Abstract
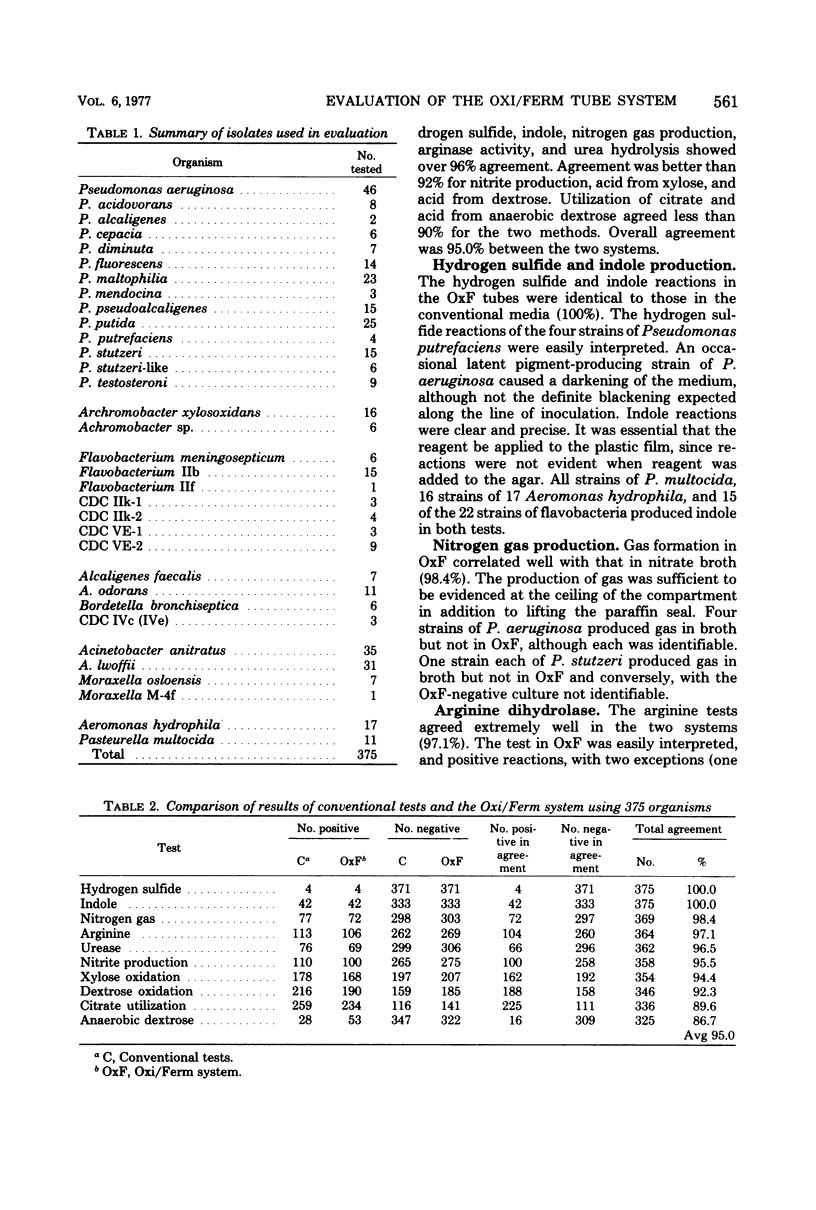
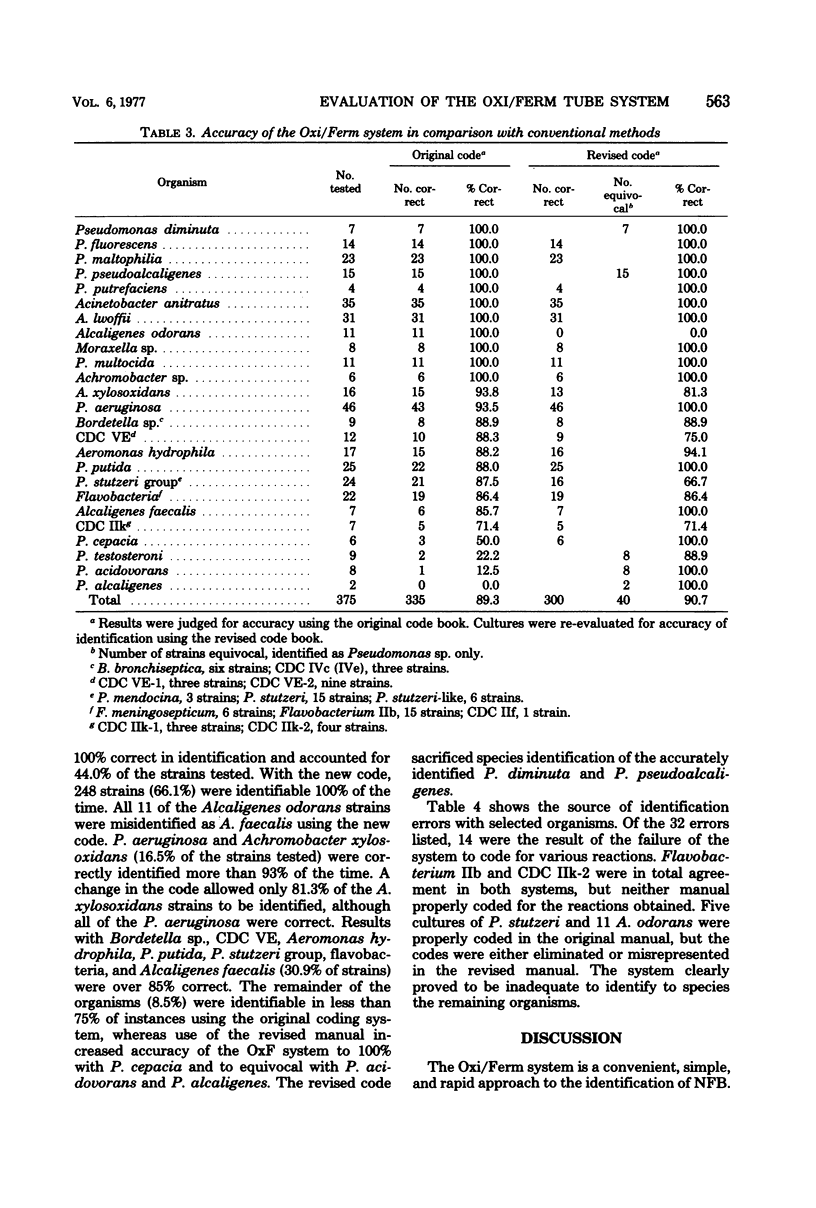
The Oxi/Ferm test system was evaluated for accuracy and reliability for identification of nonfermentative and oxidase-positive fermentative bacteria by using 375 bacterial strains obtained from stock culture and clinical specimens. The Oxi/Ferm system is a compartmentalized tube containing eight media to provide nine biochemical test results. When combined with the oxidase test, the results corresponding to the positive reactions are totaled and the composite number is located in the coding manual to identify the organisms. The 375 isolates studied were evaluated for accuracy of identification, using both the original and revised code manuals. In comparison with the conventional media used, there was 100% correlation in tests for hydrogen sulfide and indole production, over 96% for nitrogen gas, arginine, and urease, over 92% for xylose and dextrose oxidation, and less than 90% for citrate utilization and dextrose fermentation. There was an overall accuracy in identification of 89.3% using the original manual, with accuracy revised slightly upward to 90.7% using the revised manual. There was 100% accuracy in identification with 44.0% of the strains tested (11 species) using the original manual and with 66.1% (16 species) using the revised manual. Thirteen of the 40 original misidentifications and 14 of 35 revised misidentifications resulted from failure to code and were unidentifiable by Oxi/Ferm. The remainder were incorrectly identified or could not be differentiated from closely related strains. Eleven strains of Alcaligenes odorans were correctly identified using the original code, whereas no code was provided in the revised manual. The Oxi/Ferm system is both simple and rapid and is satisfactory for identification of the more common isolates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Coppel S. P., Coppel I. G. Comparison of the R-B system and the Enterotube for the identification of Enterobacteriaceae. Am J Clin Pathol. 1974 Feb;61(2):218–222. doi: 10.1093/ajcp/61.2.218. [DOI] [PubMed] [Google Scholar]
- Ederer G. M., Lund M. E., Blazevic D. J., Reller L. B., Mirrett S. Motility-indole-lysine-sulfide medium. J Clin Microbiol. 1975 Sep;2(3):266–267. doi: 10.1128/jcm.2.3.266-267.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston H. R., Baudo J. A., Stanek J. P., Schaab M. Multi-biochemical test system for distinguishing enteric and other gram-negative bacilli. Appl Microbiol. 1971 Sep;22(3):408–414. doi: 10.1128/am.22.3.408-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Pseudomonas species in clinical microbiology. Mt Sinai J Med. 1976 Nov-Dec;43(6):710–726. [PubMed] [Google Scholar]
- Isenberg H. D., Sampson-Scherer J. Clinical laboratory evaluation of a system approach to the recognition of nonfermentative or oxidase-producing gram-negative, rod-shaped bacteria. J Clin Microbiol. 1977 Mar;5(3):336–340. doi: 10.1128/jcm.5.3.336-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Yu P. K., Washington J. A., 2nd Evaluation of the enterotube system for identification of members of the family Enterobacteriaceae. Appl Microbiol. 1971 Jul;22(1):96–99. doi: 10.1128/am.22.1.96-99.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Rowen J. W., Cunningham G. F. Characterization and identification of gram-negative, nonfermentative bacteria. J Clin Microbiol. 1977 Feb;5(2):208–220. doi: 10.1128/jcm.5.2.208-220.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Rowen J. W., Higbee J. W., Johns R. W. Evaluation of the rapid decarboxylase and dihydrolase test for the differentiation of nonfermentative bacteria. J Clin Microbiol. 1976 Feb;3(2):137–142. doi: 10.1128/jcm.3.2.137-142.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter B. G., Isenberg H. D. Clinical laboratory experience with the improved Enterotube. Appl Microbiol. 1973 Jun;25(6):896–899. doi: 10.1128/am.25.6.896-899.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Characterization of saccharolytic nonfermentative bacteria associated with man. Can J Microbiol. 1970 May;16(5):351–362. doi: 10.1139/m70-062. [DOI] [PubMed] [Google Scholar]
- Tomfohrde K. M., Rhoden D. L., Smith P. B., Balows A. Evaluation of the redesigned enterotube--a system for the identification of Enterobacteriaceae. Appl Microbiol. 1973 Feb;25(2):301–304. doi: 10.1128/am.25.2.301-304.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


