Abstract
In this report, the development of multi-channel Anti-Stokes luminescent Y2O3 nanoparticles for application to in vivo upconversion imaging is detailed.
Luminescent upconverting materials have been known for over 50 years. Only recently has interest focused on preparing these compounds on a nanoscale for applications in biotechnology.1 Current optical imaging and assay technologies primarily are based on the use of organic fluorophores or semiconducting quantum dots.2 Materials, such as yttrium oxide (Y2O3) doped with rare earth elements offer an emerging alternative for use in optical imaging. Erbium and ytterbium containing Y2O3 particles absorb near infrared light at 980 nm and can emit higher energy, shorter wavelength photons in an Anti-Stokes emission process.3
For bioimaging applications Anti-Stokes luminescence offers key advantages over traditional downconversion emission observed with organic fluorphores and quantum dots. One issue concerning the use of small molecule fluophores is a lack of photostability under prolonged excitation. Unlike organic dyes, upconverting Y2O3 nanomaterials show excellent photostability and low toxicity.4 Quantum dots also have excellent photostability, but there are potential cytotoxicity issues associated with their use in vivo due to their inclusion of highly toxic metals such as cadmium,5 which is released in the presence of the biological oxidant hypochlorous acid.6 Tissue autofluorescence is another concern with the use of small molecule fluorophores for in vivo imaging. There are few if any intrinsic biological materials that display upconversion emission, therefore, upconversion emission processes may significantly limit this source of interference.
For use of upconverting nanomaterials as in vivo imaging agents, several criteria must be met. These include near infrared excitation and emission, water solubility, biocompatibility, and means for attachment of additional optical reporters or targeting molecules. In the past few years, there have been reports of upconverting nanoparticle preparations that meet some of these criteria.7
In vivo optical imaging focuses on the use of luminescent reporters that have excitation and emission in the NIR (∼600−1000 nm) where light absorption and scattering from biological tissues is minimized.8 Excitation and emission in this wavelength range can be achieved by use of Y2O3 nanoparticles co-doped with erbium and ytterbium.3 These particles may be excited using simple 980 nm diode lasers and show upconversion emission in the green or far-red/NIR depending on the concentrations of the dopants. Here we detail the preparation of surface modified upconverting Y2O3 nanoparticles containing erbium and ytterbium, which are suitable for conjugation to additional optical reporters or targeting groups. Importantly these materials can be excited with non-harmful doses of light and have luminescence emission centered at 660 nm. The utility of these particles for in vivo blood pool imaging is demonstrated by visualization of the vasculature in a mouse model after intravenous injection of the nanoparticles.
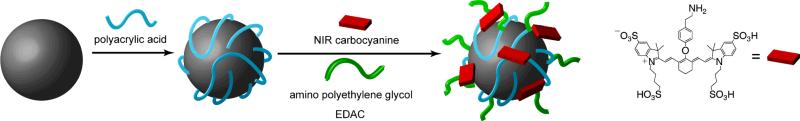
Although there are some reports of water dispersable nanoparticles,2a, 2c, 7b, 9 most methods to produce these materials yield either uncoated or hydrophobic particles that aggregate strongly or do not suspend in water. As a general approach to preparation of water-soluble upconverting nanoparticles, we developed a simple procedure for the surface coating of unmodified Y2O3 particles based on carboxylic acid coordination to the particles (Scheme 1). The surface modification of Y2O310 and isostructural Gd2O311 nanoparticles with simple carboxylic acids has been investigated extensively. To prepare stable coatings, upconverting Y2O3 nanoparticles are first coated with the polydentate polymer, polyacrylic acid (PAA). This is accomplished by sonication of the uncoated nanoparticles in ethylene glycol solution containing polyacrylic acid. Nanoparticles coated with carboxylic acid groups are not ideal for vascular imaging as they may show pronounced non-specific tissue binding12 or shortened circulation half-lives in the blood.13 To minimize the potential for non-specific binding, the carboxylic acid coated Y2O3 nanoparticles were coupled to 2000 MW mPEG-NH2 polyethylene glycol (PEG) polymers using standard amide coupling chemistry (Scheme 1). The presence of the PEG coating on the particles was verified by FTIR spectroscopy (Fig. S1, supporting information). For broader application to in vivo imaging, it will ultimately be necessary to functionalize the nanoparticle surfaces with additional optical handles or targeting moieties. To demonstrate the ability to couple multiple species to the nanoparticle surfaces, an amine modified carbocyanine fluorophore, synthesized via nucleophilic attack of a BOC protected tyramine on a chloro-substituted carbocyanine dye (Scheme S1, supporting information), was coupled to the nanoparticle surface simultaneously with the amine-modified PEG.
Scheme 1.
Surface modification of Y2O3 upconverting nanoparticles with polyacrylic acid, polyethylene glycol and NIR emitting fluorophores.
The PEG and NIR fluorophore modified nanoparticles are easily dispersed in water. The measured mean hydrodynamic diameter of the particles is 101 nm as determined by Dynamic Light Scattering (DLS) experiments (Fig. 1a). In addition, the Y2O3 cores of the coated particles were investigated by SEM (Figure S2, supporting information). The particles show excellent stability in water with no particle aggregation observed even after three weeks in solution (Fig. 1a, inset). One concern with the use of new nanomaterials is their potential cytotoxicity. Lanthanide oxides, however, have been shown to have low cytotoxicity4b and recently Y2O3 nanoparticles were demonstrated to function as neuroprotective agents.4a To investigate the cytotoxicity of the PEG coated Y2O3 upconverting nanoparticles, increasing concentrations of the particles were incubated with RAW cells and cell viability was assessed (Fig. 1b). These data show little or no cytotoxicity up to a nanoparticle concentration of 500 μg / mL. This agrees with recent reports on the cytotoxicity of similar upconverting nanomaterials based on a NaYF4 matrix.2a, 14
Fig. 1.
Characterization of the water-soluble Y2O3 upconverting nanoparticles; a) dynamic light scattering data indicating a hydrodynamic diameter of 101 nm and long term stability of the particles in aqueous solution (inset), b) viability of RAW cells after incubation with increasing concentrations of the nanoparticles as determined by the MTS assay, and c) normailzed emission spectra of the nanoparticles in 0.1 M pH 7.4 bicarbonate buffer. The particle concentration is 500 μg/mL (315 pM). The emission maxima are 660 and 797 nm for the upconversion and carbocyanine signals (solid and dashed lines, respectively). Excitation for the upconverting particles is at 980 nm with a power density of 500 mW/cm2, the carbocyanine dye is excited at 750 nm.
The upconversion emission centered at 660 nm is readily detected upon excitation of a dilute (500 μg/mL) aqueous solution of the nanoparticles with a 980 nm diode laser at an excitation power density of 500 mW/cm2 (Fig. 1c). The 980 nm excitation and 660 nm emission from the particles fall well within the wavelength range where biological tissue has increased optical transparency. When excited at 750 nm, the particles also display strong fluorescence signal originating from the attached carbocyanine fluorophores (Fig. 1c). The dye loading on the nanoparticle surface was determined to be 1100 dyes/nanoparticle. One concern with attachment of organic fluorophores to nanoparticle surfaces is the potential for significant quenching of the fluorophore emission resulting from interactions with the nanoparticle core. Significant nanoparticle mediated fluorophore quenching has previously been observed with iron oxide15 and gold16 nanoparticles. The fluorescence emission of the fluorophore modified Y2O3 nanoparticles was investigated by comparing the emission of the nanoparticle-conjugated particles to an equimolar solution of the free carbocyanine dye in aqueous solution. Less than 5% fluorescence quenching of the dye loaded particles was observed (Fig. S4, supporting information), indicating little or no quenching by the Y2O3 nanoparticle cores. These data show the Y2O3 upconverting nanoparticle platform is a capable carrier for organic fluorophores and may be used as a multichannel optical probe.
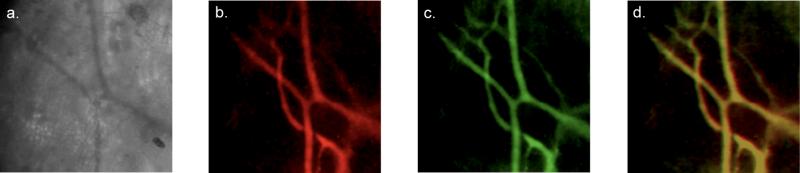
The application of the dual channel upconverting nanoparticles to in vivo vascular imaging was studied. Small animal imaging was performed following tail vein injection of the nanoparticles intonudemice. For the imaging experiments a custom built microscope system containing a 980 nm diode laser, for upconversion excitation, and a 737 nm laser, for excitation of the NIR fluorophore, was used. Following probe injection, blood vessels in the mouse ear were imaged. The probe is clearly visible through a 750 nm short-pass filter using the 980 nm diode laser for excitation at a measured laser power density of 550 mW/cm2 (Fig. 2). This power density is below the conservative limit set for human skin exposure at 980 nm of 726 mW/cm2.17 Furthermore no harmful effects from laser exposure at this power density were observed in the mice even after prolonged exposure. The particles are long circulating and can be easily imaged in the blood more than two hours post injection. Excellent co-localization of the carbocyanine fluorescence and upconversion luminescence signals are observed (Fig. 2) indicating the organic polymer coating of the nanoparticles remains intact in vivo. In addition to their use for the acquisition of static vascular images, the particles are sufficiently bright to enable real-time imaging (Supporting materials). To accomplish this, the mouse ear was irradiated at 980 nm with a power density of 550 mW/cm2 and images were recorded at a rate of 5 frames per second. This demonstration of in vivo upconversion imaging opens the possibility for future use of this nanoparticle platform for angiography, intraoperative imaging, or other bioimaging applications.
Fig. 2.
Optical imaging of blood vessels in the mouse ear following tail vein injection of the nanoparticles (10 mg); a) blood vessels imaged with a blue light filter, b) upconversion image with excitation at 980 nm and a laser power density of 550 mW/cm2, c) fluorescence image of the carbocyanine dye with excitation at 737 nm, d) merged image of the upconversion and fluorescence signals. Both the upconversion and carbocyanine fluorescence images were taken with an exposure time of 10 s.
In conclusion, the ability of lanthanide-based nanomaterials to undergo upconversion luminescence emission has been known for many years, however, only recently have efforts been put forth to develop these materials into biocompatible agents. In this report, an efficient route for preparation of upconverting Y2O3 nanoparticles suitable for in vivo imaging is demonstrated. The aqueous dispersible nanoparticles are coated with PEG polymers and NIR emitting carbocyanine fluorophores. Little or no quenching of the attached carbocyanine fluorophores is observed even with a dye loading of over 1000 dyes per nanoparticle. Preliminary data with these materials show they have low cytotoxicity. The upconversion emission signal from the particles is readily visible in vivo with excitation using a clinically relevant laser power density at 980 nm. Practical utility for imaging the vasculature in live mice is demonstrated with the nanoparticles. These initial studies indicate that this functionalized Y2O3-based upconverting nanomaterial is a promising platform for in vivo optical-based diagnostic imaging.
Supplementary Material
Acknowledgments
This research was supported in part by NIH grants U01-HL080731, R01-EB001872, U54-CA119349, P50-CA86355, R24-CA92782, and T32-EB002102.
Footnotes
Electronic Supplementary Information (ESI) available: Detailed synthetic and imaging procedures including full characterization data for all compounds and materials.
References
- 1.a Corstjens PLAM, Li S, Zuiderwijk M, Dardos K, Abrahms WR, Niedbla RS, Tanke HJ. IEEE Proc. Nanobiotechnol. 2005;152:64. doi: 10.1049/ip-nbt:20045014. [DOI] [PubMed] [Google Scholar]; b Kunigas K, Pakkila H, Ukonaho T, Rantanen T, Lovgren T, Soukka T. Clinical Chem. 2007;53:145. doi: 10.1373/clinchem.2006.076687. [DOI] [PubMed] [Google Scholar]; c Chen Z, Chen H, Hu H, Yu M, Li F, Zhang Q, Zhou Z, Yi T, Huang C. J. Am. Chem. Soc. 2008;130:3023. doi: 10.1021/ja076151k. [DOI] [PubMed] [Google Scholar]; d Rantanen T, Jarvenpaa M-L, Vuojola J, Kuningas K, Soukka T. Angew. Chem. Int. Ed. 2008;47:3811. doi: 10.1002/anie.200705861. [DOI] [PubMed] [Google Scholar]
- 2.a Chatterjee DK, Rufaihah AJ, Zhang Y. Biomaterials. 2008;29:937. doi: 10.1016/j.biomaterials.2007.10.051. [DOI] [PubMed] [Google Scholar]; b Lim SF, Riehn R, Ryu WS, Khanarian N, Tung C-K, Tank D, Austin RH. Nano Lett. 2006;6:169. doi: 10.1021/nl0519175. [DOI] [PubMed] [Google Scholar]; c Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN. Nano Lett. 2008;8:3834. doi: 10.1021/nl802223f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vetrone F, Boyer JC, Capobianco JA, Speghini A, Bettinelli M. J. Phys. Chem. C. 2003;107:1107. [Google Scholar]
- 4.a Schubert D, Dargusch R, Raitano J, Chan S-W. Biochem. Biophys. Res. Commun. 2006;342:86. doi: 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]; b Palmer RJ, Butenhoff JL, Stevens JB. Environ. Res. 1987;43:142. doi: 10.1016/s0013-9351(87)80066-x. [DOI] [PubMed] [Google Scholar]
- 5.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini MC, Kairdolf BA, Smith AM, Nie S. J. Am. Chem. Soc. 2008;130:10836. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a Kamimura M, Miyamoto D, Saito Y, Soga K, Nagasaki Y. Langmuir. 2008;24:8864. doi: 10.1021/la801056c. [DOI] [PubMed] [Google Scholar]; b Schäfer H, Ptacek P, Kömpe K, Haase M. Chem. Mater. 2007;19:1396. [Google Scholar]
- 8.Weissleder R, Ntziachristos V. Nat. Med. 2003;9:123. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 9.Traina CA, Schwartz J. Langmuir. 2007;23:9158. doi: 10.1021/la701653v. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen H, Söderlind F, Petoral RMJ, Uvdal K, Käll P-O, Ojamäe L. Surf. Sci. 2005;592:124. doi: 10.1016/j.jcis.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 11.Söderlind F, Pedersen H, Petoral RMJ, Käll P-O, Uvdal K. J. Colloid Interface Sci. 2005;288:140. doi: 10.1016/j.jcis.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 12.Bentzen EL, Tomlinson ID, Mason J, Gresch P, Warnement MR, Sanders-Bush E, Blakely R, Rosenthal SJ. Bioconjugate Chem. 2005;16:1488. doi: 10.1021/bc0502006. [DOI] [PubMed] [Google Scholar]
- 13.Ballou B, Lagerholm C, Ernst LA, Bruchez MP, Waggoner AS. Bioconjugate Chem. 2004;15:79. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 14.Jalil RA, Zhang Y. Biomaterials. 2008;29:4122. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.a Josephson L, Kircher MF, Mahmood U, Tang Y, Weissleder R. Bioconjugate Chem. 2002;13:554. doi: 10.1021/bc015555d. [DOI] [PubMed] [Google Scholar]; b Turro NJ, Lakshminarasimhan PH, Jockusch S, O'Brien SP, Grancharov SG, Redl FX. Nano Lett. 2002;2:325. [Google Scholar]
- 16.Nerambourg N, Werts MHV, Charlot M, Blanchard-Desce M. Langmuir. 2007;23:5563. doi: 10.1021/la070005a. [DOI] [PubMed] [Google Scholar]
- 17.American National Standard for Safe Use of Lasers ANSI Z136.1−2000. American National Standard Institute; Orlando, FL: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.