Abstract
Primary adenocarcinoma of the appendix is a rare malignancy that constitutes < 0.5% of all gastrointestinal neoplasms. Moreover, primary signet ring cell carcinoma of the appendix is an exceedingly rare entity. We have encountered 15 cases of primary appendiceal cancer among 3389 patients who underwent appendectomy over the past 18 years. In the present report, we describe a rare case of primary signet ring cell carcinoma of the appendix with ovarian metastases and unresectable peritoneal dissemination occurring in a 67-year-old female patient. She underwent appendectomy and bilateral salpingo-oophorectomy with a laparoscopy procedure. She then received palliative systemic chemotherapy with 12 cycles of oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX-4). The patient currently is well without progression of disease 12 mo after beginning chemotherapy.
Keywords: Appendiceal neoplasms, Carcinoma, Signet ring cell, Chemotherapy
INTRODUCTION
Primary adenocarcinoma of the appendix, first described in 1882, is an uncommon malignancy of the gastrointestinal tract that constitutes approximately 0.12 cases per one million people per year[1]. Moreover, primary signet ring cell carcinoma of the appendix is an exceedingly rare entity and little information on the discrete characteristics of this tumor has been published[2]. The clinical presentation is usually non-specific, although right lower abdominal pain is the most common symptom, which is indistinguishable from acute appendicitis[3,4]. Therefore, it is invariably difficult to diagnose primary signet ring cell carcinoma of the appendix preoperatively. Usually the diagnosis is made only after histologic examination of a surgically-removed inflamed appendix.
The treatment options for metastatic disease include systemic chemotherapy alone, hyperthermic intraoperative intraperitoneal chemotherapy, cytoreductive surgery with a peritonectomy, and a combination of treatments. However, whether debulking surgery and intraperitoneal chemotherapy are worthwhile for all aggressive, advanced disease cases remains controversial.
There are a few reported cases of primary signet ring cell appendiceal carcinoma[5]. In the case reports published, the patients underwent cytoreductive surgery and intraperitoneal chemotherapy. However, this case report documents the use of systemic chemotherapy with oxaliplatin, and 5-fluorouracil (5-FU) chemotherapy (FOLFOX-4) for the female patient with primary signet ring cell appendiceal carcinoma with ovarian metastases and unresectable peritoneal dissemination. The indications for systemic chemotherapy for aggressive primary appendiceal carcinoma were discussed. A case report is presented with a review of our 18-year experience at Kangnam St. Mary’s Hospital.
CASE REPORT
A 67-year-old female visited her primary physician because of an increasing abdominal distention for 3 mo. She was then referred to our hospital for further evaluation and treatment. Cytology of ascitic fluid showed atypical cells. Her past history was unremarkable, as was her family history.
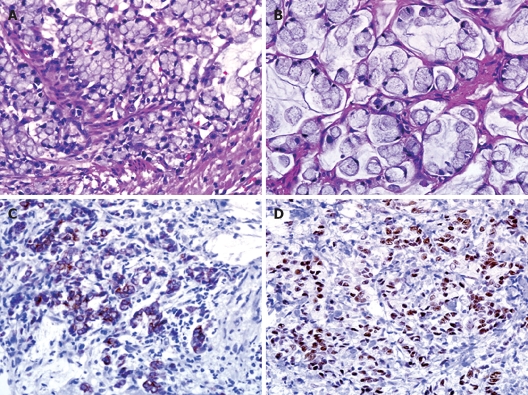
On examination, the patient appeared well with no signs of anemia. Physical examination performed on admission showed moderate distension of the abdomen without hepatomegaly. Gynecological examination was completely unremarkable. Gastrofibroscopy revealed a gastric ulcer and a biopsy showed chronic gastritis. Colonoscopy showed no positive findings. Abdominal computed tomography showed a moderate amount of fluid collection in the abdomen and pelvis with diffuse omental thickening (Figure 1). However, no other evidence of masses was seen within the abdomen and pelvis. The following tumor markers measured were 13 U/mL carbohydrate antigen (CA) 125, 12 U/mL CA19-9, and 2.55 mg/mL carcinoembryonic antigen (CEA). The results of tumor markers were normal. A diagnostic laparoscopy was performed and intra-operative frozen section analysis of a tumour of the appendix showed a signet ring cell carcinoma. The metastatic deposits extended from the pelvic cavity into the upper abdomen along the surface of the liver and diaphragm. The patient underwent appendectomy and bilateral salpingo-oophorectomy. The final pathologic examination was reported as a signet ring cell carcinoma infiltrating the vermiform appendix and metastasizing to the ovaries. Diffuse, strong immunoreactivity against CEA, cytokeratin 20, MUC2, and CDX-2 was observed in the tumor cells. Focal immunopositivity for MUC-5AC also was detected (Figure 2).
Figure 1.
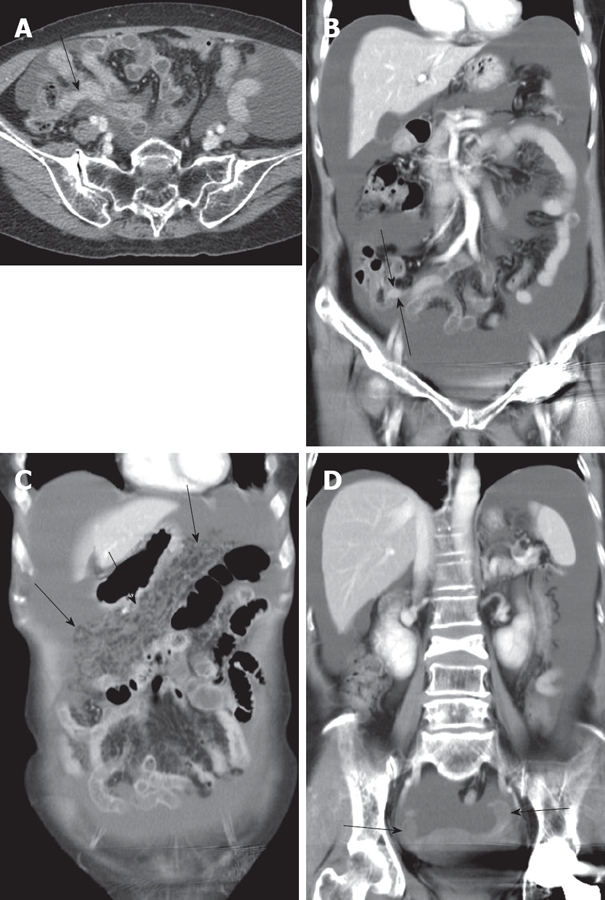
Contrast enhanced CT axial scan (A) and coronal reformatted image (B) showing massive ascites in abdominal cavity. Appendix is prominently seen with mild thickening (arrowed). Coronal reformatted images (C and D) showing increased reticulonodular densities (arrows in C) along the omentum representing carcinomatosis peritonei with no definite evidence of mass like lesion in adnexa (arrows in D).
Figure 2.
Histologic examination showing a signet ring cell carcinoma infiltrating vermiform appendix (A) and metastasizing to the ovary (B) (HE, × 400) with diffuse, strong immunoreactivity against cytokeratin 20 (C) and CDX-2 (D) in the tumor cells.
The patient received twelve cycles of FOLFOX-4 and is currently well without progression of disease, 12 mo after beginning chemotherapy.
Over an 18-year period from January 1990 to December 2007, there were 15 cases (an incidence rate of 0.4%) of primary appendiceal carcinoma among 3389 appendectomies performed in our hospital (Table 1). The mean age of patients with mucinous adenocarcinoma and patients with colonic type carcinomas was 58.0 years (range, 43-68 years) and 61 years (range, 42-73 years), respectively. Among the 15 patients, abdominal pain was the most common symptom of primary appendiceal cancer in 9 patients, and 7 of these 9 patients underwent emergency surgery. Histologically, 2 patients had low grade mucinous epithelial tumors, 8 patients had mucinous adenocarcinomas, 4 patients had colonic adenocarcinomas, and 1 patient had a signet ring cell carcinoma. In our series of 10 patients with regular follow-up, 4 patients developed relapses after debulking surgery. Of the four relapsed patients, two had a high grade colonic type adenocarcinoma and one had a mucinous adenocarcinoma with distant metastases on initial presentation. Ovarian metastases occurred in four of the 7 female cases, including the present case. Extra-abdominal metastatic sites were documented in two patients. Patient 8 had a mucinous adenocarcinoma (well-differentiated) and developed lung metastases and patient 11 had a colonic type adenocarcinoma (poorly differentiated) with liver metastases to the abdominal cavity. Of the six patients with peritoneal metastases, four underwent cytoreductive surgery, including right hemicolectomy. Palliative 5-FU-based chemotherapy was administered in three of seven cases with distant metastases.
Table 1.
Clinical characteristics, tumor features, treatment, and survival of 15 out of 3389 patients who underwent appendectomy over an 18-year period
| Patient’s No. | Age /sex | Symptom | Type of treatment | Histology | Grade | Stage | Tumor marker elevated | Surgery | Additional treatment | Metastatic sites | Relapse |
| 1 | 62/F | No | EL | Mucinous epithelial tumor | Low grade | D | CEA | RHC, TAH, BSO, Appendicectomy | Chemotherapy (CP) | Ovary, Peritoneum, Omentum | No |
| 2 | 58/F | Pain | EM | Mucinous epithelial tumor | Low grade | D | - | RHC, Appendicectomy | No | Rupture of appendix | No |
| 3 | 43/F | Pain | EL | Mucinous adenocarcinoma | WD | L | NA | BSO, Appendicectomy | No | No | Loss |
| 4 | 43/M | Pain | EL | Mucinous adenocarcinoma | Low grade | L | NA | BSO, Appendicectomy | No | No | No |
| 5 | 69/F | Pain | EL | Mucinous adenocarcinoma | WD | D | NA | TAH, BSO, Appendicectomy | No | Ovary, Peritoneum | Loss |
| 6 | 68/M | Pain | EM | Mucinous adenocarcinoma | WD | L | - | RHC, Appendicectomy | No | No | No |
| 7 | 58/M | Pain | EM | Mucinous adenocarcinoma | WD | D | - | Appendicectomy Omentectomy | No | Omentum | Yes |
| 8 | 61/M | Pain | EM | Mucinous adenocarcinoma | WD | L | - | RHC, Appendicectomy | Chemotherapy (FL) | Lung | Yes |
| 9 | 63/F | No | EL | Mucinous adenocarcinoma | WD | D | - | TAH, BSO, Appendicectomy | Chemotherapy (FOLFOX) | Ovary, Peritoneum, Omentum | No |
| 10 | 59/M | No | EL | Mucinous adenocarcinoma | WD | L | - | RHC, Appendicectomy | No | No | No |
| 11 | 42/M | Pain | EM | Colonic type adenocarcinoma | PD | L | NA | RHC, Appendicectomy | No | Liver, Peritoneum | Yes |
| 12 | 73/M | Pain | EM | Colonic type adenocarcinoma | MD | R | CEA | RHC, Appendicectomy | No | No | Yes (colon cancer) |
| 13 | 62/F | Abdominal distension | EL | Colonic type adenocarcinoma | MD | L | CEA | RHC, Appendicectomy | No | No | No |
| 14 | 67/M | Pain | EM | Colonic type adenocarcinoma | MD | L | NA | RHC, Appendicectomy | No | No | No |
| 15 | 67/F | Abdominal distension | EL | Signet ring cell carcinoma | D | - | Appendicectomy, BSO | Chemotherapy (FOLFOX) | Ovary, Peritoneum, Omentum | - |
EL: Elective; EM: Emergency; WD: Well differentiated; MD: Moderately differentiated; L: Localized; R: Regional; D: Distant; NA: Not available; RHC: Right hemicolectomy; TAH: Transabdominal abdominal hysterectomy; BSO: Bilateral salpingo-oophorectomy; CP: Cyclophosphamide/cisplatin; FL: 5-fluorouracil/leucovorin; FOLFOLX: Oxaliplatin/5-fluorouracil/leucovorin.
DISCUSSION
Primary adenocarcinoma of the appendix is a rare malignancy of the gastrointestinal tract[1]. Smeenk et al[6] reported a 0.3% prevalence of primary appendiceal mucinous epithelial neoplasms identified from 167 744 patients who underwent appendectomy. Our 18-year-experience showed a similar incidence rate (0.4%) of primary appendiceal carcinoma after appendectomy. Moreover, a primary signet ring cell carcinoma of the appendix (the case reported herein, was the first of the 15 cases) is an exceedingly rare entity, comprising only 4% of all appendiceal neoplasms[2].
The demographic characteristics of patients with cancer of the appendix vary by histology. According to report of McCusker et al[1], those diagnosed with malignant carcinoids are significantly younger (mean age, 38 years) than those diagnosed with any of the other cancer types. The mean age of patients at diagnosis of mucinous adenocarcinoma, colonic type adenocarcinoma, and signet ring cell carcinoma is approximately 60 years, 62 years, and 58 years, respectively, and an equal number of males and females developed goblet cell carcinoid, mucinous adenocarcinoma, and signet ring cell carcinoma, except for colonic adenocarcinoma which had a male predominance[1]. Our results also showed similar demographic findings.
Reported series of appendiceal adenocarcinoma are difficult to compare, as terminology and classification of these lesions are not consistent[7,8]. The International Classification of Diseases for Oncology (ICD-O) divides the tumors of appendix into five categories: colonic type adenocarcinoma, mucinous adenocarcinoma, signet ring cell carcinoma, goblet cell carcinoma, and malignant carcinoid/adenocarcinoid[1,9]. These carcinomas arise in pre-existing adenomas, either by a cystic and colonic growth pattern[10,11]. On the other hand, signet ring cell carcinomas, usually frequent in stomach and intestine, are adenocarcinomas with mucus-producing tumor cells. In this case, the tumor cells showed diffuse, strong immunoreactivity against cytokeratin 20, CDX-2, MUC-2, and CEA, and focal immunopositivity for MUC-5AC (Figure 2). CDX-2 is a useful marker to confirm an appendiceal origin of pseudomyxoma peritonei, particularly when used in conjunction with CK20, MUC-2, and MUC-5AC[12].
Most appendiceal cancers are low-grade neoplasms that are typically relatively indolent. The overall 5-year survival rate for mucinous appendiceal adenocarcinomas reported by Park et al[13] is 20.5%. According to report of McCusker[1], except for signet ring cell carcinoma and malignant carcinoid, the histologic type does not have a significant impact on survival. In addition, the extent of disease at diagnosis is a more important predictor of survival than histology. Ronnett et al[7,14] reported that the long-term survival of patients with diffuse, peritoneal metastases arising from these carcinomas is poor, with a reported 5-year survival as low as 6.7%-14%. Moreover, in a study by McGory et al[2], poorly differentiated adenocarcinoma and signet ring cell carcinoma of the appendix had the highest proportion of distant disease with a 5-year survival rate of 7%. Therefore, signet ring cell carcinoma may be a separate tumor type in the appendix that should be considered apart from other carcinomas, largely because of its poor prognosis.
Right hemicolectomy is considered the optimal treatment for most histologic types of primary appendiceal carcinoma even in the presence of perforation and in Dukes A tumors[15]. The treatment options for metastatic disease include systemic chemotherapy alone, hyperthermic intraoperative intraperitoneal chemotherapy, cytoreductive surgery with peritonectomy, and combination of treatments. Of those treatment options, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy have recently become the treatment of choice for metastatic diseases at most large centers[16]. In the present case, laparoscopic exploration allowed assessment of the pelvic metastasis which was unresectable and the final pathologic diagnosis was a signet ring cell carcinoma, with aggressive biologic behaviors. Therefore, we did not perform cytoreduction or intraperitoneal chemotherapy, but proceeded with an appendicectomy and bilateral salpingo-oophorectomy followed by systemic chemotherapy. Routine oophorectomy should be considered in all females, especially if post-menopausal. An oophorectomy itself may assist with tumour staging, reduce the likelihood of symptomatic metastases and also provide a survival advantage[17].
Given the predilection for exclusive peritoneal metastasis of mucinous adenocarcinomas of the appendix, in the case of low-grade tumors, cytoreductive surgery and regional chemotherapy are very important. Aggressive cytoreductive surgery for mucinous-type tumours is known to improve the survival rate and reduce the recurrence rate in patients with generalized pseudomyxoma peritonei compared with simple appendectomy[18]. In addition, the most consistent prognostic factor for survival is the completeness of cytoreduction[19,20]. However, whether debulking surgery for all patients with aggressive, advanced disease is worthwhile has not been elucidated. In fact, only limited data are available on surgical debulking for diffuse, peritoneal dissemination from aggressive appendiceal carcinomas, in part because the disease is so rare. Incomplete cytoreduction plus perioperative intraperitoneal chemotherapy for peritoneal dissemination from aggressive appendiceal malignancies can achieve a limited long-term survival[5,20]. Furthermore, Gonzalez-Moreno and Sugarbaker demonstrated that right hemicolectomy does not confer a survival advantage in patients with mucinous appendiceal tumors with peritoneal seeding[21]. These data suggest that in patients with aggressive appendiceal malignancy, routine right hemicolectomy should not be performed unless in conjunction with complete cytoreduction in combination with intraperitoneal chemotherapy.
Considering systemic chemotherapy, an alternative option to metastatic appendiceal carcinomas, although limited, the available data favor integration of systemic chemotherapy, including mitomycin C, fluoropyrimidines and platinum compounds, which has been used as an intraperitoneal chemotherapy[20,22]. Ishibashi et al[22] described a case of pseudomyxoma peritonei caused by carcinoma of the appendix, which was successfully treated with multidisciplinary treatment, including oxaliplatin, 5-FU, and leucovorin (modified FOLFOX6 regimen) combination systemic chemotherapy followed by cytoreduction. In a recent randomized phase III trial comparing debulking alone to debulking with continuous hyperthermic peritoneal perfusion of cisplatin in patients with low-grade, mucinous tumors of the gastrointestinal tract, all patients who had surgical debulking received four courses of postoperative intravenous chemotherapy (5-FU, leucovorin, and oxaliplatin)[23].
Recently, many targeted drugs have been used as a combination therapy to improve the survival of patients with gastrointestinal malignancies[24]. Mucin genes, which play an important role in the pathogenesis of pseudomyxoma peritonei, are regulated in part by epidermal growth factor receptor (EGFR) signaling[25,26]. Andreopoulou et al[27] recently presented that heavily pretreated patients who underwent cetuximab monotherapy showed a transient decrease in tumor markers, with an encouraging time-to-progression (3 mo) in a phase II trial of cetuximab in mucinous peritoneal carcinomatosis. In addition, Logan-Collins et al[28] demonstrated that in patients who undergo cytoreductive surgery and intraperitoneal hyperthermic perfusion for mucinous adenocarcinoma of the appendix, (n = 32), the average vascular endothelial growth factor (VEGF) counts correlate with survival (P = 0.017), and for patients with recurrence, this correlation is stronger (P = 0.002), indicating that VEGF may predict the survival of patients with peritoneal surface metastases from mucinous adenocarcinomas and that anti-angiogenic therapies may be effective in patients with this devastating disease. In the future, novel targeted therapies, including EGFR inhibitor or anti-angiogenic agents, in combination with regional treatment or systemic chemotherapy should be evaluated.
Footnotes
Peer reviewer: Toru Ishikawa, MD, Department of Gastro-enterology, Saiseikai Niigata Second Hospital, Teraji 280-7, Niigata, Niigata 950-1104, Japan
S- Editor Li DL L- Editor Wang XL E- Editor Ma WH
References
- 1.McCusker ME, Cote TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307–3312. doi: 10.1002/cncr.10589. [DOI] [PubMed] [Google Scholar]
- 2.McGory ML, Maggard MA, Kang H, O'Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264–2271. doi: 10.1007/s10350-005-0196-4. [DOI] [PubMed] [Google Scholar]
- 3.Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41:75–80. doi: 10.1007/BF02236899. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell ME, Badger SA, Beattie GC, Carson J, Garstin WI. Malignant neoplasms of the appendix. Int J Colorectal Dis. 2007;22:1239–1248. doi: 10.1007/s00384-007-0304-0. [DOI] [PubMed] [Google Scholar]
- 5.Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg. 2004;240:278–285. doi: 10.1097/01.sla.0000133183.15705.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei". Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Misdraji J, Young RH. Primary epithelial neoplasms and other epithelial lesions of the appendix (excluding carcinoid tumors) Semin Diagn Pathol. 2004;21:120–133. doi: 10.1053/j.semdp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Percy C, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology (ICD-O). 2nd edition. Geneva: World Health Organization; 1990. Available from: URL: http://www.who.int/classifications/icd/en/ [Google Scholar]
- 10.Higa E, Rosai J, Pizzimbono CA, Wise L. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix. A re-evaluation of appendiceal "mucocele". Cancer. 1973;32:1525–1541. doi: 10.1002/1097-0142(197312)32:6<1525::aid-cncr2820320632>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Djuranovic SP, Spuran MM, Kovacevic NV, Ugljesic MB, Kecmanovic DM, Micev MT. Mucinous cystadenoma of the appendix associated with adenocarcinoma of the sigmoid colon and hepatocellular carcinoma of the liver: report of a case. World J Gastroenterol. 2006;12:1975–1977. doi: 10.3748/wjg.v12.i12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nonaka D, Kusamura S, Baratti D, Casali P, Younan R, Deraco M. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology. 2006;49:381–387. doi: 10.1111/j.1365-2559.2006.02512.x. [DOI] [PubMed] [Google Scholar]
- 13.Park IJ, Yu CS, Kim HC, Kim JC. [Clinical features and prognostic factors in primary adenocarcinoma of the appendix] Korean J Gastroenterol. 2004;43:29–34. [PubMed] [Google Scholar]
- 14.Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Nitecki SS, Wolff BG, Schlinkert R, Sarr MG. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg. 1994;219:51–57. doi: 10.1097/00000658-199401000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy EM, Farquharson SM, Moran BJ. Management of an unexpected appendiceal neoplasm. Br J Surg. 2006;93:783–792. doi: 10.1002/bjs.5385. [DOI] [PubMed] [Google Scholar]
- 17.Cortina R, McCormick J, Kolm P, Perry RR. Management and prognosis of adenocarcinoma of the appendix. Dis Colon Rectum. 1995;38:848–852. doi: 10.1007/BF02049842. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker PH. Cytoreductive surgery and intraperitoneal chemotherapy with peritoneal spread of cystadenocarcinoma. Eur J Surg Suppl. 1991;38:75–82. [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27:239–243. doi: 10.1053/ejso.2000.1038. [DOI] [PubMed] [Google Scholar]
- 20.Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007;14:2289–2299. doi: 10.1245/s10434-007-9462-0. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi K, Sobajima J, Okada N, Ishizuka N, Yokoyama M, Mitsuhashi T, Miyazaki T, Nakada H, Gonda T, Ishida H. [A case of pseudomyxoma peritonei successfully treated with multidisciplinary treatment including modified FOLFOX6 regimen] Gan To Kagaku Ryoho. 2007;34:2047–2049. [PubMed] [Google Scholar]
- 23.James P. Phase III randomized study of operative debulking and systemic chemotherapy with or without intra- and peri-operative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from low-grade gastrointestinal adenocarcinoma. Available from: URL: http://www.cancer.gov/clinicaltrials/NCI-03-C-0085.
- 24.Rosen LS. VEGF-targeted therapy: therapeutic potential and recent advances. Oncologist. 2005;10:382–391. doi: 10.1634/theoncologist.10-6-382. [DOI] [PubMed] [Google Scholar]
- 25.O'Connell JT, Tomlinson JS, Roberts AA, McGonigle KF, Barsky SH. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002;161:551–564. doi: 10.1016/S0002-9440(10)64211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;277:32258–32267. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- 27.Andreopoulou E, Muggia F, Safa M, Escalon J, Downey A, Newman E, Hochster H, Wojtaszek C, Mauro D, Lowy A. Phase II study of cetuximab in mucinous peritoneal carcinomatosis. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings Part I. Vol 24, No. 18S (June 20 Supplement) 2006. p. 14020. org/cgi/content/abstract/24/18_suppl/14020. [Google Scholar]
- 28.Logan-Collins JM, Lowy AM, Robinson-Smith TM, Kumar S, Sussman JJ, James LE, Ahmad SA. VEGF expression predicts survival in patients with peritoneal surface metastases from mucinous adenocarcinoma of the appendix and colon. Ann Surg Oncol. 2008;15:738–744. doi: 10.1245/s10434-007-9699-7. [DOI] [PubMed] [Google Scholar]