Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) selectively kills tumor cells. However, its short half-life, poor delivery and TRAIL-resistant tumor cells have diminished its clinical efficacy. In this study, we explored whether novel delivery methods will represent new and effective ways to treat gliomas and if adjuvant therapy with the chemotherapeutic-agent temozolomide (TMZ) would enhance the cytotoxic properties of TRAIL in glioma lines resistant to TRAIL monotherapy. We have engineered adeno-associated virus (AAV)-vectors encoding recombinant secreted TRAIL (S-TRAIL) and bioluminescent-fluorescent marker fusion proteins and show that AAV delivered S-TRAIL leads to varying degrees of killing in multiple glioma lines, which correspond with caspase-3/7 activation. In vivo, dual-bioluminescent imaging revealed efficient delivery of therapeutic AAV-vectors directly into the tumor mass, which induced marked attenuation of tumor progression. Treatment of glioma cells with the chemotherapeutic agent TMZ alone lead to a significant accumulation of cells in G2/M phase, activated the cell cycle checkpoint protein Chk1, and increased death receptor expression in a time-dependent manner. Furthermore, combined treatment of TRAIL-resistant cell lines with AAV-S-TRAIL or previously engineered neural stem cell (NSC)-S-TRAIL and TMZ induced cell killing and markedly upregulated pro-apoptotic proteins. This study elucidates novel means of delivering S-TRAIL to gliomas, and suggests combination of clinically relevant TMZ and S-TRAIL may represent a new therapeutic option with increased potency for glioblastoma patients.
Keywords: Stem cell, AAV, TRAIL, temozolomide, glioma therapy, molecular imaging
Introduction
Glioblastoma multiforme (GBM) is the most common brain tumor comprising 23% of all primary adult brain tumors, and is the most deadly with a life expectancy of 9-12 month(1). Despite extensive investigation, GBM remains resistant to all current therapies, with resection, radiotherapy, and chemotherapy minimally prolonging survival and ultimate re-development of tumors leading to patient mortality(2). Recently, TRAIL has arisen as a promising new anti-glioma therapy due to it's ability to induce apoptosis in a wide variety of malignant cells with high tolerance and minimal toxicity to normal tissue(3-7). Purified TRAIL protein has been shown to induce apoptosis in a number of different glioma line in vitro(8-10), while both local and systemic injection of TRAIL protein exerts anti-tumor effects on intracranial human malignant glioma xenografts in mice(11). Despite initial clinical excitement, the rapid clearance of soluble TRAIL following systemic administration and large dose required to achieve glioma regression have limited the effectiveness of TRAIL in patients(12-14). Additionally, numerous studies have shown that a large percentage of primary glioma lines are resistant to TRAIL-induced apoptosis(15, 16). Therefore, methods to improve both the efficiency of delivery and tumoricidal activity of TRAIL are required.
Two major strategies that have been used to overcome the problems of delivery encountered with recombinant proteins are viral gene therapy and cell-based therapy. Viral vectors are advantageous in that viral gene therapy can deliver robust and long-term expression of therapeutic proteins with high specificity in local tissue, reducing the systemic dose and nonspecific toxicity(17). In particular, adeno-associated virus (AAV) has received much attention in both clinical and non-clinical research due to the ability of AAV to induce high levels of transgene expression in glioma cells with minimal associated toxicity or host immune response(18-21). Alternatively, neural stem cells (NSC) have arisen as new therapeutic agents for the treatment of diseases of the central nervous system due to their unique tumor specific homing properties, potential to differentiate into different neural cell types, and incorporate into the cyto-architecture of the brain following transplantation(3, 22-24). Capitalizing on these properties, we and others have demonstrated the feasibility of engineering therapeutic NSCs that can efficiently delivery anti-tumor proteins and result in tumor regression(3, 25-27). To improve the efficacy of TRAIL, we also created a novel form of TRAIL consisting of the extracellular domain of Flt3 ligand fused to the N-terminus of the extracellular domain of TRAIL (S-TRAIL)(28) and showed that S-TRAIL had potent by-stander effects on neighboring glioma cells as well as increased cell killing effects(4, 28). Engineering NSC with S-TRAIL, (NSC-S-TRAIL) we further demonstrated NSC selectively migrate to established gliomas where delivery of S-TRAIL by NSC inhibited progression of human glioma xenografts assessed by serial dual bioluminescence imaging(3, 23, 27).
In an effort to improve the efficacy of TRAIL therapy and overcome TRAIL resistance, TRAIL-based combination therapies have also been investigated(7, 29, 30). Temozolomide (TMZ) is an oral alkylating agent used extensively in clinics as part of the chemotherapeutic regimen for treatment of high-grade glioma(31, 32). TMZ is a small molecule that effectively traverses the blood-brain barrier where it induces glioma cell death by causing accumulations of DNA mismatch, subsequent growth arrest, and eventually apoptosis(32, 33). Recently, studies utilizing bacterially expressed TRAIL have highlighted a potential synergy between TMZ and TRAIL, showing combined bacterially expressed TRAIL and TMZ treatment had improved anti-tumor effects on cultured glioma cells(34). Further, convection-enhanced delivery of TRAIL protein combined with systemic administration of TMZ proved more effective than either therapy alone in mouse models(35). These studies suggest TMZ may sensitize cells to TRAIL-induced apoptosis, however it is unclear whether TMZ can enhance the killing effects of recombinant S-TRAIL delivered by novel and highly efficient AAVs or NSCs in glioma lines that are resistant to S-TRAIL monotherapy.
In this study: 1) we developed novel AAVs encoding S-TRAIL, and fusions between fluorescent and bioluminescent marker genes and investigated the anti-glioma properties of AAV-S-TRAIL in vitro using different human glioma lines and in vivo in human glioma xenograft models; 2) evaluated the effects of TMZ on glioma cells with varying resistance to TRAIL; and 3) evaluated the synergistic effect of therapeutic AAV-S-TRAIL and the previously developed NSC-S-TRAIL and TMZ in human glioma cell lines with varying degrees of TRAIL resistance.
Methods
Viral vector generation
The recombinant Gaussia Luciferase (Gluc)-DsRed2 and S-TRAIL were constructed in the AAV-MCS6 plasmid that was kindly provided by the Harvard Gene Therapy Initiative (Harvard Institute of Medicine, Boston, MA). To first generate the Gluc and DsRed2 fusion, a 569 bp Gluc fragment was obtained from pGluc-Basic Vector (New England Biolabs, Ipswich, MA) by pcr using the forward primer 5′-CTAGCTAGCGTCGACATGGGAGTCAAAGTTCTGTTTGCC-3′ bearing Nhel and Sall restriction sites and the reverse primer 5′-CCGCTCGAGGATATCGTCACCA CCGGCCCCCTTGAT-3′ bearing EcoRV and Xhol restriction sites. The 684 bp DsRed2 fragment was similarly generated by pcr from the vector LV-Fluc-DsRed2(36) using the forward primer 5′-CCGATATCATGGCCTCCTCCGAGAACGTC-3′ bearing an EcoRV restriction site and the reverse primer 5′-CCGCTCGAGGCGGCCGCCTACAGGAACAGGTGGTGGCG-3′ bearing Notl and Xhol restriction sites. The two fragments were ligated into Sall/Notl digested pIRES vector (Clontech Laboratories, Inc, Mountain View, CA). To generate Gluc-DsRed2, a 1898 bp fragment containing the IRES element and Gluc-DsRed2 was excised from the pIRES vector by digestion with EcoRI/Notl and ligated into EcoRI/Notl digested AAV-MCS6 vector. To generate AAV–S-TRAIL, the 1200 bp fragment encoding S-TRAIL was obtained from LV-S-TRAIL(23) by PCR using a forward primer 5′-CGGAATTCATGACAGTGCTGGCGCCAGCC-3′ containing an EcoRI restriction site and a reverse primer 5′-CGACGCGTTTAGCCAACTAAAAAGGCCCCGAA-3′ containing a Mlul restriction site. This fragment was ligated into EcoRI/Mlul digested Gluc-DsRed2 vector to obtain AAV-S-TRAIL. Packaging and purification of rAAV were performed as described previously(37), and typical titers were 1×1011particle/ml. The generation and packaging of LV-GFP-Fluc has been described previously(36).
Cell lines
Human glioma cell lines Gli36 expressing a constitutively active mutant epidermal growth factor receptor (EGFR) (Gli36-EGFRvIII), U251, U87, and 293T cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% heat-incubated fetal bovine serum, 100 μg/ml penicillin and 100 μg/ml streptomycin. Previous results confirmed expression of the mutant EGFR receptor in Gli36-EGFRvIII cells was shown to have no influence on TRAIL sensitivity(8). The neural progenitor cell line C17.2 has been described previously(23). Creation and characterization of the cell line Gli36-EGFRvIII-GFP-Fluc has been described previously(36). All cells were incubated at 37°C in a humidified 5% CO2 incubator.
In vitro fluorescence microscopy, bioluminescent imaging, and flow cytometry
For visualization of DsRed2, 1×105 293T cells were plated in 24 well plates. Twenty-hour h later, cells were transduced with 1 000 MOI of either Gluc-DsRed2 or S-TRAIL, and 48 h post-transduction DsRed2 fluorescence was visualized. To confirm a linear correlation between viral transduction and Gluc photon emission, 1×104 293T cells were seated for 24 h in 96 well plates. Cells were then transduced with increasing multiplicity of infection (MOI) of either Gluc-DsRed2 or S-TRAIL for 24 h, at which time cells were incubated with 15 μg of coelenterazine for 10 min, and photon emission was measured using a cryogenically cooled high efficiency CCD camera system (Roper Scientific, Trenton, New Jersey). For flow cytometry, Gli36-EGFRvIII glioma cells were transduced with LV-GFP-Fluc using an MOI of 1. Following expansion in culture, the cells were analyzed by flow cytometry (FACScalibur, BD Biosciences) to confirm GFP-positive cells.
S-TRAIL ELISA
To determine the concentration of S-TRAIL released by AAV-S-TRAIL transduced cells, 0.4×106 TRAIL-resistant 293T cells were transduced with increasing MOI of control virus (Gluc-DsRed2) or S-TRAIL. Twenty-four h later, media was collected and TRAIL protein concentration in the medium of transduced cells was measured using the TRAIL Immunoassay Kit (Biosource International, Camarillo, CA) according to manufacturer's protocol with wells coated with human TRAIL monoclonal antibody and soluble biotinylated TRAIL polyclonal antibody. Plates were read at 450 nm using an absorbance plate reader (Molecular Devices), and the data were analyzed by SOFTMAX.
Caspase and viability assays
To determine the effect of S-TRAIL on cell viability and caspase activation, Gli36-EGFRvIII, U87, or U251 glioma cells were seeded in 96-well plates (1×104 cells/well). Twenty-four h after plating, the cells were transduced with AAV-Gluc-DsRed2 or AAV-S-TRAIL at increasing MOI (0-10000). For viability, aggregate metabolic activity was measured using an ATP-dependent luminescent reagent (CellTiter-Glo, Promega). Caspase activity was determined with a caged, caspase-3/caspase-7-activatable DEVD-aminoluciferin (Caspase-Glo 3/7, Promega). Assays were done according to the manufacturer's instructions and plates were read in a luminometer at 5 s per well. To investigate the synergistic effects of TMZ and S-TRAIL, Gli36-EGFRvIII or U251 cells were plated as described above. Cells were treated with media containing increasing concentrations of TMZ (0-1 000 μM) alone or in combination with the viral vectors S-TRAIL (0-4 000 MOI) or Gluc-DsRed2 (4 000 MOI) or S-TRAIL-containing media from NSCs (0-600 ng/ml) for 18 h and cell viability was determined as described above. To measure the effects of TMZ pretreatment on S-TRAIL/TMZ, U251 glioma cells were plated in 96-well plates and left untreated or incubated with TMZ (100, 500 μM) for 24 h. New media containing fresh TMZ and control virus AAV-GlucDsRed2 (4 000 MOI), AAV-S-TRAIL (1 000, 4 000 MOI), or NSC-S-TRAIL (200, 600 ng/ml) was then added to both the pretreated cells and non-treated cells, and viability or caspase activation was determined 18 h later.
Cell Cycle Analysis
Following treatment with TMZ (100 μM; 24, 72 h), untreated and TMZ-treated cells (Gli36-EGFRvIII, U251) were pulsed for 1 h with BrdU (Amersham, Little Chalfont, UK). The cells were trypsinized and fixed in cold ethanol and denatured in 2 M HCI, 0.5% Triton X-100, that was neutralized in 0.1 M borate at pH 8.5. BrdU incorporation was detected by incubating cells with anti-BrdU antibody (Becton-Dickinson, San Jose, CA) for 30 min at room temperature. The cells were washed and incubated with FITC-conjugated anti-mouse antibody (Vector Labs, Burlingame, CA) for 30 min. Following a second wash, the cells were incubated with propidium iodide (10 μg ml−1 propidium iodide, Sigma) and RNase A (250 μg ml−1, Sigma) at 4 °C over night, and analyzed using a Becton-Dickinson FACSort flow cytometer and CellQuest software.
Cell implantation and in vivo bioluminescent imaging
Gli36-EGFRvIII glioma cells transduced with LV-GFP-Fluc (Gli36-EGFRvIII-GFP-Fluc) has been described previously(36). Briefly, cells were transduced with LV-GFP-Fluc in growth medium containing 12 μg/ml polybrene (Fisher Scientific, Pittsburg, PA), visualized for GFP to confirm transduction, and expanded. Under inhaled anesthesia (1% to 2% isoflurane plus 2L O2), 6×106 Gli36-EGFRvIII-GFP-Fluc cells in 50 μl saline were implanted subcutaneously into athymic nude mice (nu/nu; 6–7 weeks of age; Charles River Laboratories, Wilmington, MA, USA) using a 28 g syringe. Two days post-implantation, 1×109 particles of either AAV-Gluc-DsRed2 or AAV-S-TRAIL was directly injected into the tumor using a glass Hamilton syringe. A second injection of virus was administered 2 days after the initial injection. To serially monitor changes in glioma volume, mice were imaged for Fluc activity 1, 4, 7, and 12 days after the first injection of virus. D-luciferin (4.5 mg/animal in 150 μL saline) was administered to each mouse by intraperotoneal injection, and photon counts recorded 5 min after D-luciferin administration with a 5 min acquisition time. Gluc imaging to visualize viral transgene expression within the tumor mass was performed on mice 10 and 12 days after injection of Gluc-DsRed2. To visualize Gluc expression, mice received intravenous injection of coelenterazine (3.3 μg/g body wt), and were imaged for Gluc activity using a 5 min acquisition time 10 days post-injection of AAV. Fluc imaging to visualize tumor volume was performed as described above on day 12. Postprocessing and visualization of all images was performed as described previously(23, 38).
Immunohistochemistry
Seven days post-transduction by AAV, tumors were removed, placed in 10% formalin, and embedded in paraffin. 10 μM sections were generated and subjected to an antigen retrieval process using a sodium citrate buffer. Sections were stained for active caspase-3/active caspase-7 staining (1:50; Calbiochem, San Diego, CA) according to standard protocols, followed by processing for hematoxylin and eosin (H&E) by standard techniques.
Western blot Analysis and Immunocytochemistry
To investigate the effects of TMZ alone, U251 cells were treated with TMZ (100 μM) for 24 or 72 h, or left untreated. To determine the effects of combined TMZ and S-TRAIL treatment on cells, U251 cells were treated for 24 h with TMZ (500 μM), NSC-S-TRAIL (600 ng/ml), a combination of TMZ and S-TRAIL, or TMZ and S-TRAIL following 24 h of sensitization by TMZ alone. For all treatment groups, proteins were isolated from harvested cells, resolved by sodium dodecyl sulfate–polycrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with antibodies against cleaved PARP (Cell Signaling, Beverly, MA), DR4 (Sigma Aldrich, St. Louis, MO), Chk1 (Santa Cruz, Santa Cruz, CA), phosphorylated Chk1 (Cell Signaling) or α-tubulin (Sigma Aldrich) antibodies, and detected by chemiluminscence following incubation with HRP-conjugated secondary antibodies. An additional group of cells plated on cover slips was similarly treated with TMZ or S-TRAIL, fixed, permeabilized, and incubated with a cleaved caspase-3 antibody (1:200; Cell Signaling, Danvers, MA) for 1 h at 37°C. Cells were then washed and incubated with goat anti–rabbit Alexa dye (540 nm)–conjugated secondary antibody (Molecular Probes, Eugene, OR) for 1 h, washed again, mounted, and examined by fluorescence microscopy.
Statistical Analysis
Data are expressed as mean±SD and were analyzed by either Student's T-test or ANOVA (after Bartlett's test of homogeneity of variance), followed by the Newman-Keuls correction for multiple comparisons. Differences were considered significant at P<0.05. Synergy between S-TRAIL and TMZ was determined by the isobologram method(39). Gli36-EGFRvIII and U251 cells were treated with TMZ at increasing concentrations alone or in combination with NSC-S-TRAIL or AAV-S-TRAIL for 18 h, at which time cell viability was determined and EC50 values calculated for each drug alone or in combination with a fixed ratio of the second drug. The calculated EC50 values of each drug in combination were divided by the EC50 of each drug alone. An isobologram was generated where the dotted line connecting the EC50 of each drug alone represents the predicted additive value, values below the line indicate synergy and values above the line indicate antagonistic effects.
Results
In order to investigate the anti-glioma potential of S-TRAIL delivered by AAV and simultaneously monitor transgene expression, we developed novel bi-modal recombinant AAV vectors expressing a fusion of bioluminescent protein Gaussia luciferase (Gluc) and DsRed2 (Fig. 1A). Fluorescence microscopy of TRAIL-resistant 293T cells transduced with AAV-Gluc-DsRed2 or AAV-S-TRAIL revealed robust DsRed2 expression (Fig. 1B), while bioluminescence imaging of AAV-Gluc-DsRed2 or AAV-S-TRAIL revealed strong Gluc signal that correlated linearly with viral concentration (Fig. 1C). ELISA on culture medium of the cells transduced with AAV-S-TRAIL, demonstrated a dose-dependent increase in S-TRAIL secretion with 1000 MOI leading to 155±17 ng/ml of S-TRAIL (Fig. 1D). These results confirm the functionality of fused fluorescent and bioluminescent markers in the bi-modal AAV vectors and the secretion of S-TRAIL following transduction by AAV-S-TRAIL.
Figure 1. AAV-mediated expression of bi-modal fusion proteins and S-TRAIL.
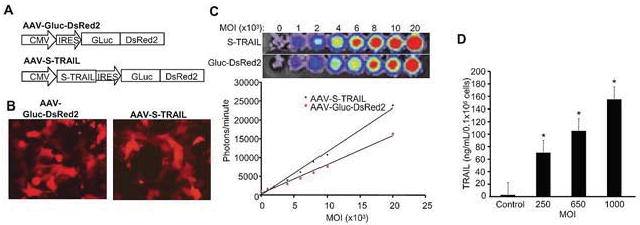
(A) Schematic representation of recombinant AAV-2 vectors encoding fluorescent-bioluminescent fusion marker and S-TRAIL. (B) TRAIL-resistant 293T cell lines were transduced with AAV-Gluc-DsRed2 or AAV-S-TRAIL (MOI 1 000) and 48 h post-infection, cells were imaged using fluorescence microscopy. (C) Representative bioluminescent images and summary graphs of 293T cell lines transduced with increasing concentrations of AAV-Gluc-DsRed2 or AAV-S-TRAIL (500-20 000 MOI). After 24 h, cells were incubated with coelenterazine and bioluminescent imaging was used to determine Gluc expression. (D) 293T cells (4×105) were transduced with control (Gluc-DsRed2) or S-TRAIL and 24 h post-infection secreted TRAIL concentration per mL of media was determined by enzyme-linked immunosorbent assay (ELISA). Maginification-10× (B).
Next, we investigated the effects of AAV-S-TRAIL on cell viability and caspase activation using three human glioma cell lines known to exhibit varying degrees of TRAIL sensitivity(8). AAV-S-TRAIL transduction induced a significant decrease in cell viability in Gli36-EGFRvIII(40), with 2000 MOI leading to a 62% reduction in viability as compared to control AAV-Gluc-DsRed2 transduced cells (Fig. 2A). Importantly, a corresponding dose-dependent upregulation in caspase activity was observed in AAV-S-TRAIL transduced Gli36-EGFRvIII cells with 2000 MOI inducing over a 3-fold increase (Fig. 2B), while no change in caspase activation was observed in control transduced cells. U87 cells exhibited an intermediate level of sensitivity to AAV-S-TRAIL with 2000 MOI inducing a 34% reduction in viability that lead to a 1.8-fold increase in caspase activation (Fig. 2C-D). U251 cell showed the highest level of TRAIL resistance with only high MOI resulting in reduction in cell viability (24% decrease; 10 000 MOI) and caspase activation (1.3-fold increase; 10 000 MOI) (Fig. 2E-F). Taken together these results suggest that S-TRAIL produced by AAV induces apoptosis in glioma cells, which correlates directly with the activation of caspases.
Figure 2. S-TRAIL induced apoptosis and caspase upregulation in human glioma cells in vitro.
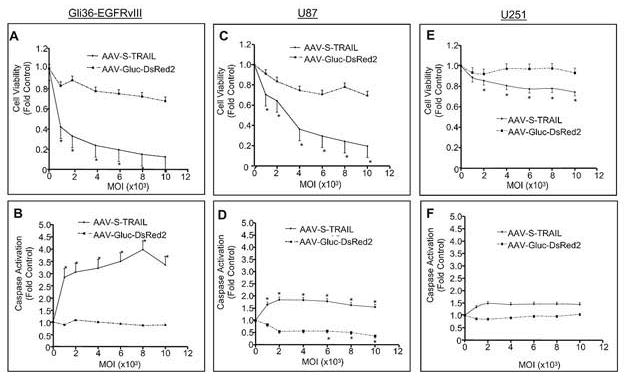
Gli36-EGFRvIII (A,B), U87 (C,D), and U251 (E,F) human glioma cells were transduced with S-TRAIL or Gluc-DsRed2 at increasing MOI (1 000-10 000) and 24 h post-transduction both cell viability (A,C,E) and caspase activation (B,D,F) were determined using bioluminescent-based assays. *p<0.05 vs. control.
Next, we utilized a glioma xenograft model to examine the effects of S-TRAIL in vivo. To permit non-invasive longitudinal monitoring of changes in tumor volume, we transduced Gli36-EGFRvIII human glioma cells with LV encoding GFP-firefly luciferase (Fluc) fusion (Gli36-EGFRvIII-GFP-Fluc). Flow cytometry analysis showed greater than 95% of the cells robustly expressed the GFP fusion (Fig. 3A). Representative images shown in Fig. 3B revealed direct delivery of AAV into established Gli36-EGFRvIII-GFP-Fluc could be followed by noninvasive Gluc bioluminescence imaging. Further utilization of serial bioluminescence imaging showed direct injection of AAV-S-TRAIL lead to marked reduction in glioma burden as early as 4 days post-injection compared to control injected gliomas (Fig. 3C). This reduction in glioma volume was sustained through day 12. Histological analysis of tumors removed 7 days post-transduction corroborated the in vivo findings showing significant increase in cleaved caspase-3 activation in gliomas treated with S-TRAIL compared to controls (Fig. 3D). These results show that AAV-S-TRAIL delivered directly into tumors has anti-tumor effects. Furthermore, bioluminescence imaging permits monitoring of both the delivery of therapeutic AAV and their efficacy in glioma models.
Figure 3. Real-time assessment of AAV transgene expression and effects of S-TRAIL on human glioma xenograft progression in vivo.
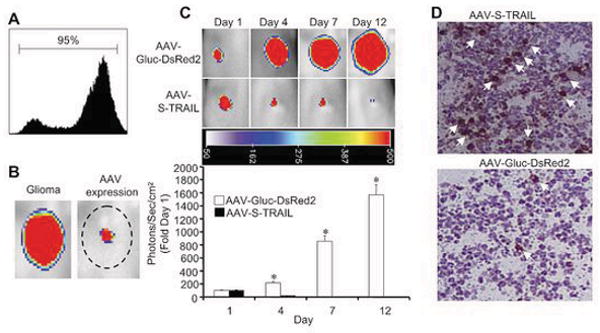
(A) Flow cytometry analysis of Gli36-EGFRvIII human glioma cells transduced with LV-GFP-Fluc. Gli36-EGFRvIII glioma cells expressing GFP-Fluc were implanted subcutaneously into nude mice and 48 and 96 h post-implantation, 1×109 particles of S-TRAIL or Gluc-DsRed2 was injected directly into the tumor mass (n=4 tumors/group, Day 1). Viral transgene expression and glioma progression were subsequently monitored by dual bioluminescent imaging. (B) 10 days after delivery of AAV-Gluc-DsRed2, mice were injected with coelenterazine and imaged for viral transgene expression. Forty-eight hours later, mice were injected with d-luciferin to visualize tumor mass. Representative images are shown to demonstrate co-localization of viral expression and glioma mass. (C) Representative images and summary data demonstrating the effects of AAV-S-TRAIL on tumor progression 1, 4, 7, and 12 days after AAV-S-TRAIL or AAV-Gluc-DsRed2 injection. (D) Seven days post-AAV-S-TRAIL or AAV-Gluc-DsRed2 injection, tumors were removed, sectioned, and stained for caspase activation. *p<0.05 vs. control.
The results of the current study (Fig. 2), coupled with previous results from our laboratory demonstrating human glioma lines exhibit varied degrees of sensitivity to S-TRAIL(8) emphasize the need for combination therapies to maximize the effectiveness of S-TRAIL therapy. The clinically utilized chemotherapeutic agent, TMZ has been suggested to enhance the toxicity of purified TRAIL (34). To first investigate the effects of TMZ alone on glioma lines, cells exhibiting high (Gli36-EGFRvIII) and low (U251) sensitivity to S-TRAIL were incubated with media containing TMZ for 24 or 72 h, or left untreated. As shown in Fig. 4A-I, TMZ treatment of both Gli36-EGFRvIII and U251 lead to a significant inhibition in cell cycle indicated by a G2/M arrest that was evident at 24 h and persisted through 72 h. This G2/M accumulation was associated with a slight increase in the subG1 population (Fig. A-I). Confirming activation of cell cycle checkpoints, Western blot analysis revealed marked increase in the levels of phosphorylated Chk1 following TMZ treatment, and no changes in total Chk1 (Fig. 4J). A marked increase in the expression of the TRAIL receptor, DR4 starting at 24 h post-treatment was observed following treatment of U251 cells with TMZ (Fig. 4K) Alternatively, TMZ did not increase DR4 expression above the already high levels observed in the highly TRAIL sensitive Gli36-EGFRvIII cell line (data not shown). These results demonstrate TMZ inhibits cell cycle progression in glioma cells, and increases the expression of death receptors, which may enhance S-TRAIL toxicity in these glioma lines.
Figure 4. TMZ induces cell cycle checkpoint arrest and upregulates death receptors on glioma cells.
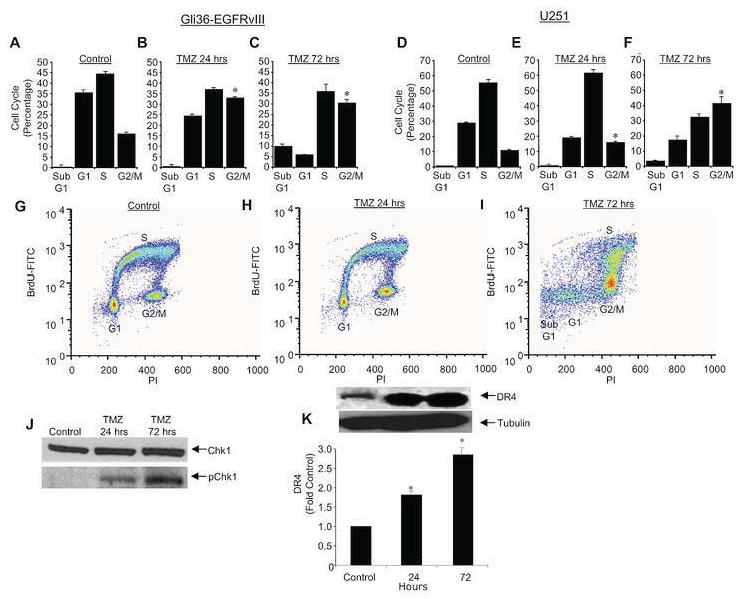
(A-F) Summary data of Gli36-EGFRvIII (A-C) and U251 (D-F) human glioma cells treated with TMZ (100 μM) for 24 (B,E), 72 h (C,F), or left untreated (A,D). Cells were pulsed for 1 h with bromodeoxyuridine, collected, stained, and cell cycle progression was analyzed by FACS. Data is expressed as percentage of cells in subG1, G1, S, or G2/M (G-I) Representative scatter plots of untreated Gli36 cells (G) and cells treated for 24 (H) or 72 h (I) with TMZ. (J) Western blot analysis demonstrating the effects of TMZ on the levels of total Chk1 and phosphorylated (p)Chk1. (K) Western blot analysis showing the expression of death receptor DR4 in U251 cells treated with TMZ for 24 or 72 h. All data is normalized to control cells (untreated). *p<0.05 vs. untreated cells.
To determine if TMZ enhances S-TRAIL induced cell killing, two approaches were used: 1) concomitant S-TRAIL and TMZ treatment; 2) pre-sensitization with TMZ prior to S-TRAIL treatment. To this end, two glioma cell lines with varying degrees of TRAIL sensitivity were co-treated with AAV-S-TRAIL (0-4 000 MOI) or NSC-S-TRAIL (0-600 ng/ml) and increasing concentrations of TMZ (0-1 000 μM). Summary data shown in Fig. 5A demonstrates that cell viability in the highly TRAIL sensitive Gli36-EGFRvIII cell line was markedly reduced by combined AAV-S-TRAIL/TMZ treatment compared to either therapy alone. A similar reduction was observed in Gli36-EGFRvIII treated with NSC-S-TRAIL/TMZ (Fig. 5B). In U251 cells that exhibited the highest resistance to S-TRAIL monotherapy, a modest yet significant increase in cell killing was observed in groups treated with AAV-S-TRAIL/TMZ or NSC-S-TRAIL/TMZ co-therapy (Fig. 5 C, D).
Figure 5. Synergistic cell killing by TMZ and S-TRAIL in human glioma cell lines.
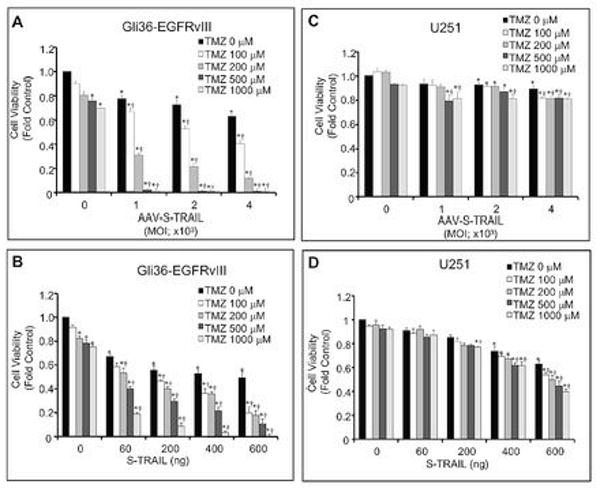
Human glioma cell lines Gli36-EGFRvIII (A,B)and U251 (C,D) were treated with increasing concentrations of TMZ alone (0-1000 μM), control virus (AAV-Gluc-DsRed2: 4 000 MOI), S-TRAIL alone (AAV-S-TRAIL: 0-4 000 MOI; NSC-S-TRAIL: 0-600 ng/mL), or a combination of TMZ and S-TRAIL. Eighteen h later, cell viability was determined by luciferase-based assay. All data is normalized to control cells (TMZ 0 μM, AAV-Gluc-DsRed2 or NSC-S-TRAIL 0 ng/mL). *p<0.05 vs. control. †p<0.05 vs. S-TRAIL alone.
To investigate if TMZ pretreatment can overcome S-TRAIL resistance and to sensitize cells to combined S-TRAIL and TMZ therapy, we first treated the minimally TRAIL sensitive U251 cells for 24 h with TMZ alone. Following this initial sensitization, TMZ was refreshed at which time AAV-S-TRAIL or NSC-S-TRAIL was added at low (1000 MOI; 200 ng/ml) and high doses (4000 MOI; 600 ng/ml). In separate groups, non-sensitized cells were treated with TMZ and S-TRAIL simultaneously. Demonstrating the potential effectiveness of TMZ sensitization for overcoming S-TRAIL resistance, summary graphs shown in Fig. 6A-B show that sensitization (s) of cells by 24 h of TMZ pretreatment significantly enhanced cell killing of S-TRAIL/TMZ treatment compared to cells not sensitized (ns) prior to S-TRAIL/TMZ addition. In U251 cells treated with NSC-S-TRAIL, enhanced killing by TMZ sensitization was associated with a marked increase in caspase 3/7 activation (Fig. 6C), as well as an increase in the levels of the apoptotic marker cleaved PARP (Fig. 6D) compared to non-sensitized cells. Additionally, immunocytochemistry confirmed a significant increase in the levels of cleaved caspase-3 in cells sensitized by TMZ (Fig. 6E). These results show pretreatment of cells with TMZ significantly increases the sensitivity of U251 glioma cells to S-TRAIL-induced apoptosis.
Figure 6. TMZ pre-treatment enhances the synergistic effects of TMZ and S-TRAIL in TRAIL-resistant glioma cells.
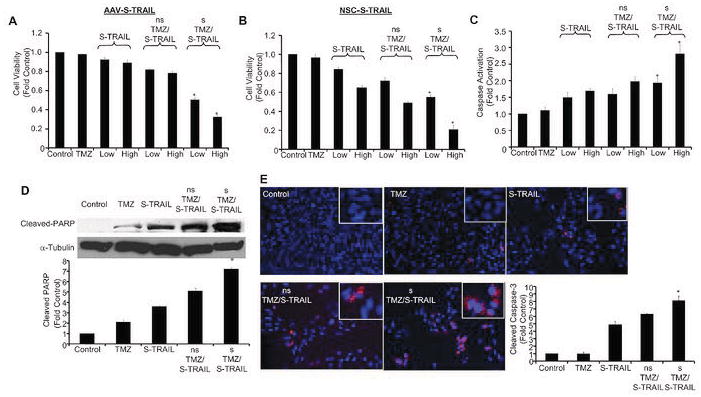
Summary data showing the viability of U251 glioma cells following concomitant TMZ and S-TRAIL treatment, or following 24 h of TMZ sensitization. Cells were left untreated (ns) or sensitized (s) with TMZ for 24 h. Both sensitized and untreated cells were subsequently treated or re-treated with TMZ alone, control virus (AAV-Gluc-DsRed2: 4 000 MOI), S-TRAIL alone (Low: AAV-S-TRAIL=1 000 MOI, NSC-S-TRAIL=200 ng/mL; High: AAV-S-TRAIL=4 000 MOI, NSC-S-TRAIL=600 ng/mL), or a combination of TMZ and S-TRAIL for an additional 18 h at which time cell viability (A,B) or caspase 3/7 activation (C) were determined. Representative images and summary data of western blot analysis showing the expression of cleaved PARP (D) and immunocytochemical analysis of cleaved caspase-3 expression (E) in U251 cells following treatment with TMZ, NSC-S-TRAIL, concomitant TMZ and S-TRAIL, or TMZ and S-TRAIL following 24 h of TMZ sensitization. All data is normalized to control cells (TMZ 0 μM, AAV-Gluc-DsRed2 or S-TRAIL 0 ng/mL). *p<0.05 vs. S-TRAIL/TMZ no pretreatment.
Discussion
Recent work in our laboratory has lead to the generation of a recombinant form of soluble TRAIL consisting of the extracellular domain of TRAIL fused to the extracellular domain of Flt3L by a leucine zipper (S-TRAIL)(5). We have shown that S-TRAIL has significantly enhanced anti-tumor effects compared to TRAIL alone(5), and that the anti-glioma effects of S-TRAIL produced by NSCs can be enhanced through synergistic inhibition of anti-apoptotic pathways(8) or upregulated microRNAs(23). In this study, we have engineered novel bi-modal AAV vectors encoding fluorescent (DsRed2) and bioluminescent (Gluc) markers and S-TRAIL, and shown the effectiveness of AAV-S-TRAIL as mono-therapy in mouse tumor models. Furthermore, we have shown that TMZ treatment sensitizes TRAIL resistant glioma cell lines to S-TRAIL produced by AAVs or NSCs.
In the endogenous form, TRAIL is expressed as a type II-membrane bound protein which functions as a key component of the immune system interacting with death receptors to aid in immune surveillance(41). Despite being a membrane bound protein, the extracellular domain of TRAIL can be cleaved leading to soluble form of TRAIL that still exhibits robust tumor cell killing in vitro and in vivo. Extending this research to the clinics, Genentech and Amgen have recently initiated clinical trials to test the tumor killing potential of soluble TRAIL in cancer patients. Current clinical trials are focused on the systemic delivery of purified TRAIL, an approach that suffers from several limitation including inefficient delivery of TRAIL to the primary glioma mass and potentially increased toxicity to normal tissue(7). Recently, other studies have focused on TRAIL delivery(3, 5, 35, 42). Previous results from our lab demonstrated that therapeutic NSC-S-TRAIL represents a novel means to selectively target glioma masses, while the capacity to differentiate into cells of neural lineage suggests therapeutic NSC may be able to replace neural tissue damaged by the glioma(3, 23, 27). Due to their tropism for tumor masses and ability to be engineered to secrete anti-tumor therapies, stem cells could be well suited for treating gliomas. However, in instances where anti-tumor therapies are toxic to stem cells, unable to be secreted, require a higher dose than can be delivered by stem cells, or the host rejects stem cell engraftment, direct injection of viral vectors into tumors may prove advantageous. As such, several viral vectors have been utilized clinically, however one of the most promising among these is AAV. AAV transduction occurs in both dividing and non-dividing cells(20), leads to high levels of transgene expression, and can persist for several months in the brain(43). Furthermore, AAV has entered clinical trials and has been shown to have several beneficial effects over other viral vectors (reviewed in (20, 28)). Based on this, we developed a novel AAV-S-TRAIL as an alternative to NSC-S-TRAIL. The results of this study demonstrate transduction of glioma cells with AAV-S-TRAIL lead to glioma cell killing in vitro and in vivo assessed by serial bioluminescence imaging. These results suggest the high transduction efficiency and prolonged transgene expression achieved by direct delivery of AAV-S-TRAIL offers the potential to deliver high levels of S-TRAIL therapy to localized regions of the glioma, thus minimizing toxicity to surrounding or systemic tissue. Further, the inclusion of Gluc-DsRed2 fusion proteins in this vector permits simple and non-invasive monitoring of viral gene expression that should allow easy determination of transduction efficiency and duration of expression in vivo, both vital criteria for achieving effective viral gene therapy. To our knowledge, this study is the first to demonstrate utility of AAV encoding TRAIL to treat malignant human glioma cells lines.
A large percentage of primary glioma lines are resistant to TRAIL-induced apoptosis(15, 16) and numerous studies have demonstrated the potential of sensitizing TRAIL-resistant tumor cell lines by combining TRAIL with chemotherapeutic agents (8, 23, 34, 44). In this study, we demonstrate that TRAIL sensitivity can be enhanced both in lines already sensitive to TRAIL, and more importantly, in TRAIL resistant cell lines. At concentrations as low as 200 μM, TMZ significantly increased the killing effects of S-TRAIL in Gli36-EGFRvIII. In U251 cell lines, the sensitization of cells required 24 h of pre-treatment with TMZ alone in order to induce TRAIL sensitivity, however following this treatment combined TMZ and S-TRAIL lead to greater than 60% reduction in cell viability in this line which showed minimal sensitivity to S-TRAIL therapy alone. The oral chemotherapy TMZ(31), S-TRAIL(7), AAV vectors(28), and cell-based(45) therapies are all being utilized in the clinics. TMZ has been implemented as part of the standard of care for patients being treated for glioblastoma. Clinical trials with TRAIL have relied primarily on the injection of purified protein, a method that has proven inefficient. The results of this study suggest that the combination of these clinically relevant therapies may improve their delivery and potency, thus leading to improved outcomes for patients.
TRAIL-induced apoptosis is a receptor-mediated pathway, triggered upon binding of TRAIL to death receptors(6, 7). Treatment of glioma cells with NSC-S-TRAIL leads to the introduction of fully functional TRAIL that can immediately activate death receptors to initiate apoptosis. In contrast, transduction of glioma cells with AAV-S-TRAIL requires cellular uptake of the virus, uncoating, integration into the cell nucleus, expression and processing of S-TRAIL which is then secreted(20). It is only after this secretion that we expect functional S-TRAIL to be present in the medium and capable of binding to the death receptor pathways to induce apoptosis. Therefore, we anticipate that the increased effectiveness of NSC-S-TRAIL is most likely due to this rapid death receptor binding and apoptotic induction compared to the delayed activation triggered by AAV-S-TRAIL transduction. However, both S-TRAIL delivered by either NSC or AAV proved to have potent anti-glioma effects that were synergistically enhanced by TMZ co-therapy.
In this study, we observed TMZ induced accumulation of cells in G2/M phase that was associated with upregulation of death receptors. A number of studies have clearly demonstrated the capacity of TMZ to induce cell-cycle arrest in glioma lines. This effect is typically mediated by activation of the ATM/ATR pathways in response to DNA damage, which subsequently activates down stream cell cycle checkpoints to inhibit progression cell cycle progression(46-48). Various therapeutic agents have been shown to increase the expression of death receptors, including radiation(48), topoisomerase inhibitors(49), and methylating agents(50). As TRAIL toxicity is mediated by receptor binding, receptor upregulation provides a clear link for the mechanism underlying the synergy observed between TRAIL and these anti-glioma therapies. Previous studies have shown upregulation of TRAIL receptors in U251 cell by combined treatment of TRAIL and TMZ(34). In this study, we observed TMZ-induced upregulation of DR4 as early as 24 h post-TMZ treatment that remained elevated through 72 h. The increase in DR4 expression was paralleled by increased accumulation of cells in G2/M phase and activation of Chk1. These results suggest TMZ and TRAIL synergism involves two mechanisms: 1) increase of death receptor expression in lines with low basal expression permitting increased TRAIL receptor binding, 2) inhibition of cell proliferation in parallel with TRAIL-induced apoptosis. As such, the first mechanism will predominate in slow proliferating cell lines with low DR4 expression (i.e. U251), while the second mechanism will predominate in faster proliferating cell lines with high DR4 expression (Gli36-EGFRvIII). Additional investigation will be required to elucidate the exact mechanism of TMZ and TRAIL synergy in various cell lines, however it is becoming increasingly clear that one of the key events underlying TMZ/TRAIL synergy is increased expression of death receptors.
In conclusion, we have shown that ant-tumor effects of S-TRAIL delivered by novel means are enhanced by co-treatment with the clinical chemotherapeutic agent TMZ. We anticipate that further investigation in vivo using S-TRAIL delivered by AAVs or NSC and TMZ will pave a way for their combined use in clinics. One could envision the integration of TRAIL therapy into TMZ and radiotherapy currently in place as the standard of care, or even eliminate the need for radiotherapy as the high level of TMZ and TRAIL synergism should permit efficient treatment of heterogenous gliomas.
Supplementary Material
Gli36-EGFRvIII and U251 cells were incubated with increasing concentrations of TMZ (0-1 000 μM) and (A, C) NSC-S-TRAIL (0-600 ng/mL) or (B,D) AAV-S-TRAIL (0-4 000 MOI). Isobologram analysis was performed as described in Materials and Methods.
Acknowledgments
We would like to thank Lee Zhou for providing us with Chk-1 antibodies and also acknowledge Patrick Wen, Claire Sauvageot, and Stephen Yip for expert advice on TMZ studies. We graciously acknowledge that this work was supported by National Institute of Health (T32 CA79443) (RW), American Brain Tumor Association (KS), Goldhirsh Foundation (KS), Catherine and Pappas award in Neuro-oncology (KS).
References
- 1.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 2.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 3.Shah K, Bureau E, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 4.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Shah K, Tung CH, Yang K, Weissleder R, Breakefield XO. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64:3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- 6.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 7.Koschny R, Walczak H, Ganten TM. The promise of TRAIL-potential and risks of a novel anticancer therapy. J Mol Med. 2007;85:923–935. doi: 10.1007/s00109-007-0194-1. [DOI] [PubMed] [Google Scholar]
- 8.Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaganathan J, Petit JH, Lazio BE, Singh SK, Chin LS. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in established and primary glioma cell lines. Neurosurgical Focus. 2002;13 doi: 10.3171/foc.2002.13.3.6. ecp1. [DOI] [PubMed] [Google Scholar]
- 10.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427:124–128. doi: 10.1016/s0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 11.Roth W, Isenmann S, Naumann U, et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 12.Kelley SK, Harris LA, Xie D, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 13.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165:2886–2894. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 14.Kagawa S, He C, Gu J, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 15.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieger J, Frank B, Weller M, Wick W. Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. Cell Physiol Biochem. 2007;20:23–34. doi: 10.1159/000104150. [DOI] [PubMed] [Google Scholar]
- 17.Rainov NG, Ren H. Gene therapy for human malignant brain tumors. Cancer J. 2003;9:180–188. doi: 10.1097/00130404-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lawler SE, Peruzzi PP, Chiocca EA. Genetic strategies for brain tumor therapy. Cancer Gene Ther. 2006;13:225–233. doi: 10.1038/sj.cgt.7700886. [DOI] [PubMed] [Google Scholar]
- 19.Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorsen F, Afione S, Huszthy PC, et al. Adeno-associated virus (AAV) serotypes 2, 4 and 5 display similar transduction profiles and penetrate solid tumor tissue in models of human glioma. J Gene Med. 2006;8:1131–1140. doi: 10.1002/jgm.939. [DOI] [PubMed] [Google Scholar]
- 22.Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx. 2005;2:215–225. doi: 10.1602/neurorx.2.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 24.Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Annu Rev Med. 58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- 25.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 26.Kim SK, Kim SU, Park IH, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 27.Shah K. Current advances in molecular imaging of gene and cell therapy for cancer. Cancer Biol Ther. 2005;4:518–523. doi: 10.4161/cbt.4.5.1706. [DOI] [PubMed] [Google Scholar]
- 28.Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- 29.Rohn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20:4128–4137. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 30.Cretney E, Takeda K, Smyth MJ. Cancer: novel therapeutic strategies that exploit the TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway. Int J Biochem Cell Biol. 2007;39:280–286. doi: 10.1016/j.biocel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 32.van den Bent MJ. Adjuvant treatment of high grade gliomas. Annals of Oncology. 2006;17 10:x186–190. doi: 10.1093/annonc/mdl258. [DOI] [PubMed] [Google Scholar]
- 33.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 34.Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on temozolomide-resistant glioma cells. J Neurosurg. 2007;106:646–651. doi: 10.3171/jns.2007.106.4.646. [DOI] [PubMed] [Google Scholar]
- 35.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 36.Shah K, Hingtgen SD, Kasmieh R, et al. Bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model. J Neurosci. 2008;28:4406–4413. doi: 10.1523/JNEUROSCI.0296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Ma C, Bass C, Terwilliger EF. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341:203–214. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 38.Shah K, Tang Y, Breakefield X, Weissleder R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22:6865–6872. doi: 10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
- 39.Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 40.Arwert E, Hingtgen S, Figueiredo JL, et al. Visualizing the dynamics of EGFR activity and antiglioma therapies in vivo. Cancer Res. 2007;67:7335–7342. doi: 10.1158/0008-5472.CAN-07-0077. [DOI] [PubMed] [Google Scholar]
- 41.Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol. 2006;84:87–98. doi: 10.1111/j.1440-1711.2005.01413.x. erratum appears in. [DOI] [PubMed] [Google Scholar]; Immunol Cell Biol. 2006 Apr;84(2):238. doi: 10.1111/j.1440-1711.2006.01421.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu W, Sun Q, Wan J, She Z, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 43.Xu R, Janson CG, Mastakov M, et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 2001;8:1323–1332. doi: 10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- 44.Sayers TJ, Murphy WJ. Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother. 2006;55:76–84. doi: 10.1007/s00262-005-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachoud-Levi AC, Remy P, Nguyen JP, et al. Lancet. 2000;356:1975–1979. doi: 10.1016/s0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- 46.Caporali S, Falcinelli S, Starace G, et al. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66:478–491. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 47.Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:5843–5849. [PubMed] [Google Scholar]
- 48.Guan B, Yue P, Clayman GL, Sun SY. Evidence that the death receptor DR4 is a DNA damage-inducible, p53-regulated gene. J Cell Physiol. 2001;188:98–105. doi: 10.1002/jcp.1101. [DOI] [PubMed] [Google Scholar]
- 49.Gibson SB, Oyer R, Spalding AC, Anderson SM, Johnson GL. Increased expression of death receptors 4 and 5 synergizes the apoptosis response to combined treatment with etoposide and TRAIL. Mol Cell Biol. 2000;20:205–212. doi: 10.1128/mcb.20.1.205-212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gli36-EGFRvIII and U251 cells were incubated with increasing concentrations of TMZ (0-1 000 μM) and (A, C) NSC-S-TRAIL (0-600 ng/mL) or (B,D) AAV-S-TRAIL (0-4 000 MOI). Isobologram analysis was performed as described in Materials and Methods.


