Abstract
AIM: To investigate the intercellular spaces between the most superficially located esophageal epithelial cells in patients with gastroesophageal reflux disease (GERD).
METHODS: Eighteen patients with erosive esophagitis, 10 patients with non-erosive reflux disease (NERD), and 18 normal asymptomatic volunteers were enrolled. Biopsy specimens were obtained from the lower esophageal mucosa without ulcer or erosion. Scanning electron microscopy was employed to investigate the tightness of the superficial cellular attachment.
RESULTS: The intercellular space between the most superficially located epithelial cells in patients with erosive esophagitis or NERD was not different from that in asymptomatic healthy individuals.
CONCLUSION: Widened luminal intercellular spaces of esophageal superficial epithelium are not responsible for the induction of reflux symptoms in patients with GERD.
Keywords: Reflux esophagitis, Non-erosive gastroesophageal reflux diseases, Electron microscopy, Questionnaire
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a symptom complex caused by the reflux of gastric contents into the esophagus with and without complications[1,2]. Patients with esophageal complications, including esophageal erosions and ulcers, are reported to have a higher incidence of gastroesophageal acid reflux than those without complications[3,4]. The esophageal mucosa, composed of stratified squamous epithelium, has limited resistance against gastric juice because its secretion of mucus and bicarbonate is negligible in comparison with the gastric mucosa. Mucus is important for protecting the mucosal surface from pepsin, a protease present in gastric juice. Bicarbonate secretion into the mucus gel layer is necessary to neutralize hydrogen ions diffusing into it[5]. Therefore, when the esophageal mucosa is exposed to acidic gastric juice for a longer period, erosions and ulcers on the esophageal mucosa can easily be formed. GERD with these mucosal complications is known as reflux esophagitis (RE) and that without such complications is referred to as non-erosive reflux disease (NERD)[2]. Patients with NERD suffer significant reflux symptoms including heartburn and acid regurgitation, even in the absence of esophageal complications[2,6]. Afferent neurons with chemoreceptors, including transient receptor potential vanilloid type 1 (TRPV-1), are reported to terminate in the esophageal epithelial intercellular spaces[7,8]. In healthy individuals, these spaces are narrow and hydrogen ions and pepsin have difficulty to penetrate into them to stimulate noxious receptors on nerve terminals[5,9]. In order for acid and pepsin to penetrate into the intercellular spaces, the intercellular adhesion of the uppermost squamous epithelial cells needs to be damaged or widened[5,9,10]. Several reports have described the widening of intercellular spaces between squamous epithelial cells in mid-stratified epithelial layers by using transmission electron microscopy (TEM)[9-12]. However, there has been no description of intercellular adhesion between cells located in the uppermost part of the stratified squamous epithelium, since intercellular spaces of esophageal superficial epithelium could not be observed by TEM. Thus, this study was designed to investigate the luminal surface of the esophageal squamous epithelium in patients with GERD by using scanning electron microscopy and to examine the relationship between the tightness of esophageal superficial epithelial adhesion and the presence or absence of reflux symptoms.
MATERIALS AND METHODS
Patients
Eighteen symptomatic patients with RE (male/female = 14/4, mean age 58), 10 patients with NERD (male/female = 3/4, mean age 61), and 18 normal volunteers without any gastrointestinal and reflux symptoms (male/female = 13/5, mean age 65) were enrolled in this study. None of the subjects had a history of gastrointestinal surgery or had been taking drugs that would influence gastric acid secretion or esophageal function. Thirteen of the patients with RE were diagnosed as grade A according to the Los Angeles classification[3], 3 as grade B, and 2 as grade C. All the patients with RE or NERD had typical reflux symptoms and their scores for the Japanese version of the Carlsson-Dent self-administered questionnaire (QUEST)[13] were over 4.
This study was performed in accordance with the declaration of Helsinki. Written informed consent was obtained from all the patients and volunteers. The protocol of this study was approved by the ethics committee of Shimane University School of Medicine.
Methods
All the enrolled subjects were investigated by upper gastrointestinal endoscopy for the possible presence of esophageal mucosal breaks and to grade any mucosal breaks according to the Los Angeles classification. One biopsy specimen was taken from the esophageal mucosa without mucosal breaks at the frontal wall 2 cm above the squamous-columnar junction (SCJ), since the evident mucosal injuries are frequently observed at the level of SCJ in patients with RE. Hiatus hernia over a length of 2 cm was not found in any of the subjects.
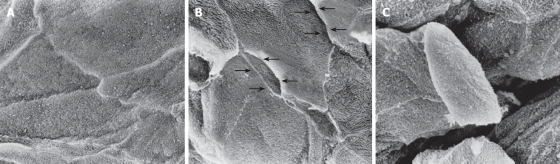
The biopsy samples were immediately immersed in fixative liquid and routinely processed for scanning electron microscopic observation as described previously[14-16]. The epithelial surface of the processed biopsy specimens was observed at a magnification of × 2000 using a Hitachi S-800 scanning electron microscope (Hitachi Co., Ltd., Tokyo, Japan). Three areas on the epithelial surface of each biopsy sample were randomly selected and electron micrographs were taken for analysis. The photographs were reviewed and graded by three gastroenterologists (KF, KK, and MM) who were blinded to the clinical diagnosis of each case. The reviewers graded the intercellular spaces between the surface epithelial cells on each of three photographs taken from a biopsy sample into 3 grades according to the sample photographs shown in Figure 1. Grade 1 represented a close and tight attachment of the surface epithelial cells. Grade 2 represented a loose attachment evident in some areas of the photo and the presence of widened intercellular spaces. Grade 3 represented looser intercellular adhesion and identifiable large intercellular spaces in over 50% of the area of intercellular attachment. If there was an inconsistency in the reading of photographs between 3 reviewers, the final diagnosis was decided by simultaneous review by the 3 authors. The mean value of these grades determined on each photo was calculated as the representative grade for each individual case. The intercellular space between the surface epithelial cells was also quantified with NIH image software (version 1.61, U.S. National Institute of Health) based on the threshold technique.
Figure 1.
Intercellular spaces between the most superficially located squamous epithelial cells were graded from 1 to 3. Grade 1 (A) represents close and tight attachment. Grade 2 (B) represents loose attachment in some areas, with widened intercellular spaces (arrows). Grade 3 (C) represents further loosening of intercellular adhesion (× 2000).
The intercellular adhesion, cellular surface structure of the uppermost epithelial cells was also observed and graded (Figure 2). When the cellular surface had regularly shaped multiple folds, the cellular surface structure was graded as 1. When no such regularly shaped folds were observed, it was graded as 3. A cellular surface intermediate between grades 1 and 3 was graded as 2.
Figure 2.
The cellular surface structure of the uppermost epithelial cells was graded from 1 to 3. Grade 1 (A) represented regularly shaped multiple folds. Grade 3 (C) represented an absence of such regularly shaped folds. A cellular surface intermediate between grades 1 and 3 was classified as grade 2 (B).
Statistical analysis
Statistical comparison between normal controls, patients with NERD, and patients with RE was performed by Kruskal-Wallis test followed by Mann-Whitney U-test. Differences at P < 0.05 were considered to be statistically significant.
RESULTS
Cellular attachments between surface squamous epithelial cells identified by scanning electron microscopy were remarkably diverse, as shown in Figure 3A. Even in normal asymptomatic individuals, grades of attachment between cells ranged from grade 1.0 to grade 2.4. Attachment grades in patients with symptomatic RE and NERD showed almost the same range of distribution as those in normal individuals. When the median and mean of each group were calculated, there were no statistically significant differences among the three groups. The percentage intercellular space quantified by NIH image in the three groups is shown in Figure 3B. Even quantitative measurement by NIH image revealed no significant difference between patients with GERD and normal individuals.
Figure 3.
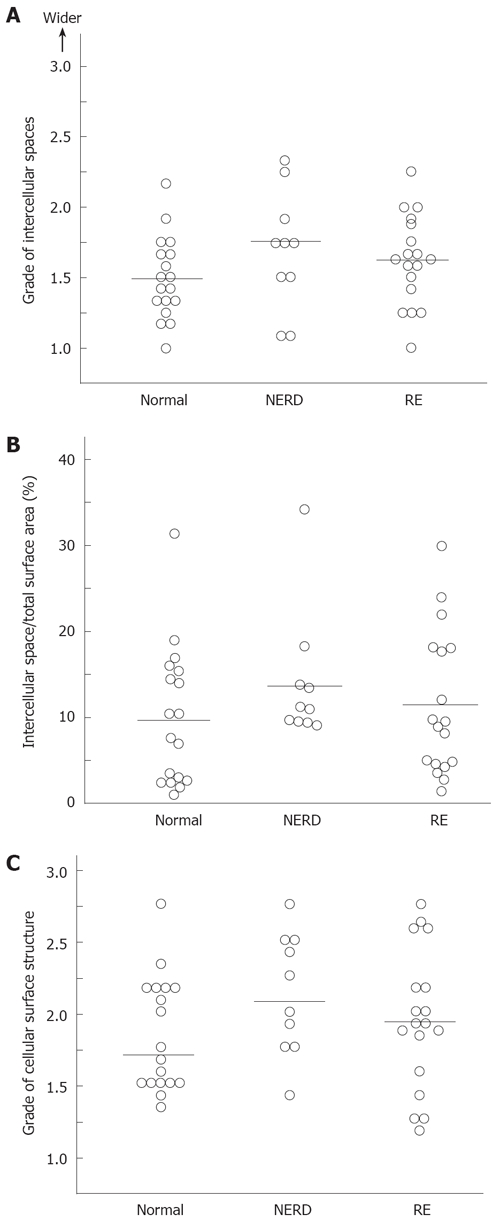
A: The intercellular spaces between the most superficially located squamous epithelial cells are shown. Each dot represents an individual case and horizontal lines show the median values. There was no statistically significant difference among the groups; B: The intercellular spaces between the most superficially located squamous epithelial cells were quantified using the NIH Image program. The proportion of intercellular space relative to total superficial area was determined in individuals with NERD and RE, and in asymptomatic healthy individuals. Each dot represents an individual case. Horizontal lines show the median values. There was no statistically significant difference among the groups; C: The regularity of the luminal cellular surface structure of the squamous cells was compared between normal individuals and patients with RE or NERD. There was no difference in the cellular surface structure between normal individuals and patients with RE or NERD. Each dot represents an individual case and horizontal lines show the median values.
Grades of epithelial cellular surface structure in individual cases are shown in Figure 3C. The grades of intercellular attachment and irregularity of the superficial cellular surface structure were distributed over a wide range. The median and mean grades among the three groups showed no statistically significant differences.
DISCUSSION
The presence of esophageal lesions and the presence of reflux symptoms are not well correlated[2]. Many patients with endoscopically evident esophageal erosions have no reflux symptoms[2,17-19]. In fact, our recent study confirmed that 74% of patients with esophageal erosions/ulcers do not have reflux symptoms, including heartburn or regurgitation[20]. Therefore, other pathological changes in the esophageal mucosa may have some role in the induction of reflux symptoms. Tobey and colleagues found widening of squamous epithelial intercellular spaces, not only in patients with RE, but also in patients with NERD[10]. In the epithelial intercellular space, many terminals of afferent neurons are present and convey various noxious stimuli to the central nervous system. When the intercellular spaces become widened, acid and pepsin refluxed from the stomach can easily penetrate the epithelial layer and stimulate noxious receptors on the afferent neurons and induce reflux symptoms[5]. An association between widened intercellular spaces and reflux symptoms has been reported[11,12]. In order to enter the mucosal intercellular spaces, acid and pepsin have to penetrate the intercellular adhesion sites of the uppermost superficial epithelial cells. In this study, the intercellular attachment of the most superficial squamous epithelial cells in the esophagus was investigated by scanning electron microscopy in asymptomatic normal individuals and symptomatic patients with RE or NERD. It was found that the gap between superficial epithelial cells varied markedly in individuals, even in healthy volunteers. Some individuals had tight attachment between superficial cells whereas others had loose intercellular attachment. The tightness of superficial cellular attachment did not differ significantly among normal volunteers, patients with NERD, and patients with RE.
Patients with NERD complain of significant reflux symptoms, even if their gastroesophageal acid reflux is limited[2,21,22]. Some investigators have reported that patients with NERD show hypersensitivity of the esophagus to esophageal extension and acid perfusion[23,24]. Loose attachment between esophageal squamous cells is the most easily suspected cause of esophageal acid hypersensitivity. In the present study, however, it was clarified that loose attachment between the most superficially located squamous cells was not a cause of acid hypersensitivity in patients with NERD.
There were several limitations to this study. The number of subjects investigated in this study was relatively small. In addition, we could not demonstrate the influence of esophageal acid reflux on the attachment of superficial squamous epithelium, since 24-h esophageal pH monitoring study was not performed in this study and the diagnosis of NERD was made by reflux symptoms and endoscopic findings. The location, from which the biopsy samples were obtained, also might influence the results of this study, since we took only one biopsy from the esophageal mucosa without mucosal breaks at the frontal wall 2 cm above the SCJ. Further study will be necessary to clarify the relationship between the attachment of superficial squamous cells and the attachment of more deeply located squamous cells. For this purpose, the combination of transmission and scanning electron microscopy might be useful.
In summary, using scanning electron microscopy, we have clarified that the intercellular adhesion of esophageal superficial squamous cells is diverse among individuals, and does not differ among normal individuals and patients with NERD and RE.
COMMENTS
Background
To investigate the intercellular spaces between the most superficially located esophageal epithelial cells in patients with gastroesophageal reflux disease (GERD).
Research frontiers
The widening of squamous epithelial intercellular spaces was demonstrated in patients with reflux esophagitis (RE), but also patients with non-erosive reflux disease (NERD), by using transmission electron microscopy (TEM), and an association between widened intercellular spaces and reflux symptoms has been reported. However, the intercellular attachment of the most superficial squamous epithelial cells in the esophagus was not investigated by using scanning electron microscopy.
Innovations and breakthroughs
The intercellular space between the most superficially located epithelial cells in patients with erosive esophagitis or NERD was not different from that in asymptomatic healthy individuals. Widened luminal intercellular spaces of esophageal superficial epithelium are not responsible for the induction of reflux symptoms in patients with GERD.
Applications
The relationship between the attachment of superficial squamous cells and the attachment of more deeply located squamous cells should be investigated in future by using the combination of transmission and scanning electron microscopy.
Terminology
The majority of patients with reflux symptoms do not have esophageal mucosal injury; these patients are referred to as NERD. Scanning electron microscopy used in this study can reveal the minimal changes of the luminal surface of the esophageal squamous epithelium.
Peer review
This is an interesting study. Authors investigated the intercellular spaces between the most superficially located esophageal epithelial cells in patients with GERD.
Acknowledgments
We wish to thank Ms. Rika Tohma and Ms. Keiko Masuzaki for their technical support.
Footnotes
Supported by The Grants-in-Aid from Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, No. 19590724
Peer reviewer: Gert De Hertogh, PhD, Department of Morphology and Molecular Pathology, University Hospitals KULeuven, Minderbroedersstraat 12, Leuven 3000, Belgium
S- Editor Li DL L- Editor Rippe RA E- Editor Lin YP
References
- 1.Dodds WJ, Hogan WJ, Helm JF, Dent J. Pathogenesis of reflux esophagitis. Gastroenterology. 1981;81:376–394. [PubMed] [Google Scholar]
- 2.Fass R, Fennerty MB, Vakil N. Nonerosive reflux disease--current concepts and dilemmas. Am J Gastroenterol. 2001;96:303–314. doi: 10.1111/j.1572-0241.2001.03511.x. [DOI] [PubMed] [Google Scholar]
- 3.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi K, Fujishiro H, Katsube T, Yuki M, Ono M, Kawamura A, Rumi MA, Watanabe M, Kinoshita Y. Predominant nocturnal acid reflux in patients with Los Angeles grade C and D reflux esophagitis. J Gastroenterol Hepatol. 2001;16:1191–1196. doi: 10.1046/j.1440-1746.2001.02617.x. [DOI] [PubMed] [Google Scholar]
- 5.Orlando RC. Review article: oesophageal mucosal resistance. Aliment Pharmacol Ther. 1998;12:191–197. doi: 10.1046/j.1365-2036.1998.00308.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiklund I, Bardhan KD, Muller-Lissner S, Bigard MA, Bianchi Porro G, Ponce J, Hosie J, Scott M, Weir D, Fulton C, et al. Quality of life during acute and intermittent treatment of gastro-oesophageal reflux disease with omeprazole compared with ranitidine. Results from a multicentre clinical trial. The European Study Group. Ital J Gastroenterol Hepatol. 1998;30:19–27. [PubMed] [Google Scholar]
- 7.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 10.Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525–532. doi: 10.1046/j.1365-2036.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, Miglioli M, Di Febo G. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100:537–542. doi: 10.1111/j.1572-0241.2005.40476.x. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson R, Dent J, Bolling-Sternevald E, Johnsson F, Junghard O, Lauritsen K, Riley S, Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 14.Ashizawa N, Niigaki M, Hamamoto N, Niigaki M, Kaji T, Katsube T, Sato S, Endoh H, Hidaka K, Watanabe M, et al. The morphological changes of exocrine pancreas in chronic pancreatitis. Histol Histopathol. 1999;14:539–552. doi: 10.14670/HH-14.539. [DOI] [PubMed] [Google Scholar]
- 15.Kaji T, Ishihara S, Ashizawa N, Hamamoto N, Endo H, Fukuda R, Adachi K, Watanabe M, Nakao M, Kinoshita Y. Adherence of Helicobacter pylori to gastric epithelial cells and mucosal inflammation. J Lab Clin Med. 2002;139:244–250. doi: 10.1067/mlc.2002.122280. [DOI] [PubMed] [Google Scholar]
- 16.Ashizawa N, Sakai T, Yoneyama T, Naora H, Kinoshita Y. Three-dimensional structure of peripheral exocrine gland in rat pancreas: reconstruction using transmission electron microscopic examination of serial sections. Pancreas. 2005;31:401–404. doi: 10.1097/01.mpa.0000181488.27399.dd. [DOI] [PubMed] [Google Scholar]
- 17.Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 18.Isolauri J, Laippala P. Prevalence of symptoms suggestive of gastro-oesophageal reflux disease in an adult population. Ann Med. 1995;27:67–70. doi: 10.3109/07853899509031939. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy T, Jones R. The prevalence of gastro-oesophageal reflux symptoms in a UK population and the consultation behaviour of patients with these symptoms. Aliment Pharmacol Ther. 2000;14:1589–1594. doi: 10.1046/j.1365-2036.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 20.Mishima I, Adachi K, Arima N, Amano K, Takashima T, Moritani M, Furuta K, Kinoshita Y. Prevalence of endoscopically negative and positive gastroesophageal reflux disease in the Japanese. Scand J Gastroenterol. 2005;40:1005–1009. doi: 10.1080/00365520510023260. [DOI] [PubMed] [Google Scholar]
- 21.Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537–545. doi: 10.1046/j.1365-2036.2003.01423.x. [DOI] [PubMed] [Google Scholar]
- 22.Shi G, Bruley des Varannes S, Scarpignato C, Le Rhun M, Galmiche JP. Reflux related symptoms in patients with normal oesophageal exposure to acid. Gut. 1995;37:457–464. doi: 10.1136/gut.37.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miwa H, Minoo T, Hojo M, Yaginuma R, Nagahara A, Kawabe M, Ohkawa A, Asaoka D, Kurosawa A, Ohkusa T, et al. Oesophageal hypersensitivity in Japanese patients with non-erosive gastro-oesophageal reflux diseases. Aliment Pharmacol Ther. 2004;20 Suppl 1:112–117. doi: 10.1111/j.1365-2036.2004.01990.x. [DOI] [PubMed] [Google Scholar]
- 24.Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7–12. doi: 10.1136/gut.37.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]