Abstract
Nearest-neighbor recognition measurements have been made for an exchangeable phospholipid (A) interacting with an exchangeable form of cholesterol (B) in host membranes derived from 1, 2-dipalmitoyl-sn-glycero-3-phosphocholine and varying concentrations of cholesterol, 7β-hydroxycholesterol (7β–OH), and 25-hydroxycholesterol (25-OH). Whereas partial replacement of cholesterol with 7β–OH strengthens the association between A and B, a similar substitution with 25-OH weakens this association. A model that accounts for this dichotomy, and the possible relevance of these findings to the cytotoxicity of 7β–OH and to Alzheimer's disease are briefly discussed.
Introduction
Cholesterol is a major building block of mammalian cell membranes.1 Because of its fluidizing and condensing effects on lipids, the structural role that this sterol plays in cell membranes has been the subject of intense investigation. One popular hypothesis is that cholesterol combines with high melting lipids to form microdomains termed, “lipid rafts”. It has also been proposed that these rafts act as organizing centers that (i) assemble signaling molecules, (ii) assist in membrane protein trafficking, and (iii) regulate neurotransmission.2-6 Despite its popularity, the lipid raft hypothesis remains controversial.3,4
In addition to clarifying its effects on membrane structure, attention has begun to focus on apparent connections between some of cholesterol's oxidation products and certain diseases.7-10 In particular, the formation of 7β-hydroxycholesterol (7β–OH)—an oxysterol that is known to be highly neurotoxic—appears to be associated with Alzheimer's disease.7 Although it is known that this disease is characterized by an accumulation of β–amyloid, and that this peptide contributes to the formation of 7β–OH, exactly how this peptide and oxysterol may affect membrane organization and cell death are not presently understood.11,12
In the work reported herein, we sought to gain fundamental insight into the effects of 7β–OH on membrane organization by quantifying its effects on nearest-neighbor interactions in the liquid-ordered, lo, phase.13 The lo phase was of particular interest because it has been widely used as models for lipid rafts, since cholesterol has been shown to associate with certain high-melting lipids in that phase.14 To place the effects of 7β-OH into perspective, we also sought to quantify the effects of a related oxysterol in which a hydroxyl group is located in the isooctyl side chain of cholesterol; that is, 25-hydroxycholesterol (25-OH).15
Results and Discussion
Quantifying Nearest-Neighbor Interactions Using The NNR Method
To measure the effects that 7β–OH and 25-OH have on membrane organization, we have employed the highly-sensitive, nearest-neighbor recognition (NNR) method.16,17 As discussed elsewhere, such experiments take molecular-level snapshots of bilayer organization by detecting and quantifying the thermodynamic tendency of exchangeable monomers to become nearest-neighbors of one another.18-21 Typically, two lipids of interest (A and B) are converted into exchangeable dimers (AA, AB and BB), which are then allowed to undergo monomer interchange via thiolate-disulfide exchange. The resulting equilibrium that is established, whereby one molecule of AA reacts with one molecule of BB to give two molecules of AB, is governed by an equilibrium constant, K= [AB]2/([AA][BB]). When monomers A and B mix ideally, this is reflected by an equilibrium constant that equals 4.0. When homo-associations are favored, the equilibrium constant is less than 4.0; favored hetero-associations are indicated by a value that is greater than 4.0. Nearest-neighbor interaction free energies between A and B are then given by ωAB= -1/2 RT ln(K/4).22 Here, K is divided by 4 because the heterodimer is statistically favored by a factor of 2, which is why random mixing is reflected by K=4. The factor of ½ arises because K governs the formation of two dimers.
Choice of Lipids
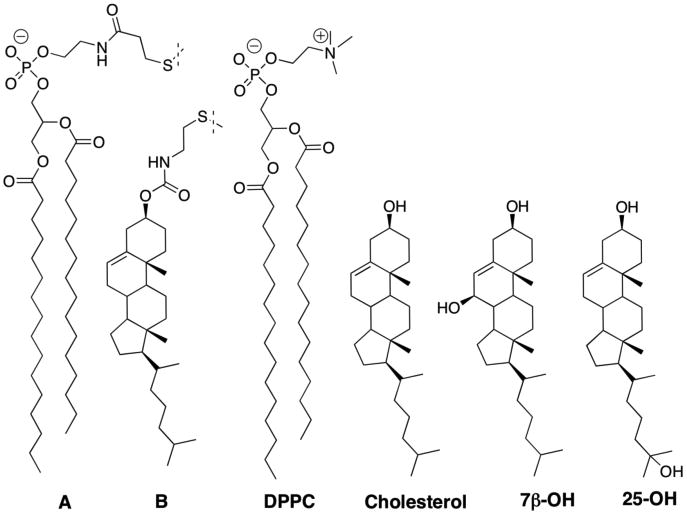
In this work, we carried out NNR exchange reactions at 45°C in cholesterol-rich liposomal membranes using 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) as the host phospholipid (Chart 1). Our choice of A and B as the exchangeable lipids was based on the fact that the gel to liquid-crystalline phase transition temperature (Tm) of AA (41.9.°C) is nearly identical to that of DPPC (41.5°C), and that the condensing behavior of BB is nearly identical to that of cholesterol.18,23
Chart 1.
Phase Properties
Before carrying out NNR experiments, we first examined whether 7β-OH and 25-OH significantly affect the phase properties of cholesterol-rich membranes of DPPC. For this purpose, we measured the fluorescence of a phase-sensitive probe, Laurdan, and determined generalized polarization (GP) values as a function of temperature. Here, GP= (I440-I490)/(I440+I490), and I440 and I490 are the emission intensities at these wavelengths (λex=350 nm).24 As disscussed elsewhere, such GP values reflect, most directly, the polarity surrounding the Laurdan moiety in the bilayer and are very sensitive to changes in phase.24
At low cholesterol concentrations (2.5 mol%), a gel to liquid-crystalline (i.e., liquid-disordered, ld) phase transition was evident with a melting temperature of ca. 41°C (Figure 1). In contrast, when 40 mol% cholesterol was included in the bilayer, which is known to maintain the lo phase from 30°C to 55°C, the GP values were found to decrease modestly and linearly with increasing temperatures.25 Incremental replacement of cholesterol with 7β–OH showed this same “signature” for the lo phase (i.e., a similar linearity and a similar slope). However, all of the GP values were lowered to a similar extent at each temperature. When the total sterol content in the bilayer consisted of 20 mol% cholesterol (which leads to a transition from a gel/lo coexistence phase to a lo/ld coexisence phase, as the temperature is increased from 30°C to 55°C), a broad but distinct inflection was observed in the plot of GP versus temperature (Figure 2).25 Significantly, a very similar shaped curve was found in bilayers containing 10 mol% of cholesterol plus 10 mol% 7β-OH, reflecting the same phase behavior. Here also, all GP values were lowered to a similar extent at each temperature. For bilayers that contained 20 mol% cholesterol plus 20 mol% 25-OH, the GP/temperature profile was virtually identical with that found with 40 mol% cholesterol (Supporting Information). Taken together, these results indicate that partial replacement of cholesterol by 7β–OH or 25-OH leaves the bilayer in the same lo phase. The lower GP values in the presence of 7β–OH are a likely consequence of close proximity of this fluorophore to the polar 7β-hydroxyl group, and not greater water accessibility from any “softening” effects. If there were any “softening” of the bilayer by the presence of 7β-OH, one would expect to observe an increase in the slope as the temperature is increased, and this is clearly not the case. It should be noted that these results are also fully consistent with those of a previous study, in which cholesterol was fully replaced by these sterols.10
Figure 1.
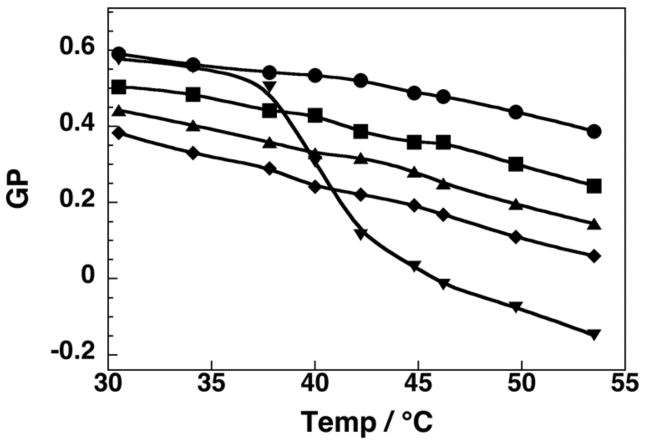
Plot of GP versus temperature in liposomes made from DPPC/DPPG/cholesterol/7β-OH (57.5/2.5/40/0,mol%) (●), (95/2.5/2.5/0, mol%) (▼), (57.5/2.5/30/10, mol%) (■), (57.5/2.5/20/20, mol%) (▲), (57.5/2.5/10/30, mol%) (◆).26
Figure 2.
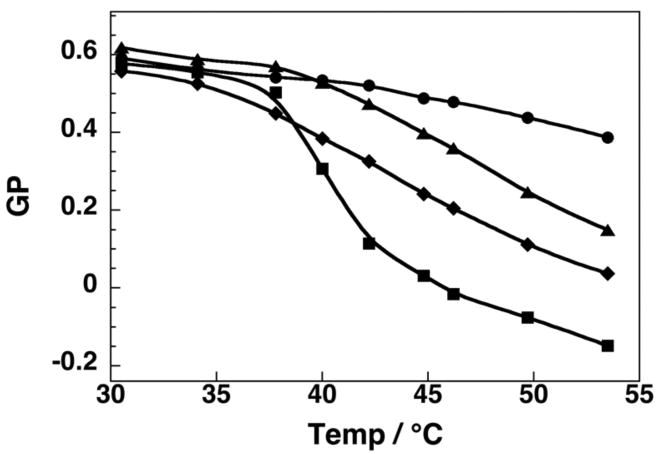
A comparison of GP values as a function of temperature for liposomes containing 20% cholesterol (▲), and 10% cholesterol + 10% 7β-OH (◆), with ones containing 40 mol% cholesterol (●) and 2.5% cholesterol (■).
NNR Measurements
Exchangeable phospholipid dimers AA, BB and AB that were used in this investigation were synthesized using established procedures.18,20 Liposomes that were prepared via extrusion had average diameters, which were 170-190 nm (dynamic light scattering; Nicomp 270, Gaussian analysis). Analysis of these dispersions after NNR exchange reactions were completed showed no significant change in their size or size distriubtion.
Figure 3 summarizes the results of our NNR measurements. In brief, incremental replacement of cholesterol by 7β–OH in membranes containing a total sterol content of 40 mol% resulted in a steady increase in K. A significant increase in K was also evident in the lo/ld coexistence region for bilayers containing 10 mol% cholesterol plus 10 mol% 7β–OH (Figure 3). In sharp contrast, replacement by 25-OH resulted in a steady decrease in K. On going from 40 mol% cholesterol to 20 mol% cholesterol+ 20 mol% dihydrocholesterol (i.e., a slightly more lipophilic sterol), no significant change in K (Figure 3) or GP values (Supporting Information) were observed.
Figure 3.
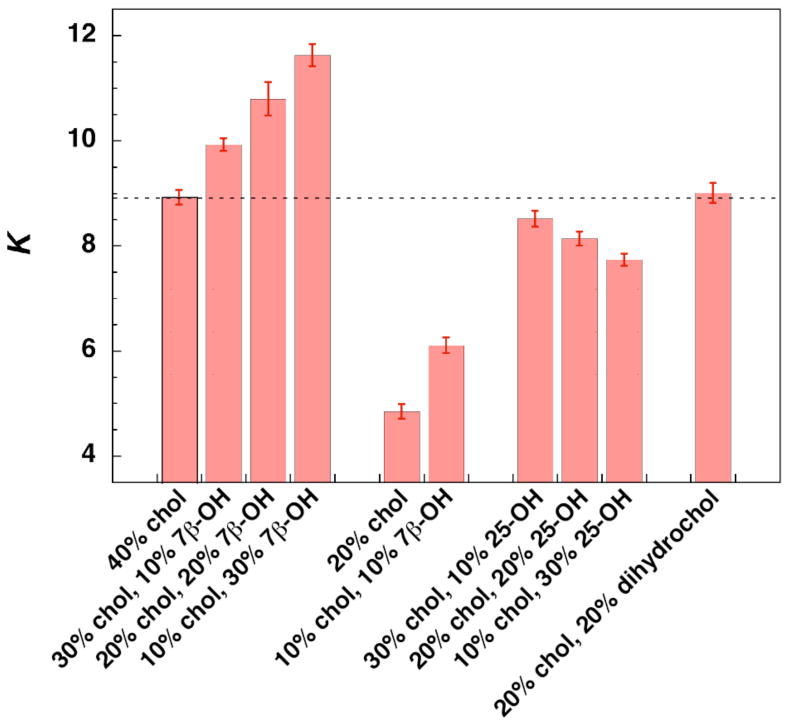
All NNR exchange reactions were carried out at 45°C in liposomes made from DPPC and the indicated mole percentages of sterols, plus 5 mol% of AB. Equilibrium was confirmed by following dimer distributions as a function of time and by carrying out analogous reactions starting with 2.5 mol% of AA and BB (Supporting Information).21 The dotted line marks the K value found in the presence of 40% cholesterol; error bars represent one standard deviation and were obtained from time course measurements.
A Model for the Efffects of 7β–OH and 25-OH on Sterol-Phospholipid Association
A simple model that can account for the strengthening of the association between A and B in the presence of 7β–OH, and also the weakening of this association in the presence of 25-OH, is based on occupied areas (or lateral pressure) near the surface of the membrane. In the monolayer leaflets of liquid phospholipid bilayers, the phospholipid head groups occupy only about half the lipid/water interface and the rest of the surface is filled with partially hydrated CH2 groups from the acyl chains.27,28 When cholesterol is present, its hydroxyl group fills in that same space and, thus, its condensing effect on phospholipid monolayers consists, exclusively, of decreasing the surface area of the phospholipids by releasing into the lipid region their methylene groups proximal to the ester groups. This results in a partial straightening of the acyl chains, which promotes chain-chain interactions and leads to the formation of the liquid-ordered phase (Figure 4). This partial straightening of the acyl groups also thickens the monolayer and renders the effective chain lengths comparable to the size of the cholesterol molecule. It is this matching of size that allows cholesterol and phospholipids to form complexes, which is detected by our NNR measurements and is the hallmark of the liquid-ordered phase.29-31
Figure 4.
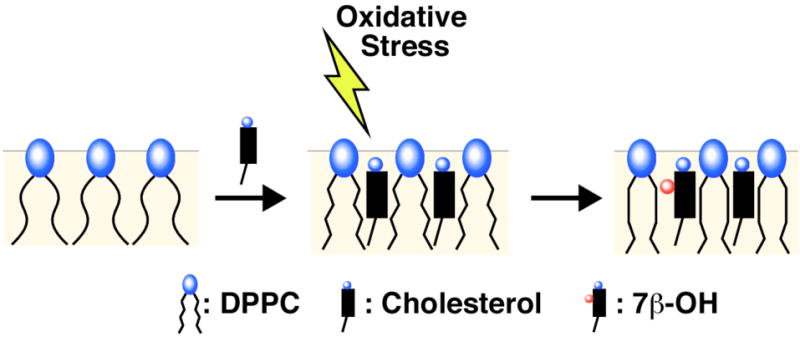
A model showing an enhanced condensing effect on going from cholestrol to 7β-OH.
A corollary of this mechanism is that if a cholesterol derivative (i.e., an oxysterol) occupies a surface area that is larger than that of cholesterol, then it should be more effective in displacing the acyl chains and, hence, produce a more stable liquid-ordered phase. The results of our NNR experiments with 7β-OH support this hypothesis; that is, 7β-OH, which prefers a surface conformation that has a larger surface area than cholesterol, produces a better stabilization of the heterodimer.27
The reduction in K that results from the partial replacement of cholesterol with 25-OH can then be accounted for if one assumes that the sterol inserts into the monolayer (either partially or completely) in an “upside down” fashion, which exposes the side-chain hydroxyl group to the interface (not shown).15 It should be noted that since 25-OH contains one small polar head at each end of the sterol, both upside down as well as “right-side-up” orientations are possible.15 This would result in an interfacial area that is less than that of cholesterol, a sterol that still possesses the same overall cross-sectional area, but reduced stabilization of the heterodimer, AB. Alternatively, this weakening effect by 25-OH may be due to repulsive interactions between the polar 25-hydroxyl group (buried deep within the membrane) and the acyl chains of neighboring phospholipids, which are strongly lipophilic.
Biological Implications
The magnitude of the effects reported herein are small but significant. Thus, a change in K from 8.95 (ωAB= 254 cal/mol, 40% cholesterol) to 11.63 (ωAB= 337 cal/mol, 10% cholesterol + 30% 7β-OH) corresponds to a free energy difference of 83 cal/mol. It is noteworthy, in this regard, that recent Monte Carlo simulations have shown that for ternary mixtures of sphingomyelin/1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphocholine/cholesterol (35/30/35, mol/mol/mol), a change in nearest-neighbor interactions energies of only 50 cal/mol is sufficient to transform large domains into phase-separated regions.22 Thus, changes in interaction energies on the order of tens of calories per mole of lipid are sufficient to cause major changes in membrane structure. The fact that the effects of 7β-OH are directly proportional to its concentration further implies that small but significant effects should exist even at much lower concentrations. How much of a change in interaction energy is needed to cause a cell membrane to malfunction, however, is a question that remains to be answered.
If the primary role of amyloid beta-peptide in the development of Alzheimer's disease is to oxidize cholesterol to 7β–OH, then the present findings suggest that modest changes in the distribution of cholesterol throughout the membrane may be a key factor in the loss of proper celluar function. Specifically, it is conceivable that even a modest excess of 7β-OH due to oxidative stress may increase the percentage of cholesterol that becomes nearest-neighbors of high-melting lipids, and reduce the percentage of cholesterol that is available for becoming nearest-neighbors of transmembrane proteins; e.g., signaling proteins. To the extent that a cholesterol microenvironment is necessary for the proper functioning of such proteins, this redistribution would be expected to have negative consequences.
Conclusions
Nearest-neighbor recognition measurements that have been carried out in the liquid-ordered phase have revealed a dichotomy between 7β-OH and 25-OH, in terms their effects on sterol-phospholipid association relative to cholesterol. Thus, whereas partial replacement of cholesterol with 7β–OH strengthens the association between A and B, a similar replacement with 25-OH weakens this association. These findings demonstrate how the location of a hydroxyl group that has been added to cholesterol can control the directionality of an oxysterol's effects on nearest-neighbor interactions in the lo phase. In a broader context, these results suggest that changes in the lateral distribution of cholesterol in the membranes of neurons, resulting from an excess of 7β-OH, may have relevance to Alzheimer's disease.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (PHS GM56149). We thank Drs. Vaclav Janout and Serhan Turkyilmaz (Lehigh) for valuable discussions.
Footnotes
SUPPORTING INFORMATION. Experimental procedures and raw data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gennis RB. Biomembranes: Molecular Structure and Function. Springer-Verlag; New York: 1989. [Google Scholar]
- 2.Simons K, Ikonen E. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Edidin M. Annu Rev Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 4.Munro S. Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 5.Simons K, Vaz WLC. Annu Rev Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh TJ, editor. Lipid Rafts. Springer-Verlage; New York, N.Y: 2007. [Google Scholar]
- 7.Nelson TJ, Alkon DL. J Biol Chem. 2005;280:7377–7387. doi: 10.1074/jbc.M409071200. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela A, Sanhueza J, Nieto S. Biol Res. 2003;36:291–302. doi: 10.4067/s0716-97602003000300002. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Frangos JA. Biophys J. 2008;95:620–628. doi: 10.1529/biophysj.107.114983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massey J, Pownall HJ. Biochemisry. 2006;45:10747–10758. doi: 10.1021/bi060540u. [DOI] [PubMed] [Google Scholar]
- 11.Taylor BM, Sarver RW, Fici G, Poorman RA, Lutzke BS, Molinari A, Kawawbe T, Kappenman K, Buhl AE, Epps DE. J Protein, Chem. 2003;22:31–40. doi: 10.1023/a:1023063626770. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein SL, Wyttenback T, Baumketner A, Shea JE, Bitan G, Teplow DB, Bowers MT. J Am Chem Soc. 2005;127:2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 13.Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zukeermann MJ. Biochim Biophys Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 14.Quinn PJ, Wolf C. Biochim Biophys Acta. 2009;1788:33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Olsen BN, Schlesinger PH, Baker NA. J Am Chem Soc. 2009;131:4854–4865. doi: 10.1021/ja8095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson SK, Regen SL. Chem Rev. 1997;97:1269–1280. doi: 10.1021/cr960381s. [DOI] [PubMed] [Google Scholar]
- 17.Regen SL. Current Opinion in Chem Biol. 2002;6:729–735. doi: 10.1016/s1367-5931(02)00398-8. [DOI] [PubMed] [Google Scholar]
- 18.Sugahara M, Uragami M, Yan X, Regen SL. J Am Chem Soc. 2001;123:7939–7940. doi: 10.1021/ja016199c. [DOI] [PubMed] [Google Scholar]
- 19.Sugahara M, Uragami M, Regen SL. J Am Chem Soc. 2003;125:13040–13041. doi: 10.1021/ja038102n. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Zhang J, Jing B, Regen SL. J Am Chem Soc. 2005;127:8813–8816. doi: 10.1021/ja0513988. [DOI] [PubMed] [Google Scholar]
- 21.Mitomo H, Chen WH, Regen SL. Langmuir. 2009;25:4328–4330. doi: 10.1021/la900346p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida PFF. Biochim Biophys Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Krisovitch SM, Regen SL. J Am Chem Soc. 1992;114:9828–9835. [Google Scholar]
- 24.Parassi T, DiStefano M, Loiero M, Ravagnan G, Gratton E. Biophys J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang YW, Costa-Filho J, Freed JH. J Phys Chem, B. 2007;111:11260–11270. doi: 10.1021/jp0732110. [DOI] [PubMed] [Google Scholar]
- 26.1,2-Dipalmitoyl-sn-glycero-3-phospho-(1′rac-glycerol) (sodium salt) (i.e., DPPG) (2.5 mol% was used to simulate NNR conditions since A has a negative charge, although GP vaules were found to be independent of charge (not shown).
- 27.Kaufmann JM, Westerman PW, Carey MC. J Lipid Res. 2000;41:991–1003. [PubMed] [Google Scholar]
- 28.Cantor RS. Biochemistry. 1997;36:2339–2344. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 29.Radakrishnan A, McConnell HM. J Am Chem Soc. 1999;121:486–487. [Google Scholar]
- 30.McConnell HM, Radhakrishnan A. Biochim Biophys Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 31.Veatch SL, Keller SL. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


