Abstract
AIM: To review the surgical outcomes in terms of the surgical indications and relevant prognostic factors.
METHODS: Sixteen patients underwent therapeutic lung surgery between March 1999 and May 2006. The observation period was terminated on May 31, 2007. The surgical outcomes and the clinicopathological factors were compared.
RESULTS: There was no mortality or major morbidity encountered in this study. The mean follow-up period after metastasectomy was 26.7 ± 28.2 (range: 1-99 mo), and the median survival time was 20 mo. The 1- and 5-year survival rates were 56% and 26%, respectively. At the end of the follow-up, 1 patient died from hepatic failure without recurrence, 6 died from hepatic failure with a recurrent hepatocellular carcinoma (HCC), and 4 died from recurrent HCC with cachexia. Among several clinical factors, Kaplan-Meier analysis revealed that liver transplantation as a treatment for the primary lesion, grade of cell differentiation, and negative evidence HBV infection were independent predictive factors. On Cox’s proportional hazard model, there were no significant factors affecting survival after pulmonary metastasectomy in patients with HCC.
CONCLUSION: A metastasectomy should be performed before other treatments in selected patients. Although not significant, patients with liver transplantation of a primary HCC survived longer. Liver transplantation might be the most beneficial modality that can offer patients better survival. A multi-institutional and collaborative study would be needed for identifying clinical prognostic factors predicting survival in patients with HCC and lung metastasis.
Keywords: Hepatocellular carcinoma, Pulmonary metastasis, Metastasectomy, Liver transplantation, Thoracoscopy
INTRODUCTION
Pulmonary metastasis from a hepatocellular carcinoma (PM-HCC) is quite frequent occurring in approximately 10% of patients treated with curative surgery for HCC[1-3]. However, its incidence varies according to the diagnostic modality, stage of the HCC, treatment plan, and observation time[1,4,5]. Unlike a PM from other solid tumors, it is difficult to manage PM-HCC, partly because it is commonly associated with advanced liver disease, or the patients cannot tolerate systemic chemotherapy for an intrahepatic recurrence or extrahepatic metastasis (EHM)[6,7]. In terms of tumor staging, patients who develop an EHM often have a tumor that is too advanced for surgical treatment and are offered systemic chemotherapy with or without combined locoregional therapy for the concurrent intrahepatic tumor[7,8]. Although few experiences have been reported, an active treatment strategy for better outcomes was proposed for an EHM with or without an intrahepatic recurrence[3,9,10]. Regarding the surgical indications for PM, the primary solid tumors should be controlled before or soon after performing a metastasectomy. However, transcatheter or percutaneous local therapy for a primary HCC has excellent therapeutic results. Therefore, a pulmonary metastasectomy with diagnostic or therapeutic intent may provide adequate management plans, such as liver transplantation or local therapy[10,11]. The treatment plans for a PM-HCC can be individualized by taking these factors into consideration. A large series of surgical treatments for PM from solid tumors has increasingly shown that a pulmonary metastasectomy is effective in prolonging the survival rate. Similarly, surgical experience for PM-HCC has increased, and favorable results on a metastasectomy have been reported even though they were from a select group of cases or a small series[2,3,10,12]. Therefore, a more aggressive approach to PM or extended surgical indication has been adopted to extend the survival rates of oncology patients. The aim of this study was to identify the appropriate candidates for aggressive intervention as well as to assess the clinical prognostic factors in patients with PM-HCC.
MATERIALS AND METHODS
From March 1999 to May 2006, 23 patients with a primary HCC were referred to our department for an evaluation of indeterminate pulmonary lesion(s) that had been resected by an open thoracotomy, or thoracoscopy. Patients with benign pulmonary lesions were excluded from the analysis of the survival rate and prognostic factors. The primary HCC was treated or planned to be treated by a hepatic resection, percutaneous or transcatheter local therapy before or after the pulmonary resection[13]. The surgical indications, which were similar to the previously described guidelines at our institution, included those patients who had resectable pulmonary metastases from the HCC before or after the treatment for the primary HCC, with the potential of complete control of the HCC after the metastasectomy or with a lower surgical risk (normal or mild liver dysfunction, acceptable lung function for partial resection or extended resection, normal cardiac function shown by Doppler echocardiography) for a metastasectomy (Table 1). Sixteen of 23 (60%) patients showed a true PM-HCC on the pathologic diagnosis. None of the patients were lost to follow-up and all patients were observed until May, 2007 after the pulmonary metastasectomy. The clinical outcomes were evaluated at regular 3-mo intervals with laboratory tests, which included serum α-feto protein and radiological imaging studies, such as a conventional or spiral chest computed tomography (CT) scan. In particular, the PET-CT scan was performed to evaluate the other EHM before thoracic surgery in selective cases. Although general adjuvant chemotherapy after a metastasectomy was not applied to the patients with the exception of one, locoregional therapy, such as percutaneous ethanol injection therapy (PEIT), radiofrequency ablation (RFA) or transcatherter arterial chemotherapy for the primary HCC, or radiation therapy, including tomotherapy for recurrent metastatic tumors in the lung, was used where needed. The overall survival time after the pulmonary metastasectomy was evaluated with respect to the disease-free interval (DFI), which is defined as the interval between the completion of the HCC treatment and the time the PM was diagnosed, the number of lung metastases, the therapeutic modality used for the primary lesion, such as surgical resection or liver transplantation, the number of pulmonary metastasectomies performed, and other clinical factors.
Table 1.
Guidelines for pulmonary metastasectomy1
| Primary lesion | Metastatic lesion | Patient factor |
| Completely cured | Therapeutic intents | Tolerable margin of safety in lung function |
| Controllable surgically or with other modalities after metastasectomy | Diagnostic intents (true malignancy, evaluate chemotherapy) | Good risk for other organ functions (heart and cerebral status) |
Surgical treatment for PM-HCC
The surgical plan for PM was considered based on the anatomical characteristics of the lung lesions, patients’ medical status including the liver function values or potential risk factors for general anesthesia and operation. The tumor was resected conservatively by a pulmonary wedge resection using either a thoracoscopy or an open thoracotomy in order to preserve the lung function and minimize the surgical risk to the patients with chronic liver diseases. There was only one case of an anatomical lobectomy. For such reasons, a limited resection through a thoracoscopy was applied widely to the patients in this study. However, the thoracoscopic technique was modified in that for difficult lesions, such as a small nodule or deep seated pulmonary lesion, barium sulfate or microcoils were injected into or around the nodule under CT guidance[14]. Moreover, metastatic nodules localized using radiopaque materials, in which the radiopacity was visualized on the portable fluoroscopic monitor and was helpful to guide thoracoscopic resection, could be detected and resected easily. This technique enabled us to avoid an open thoracotomy and allowed for an additional repeat thoracotomy with minimal morbidities. In addition, an open thoracotomy was adopted for some specific lesions that were centrally located or recurrent lesions with possible dense pleural adhesion caused by the previous thoracotomy.
Treatment of primary HCC
The treatment for the primary HCC or intrahepatic recurrent tumor was performed before (n = 21) or after (n = 2) the pulmonary metastasectomy: hepatic resection, liver transplantation and local therapy for 5, 7 and 5 patients with an intrahepatic recurrence or concurrent PM-HCC, respectively.
Statistical analysis
The survival curves of 16 patients who were pathologically proven to have a metastatic lesion were calculated using the Kaplan-Meier method, and the survival according to the prognostic factors was compared using a log-rank test. Using Cox’s proportional hazard model, clinical prognostic indicators predicting increased survival of patients with HCC and pulmonary metastasectomy were tested. The statistical study was performed with SAS statistical software package (version 6.12, SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Sixteen of 23 (60%) patients, who had undergone a pulmonary resection, were diagnosed with a PM-HCC on pathology examination. Of the 16 PM-HCC, 12 patients had a large primary tumor or multicentricity, and 9 patients showed poor differentiation according to the Edmonson-Steiner grading system, which determines the grade of HCC differentiation. Table 2 summarizes the clinical features of the 16 PM-HCC before or after the pulmonary metastasectomy. Of the 16 patients, 2 had a concurrent PM-HCC at the time of the initial presentation, and the PM-HCC was developed in sequence in 14 patients after treatment for the primary lesion. There was a mean 14 ± 4 mo (range: 0-38 mo) DFI between the completion of the HCC treatment and the time the PM was diagnosed. Twenty-five thoracic procedures were performed for 30 pulmonary lesions of PM-HCC. Among them, 4 concurrent nodules showed benign characteristics. There were no surgery-related deaths or major complications but one patient had a prolonged hospital stay in consequence of air leakage after a repeated resection by an open thoracotomy.
Table 2.
Clinical findings of 16 patients with HCC who underwent pulmonary metastasectomy
| Clinical findings | Number of patients (n = 16) | Percents of patients (%) |
| Gender: male/female | 14/2 | 87.5/12.5 |
| Age at pulmonary resection (yr) | 45 ± 9.8 (31-67)1 | |
| Etiology of liver diseases | ||
| Cirrhosis + HCC + HBV | 5 | 31.3 |
| Cirrhosis + HCC | 2 | 12.5 |
| Alcoholic liver disease + HCC | 2 | 12.5 |
| HCC + HBV | 7 | 43.8 |
| Maximal diameter of lung lesions | 13 ± 10 (3-50 mm) | |
| Treatment of primary lesion2 | ||
| Hepatic resection | 5 | 31.3 |
| Liver transplantation | 7 | 43.8 |
| Local therapy | 5 | 31.3 |
| Treatment of lung lesions2 | ||
| Thoracoscopic resection | 15 | 93.8 |
| Open wedge resection | 9 | 56.3 |
| Lobectomy | 1 | 6.3 |
| Tomotherapy after recurrence | 2 | 12.5 |
| Maximal diameter of lung lesions | 13 ± 10 (3-50 mm) | |
| Number of lung metastasectomy | ||
| 1 | 9 | 56.2 |
| ≥ 2 | 7 | 43.8 |
| HBV hepatitis | ||
| Yes | 12 | 75.0 |
| No | 4 | 25.0 |
| HCC pathologic finding | ||
| Well differentiated | 7 | 43.8 |
| Poorly differentiated | 9 | 56.2 |
| Alpha fetoprotein | ||
| > 20 ng/mL | 8 | 50.0 |
| < 20 ng/mL | 8 | 50.0 |
| Liver cirrhosis | ||
| Yes | 7 | 43.8 |
| No | 9 | 56.3 |
mean ± SD (range);
Multiple therapies were performed.
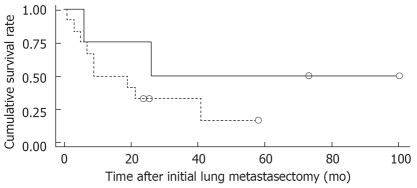
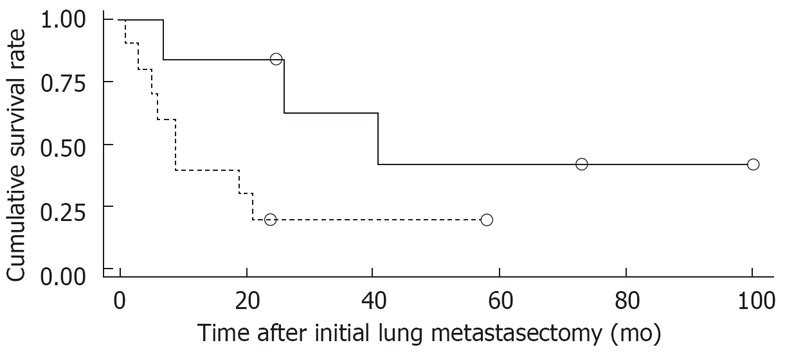
Figure 1 shows the overall survival rates after the initial diagnosis of the primary HCC and pulmonary metastasectomy. The median survival time after the pulmonary metastasectomy was 20 mo. The 1- and 5 year survival rates after the pulmonary metastasectomy were 56% and 26%, respectively. When the prognosis after the pulmonary metastasectomy was compared according to the therapeutic modality for primary HCC, the survival was found to be significantly higher in patients who had undergone liver transplantation than in those who had received other treatments, such as a surgical resection, locoregional therapy (Figure 2). In addition, among clinical factors, liver transplantation, negative evidence of an HBV infection (Figure 3) and well differentiation of HCC (Figure 4) showed longer survival by log rank test.
Figure 1.
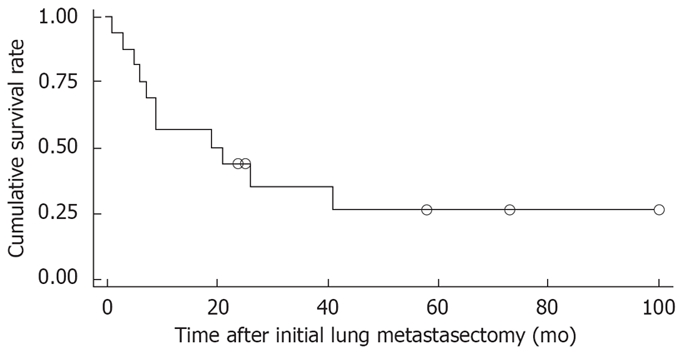
Overall cumulative survival curves of 16 patients with metastasectomy (Open circles indicate censored cases).
Figure 2.
Comparison of survival in patients with HCC after pulmonary metastasectomy by liver transplantation. Patients who had undergone liver transplantation survived longer significantly (log-rank test, P = 0.014).
Figure 3.
Comparison of survival in patients with HBV hepatitis-related HCC after pulmonary metastasectomy. A significant difference was found between the two groups (log-rank test, P = 0.0386).
Figure 4.
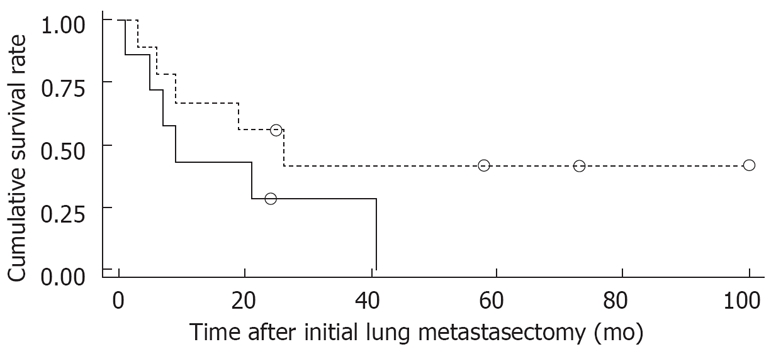
Comparison of survival in patients with HCC after pulmonary metastasectomy by pathologic findings for histologic differentiation. A significant difference was found between the two groups (log-rank test, P = 0.0392).
A total of 11 patients died during the follow-up period (Table 3). Six patients developed an intrahepatic recurrence during the follow-up after the metastasectomy: one patient was treated successfully by local therapy but another two developed an extrahepatic metastasis during treatment. This suggests that control of the primary lesion can result in a favorable prognosis even in the advanced stage. Among the 9 patients with recurrent PM-HCC after the pulmonary metastasectomy, they all underwent repeated pulmonary metastasectomy more than twice, of whom two received tomotherapy for recurrence. Five of these patients are still alive, four of whom show no evidence of recurrent disease (98, 73, 25, 58 mo, respectively).
Table 3.
Follow-up results of 16 patients with HCC after pulmonary metastasectomy
| Number of patients (n = 16) | Percentages of patients (%) | |
| Status at the last observation period | ||
| Survival without recurrence | 4 | 25.0 |
| Survival with recurrence | 1 | 6.25 |
| Died of recurrence, cachexia | 4 | 25.0 |
| Died of hepatic failure + recurrence | 6 | 37.5 |
| Died of hepatic failure | 1 | 6.25 |
DISCUSSION
HCC is a major public health problem in Asian countries, including Korea, where HBV infections are endemic[2,6,14]. A longer survival of HCC has been achieved with the recent advances in diagnostic and therapeutic modalities. However, the presence of an extrahepatic metastasis (EHM) of an HCC, which frequently occurs in the lung, regional nodes, bone and brain[4,6], is an indicator of a poor prognosis with a very low 1-year survival rate[6,10]. Despite the advances in therapeutic strategies, an EHM is still a major obstacle for further improving the overall survival and prognosis of the patients. Unfortunately, patients with an EHM do not tolerate systemic chemotherapy well, which is first considered as the therapeutic modality for EHM that finally leads to a shorter survival time[6,8,15,16]. For these reasons, the surgical management of PM-HCC might be a good option. With increasing experience[14], a pulmonary metastasectomy has become more acceptable. Hence, we have developed new guidelines by modifying previous results[17,18] (Table 1).
The incidence or prevalence of PM-HCC depends on the T category, imaging technique, observation time, and therapeutic modality[1,4,5,9]. Because most patients with PM-HCC do not show pulmonary symptoms until they progress to full-blown disease, spiral CT scan is essential for making an early and accurate diagnosis of a PM-HCC. Although PET is effective in detecting distant metastasis in other solid tumors, the diagnostic accuracy of primary HCC showed limited clinical significance[19]. In the present series, 5 true metastatic lesions in 3 patients were confirmed by pathological examination but PET-CT did not show any diagnostic accuracy for these lesions, which might be due to the small lesions or the low standardized uptake values (SUV). By contrast, serial monitoring of the α-feto protein level might help to confirm PM-HCC in the absence of a primary HCC or detect an early recurrence or EHM. However, in this study, the α-feto protein level was elevated by more than 20 ng/mL in only 8 (50%) of the 16 patients with a PM-HCC. The reason why only 50% of patients with an EHM had an elevated α-feto protein level might be due to a small lesion (< 1 cm) or severe liver dysfunction.
The pathological characteristics of the concurrent or sequential pulmonary lesions in patients with a primary HCC can affect the treatment plan and prognosis[15]. Of the 23 patients with a preoperative suspicious metastasis in this series, only 16 (70%) had a true PM-HCC. In addition, the therapeutic plan was consequently changed in 9 of the 23 (39%) patients according to the pathology results. A less invasive or conservative pulmonary resection is recommended for treating a PM from a solid tumor in order to preserve the lung function in the case of a possible PM in future. As shown in this series, the thoracoscopic resection was performed in 10 of 25 (40%) procedures. Although thoracoscopy did not affect the surgical outcome or survival compared with an open thoracotomy in this and other series, it may allow for better satisfaction and an additional pulmonary metastasectomy[14,20].
The prognosis of patients with a PM-HCC has been reported to be as low as 20%-30% at 1 yr despite receiving systemic chemotherapy[6,16]. A pulmonary metastasectomy of an HCC increases the median survival or long-term survival[2,10]. Although there were no consistent and complete prognostic indicators, several clinical or pathological factors have been reported[2,3,5,16,20]. Generally, patient selection according to the prognostic factors, such as single metastasis and small primary HCC, is essential for a survival benefit by surgery. In this series, liver transplantation, negative evidence of an HBV infection, and well differentiation of HCC were significant prognostic indicators among the other clinical factors. Accordingly, this finding suggests that hepatic factors indicating a complete resection and good liver function might be essential for better survival[11,16]. However, Cox’s proportional hazard model (Tables 4 and 5) showed no significant clinical indicators, which might be attributed to small numbers of patients and poor survival due to high virulence of HCC. More importantly, a brain metastasis of HCC is not common but life threatening, and should be detected early, especially in patients with PM-HCC[5,20,21].
Table 4.
Cox’s proportional hazards model of all factors predicting increased survival after pulmonary metastasectomy in patients with HCC
| Prognostic factors | Parameter estimate (β) | P | HR | 95% confidence interval |
| Disease-free interval (≤ 12 vs > 12 mo) | 1.97545 | 0.4005 | 7.21 | 0.072-721.009 |
| HBV viral hepatitis (vs no) | -33.8718 | 0.9906 | 0 | 0-. |
| Well differentiation grade (vs poorly) | -18.73237 | 0.9896 | 0 | 0-. |
| α-feto protein > 20 ng/mL (yes vs no) | -4.49444 | 0.0748 | 0.011 | .-1.569 |
| Liver transplantation (vs no) | 32.71851 | 0.9818 | 1.62E + 14 | 0-. |
| Single metastasectomy (vs multiple) | -15.0999 | 0.0572 | 0 | 0-1.83 |
| Thoracoscopy (vs others) | -13.02123 | 0.9928 | 0 | 0-. |
| Age | 0.55293 | 0.0419 | 1.738 | 1.021-2.961 |
Table 5.
Cox’s proportional hazards model of liver transplantation, HBV viral hepatitis, well differentiation grade predicting increased survival after pulmonary metastasectomy in patients with HCC
| Prognostic factors | Parameter estimate (β) | P | HR | 95% confidence interval |
| HBV viral hepatitis (vs no) | -0.33272 | 0.7674 | 0.717 | 0.079-6.504 |
| Well differentiation grade (vs poorly) | -0.7663 | 0.3007 | 0.465 | 0.109-1.984 |
| Liver transplantation (vs no) | 1.26672 | 0.1568 | 3.549 | 0.615-20.495 |
During the follow-up after the metastasectomy, EHM or intrahepatic recurrence may be controlled by irradiation therapy[20,22], local therapy[10,13] as well as by a repeated metastasectomy[10,12,20,23]. In this series, a repeated pulmonary resection for a recurrent PM-HCC after a metastasectomy was performed in 6 patients. Although patients with a single metastasis or single pulmonary surgery might show better survival[2,20], multiple metastases or repeated metastasectomies are not risk factors for long-term survival, as shown in this and previous studies[12,24].
When considering the cause of death in this and other reports, hepatic dysfunction complicating liver cirrhosis was one of the most important factors for the long-term survival[2,16,25]. Considerable attention should be paid to patient selection, which may be essential for achieving long-term survival in patients with a PM-HCC according to prognostic analysis. As shown in this study and suggested by others, the prognosis may be affected by many clinical or pathologic parameters, which may be major limitations in clinical reports including these results.
In summary, selected patients without an intrahepatic HCC and with a good hepatic reserve showed that a pulmonary metastasectomy is effective in controlling a PM-HCC and can offer the only chance for long-term survival.
COMMENTS
Background
Treatment of patients with primary hepatocellular carcinoma (HCC) and concurrent or subsequent pulmonary metastasis (PM) has no definite or proper guidelines, but it has been reported that pulmonary metastasectomy has been proven to prolong the survival in a selected group or a small series. Local therapy and surgical resection have been effective in treating the primary HCC. However, systemic chemotherapy for distant metastasis had limitations, such as poor toleration and aggravation of liver function. Although a few clinical prognostic factors affected the survival rate in patients after pulmonary metastasectomy, there were limitations in terms of small number and heterogenosity of patients.
Research frontiers
Diagnostic and therapeutic surgical interventions for PM in patients with HCC affected the treatment plans depending on the result of pathology. In fact, surgical metastasectomy of PM may provide perioperative and anaesthetic risk, particularly in patients with hepatic dysfunction. Moreover, repeat metastasectomy for patients with recurrent PM who had no other distant metastasis may have technical difficulties despite of good results. Adequate guidelines for management of PM-HCC have been proposed based on significant prognostic factors increasing survival. Pulmonary metastasectomy may be a good option for PM-HCC.
Innovations and breakthroughs
A better result on surgical management of PM would be achieved with collaborative investigation and adequate guidelines. This strategy provided better survival and none to minimal surgical risk. Moreover, significant prognostic factors, such as liver transplantation as a treatment modality for primary disease, absence of HBV infection, and well differentiation in pathology, were identified despite of small numbers of patients.
Applications
Pulmonary metastasectomy or more extended resection should be considered for diagnostic or therapeutic surgical interventions in managing patients with collaborative approaches, otherwise most of them might survive for less than one year. When considering surgery, the adequate guidelines, as proposed in this series should be adopted for better results. Firstly, adequate control over primary liver cancer is prerequisite. Aggressive management has the possibility of better results in patients who had undergone liver transplantation. Secondly, conservative resection or minimally invasive surgery may help to treat recurrent lung lesions or to provide less risk. Lastly, PM usually indicates advanced stage in patients with HCC, therefore other distant metastasis, particularly brain metastasis, should be excluded in surgical indications and be carefully detected by MRI on follow-up.
Peer review
This paper reported the clinical significance of pulmonary metastasectomy to improve the prognosis of patients with HCC. This manuscript arouses interest for readers and provides an important clue to treat such patients and alter the survival rate.
Footnotes
Supported by 21C Frontier Functional Human Genome Project from the Ministry of Science & Technology in Korea, No. FG06-11-11
Peer reviewer: Akihito Tsubota, Assistant Professor, Institute of Clinical Medicine and Research, Jikei University School of Medicine, 163-1 Kashiwa-shita, Kashiwa, Chiba 277-8567, Japan
S- Editor Li DL L- Editor Kumar M E- Editor Lin YP
References
- 1.Inagaki Y, Unoura M, Urabe T, Ogino H, Terasaki S, Matsushita E, Kaneko S, Morioka T, Furusawa A, Wakabayashi T. Distant metastasis of hepatocellular carcinoma after successful treatment of the primary lesion. Hepatogastroenterology. 1993;40:316–319. [PubMed] [Google Scholar]
- 2.Lam CM, Lo CM, Yuen WK, Liu CL, Fan ST. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198–1200. doi: 10.1046/j.1365-2168.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 3.Tomimaru Y, Sasaki Y, Yamada T, Eguchi H, Takami K, Ohigashi H, Higashiyama M, Ishikawa O, Kodama K, Imaoka S. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. Am J Surg. 2006;192:46–51. doi: 10.1016/j.amjsurg.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 5.Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 6.Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka H, Ikeda K, Yoshimori M. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology. 1997;44:251–257. [PubMed] [Google Scholar]
- 7.Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma. Adriamycin versus quadruple chemotherapy. Cancer. 1984;53:401–405. doi: 10.1002/1097-0142(19840201)53:3<401::aid-cncr2820530306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Jang JW, Park YM, Bae SH, Choi JY, Yoon SK, Chang UI, Nam SW, Kim BS. Therapeutic efficacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:415–420. doi: 10.1007/s00280-004-0829-7. [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Takenaka K, Gion T, Fujiwara Y, Kajiyama K, Maeda T, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology. 1996;111:720–726. doi: 10.1053/gast.1996.v111.pm8780578. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, O'Suilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002;195:311–318. doi: 10.1016/s1072-7515(02)01226-7. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol. 2005;43:584–589. doi: 10.1016/j.jhep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 12.O'Suilleabhain CB, Poon RT, Lau CW, Fan ST. Repeated resections of extrahepatic metastases after hepatic resection: an aggressive approach to hepatocellular carcinoma. Hepatogastroenterology. 2004;51:825–829. [PubMed] [Google Scholar]
- 13.Jang JW, Choi JY, Bae SH, Kim CW, Cho SH, Yoon SK, Yang JM, Han JY, Lee YS, Kim DG. The best candidates for transarterial chemotherapy in patients with hepatocellular carcinoma awaiting liver transplantation: a cohort-based characterization of dropout times. Aliment Pharmacol Ther. 2007;26:87–94. doi: 10.1111/j.1365-2036.2007.03345.x. [DOI] [PubMed] [Google Scholar]
- 14.Moon SW, Wang YP, Jo KH, Kwack MS, Kim SW, Kwon OK, Jang HS. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg. 1999;68:1815–1820. doi: 10.1016/s0003-4975(99)00764-x. [DOI] [PubMed] [Google Scholar]
- 15.Utsunomiya T, Shimada M, Shirabe K, Kajiyama K, Gion T, Takenaka K, Sugimachi K. Clinicopathological characteristics of patients with extrahepatic recurrence following a hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2001;48:1088–1093. [PubMed] [Google Scholar]
- 16.Arii S, Monden K, Niwano M, Furutani M, Mori A, Mizumoto M, Imamura M. Results of surgical treatment for recurrent hepatocellular carcinoma; comparison of outcome among patients with multicentric carcinogenesis, intrahepatic metastasis, and extrahepatic recurrence. J Hepatobiliary Pancreat Surg. 1998;5:86–92. doi: 10.1007/pl00009956. [DOI] [PubMed] [Google Scholar]
- 17.Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg. 1965;49:357–363. [PubMed] [Google Scholar]
- 18.McCormack P. Surgical resection of pulmonary metastases. Semin Surg Oncol. 1990;6:297–302. doi: 10.1002/ssu.2980060513. [DOI] [PubMed] [Google Scholar]
- 19.Hustinx R. PET imaging in assessing gastrointestinal tumors. Radiol Clin North Am. 2004;42:1123–39, ix. doi: 10.1016/j.rcl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima J, Tanaka M, Matsumoto J, Takeuchi E, Fukami T, Takamoto S. Appraisal of surgical treatment for pulmonary metastasis from hepatocellular carcinoma. World J Surg. 2005;29:715–718. doi: 10.1007/s00268-005-7687-2. [DOI] [PubMed] [Google Scholar]
- 21.Tunc B, Filik L, Tezer-Filik I, Sahin B. Brain metastasis of hepatocellular carcinoma: A case report and review of the literature. World J Gastroenterol. 2004;10:1688–1689. doi: 10.3748/wjg.v10.i11.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aramaki M, Kawano K, Sasaki A, Matsumoto T, Kai S, Iwashita Y, Himeno Y, Kitano S. Prolonged survival after repeat resection of pulmonary metastasis from hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2002;9:386–388. doi: 10.1007/s005340200046. [DOI] [PubMed] [Google Scholar]
- 23.Gwak GY, Jung JO, Sung SW, Lee HS. Long-term survival after pulmonary metastatectomy of hepatocellular carcinoma; treatment outcome or natural history? Hepatogastroenterology. 2004;51:1428–1433. [PubMed] [Google Scholar]
- 24.Koide N, Kondo H, Suzuki K, Asamura H, Shimada K, Tsuchiya R. Surgical treatment of pulmonary metastasis from hepatocellular carcinoma. Hepatogastroenterology. 2007;54:152–156. [PubMed] [Google Scholar]
- 25.Chen YJ, Hsu HS, Hsieh CC, Wu YC, Wang LS, Hsu WH, Huang MH, Huang BS. Pulmonary metastasectomy for hepatocellular carcinoma. J Chin Med Assoc. 2004;67:621–624. [PubMed] [Google Scholar]