p120 loss was associated with down-regulation of all members of the complex. Thoreson et al[30] suggested that it is possible that morphologic and behavioral changes in some tumors are due to p120 loss and consequent destabilization of E-cadherin. The roles for p120 as either tumor suppressor or metastasis promoter during tumor progression differ with the order, in which p120 or E-cadherin was down-regulated first. If p120 is lost first, E-cadherin level will fall significantly[36], which is likely to be paralleled by reduced levels of α - and β -catenins[37]. If E-cadherin is lost first, p120 may directly and actively promote metastasis.
With regard to the relationship between the E-cadherin/catenin complex expression and patients’ survival in HCC, we found that patients with low expression of these five molecules had poorer prognosis than those with normal expression. Unlike most previous reports[38-41], which revealed only one or a few members of this complex were associated with patients’ survival, our result demonstrated that all molecules of E-cadherin/catenin complex were significantly correlated with HCC patients’ survival. However, when E-cadherin/catenin complex status and other clinicopathological parameters were analyzed by the Cox regression model, abnormal expression of the E-cadherin/catenin complex was not found to be an independent prognostic factor. Thus, it may be more exact and valuable to detect the co-expression of E-cadherin/catenin complex than to explore only one of them.
In conclusion, these data indicated that abnormal expression of E-cadherin/catenin complex is common in HCC. The complex expression is mostly located in the cytoplasm of HCCs and correlated with tumor differentiation, IM and patients’ survival. E-cadherin/catenin complex may play an important role in development and progression of human HCC.
COMMENTS
Background
The E-cadherin/catenin complex plays a critical role in the control of epithelial differentiation and intercellular adhesion. Altered expression and/or function disruption of its components have been implicated in tumor development and progression.
Research frontiers
The expression of E-cadherin/catenin complex has been shown to associate with tumor histological features in several cancers. E-cadherin gene methylation or β-catenin exon 3 mutation occurred frequently in liver cancer and there was significant correlations between these abnormal biologic behavior and tumor development and progression. Some studies also have explored the relationship between hepatocellular carcinoma (HCC) and the expression of E-cadherin/catenin complex, but the conclusions are still controversial.
Innovations and breakthroughs
The results suggest that down-regulated expressions of E-cadherin, catenins and p120 occurred frequently in HCCs and contributed to the progression and development of tumor. It may be more exact and valuable to detect the co-expression of E-cadherin/catenin complex than to explore only one of them in predicting tumor invasion, metastasis and patient’s survival.
Applications
Because down-regulated expression of E-cadherin/catenin complex contributes to the progression and development of HCC, this complex can be used as a valuable biologic marker for predicting the invasion and metastasis of HCC.
Peer review
The authors have shown reduced expression of E-cadherin/catenins in HCC. It is a good study which prognosticates the invasiveness of HCC. The study indicated that E-cadherin/catenin complex may be valuable biological markers for predicting tumor invasion and metastasis. However, its clinical application should be further studied.
Footnotes
Peer reviewer: Yogesh K Chawla, PhD, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Li DL L- Editor Ma JY E- Editor Zhang WB
References
- 1.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 2.Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- 3.Yap AS. The morphogenetic role of cadherin cell adhesion molecules in human cancer: a thematic review. Cancer Invest. 1998;16:252–261. doi: 10.3109/07357909809039774. [DOI] [PubMed] [Google Scholar]
- 4.Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- 5.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72 Suppl 1:30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 6.Tommasi S, Pinto R, Pilato B, Paradiso A. Molecular pathways and related target therapies in liver carcinoma. Curr Pharm Des. 2007;13:3279–3287. doi: 10.2174/138161207782360663. [DOI] [PubMed] [Google Scholar]
- 7.Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, Herault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 8.Sarrio D, Moreno-Bueno G, Sanchez-Estevez C, Banon-Rodriguez I, Hernandez-Cortes G, Hardisson D, Palacios J. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum Pathol. 2006;37:1042–1049. doi: 10.1016/j.humpath.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Tang WW, Stelter AA, French S, Shen S, Qiu S, Venegas R, Wen J, Wang HQ, Xie J. Loss of cell-adhesion molecule complexes in solid pseudopapillary tumor of pancreas. Mod Pathol. 2007;20:509–513. doi: 10.1038/modpathol.3800764. [DOI] [PubMed] [Google Scholar]
- 10.Nozawa N, Hashimoto S, Nakashima Y, Matsuo Y, Koga T, Sugio K, Niho Y, Harada M, Sueishi K. Immunohistochemical alpha- and beta-catenin and E-cadherin expression and their clinicopathological significance in human lung adenocarcinoma. Pathol Res Pract. 2006;202:639–650. doi: 10.1016/j.prp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Bukholm IR, Nesland JM, Bukholm G. Expression of adhesion proteins E-cadherin, alpha-catenin, beta-catenin and gamma-catenin is different in T1 and T2 breast tumours. Pathology. 2006;38:403–407. doi: 10.1080/00313020600922520. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura E, Sugihara H, Bamba M, Hattori T. Dynamic alteration of the E-cadherin/catenin complex during cell differentiation and invasion of undifferentiated-type gastric carcinomas. J Pathol. 2005;205:349–358. doi: 10.1002/path.1718. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura T, Makino R, Mitamura K. Frequent down-regulation of E-cadherin by genetic and epigenetic changes in the malignant progression of hepatocellular carcinomas. Clin Cancer Res. 2001;7:594–599. [PubMed] [Google Scholar]
- 14.Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161:1015–1022. doi: 10.1016/S0002-9440(10)64262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, House MG, Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol. 2005;18:412–420. doi: 10.1038/modpathol.3800287. [DOI] [PubMed] [Google Scholar]
- 16.Endo K, Ueda T, Ueyama J, Ohta T, Terada T. Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum Pathol. 2000;31:558–565. doi: 10.1053/hp.2000.6683. [DOI] [PubMed] [Google Scholar]
- 17.Ihara A, Koizumi H, Hashizume R, Uchikoshi T. Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology. 1996;23:1441–1447. doi: 10.1053/jhep.1996.v23.pm0008675162. [DOI] [PubMed] [Google Scholar]
- 18.The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 19.Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 20.Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- 21.Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- 22.Nuruki K, Toyoyama H, Ueno S, Hamanoue M, Tanabe G, Aikou T, Ozawa M. E-cadherin but not N-cadherin expression is correlated with the intracellular distribution of catenins in human hepatocellular carcinomas. Oncol Rep. 1998;5:1109–1114. doi: 10.3892/or.5.5.1109. [DOI] [PubMed] [Google Scholar]
- 23.Kozyraki R, Scoazec JY, Flejou JF, D'Errico A, Bedossa P, Terris B, Fiorentino M, Bringuier AF, Grigioni WF, Feldmann G. Expression of cadherins and alpha-catenin in primary epithelial tumors of the liver. Gastroenterology. 1996;110:1137–1149. doi: 10.1053/gast.1996.v110.pm8613003. [DOI] [PubMed] [Google Scholar]
- 24.Ishizaki Y, Ikeda S, Fujimori M, Shimizu Y, Kurihara T, Itamoto T, Kikuchi A, Okajima M, Asahara T. Immunohistochemical analysis and mutational analyses of beta-catenin, Axin family and APC genes in hepatocellular carcinomas. Int J Oncol. 2004;24:1077–1083. [PubMed] [Google Scholar]
- 25.Park JY, Park WS, Nam SW, Kim SY, Lee SH, Yoo NJ, Lee JY, Park CK. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005;25:70–76. doi: 10.1111/j.1478-3231.2004.0995.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim YD, Park CH, Kim HS, Choi SK, Rew JS, Kim DY, Koh YS, Jeung KW, Lee KH, Lee JS, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:110–118. doi: 10.1111/j.1440-1746.2007.05250.x. [DOI] [PubMed] [Google Scholar]
- 27.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 29.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 31.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsunobu M, Toyosaka A, Oriyama T, Okamoto E, Nakao N. Intrahepatic metastases in hepatocellular carcinoma: the role of the portal vein as an efferent vessel. Clin Exp Metastasis. 1996;14:520–529. doi: 10.1007/BF00115112. [DOI] [PubMed] [Google Scholar]
- 33.Maeda T, Adachi E, Kajiyama K, Takenaka K, Sugimachi K, Tsuneyoshi M. Proliferative activity in intrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol Hepatol. 1996;11:752–757. doi: 10.1111/j.1440-1746.1996.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 34.Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460–1467. doi: 10.1053/jhep.1996.v24.pm0008938181. [DOI] [PubMed] [Google Scholar]
- 35.Asayama Y, Taguchi Ki K, Aishima Si S, Nishi H, Masuda K, Tsuneyoshi M. The mode of tumour progression in combined hepatocellular carcinoma and cholangiocarcinoma: an immunohistochemical analysis of E-cadherin, alpha-catenin and beta-catenin. Liver. 2002;22:43–50. doi: 10.1046/j.0106-9543.2001.01580.x. [DOI] [PubMed] [Google Scholar]
- 36.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- 38.Han SA, Chun H, Park CM, Kang SJ, Kim SH, Sohn D, Yun SH, Lee WY. Prognostic significance of beta-catenin in colorectal cancer with liver metastasis. Clin Oncol (R Coll Radiol) 2006;18:761–767. doi: 10.1016/j.clon.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, Jass JR. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. 2007;50:453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 40.Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, Lopes CS. Loss of beta-catenin is associated with poor survival in ovarian carcinomas. Int J Gynecol Pathol. 2004;23:337–346. doi: 10.1097/01.pgp.0000139711.22158.14. [DOI] [PubMed] [Google Scholar]
- 41.Paredes J, Correia AL, Ribeiro AS, Milanezi F, Cameselle-Teijeiro J, Schmitt FC. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J Clin Pathol. 2008;61:856–862. doi: 10.1136/jcp.2007.052704. [DOI] [PubMed] [Google Scholar]