Abstract
The serine/threonine protein kinase B (PKB, also known as Akt) plays a pivotal role in diverse cellular functions. Elevated expression of activated Akt has been detected in a wide variety of human cancers; however, the mechanism of Akt protein stability regulation remains unclear. In this study, we showed a strong correlation between the expression levels of an oncogenic peptidyl-prolyl cis/trans isomerase Pin1 and levels of Akt phosphorylation at S473 in multiple cancer types (P<0.0001). Akt-pS473 status combined with Pin1 expression levels predicted a poorer prognosis than did either one alone in patients with breast cancer (P = 0.0052). We further showed that Pin1 regulated Akt stability and phosphorylation on S473 through the phosphorylated Thr-Pro motifs of Akt. These motifs are conserved evolutionary and are required for the maintenance of Akt stability and its interaction with Pin1. In addition, repressing Pin1 expression through either homologue Pin1 knockout or small interfering RNA-mediated knockingdown compromised its ability to protect Akt from degradation. Our results show how Akt protein stability is regulated by the peptidyl-prolyl cis/trans isomerase Pin1 and highlight the importance of this oncogenic network in human disease pathogenesis.
Keywords: PKB/Akt, Pin1, peptidyl-prolyl cis/trans isomerase, breast cancer
Introduction
The serine/threonine protein kinase B (PKB, also known as Akt) plays a pivotal role in diverse cellular functions (Brazil et al., 2004; Yang et al., 2004; Testa and Tsichlis, 2005; Dummler and Hemmings, 2007). Activation of Akt requires phosphorylation of Akt at two critical sites: one within the activation loop (T308 for Akt1) and the other with the C-terminus (S473 for Akt1) (Alessi et al., 1996; Bellacosa et al., 1998; Chan and Tsichlis, 2001; Bayascas and Alessi, 2005; Du and Tsichlis, 2005; Sarbassov et al., 2005). Aberrant activation of the Akt pathway has been widely implicated in human cancers (Luo et al., 2003; Testa and Tsichlis, 2005; Bellacosa et al., 2005; Dillon et al., 2007; Dummler and Hemmings, 2007; Tokunaga et al., 2008). As a direct downstream target of phosphatidylinositol 3-kinase (PI3K), elevated Akt activation in human cancers can be a result of enhanced activation phosphorylation of Akt on the Ser473 site because of enhanced PI3K activation resulting from the amplification/overexpression of growth factor receptors (such as Her-2/neu, EGFR), somatic mutation of ras oncogenes, somatic mutation as well as amplification/overexpression of PI3K (PI3CA or p85 subunit), or the loss-of-function mutation (somatic or germ line) and decreased expression (loss of heterozygosity or methylation) of a 3′ phosphatase PTEN (phosphatase with tensin homology), which converts PIP3 to PIP2 and thus shuts off PI3K signaling (Luo et al., 2003; Bellacosa et al., 2005; Guertin and Sabatini, 2005; Kang et al., 2005; Cully et al., 2006). Amplification and overexpression, as well as somatic mutation (at a very low frequency) of Akt itself, also contribute to the altered activation of Akt in human cancers (Bellacosa et al., 2005; Soung et al., 2006; Carpten et al., 2007). Furthermore, altered expression of the Ser473-specific protein phosphatase PHLPP (PH domain leucine-rich repeat protein phosphatase) also affects Akt activity, because reduced PH domain leucine-rich repeat protein phosphatase in certain cancer cell lines correlates with Akt activity (Gao et al.,2005; Brognard et al., 2007). Although significant progress has been made in our understanding of the mechanisms underlying Akt activation phosphorylation, particularly, on S473 at the hydrophobic motif (Bhaskar and Hay, 2007), few reports have documented how Akt phosphorylation per se regulates Akt stability in cells.
Recent identification and characterization of a peptidyl-prolyl cis/trans isomerase (PPIase), Pin1, has led to the discovery of a new post-phosphorylation regulatory mechanism that may modulate the balance between protein phosphorylation and dephosphorylation as well as protein stability at the cellular level (Lu et al., 2006; Lu and Zhou, 2007; Yeh and Means, 2007; Finn and Lu, 2008; Takahashi et al., 2008). Pin1 has been shown to regulate the stability of its substrate proteins, such as cyclin D1 and many others (Yeh et al., 2004; Lu et al., 2006; Lu and Zhou, 2007; Yeh and Means, 2007). Pin1 is overexpressed in a wide range of human cancers, and its overexpression closely correlates with the higher levels of cyclin D1; inactivation of which could protect against breast cancer induced by oncogenic neu or ras (Bao et al., 2004; Wulf et al., 2004; Lu and Zhou, 2007; Yeh and Means, 2007). As one of the major downstream effectors of oncogenic neu and ras, the PI3K-Akt pathway is also a critical regulator of cyclin D1 expression through Akt-mediated inhibition of the GSK3 and FOXO proteins, which repress cyclin D1 expression or promote the degradation of cyclin D1 or both (Muise-Helmericks et al., 1998; Kops and Burgering, 1999; Burgering and Medema, 2003; Dillon et al., 2007). In addition, recent studies on mammary tumorigenesis in a Pin1 or Akt1 knockout setting unveiled a striking similarity in a dependence on either Pin1 or Akt1 for the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu, MMTV-v-Ha-Ras, or MMTV-polyoma middle T (PyMT) transgenic mice because ablation of either Pin1 or Akt1 effectively blocks the induction of cyclin D1 by neu, ras, or PyMT (Wulf et al., 2004; Maroulakou et al., 2007). Furthermore, many of the Pin1 substrates, such p53, NFκB, cyclin D1, and β-catenin, are also under regulation by Akt (Cully et al., 2006; Manning and Cantley, 2007).
Given the close functional relationship between Akt and Pin1 as described above (Wulf et al., 2004; Maroulakou et al., 2007), we asked whether Pin1 and Akt might interact with each other and collaboratively regulate tumorigenesis. In the current report, we first explored whether a pathological relationship exists between the expression of Akt and Pin1 in human cancer by reviewing our earlier studies on cyclin D1 expression, Akt activation (pS473), and Her-2/neu expression in a cohort of human breast cancer tissue samples (Lin et al., 2000; Xia et al., 2004; Cha et al., 2005). We were able to unveil a strong correlation between the expression levels of an oncogenic peptidylprolyl cis/trans isomerase Pin1 and levels of Akt phosphorylation at S473 in this cohort of human breast cancer tissue samples as well as in multiple cancer types from tissue microarray slides. We then investigated whether Pin1 and Akt interact with each other by co-immunoprecipitation and glutathione-S-transferase (GST) pull-down assay. Next, we mapped the motifs in Akt that are required for Akt interaction with Pin1 by site-directed mutagenesis. We further explored how Akt stability and activation phosphorylation are regulated by Pin1 through either homologous deletion of Pin1 or small interfering RNAs-mediated knockdown of Pin1. We showed that phosphorylation of Akt at the Thr-Pro motifs, which are required for its interaction with Pin1, is critical for the maintenance of Akt protein stability and activation phosphorylation. Our findings will help to understand the roles of Akt and Pin1 in oncogenesis and may open a new avenue for the development of targeted therapeutics for human diseases with aberrant Akt activation.
Results
Pathological correlations between the expression levels of Pin1 and Akt-pS473 in human cancers
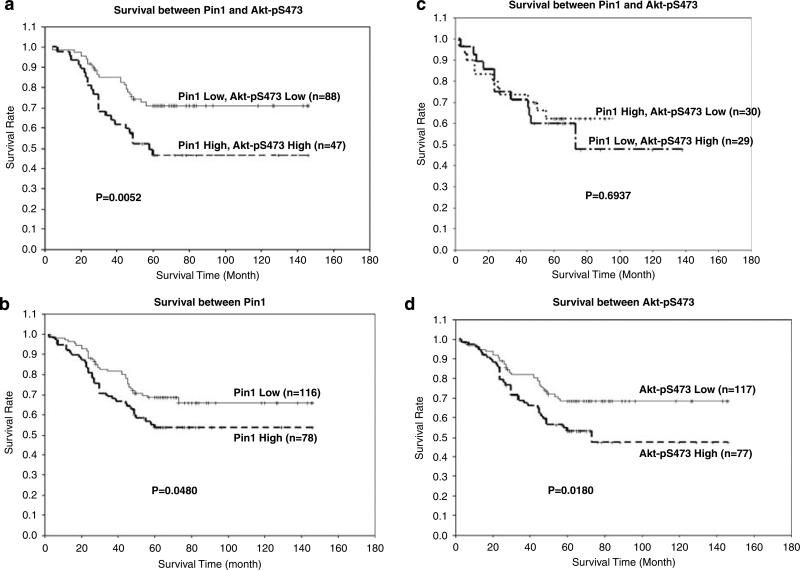
To explore whether a pathological relationship exists between the expression of Akt and Pin1 in human cancer, we reviewed our earlier studies on cyclin D1 expression, Akt activation (pS473), and Her-2/neu expression in a cohort of human breast cancer tissue samples (Lin et al., 2000; Xia et al., 2004; Cha et al., 2005). As expected, we observed a close correlation between the expression levels of Pin1 and cyclin D1 in these breast tumor tissues (Supplementary Figure S1A). Interestingly, we also observed that the expression of Akt-pS473 was strongly correlated with Pin1 expression in this cohort (P<0.0001, Figures 1a and b) and in different types of human tumor tissues as well (P<0.0001, Figure 1c; Supplementary Figure S1B). The expression levels of Pin1 and Akt-pS473 were also associated with tumor stages in human breast cancer (both P<0.05, Figure 1d). In addition, a combination of Akt-pS473 status and Pin1 expression levels predicts a poorer prognosis than does either one alone in patients with breast cancer (P = 0.0052, Figures 2a versus b–d). These results suggest the existence of a clinical pathological link between Pin1 expression and Akt phosphorylation on S473.
Figure 1.
Elevated expression of Pin1 correlates with expression of Akt-ps473 in tumor tissues. (a) A representative immunohistochemical staining of Pin1, cyclin D1, Akt-pS473 protein expression in human breast cancer. (b) Correlations between the expressions of Pin1 and Akt-pS473 in breast cancer. (c) Co-expression of Akt-pS473 and Pin1 in different types of tumors. (d) Expression of Akt-pS473 and Pin1 correlates with tumor stages in human breast cancer. L, low (immunoreactive score 0 and 1+); H, high (score 2+ and 3+)
Figure 2.
Expression levels of Pin1 and Akt-pS473 and their correlations with prognosis of patients with breast cancer. (a) Difference of survival rate between low levels versus high levels of Pin1 and Akt-pS473 phosphoryation expression in patients with breast cancer. (b) Difference of survival rate between low levels versus high levels of Pin1 expression in patients with breast cancer. (c) Difference of survival rate between high levels of Pin1//low levels of Akt-pS473 and low levels of Pin1//high levels of Akt-pS473 expression in patients with breast cancer. (d) Difference of survival rate between low levels versus high levels of Akt-pS473 phosphoryation expression in patients with breast cancer.
Interactions between Akt and Pin1 are independent of Akt phosphorylation on either T308 or S473
Phosphorylation of proteins on serine or threonine residues preceding proline (pSer/Thr-Pro) by various proline-directed protein kinases is a principal regulatory mechanism in cell proliferation and transformation (Lu et al., 2006; Lu and Zhou, 2007; Yeh and Means, 2007). To uncover whether Akt contains a phosphorylated Ser/Thr-Pro motif, we examined phosphorylation of Akt at the Ser/Thr-Pro sites using a monoclonal antibody MPM2, which recognizes specifically the phosphorylated Ser/Thr-Pro (pSer/Thr-Pro) motifs (Lu et al., 1999). Immunoprecipitated Akt protein can be recognized by MPM2 antibody specifically, and a protein immunoprecipitated with MPM2 antibody can also be recognized by a specific antibody against Akt, suggesting that Akt contains pSer/Thr-Pro motifs (Figure 3a). As, most MPM2 antigens can interact with the peptidyl-prolyl isomerase Pin1, which specifically isomerizes the pSer/Thr-Pro peptide bond (Lu et al., 1999), we therefore tested whether Akt associates with Pin1 in vivo by co-immunoprecipitation of endogenous Akt or Pin1. Akt was detected in immunoprecipitated Pin1 proteins, and Pin1 was also detected in the protein complex immunoprecipitated by an anti-Akt antibody (Figure 3b), suggesting that Akt and Pin1 may interact with each other. To further characterize the interaction of Akt and Pin1, we used GST-Pin1 fusion protein to pull down endogenous Akt protein. When examined, Akt was pulled down by the wild-type Pin1 but not with GST or a mutant Pin1 (W34A), which disrupts Pin1 from interacting with its substrate (Figures 3c and d). This interaction can also be detected by using a purified recombinant human Akt1 protein (rhAkt1) and GST-Pin1 fusion protein, suggesting the interaction is direct (Supplementary Figure S2A). As expected, the Pin1 pulled down Akt protein was immunoreactive with the MPM2 antibody that recognized the pSer/Thr-Pro motifs (Figure 3c). Interestingly, the Pin1-associated Akt protein was also phosphorylated at both T308 and S473 (Figure 3c), which were not immediately followed by a proline residue. We therefore investigated whether Akt phosphorylation at the T308 and S473 sites might be required for its immunoreactivity with MPM2 and therefore its interaction with Pin1. This possibility was ruled out as increases or decreases in pS473 and pT308 of Akt induced by insulin-like growth factor -1 and a PI3K inhibitor LY294002, respectively, did not affect MPM2 immunoreactivity of Pin1-associated Akt or Pin1/Akt association (Figure 3e). Thus, Akt phosphorylation at the T308 and S473 sites was not required for its immunoreactivity with MPM2 or its interaction with Pin1.
Figure 3.
Interactions between Pin1 and Akt proteins. (a) Immunoprecipitated Akt can be recognized by anti-MPM2 antibody and vice versa. (b) Co-immunoprecipitation of endogenous Akt and Pin1 proteins in breast cancer MDA-MB-468 cell lysate. Approximately 4 mg of whole cell lysate was used for each co-immunoprecipitation, and about 50 μg of whole cell lysate (WCL) was loaded as control. (c) Precipitation of Akt by (glutathione-S-transferase) GST-Pin1 fusion protein pull-down assay (right panel) and (d) gel staining of purified GST, GST-Pin1, and GST-Pin1 (W34A) fusion proteins (left panel). (e) Co-immunoprecipitation of Pin1 and Akt proteins in MDA-MB-468 cells in the presence or absence of insulin-like growth factor (IGF)-1 (50 ng/ml) for 2 h and with or without addition of PI3 K inhibitor LY294002 (LY, 20 μm). IP, immunoprecipitation; WB, western blot; MPM2, antibody against phosphorylated Ser/Thr-Pro motifs.
Phosphorylation of Akt at the Thr92-Pro and Thr450-Pro motifs are required for its interaction with Pin1
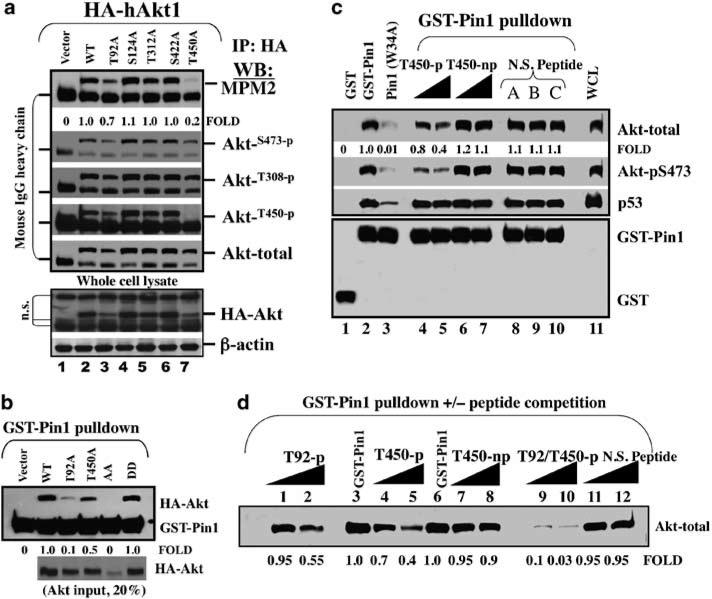
To further address functionality of the Akt/Pin1 interaction and its relationship with phosphorylation of the Ser/Thr-Pro motifs, we asked whether Pin1 and Akt interaction might require phosphorylation of the Ser/Thr-Pro motifs. To this end, we mutated Ser/Thr to Ala in the five Ser/Thr-Pro sites of human Akt1 (Supplementary Table 1). Single mutations of T312A, S124A, and S422A did not attenuate Akt immunoreactivity with MPM2 antibody, their expression levels (total Akt or HA-Akt1 in the whole cell lysate), or activation phosphorylation on S473 and T308 sites (Figure 4a). A point mutation of either T92A or T450A did reduce Akt immunoreactivity with MPM2 (Figure 4a), suggesting that phosphorylated T92-P93 and T450-P451 motifs are responsible for MPM2 immunoreactivity. In addition, the expression levels of both T92A and T450A mutants were reduced in the whole cell lysates (Figure 4a, lower panel, lanes 3 and 7), which was further confirmed by another independent experiment in 293T cells (Supplementary Figure S2B).
Figure 4.
Phosphorylation of Akt on the T92-Pro and T450-Pro sites is critical for the interaction between Pin1 and Akt. (a) Point mutation analysis of Akt Ser/Thr-Pro motifs identifies that mutation of T92 or T450 to T92A or T450A reduces Akt immunoreactivity toward anti-phosphorylated Ser/Thr-Pro motif antibody, MPM2, and anti-phospho-Akt antibodies (pS473 and pT308) in Akt1 (−/−) mouse embryonic fibroblasts (MEF) cells. (b) A (glutathione-S-transferase) GST-Pin1 pull-down assay of HA-tagged wild-type human Akt1 or Akt1 with mutation of T92 or T450 or both. (c) GST-Pin1 pull-down assay of Akt in MDA-MB-468 cell lysate in the presence or absence of peptide competition with phosphorylated or non-phosphorylated T450 peptide as well as non-specific phosphopeptides (N.S. peptide A, B, and C). (d) GST-Pin1 pull-down and competition with a phosphorylated T92 peptide and/or T450 peptide.
We then investigated whether these two sites might be important for the Akt-Pin1 interaction by GST-Pin1 pull-down assay. Indeed, a GST-Pin1 pull-down assay of HA-tagged Akt1 proteins indicated that a single (T92A or T450A) mutation reduced Akt binding with GST-Pin1 (Figure 4b), and the double mutation of T92A/T450A completely abolished the binding activity (Figure 4b). It is worthwhile to mention that the double mutation of T92D/T450D mimicking phosphorylation of T92 and T450 rescued its binding activity with GST-Pin1, suggesting that phosphorylation of T92 and T450 was critical for its interaction with Pin1. This result was further supported by phosphopeptide competition experiments (Figures 4c and d). When T92 or T450 phosphopeptides or both were used to specifically compete GST-Pin1 binding with Akt, the T450 phosphopeptide partially competed GST-Pin1 binding with Akt (Figure 4c, lane 2 versus 4, 5), whereas a T450 non-phosphopeptide did not compete with GST-Pin1 binding with Akt (Figure 4c, lane 2 versus 6, 7).
The interaction of Pin1 with p53 is known (Zacchi et al., 2002) and was used in our study as a control to indicate the specificity of a Pin1 phosphopeptide competition that affects Pin1/Akt but not Pin1/p53 interaction (Figure 3c). Similarly, the T92 phosphopeptide also partially competed GST-Pin1 binding with Akt (Figure 4d, lanes 1, 2 versus 3), and a combination of the T92 and T450 phosphopeptides almost completely abolished Akt binding with GST-Pin1 (Figure 4d, lane 3 versus 10). Taken together, these results suggested that the interaction of Akt with Pin1 depended on the phosphorylated Ser/Thr-Pro motifs (Thr92-Pro and Thr450-Pro for human Akt1). Sequence alignment analysis revealed that the Thr92-Pro and Thr450-Pro motifs were highly conserved in different Akt isoforms and species, from Caenorhabditis elegans to human (Supplementary Table 2). Consistently, a GST-Pin1 pull-down assay showed that isoforms of human Akt2 and Akt3 could also be pulled down by GST-Pin1 (Supplementary Figure S2C).
Phosphorylation of Akt1 on T92-Pro and T450-Pro motifs (Akt1-T92A/T450A) is critical for the maintenance of Akt stability
We noticed that mutation of either T92 or T450 affected expression levels of Akt protein by either immunoprecipitation (Figure 4a) of HA-tagged Akt1 or western blot of HA-Akt1 in the whole cell lysates (Figure 4a, lower panel; Supplementary Figure S2A). Given that phosphorylated Thr-Pro motifs are critical for Akt interaction with Pin1 and that Pin1-mediated cis/trans conformational modifications have been reported to affect stability of its substrate proteins (Lu et al., 2006; Lu and Zhou, 2007; Yeh and Means, 2007), we investigated whether the Thr/Ser-Pro motifs of Akt are required for the maintenance of its stability. To this end, we transfected complementary DNA (cDNA) constructs with either alanine or an aspartic acid substitution of Akt on Thr92 and/or Thr450 into 293 cells, respectively. When examined, the levels of total Akt protein as well as activation phosphorylation on S473 sites in mimicking phosphorylation mutants (T92D, T450D, and T92D/T450D) were slightly higher than those of the wild-type Akt (Figure 5a, lanes 3, 5, and 8 versus 2). Similarly, the immunoreactivity against the MPM2 antibody was also slightly higher in the aspartic acid mimicking phosphorylation mutants than they were in the alanine mutants. Again, the alanine mutants (T92A, T450A, and T92A/T450A) expressed lower levels of Akt proteins than wild-type Akt (Figure 5a, lanes 4, 6, 7, and 9 versus 2), which is consistent with the data shown in Figure 4a. Together, the data suggest that the T92A/T450A mutant may be unstable (Figure 5a).
Figure 5.
Phosphorylation of Akt on the T92- and T450-Pro motifs is critical for the maintenance of Akt stability. (a) Relative Akt protein and phosphorylation levels on the Thr-Pro motifs were measured by anti-Akt antibody, anti-MPM2 antibody, and a phosphospecific antibody against the phosphorylated Thr450-Pro motif of human Akt1 (Akt-pT450). Expression of tubulin in the whole cell lysate (10% of input) was used as an input loading control for the IP. Fold changes of Akt-total and Akt-pS473 levels of each Akt mutant were normalized with that of the levels of wild-type Akt. (b) Cycloheximide chase of exogeneous, HA-tagged wild-type human Akt1 (Akt1-WT), and Akt1-T92A/T450A (Akt1-AA). (c) Quantification of Akt proteins from three independent experiments with cycloheximide chasing. WT, wild-type Akt1; AA, Akt1-T92A/T450A mutant. (d) Basal and IGF-1 stimulated Akt protein and activation phosphorylation at T308 and S473 sites in Pin1 wild type (+/+) or Pin1 (−/−) MEFs.
To test whether the unstable Akt1 mutant is because of the protein half-life alteration, we did a cycloheximide chase labeling to assay the protein stability of wild-type Akt1 and Akt1-T92A/T450A (Akt-AA) double mutant. Compared with wild-type Akt1, the double mutant Akt1-AA protein level was reduced much faster (<30 min) than was wild-type Akt1 (>6 h) (Figures 5b and c). The results suggest that the phosphorylated Thr-Pro motifs of Akt are critical for the maintenance of Akt stability.
As phosphorylation of Akt on the Thr92- and Thr-450-Pro motifs is required for both interaction with Pin1 and maintenance of Akt stability, we then investigated whether Pin1 affects the stability of Akt protein. To this end, we examined the expressing levels of Akt total protein in Pin1 wild type and Pin1 null mouse embryonic fibroblasts (MEFs) in the presence or absence of insulin-like growth factor-1 stimulation. Indeed, reduced levels of total Akt protein were detected in Pin1-null MEF cell lysates with or without stimulation of insulin-like growth factor-1 (Figure 5d). Similarly, reduced levels of Akt total protein were also detected when Pin1 expression was knocked down by specific small interfering RNAs against Pin1 (Supplementary Figure S3). As experimental controls, the expression levels of cyclin D1, but not β-actin, were also reduced on knocking down Pin1 expression (Supplementary Figure S3). These results indicate that Pin1 is required for the maintenance of Akt protein stability in vivo.
Discussion
Our results show that phosphorylation of Akt on both Thr92-Pro and Thr450-Pro motifs is critical for the maintenance of Akt stability. Phosphorylation of Akt on these Thr-Pro motifs renders cis/trans conformational modifications that are catalyzed by the prolyl isomerase Pin1, deletion or inactivation of which will result in Akt degradation. In addition, loss of Akt phosphorylation on the Thr-Pro motifs by double mutation of T92 and T450 sites also results in a labile Akt mutant protein.
The biological activities as well as the oncogenic activities of Akt are largely dependent on phosphorylation at T308 and S473 residues (for human Akt1). Until recently, few reports have documented how Akt protein stability is regulated by its phosphorylation (Bhaskar and Hay, 2007; Manning and Cantley, 2007). Earlier studies by Bellacosa et al. (1998) showed that in serum-starved cells, Akt is constitutively phosphorylated at Ser124 and Thr450, which is independent of PI3K; neither serum starvation nor treatment of cells with the PI3K inhibitor Wortmannin interferes with phosphorylation of these sites. In addition, inactive mutation of these two sites into Ala (S124A and T450A) only marginally inhibits the activation of Akt by growth factors; therefore, it has been proposed by Tsichlis et al. that phosphorylation of Akt on these sites is the first step for a full activation of Akt (Alessi et al., 1996; Chan et al., 1999; Chan and Tsichlis, 2001). Consistent with their study, in our current report, we also observed that mutation of Ser124-Pro motif to Ala124-Pro did not affect Akt protein stability and activation phosphorylation. Rather, we found that mutation of both T92- and T450-Pro motifs to Ala (T92A/T450A) drastically reduced Akt stability. Thus, we uncovered a plausible molecular mechanism that contributes to the regulation of Akt stability.
The Thr450-Pro motif is not only conserved within the Akt family and among different species but also in other AGC kinases such as PRK2, p70S6K, PKA, and PKC family. Structurally, the Thr450 residue happens to be located in the ‘turn motif’, which anchors the C-terminus hydrophobic motif at the top of the upper lobe of the kinase domain, with the phosphorylated threonine residues at the apex of a tight turn (Newton, 2003). Although mutation of this residue (T450) to alanine (T450A) did not dramatically affect Akt stability, activation phosphorylation, and kinase activity, as showed in thise study as well as recent reports by other groups (Facchinetti et al., 2008; Ikenoue et al., 2008), mutation of a conserved site in PKA as well as a compensating site in PKC βII destabilizes the kinase domain and abolishes kinase activity of PKA and PKC βII. Similarly, knockdown or knockout components of mTORC2 kinase (for example, Rictor and Sin1) drastically reduced the stability of PKCα protein, but not the stability of Akt, as showed by Ikenoue et al. and Facchinetti et al., even though mTORC2 has been shown as a ‘turn motif’ kinase for both PKC and Akt (T450) (Facchinetti et al., 2008; Ikenoue et al., 2008). The differences between the stability of PKC and Akt in response to knockdown of mTORC2 indicate that additional mechanisms may be involved in the regulation of Akt stability (that is, phosphorylation or dephosphorylation of T450 alone is not sufficient to either stabilize or destabilize Akt). Alternatively, compensating site(s) may exist for the stabilization of Akt.
As the PKA and family members of PKC do not have a PH domain, which has an inhibitory effect on activation phosphorylation of Akt, we speculate that mutation of T450 alone in Akt is not sufficient to destabilize the kinase domain as in PKA or PKC. We also think that phosphorylation of Akt on the T92 residue in the PH domain of Akt may release the inhibitory effect of PH domain on the kinase domain through binding with Pin1, whereas phosphorylation of Akt on the T450 residue in the turn motif may be critical not only for the folding and maturation of the Akt protein but also for the access of the S473 kinase in the hydrophobic motif by a likely conformational modification catalyzed by Pin1. Therefore, mutation of both T92A and T450A in Akt1 generates an inactive, labile Akt mutant, as both active sites in the kinase domain and the hydrophobic motif will be blocked and presumably inaccessible to upstream kinases and the prolyl isomerase Pin1.
Taken together, the current report uncovers that phosphorylation of Akt on Thr92- and Thr450-Pro motifs is critical for the maintenance of Akt stability and activation. As discussed above, phosphorylation of Akt at T450 site has been identified earlier by mass spectrum and the kinases that phosphorylate Akt on this site have also been reported recently (Facchinetti et al., 2008; Ikenoue et al., 2008); however, kinases that phosphorylate Akt on the T92-Pro are still unknown. Therefore, identification of additional kinases that phosphorylate Akt on the Thr92-Pro and/or Thr450-Pro motifs and further elucidation of the mechanisms of Akt phosphorylation at the Thr-Pro motifs will be important for future directions. Furthermore, the findings described in this report also open a new avenue for the design of targeted interventions of aberrant Akt activation for the treatment of human diseases, such as cancer.
Materials and methods
Cell lines, cell lysate, primary antibodies, and chemicals
The Pin1 wild type and knockout MEFs were obtained as a kind gift from Dr Kunping Lu (Harvard Medical School, Boston, MA, USA). Mouse Akt1 (−/−) MEFs were obtained from Dr Nissim Hay (University of Illinois, Chicago, IL, USA). The rest of the cell lines used were routinely maintained in this laboratory. Rabbit polyclonal antibodies against phospho-Akt (Ser-473, Cat. No. 9271, Cat. No. 2967, and Cat. No. 4058; Thr-308, Cat. No. 9275 and Cat. No. 4056; Ser-473, IHC Specific, Cat. No. 9277; Thr-450, Cat. No. 9267), non-phosphorylated total Akt (Cat. No. 9272), and a monoclonal mouse anti-human phospho-Akt (Ser-473, Cat. No. 4051), and cyclin D1 (DCS6, Cat. No. 2926) were purchased from Cell Signaling Technology (Beverly, MA, USA). Immobilized Akt antibody agarose beads for immunoprecipitation of Phospho-Akt (Ser-473, McAb, Cat. No. 9272) and total Akt1 (Akt1, C-20, goat polyclonal, Cat. No. sc-1618) were obtained from Cell Signaling and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. Monoclonal antibodies against phosphorylated Ser/Thr-Pro motif (MPM2 antibody, Cat. No. 05−368) and rabbit polyclonal antibodies against Pin1 (Cat. No. 07−091) were obtained from Upstate (Lake Placid, NY, USA). A monoclonal mouse antibody against human α-Tubulin (clone B-5−1−2, Cat. No. T5168) or rabbit polyclonal antibodies against β-actin were used as a loading control for western blot; both antibodies were purchased from Sigma (St Louis, WA, USA). To detect HA-tagged proteins, a monoclonal anti-HA antibody was used (Boerhinger Mannheim, Indianopolis, IN, USA; Cat. No.1583816). PI3K inhibitors (Wortmannin and LY294002) were from Calbiochem (EMD Biosciences, Inc., La Jolla, CA, USA). Cycloheximide was purchased from Calbioche. Pin1-specific small interfering RNAs (Cat. No. LQ-003291−00) and non-specific control small interfering RNAs (Cat. No. D-001140−01−05) were purchased from Dpharmacon Inc. (Lafayette, CO, USA).
Plasmids and site-directed mutagenesis
To detect the expression of exogenous Akt proteins (wild type or mutants), the full length of the human Akt1 cDNA was subcloned into pcDNA6A vectors (Invitrogen, Carlsbad, CA, USA) with a hemagglutinin (HA) epitope tag. All Akt1 mutants with point mutations of the Ser/Thr-Pro motifs were generated by a QuikChange site-directed mutagenesis kit (Cat. No.200514 and 200523, Stratagene, La Jolla, CA, USA) according to the protocol provided by the manufacturer. The full length of the human Pin1 cDNA was subcloned into pcDNA3 vectors (Invitrogen). The pcDNA1-Pin1 mutants (W34A and R68,69A), pGEX-2TK-GST-Pin1, GST-Pin1 mutants (W34A, R68,69A), and Escherichia coli bacteria were obtained from Xiao Z and Zheng H (Boston University School of Medicine). All DNA constructs were sequenced to confirm the mutation as well as to verify the accuracy of the full-length cDNA sequence.
Western blot
To detect expression levels of Pin1 and Akt proteins as well as Akt phosphorylation on different sites, standard western blot analysis was performed. MEFs (with or without either Pin1 or Akt knockout), human embryonic kidney (HEK) 293 cells, and breast cancer (MDA-MB-231, MDA-MB-453, and MDA-MB-468) cells were all maintained in 10% bovine serum containing Dulbecco modified Eagle's medium (DMEM) supplemented with penicillin and streptomycin at 37 °C under humidified atmosphere with 5% CO2. Cells were washed twice with cold PBS and lysed in a lysis buffer A containing 20 mm Tris–HCl (pH 7.5), 150 mm NaCl, 5 mm EDTA, 10 mm NaF, 1% Nonidet P-40 (NP-40), 1.0 mm phenylmethylsulfonyl fluoride, 1.0 mM sodium orthovanadate (NaVO3), and 1.5% aprotinin. The cell extracts were cleared by centrifugation, and the protein concentration was determined using a Bio-Rad (Hercules, CA, USA) protein assay reagent and analyzed in a spectrophotometer using bovine serum album (Sigma) as the protein standard. Aliquots of protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes (Millipore Corp., Bedford, MA, USA) using standard procedures. The membranes were then subjected to western blotting, and the blots were developed with the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Immunoprecipitation
To detect whether endogeneous Akt and Pin1 interacts with other, a co-immunoprecipitation experiment was performed. Exponentially growing breast cancer MDA-MB-468 cells maintained in Dulbecco modified Eagle's medium, as described above, were washed twice with ice-cold PBS and lysed by sonication in modified RIPA (radioimmunoprecipitation) B buffer, which contains 50 mm Tris–HCl (pH 7.4), 0.5% NP-40, 0.25% sodium deoxycholate, 1 mm ethylene glycol tetraacetic acid, and a cocktail of protease inhibitors. After centrifugation of the cell lysate at 14 000 × g in a pre-cooled centrifuge for 30 min, cell pellet was discarded and supernatant was transferred to fresh centrifuge tube. The supernatant was pre-cleared by incubation with 100 μl of protein G agarose bead slurry (50%, Roche Diagnostics, Indianapolis, IN, USA, Cat No. 1134515) per 1 ml of cell lysate at 4 °C for 30 min on an orbital shaker. Aliquot of pre-cleared supernatant adjusted to a concentration of 4 mg/ml was incubated with anti-Akt1 (total Akt), anti-Pin1, and anti-phosphorylated Ser/Thr-Pro motif antibody (MPM2), respectively, at 4 °C overnight on an orbiter shaker. Capture the immunocomplex by adding 100 μl protein G agarose bead slurry and gently shacking for 1 h at 4 °C. Collect the agarose beads by pulse centrifugation and discard the supernatant and wash the beads three times with 800 μl ice-cold RIPA B buffer. Resuspend the agarose beads in 60 μl 2× sample buffer and mix gently. Boil the agarose beads for 5 min to dissociate the immunocomplexes from the beads. The beads were collected by centrifugation and SDS–PAGE is performed with the supernatant. The association of Pin1 in the anti-Akt immunocomplex or the association of Akt in the anti-Pin1 as well as anti-MPM2 immunocomplex was determined by western blot.
To determine which Ser/Thr-Pro motif of Akt responsible for the immunoreactivity to the anti-MPM2 antibody as well as its involvement in Akt interacting with Pin1, exogenous HA-tagged Akt plasmid constructs were transfected into either Akt1 (−/−) MEFs or HEK293 cells with FuGene 6 (Cat. No. 1814443, Roche). Proteins were pre-cleared through the addition of normal mouse immunoglobulin G (IgG) and immunoprecipitated by anti-HA antibody by a similar immunoprecipitating procedure as described above.
Glutathione-S-transferase pull-down assay
To further determine whether exogenous human Pin1 protein may also interact with wild-type Akt protein as well as Akt protein with a point mutation in the Ser/Thr-Pro motifs, a GST pull-down assay was used. Again, AKt1 (−/−) MEF cells after transfection with HA-tagged human Akt1 (wild type or mutants) in pcDNA6A vector for 36 h or MDA-MB-468 cells maintained in Dulbecco modified Eagle's medium were washed with ice-cold PBS and lysed by sonication in HEPES buffer (HEPES-KOH, pH 7.5, 10 mm KCl, 10 mm MgCl2, and 0.5 mm dithiothreitol). Cell lysates were centrifuged at 10 000 r.p.m. for 30 min, and the supernatants were then transferred to a fresh tube. Aliquots of cell lysates (typically 200 μg of lysate/reaction) were incubated with purified recombinant human GST-Pin1 or GST-Pin1 mutant fusion proteins (2.5 μg per reaction), with or without phospho-T92 and/or Phospho-T450 peptide (1−5 μg per reaction) competition, at 4°C for 3 h or a specified time. GST proteins were then brought down by Glutathione Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ, USA) and washed with PBS three times and eluted with fresh elution buffer (Amersham Biosciences). Bacterial-expressed GST proteins were purified by using a Bulk GST purification kit (Cat. No. 27−4570−01, Amersham Biosciences) following the instructions provided by the manufacturer.
Cycloheximide pulse-chase experiment
To monitor changes in the stability of the wild-type Akt1 and the double mutant Akt1 (T92A/T450A), expression constructs of pcDNA-6A HA-tagged wild-type Akt1 (Akt1-WT, 2.5 μg) and a double mutant Akt1-T92A/T450A (Akt1-AA, 10 μg) were transfected into HEK 293 cells by using the Lipofectamine reagent (Invitrogen). Owing to the lower-level expression of the Akt1-T92A/T450A double mutant, a quadrupled amount (10 μg) of plasmid DNA of this mutant was used to secure a detectable level of this double mutant at the starting point. Cycloheximide chase experiments were performed on cells transfected (as indicated above) with cycloheximide (20 μg/ml) added to the culture 24 h after transfection (time 0). Proteins were prepared at the indicated times (chasing time 0−8 h), and equal amounts were subjected to immunoprecipitate with anti-HA antibody and followed with immunoblot analysis. A monoclonal anti-HA antibody detected expression of HA-tagged Akt1 at various time points after exposure to cycloheximide; β-actin was used as a loading control.
Tissue microarray and immunohistochemistry
Tissue microarry slides (HistoArray #IMH-371, IMH-326∼8, 3/BA2, IMH-304/CB2, and VA1) were purchased from IMGENEX (San Diego, CA, USA). Detailed information about each slide is available on-line at (http://www.imgenex.com/tissue_array). Slide procession and immunohistochemistry staining were performed according to the manufacturer's protocol and as described earlier (Liao and Hung, 2003). One representative slides per case was evaluated with the antibodies mentioned above. The intensities of staining seen in different areas of the same slide were analyzed according to criteria described earlier (Liao and Hung, 2003).
Statistical analysis
Statistical analysis was carried out using SPSS, version 10.0. Fisher's exact and Student's t-tests were applied to assess the statistical significance of the associations between expression of Pin1, Akt, and various clinicopathologic variables. Correlations were calculated according to Spearman rank correlation. Univariate survival analysis was carried out according to Kaplan–Meier method, whereas differences in survival curves were assessed with the log-rank test (Mantel–Cox test) using SSPS 12.1 software. All P-values were two sided, and P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This project has been supported by funds from the Department of Defense Breast Cancer Research Program (DAMD17-01-1-0300), the Susan G Komen Foundation (BCTR0504146), a Research Development Award from the SPORE in Ovarian Cancer (P50-CA83639-A01) (to LY), and by the grant support from the NIH P01 099031, P20 CA101936 (MDACC Pancreatic Cancer SPORE), P50 CA116199 (MDACC Breast Cancer SPORE), P50 CA83639 (MDACC Ovarian Cancer SPORE), Marcus Foundation, National Breast Cancer Foundation, Inc., and Kadoorie Charitable Foundations (to M-C H). We acknowledge Dr Martin L Campbell at the Synthetic Antigen Laboratory, The University of Texas MD Anderson Cancer Center, for the synthesis of the phospho- and non-phospho-peptides as well as the NIH core grant to MD Anderson Cancer Center (CA16672). We thank Drs Xiangho He, Tiebang Kang, Bo Ping, Pingyu Zhang, Weiya Xia, and Mr William Spohn for their participation in the earlier development of this project. We also appreciate Dr Hay N (University of Illinois, Chicago, IL, USA), Dr Lu KP (Department of Medicine, Beth Deaconess Medical Center, Harvard Medical School, Boston, MA, USA), and Dr Xiao Z (Department of Biochemistry and Department of Medicine, Boston University School of Medicine, Boston, MA, USA) for their kindness in providing us the Akt1 MEF cells, Pin1 MEF cells, and Pin1 constructs. We are grateful for the technical assistance from Dr Jeng C Cheng and Dr Stephanie A Miller during the preparation of this paper. We also greatly appreciate the editing and language improvement of this paper by Ms Kristi Speights at the Department of Scientific Publication, The University of Texas MD Anderson Cancer Center.
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol. 2004;164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Chan TO, Tsichlis PN. PDK2: a complex tail in one Akt. Sci STKE. 2001;2001:PE1. doi: 10.1126/stke.2001.66.pe1. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3 K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene. 2005;24:7401–7409. doi: 10.1038/sj.onc.1209099. [DOI] [PubMed] [Google Scholar]
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn G, Lu KP. Phosphorylation-specific prolyl isomerase Pin1 as a new diagnostic and therapeutic target for cancer. Curr Cancer Drug Targets. 2008;8:223–229. doi: 10.2174/156800908784293622. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med. 1999;77:656–665. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- Liao Y, Hung MC. Regulation of the activity of p38 mitogen-activated protein kinase by Akt in cancer and adenoviral protein E1A-mediated sensitization to apoptosis. Mol Cell Biol. 2003;23:6836–6848. doi: 10.1128/MCB.23.19.6836-6848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KP, Suizu F, Zhou XZ, Finn G, Lam P, Wulf G. Targeting carcinogenesis: a role for the prolyl isomerase Pin1? Mol Carcinog. 2006;45:397–402. doi: 10.1002/mc.20216. [DOI] [PubMed] [Google Scholar]
- Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Muise-Helmericks R, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, et al. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Soung YH, Lee JW, Nam SW, Lee JY, Yoo NJ, Lee SH. Mutational analysis of AKT1, AKT2 and AKT3 genes in common human carcinomas. Oncology. 2006;70:285–289. doi: 10.1159/000096289. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Uchida C, Shin RW, Shimazaki K, Uchida T. Prolyl isomerase, Pin1: new findings of post-translational modifications and physiological substrates in cancer, asthma and Alzheimer’s disease. Cell Mol Life Sci. 2008;65:359–375. doi: 10.1007/s00018-007-7270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- Wulf G, Garg P, Liou YC, Iglehart D, Lu KP. Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. EMBO J. 2004;23:3397–3407. doi: 10.1038/sj.emboj.7600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y, et al. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res. 2004;10:3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- Yeh ES, Means AR. PIN1, the cell cycle and cancer. Nat Rev Cancer. 2007;7:381–388. doi: 10.1038/nrc2107. [DOI] [PubMed] [Google Scholar]
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.