Abstract
Objective
To determine whether the intrauterine environment affects the postnatal vascular response to stress in a model of fetal programming induced by endothelial nitric oxide synthase (NOS3) deficiency.
Methods
Homozygous NOS3 knockout and wild type controls were cross-bred to obtain maternally- (KOM) and paternally- (KOP) derived heterozygous offspring. At 14 weeks of age, in-vivo blood pressure (BP) measurements by telemetry, and in-vitro carotid arteries vascular reactivity studies were performed in male offspring after subjecting them to shaker stress.
Results
KOM offspring, compared to KOP, had significantly higher systolic BP, mean arterial BP and pulse pressure before, as well as after introduction of the shaker stress. The difference in the latter between KOM and KOP was accentuated after stress. KOM offspring also had significantly higher contractile responses to phenylephrine when compared to KOP, and this was abolished after incubation with L-NAME.
Conclusion
The adverse uterine environment affects the postnatal vascular response to stress
Keywords: Fetal programming, vascular phenotype, shaker stress, postnatal
INTRODUCTION
Fetal exposure to a suboptimal intrauterine environment increases its risk of acquiring cardiovascular and other diseases in adult life through a process known as “developmental origin of adult diseases.” This concept was popularized by Dr David Barker who through a series of epidemiological studies in the late 1980’s showed that the geographical differences in average blood pressure and mortality from cardiovascular disease in Britain partly reflected past differences in the intrauterine environment.1 These and other studies confirm that adverse changes, or insults, in the intrauterine environment during the critical periods of development lead to fetal programming with long lasting adaptive changes in the fetal structure, physiology and metabolism.2,3 Although most of the impact is thought to occur during fetal development, the postnatal period is believed to also play a crucial role. We have previously shown that postnatal interventions in the first generation heterozygotes, such as cross-fostering to mothers with the opposite genetic makeup of NOS3, may alter the adult vascular profile.4
However, these observed associations between an unfavorable intrauterine fetal environment and diseases in later life may be confounded by the presence of a genetic predisposition for the particular disease. In order to circumvent this potential problem in inducing an unfavorable uterine milieu, we used a transgenic mouse model deficient in endothelial nitric oxide synthase (e-NOS or NOS-3). This model combines the effects of a hostile uterine environment as well as a genetic abnormality affecting vascular function, and thus is ideal to investigate the contribution of each on the vascular function in adult life. NOS-3 is the rate limiting enzyme responsible for the generation of nitric oxide (NO) in endothelial cells from its substrate L-arginine. NO is a potent smooth muscle relaxant and one of the primary modulators of vascular tone.5,6,7 It plays a vital role in maintaining adequate utero-placental perfusion through its vasodilatory properties. Inhibition of NOS3 results in maternal hypertension and fetal growth restriction.8,9 By cross-breeding knockout mice lacking NOS-3 (NOS3−/−KO) to wild type ones (NOS3+/+WT), we previously showed that heterozygous offspring born to NOS3−/−KO mothers (and therefore exposed to an abnormal maternal uterine environment) have abnormal litter size, in addition to abnormal vascular function and blood pressure profile later in life compared to the genomically-similar heterozygous offspring born to wild type mothers with normal intrauterine environment.10
We hypothesize that the effects of the intrauterine environment on the vascular phenotype and blood pressure in the NOS-3 mouse model affect the responsiveness of these animals to postnatal stress.
Our objective was to characterize the effect of development in an adverse uterine environment on the vascular response to postnatal stress in adult offspring in this previously characterized model of fetal programming.
MATERIALS AND METHODS
Animals
Mature cycling female and male mice (4–6 weeks old) homozygous for disruption of the NOS3 gene (NOS3-knockout, strain B6.129P2-Nos3tm1Unc, stock no. 002684, NOS3−/−KO) and their age-matched wild-type controls (NOS3-wild-type, strain C57BL/6J, stock no. 000664, NOS3+/+WT) were purchased from Jackson Laboratory (Barharbour, Maine). Animals were maintained and bred in the animal care facility at the University of Texas Medical Branch. They were housed separately in temperature- and humidity-controlled quarters with constant 12-hour light:dark cycles and provided with food and water ad libitum. Regular maintenance and care was provided by certified personnel and veterinary staff according to the guidelines of the Animal Care and Use Committee (ACUC) of the University of Texas Medical Branch. All surgical procedures were carried out by trained personnel according to the ACUC guidelines also.
Homozygous male and female NOS3 knockout (NOS3−/−KO) and wild-type mice (NOS3+/+WT) were cross-bred to obtain first generation heterozygous NOS3 offspring born to wild-type mothers (abnormal allele inherited from father, or paternally-derived heterozygous; KOP) and first generation heterozygous NOS3 offspring born to knockout mothers (abnormal allele inherited from mother, or maternally-derived heterozygous; KOM). The 2 groups of heterozygous offspring are genomically-similar but developed in normal mothers or mothers lacking functional NOS3, respectively. All animals were genotyped by PCR on DNA isolated from their tail.
Blood pressure measurement
In vivo blood pressure was measured by telemetry in conscious unrestrained offspring at 14 weeks of age. Only male first generation offspring (n=8–9 per group) were used. Animals were anesthetized with a mixture of ketamine (Ketalar, Parke-Davis, Morris Plains, NJ) and xylazine (Gemini, Rugby, Rockville Center, NY), then the left common carotid artery was located and carefully isolated through a vertical midline skin incision along the neck. A 0.4 mm catheter was then introduced into the carotid artery through a small incision in the vessel wall, and the body of the transducer (PA-C10 model, Data Sciences, St. Paul, MN) was secured in a subcutaneous pouch. The catheter was then tunneled to a telemetric transducer encased in a miniature capsule. The neck incision was closed using 6–0 silk. Mice were kept warm on a heating pad and monitored closely until fully recovered from anesthesia. Blood pressure was continuously measured for 6 days in the unrestrained conscious animal. Daily average of systolic (SBP), diastolic (DBP) and pulse pressure (PP) were obtained. Mean arterial blood pressure (MAP) was calculated as (SBP + 2xDBP)/3 from the recorded data and then averaged over the same 24 hr periods.
Shaker Stress
Between the 3rd and 4th day of telemetry, male heterozygous offspring were exposed to shaker stress. This was introduced through a specially designed caging system attached to a shaking platform (Model 5901; Eberbach Inc., Ann Arbor, MI). Mice were exposed to 2-minute shaking sessions (150 cycles/min; 2.86 cm stroke) intermittently every 13 to 45 minutes, for a total of 14 shaking sessions with a total interval time of 6 hrs in a 24 hour period. The variable rest periods between stress sessions were chosen with the aim of introducing an unpredictable stress stimulus. This method of the shaker stress is a mild, pain-free stimulus that elicits reproducible changes in BP, HR, sympathetic, locomotor and behavioral activities in a more stable environment.11
Vascular reactivity experiments
At the end of the six days of blood pressure measurement period, male heterozygous offspring (n=8–12 per group) were sacrificed by CO2 inhalation in a closed chamber according to the ACUC and the American Veterinary Medical Association guidelines. Carotid arteries were careful dissected, isolated, and 2-millimeter segments obtained and mounted in a wire myograph (Model 410A, J.P. Trading I/S, Aarhus, Denmark) over 25 Pm tungsten wires. The preparations were bathed in physiological salt solution maintained at 37°C, pH ~7.4 and a mixture of 95% O2 and 5% CO2 was bubbled continuously through the solution. The force was recorded continuously using an isometric force transducer and analyzed using Power laboratory data acquisition and playback software (DataQ Instruments, Inc, Akron, OH). After stabilization of the tone, the vessels were contracted twice with 60 mmol/L KCl for 30 minutes to enhance reproducibility of responses. The second response to KCl was used as a reference to calculate the percent of contraction achieved by the contractile agent studied. After 1 hour equilibration in physiologic salt solution, contractile responses to cumulative concentrations of the α1-adrenergic agonist phenylephrine hydrochloride (PE; 10−10to 10−5 mmol/L), in the presence and absence of the non selective NOS inhibitor L-NAME (10−5 mmol/L), were assessed. In addition, relaxant responses to cumulative concentrations of sodium nitroprusside (SNP; 10−10 to 10−5 mmol/L) were evaluated after precontraction of the vessels with 2 doses of PE to achieve final concentrations of 10−7–10−6 mmol/L. This will produce matching contractions in the study groups. PE precontraction was used to calculate the percent relaxation induced by the vasorelaxant agent used. After each agent tested, the vessels were washed with Krebs solution and left to recover for ≥30 minutes until they returned to their basal passive tension.
Drugs and solutions
PE, L-NAME and SNP were purchased from Sigma Chemical Company (St. Louis, MO). Stock solutions of all of the drugs (10−2 mol/L) were prepared in deionized water and stored at −20°C. The composition of physiologic salt solution was NaCl, 119 mmol/L; KCl, 4.7 mmol/L; NaH2PO4, 1.2 mmol/L; NaHCO3, 25 mmol/L; MgCl2, 1.2 mmol/L; CaCl2, 2.5 mmol/L; ethylenediaminetetraacetic acid, 0.026 mmol/L; glucose, 11.5 mmol/L.
Data analysis
Blood pressure was recorded continuously for 6 days. Data were averaged over 24 hour periods for each day of the 6 days of recording period. MAP, SBP, DBP and PP were collected, expressed as mean ± SEM and compared between KOM and KOP offspring. For the vascular reactivity studies, the maximal effect was calculated and expressed as mean ± SEM. Response to KCL was used as a reference to calculate the percent of contraction achieved by the contractile agents studied, while PE precontraction was used to obtain the percent relaxation induced by SNP. Continuous data were tested for normality using Kolmogorov-Smirnov test, and then compared between KOP and KOM using the Student-t or Mann-Whitney U tests as appropriate. Repeated measure ANOVA was used for within-group comparison. A p < 0.05 was considered statistically significant.
RESULTS
Offspring male mice were weighed at 14 weeks of age, and there was no difference in the weight between paternally-derived (KOP) and maternally-derived (KOM) heterozygous offspring (26 ± 1.8 vs. 26.13 ± 1.9 gm).
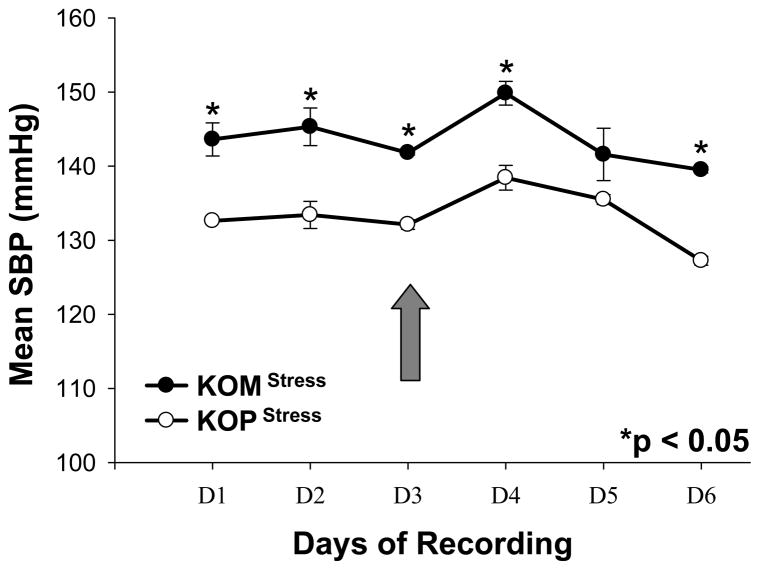
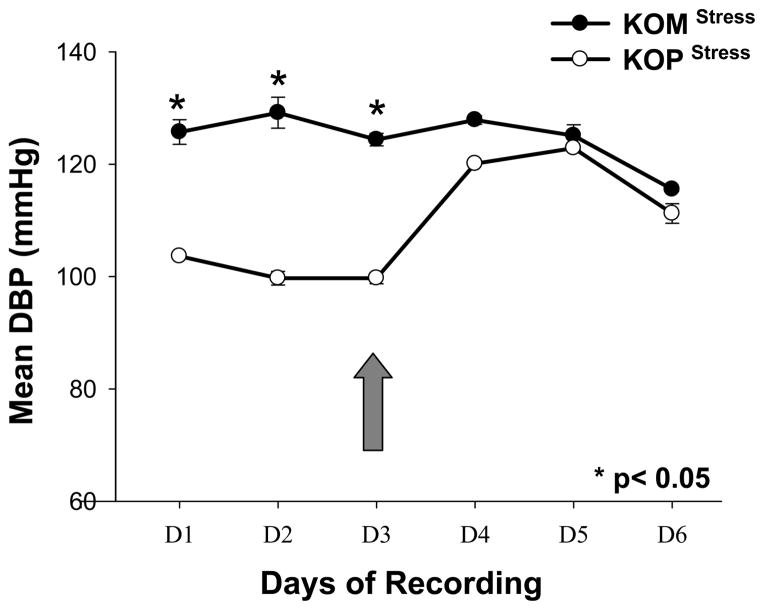
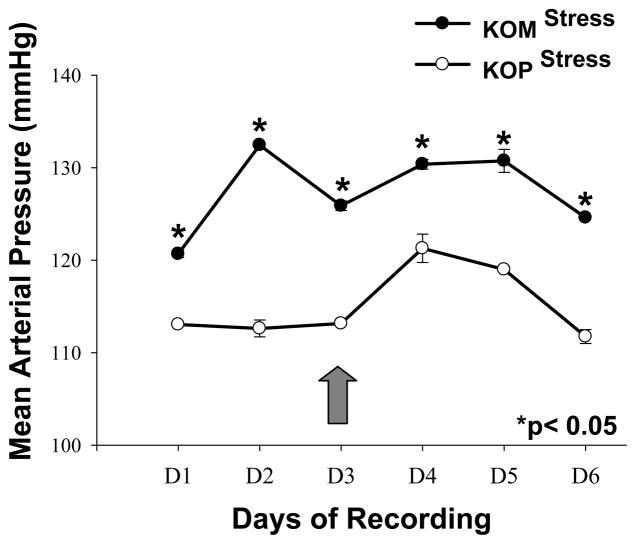
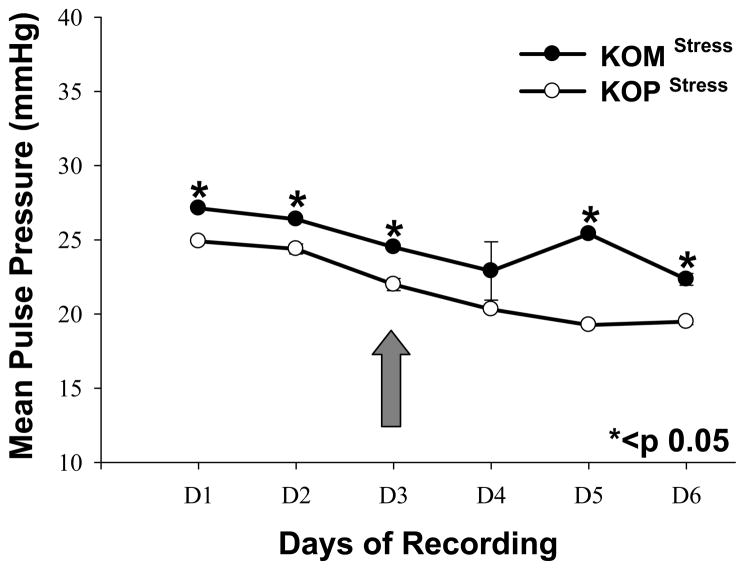
First generation male mice offspring had significantly higher SBP in the KOM compared to the KOP group for every day before and after introduction of the shaker stress (Figure 1). DBP was also significantly higher in the KOM group compared to the KOP during the first 3 days of recording; however that difference was abolished after shaker stress introduction. The DBP significantly increased in the KOP offspring following stress, but remained unchanged in the KOM (Figure 2). MAP and PP were also significantly different between KOM and KOP offspring for all 6 days of recording before and after stress (Figures 3, 4). The difference in PP however, was further exacerbated by the introduction of stress.
Figure 1.
Mean and SEM of daily systolic blood pressure in maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring at 14 weeks of age. Blood pressure was measured in the carotid artery before and after introduction of stress stimuli (large arrow) (n=8–9 mice per group).
Figure 2.
Mean and SEM of daily diastolic blood pressure in maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring at 14 weeks of age. Blood pressure was measured in the carotid artery before and after introduction of stress stimuli (large arrow) (n=8–9 mice per group).
Figure 3.
Mean and SEM of daily mean arterial blood pressure in maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring at 14 weeks of age. Blood pressure was measured in the carotid artery before and after introduction of stress stimuli (large arrow) (n=8–9 mice per group).
Figure 4.
Mean and SEM of daily pulse pressure in maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring at 14 weeks of age. Blood pressure was measured in the carotid artery before and after introduction of stress stimuli (large arrow) (n=8–9 mice per group).
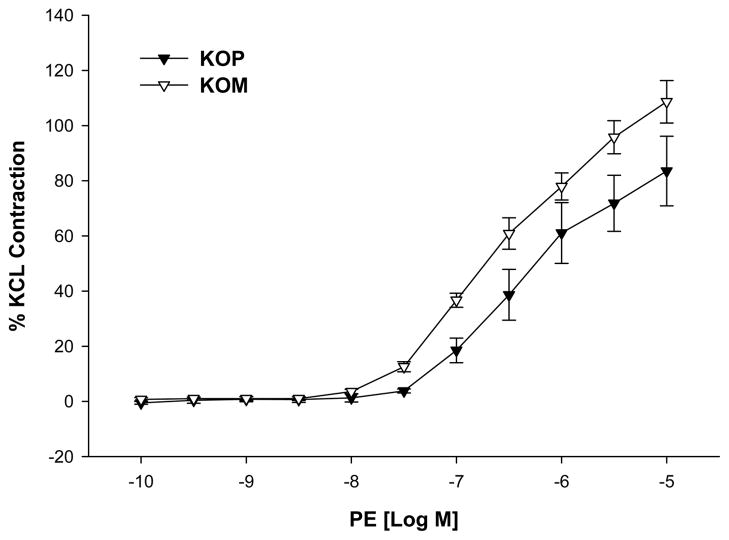
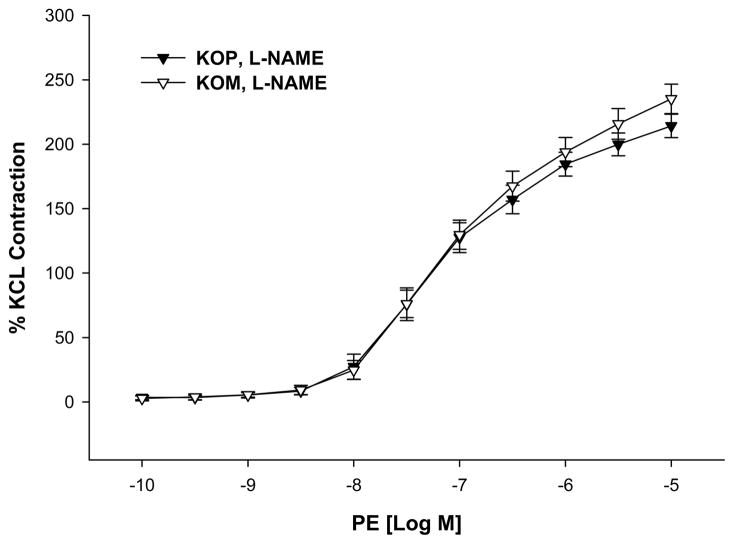
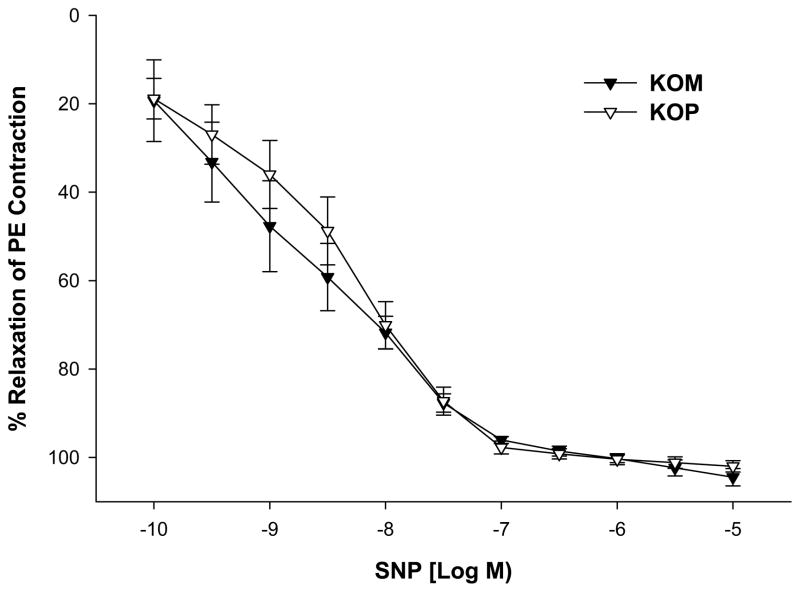
KOM mice offspring exposed to shaker stress had significantly higher contractile responses to PE when compared to KOP offspring (maximal contractile response 108.65 ± 7.70 vs. 83.52 ± 12.61; P=0.04; Figure 5). However, this difference was abolished after incubation with L-NAME (maximal contractile response 235.19 ± 11.46 vs 214.19 ± 9.04; p=0.22; Figure 6). On the other hand, the vasorelaxant responses to SNP did not differ between KOM and KOP groups (maximal relaxation response 102.00 ± 1.29 vs. 104.46 ± 1.97; p=0.94; Figure 7).
Figure 5.
Concentration-response curves to phenylephrine (PE, 10−10–10−5 M) in the carotid artery of maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring exposed to shaker stress at 14 weeks of age (n=8–12 mice per group).
Figure 6.
Concentration-response curves to phenylephrine (PE, 10−10–10−5 M) in the presence of L-NAME in the carotid artery of maternally- (KOM) and paternally- (KOP) derived male heterozygous offspring exposed to shaker stress at 14 weeks of age (n=8–12 mice per group).
Figure 7.
Relaxation-response curves to sodium nitroprusside (SNP, 10−10–10−5 M) in the carotid artery of maternally- (KOM) and paternally (KOP) derived male heterozygous offspring exposed to shaker stress at 14 weeks of age (n=8–12 mice per group).
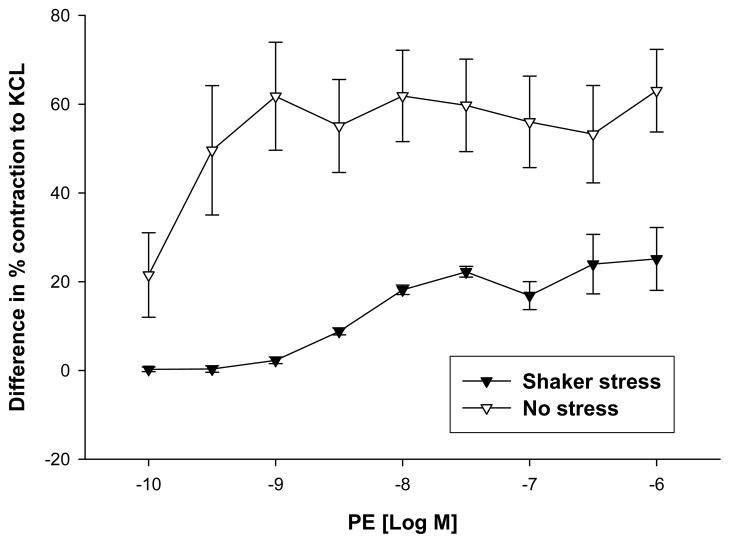
We then compared the differences in the contractile responses to PE between KOP and KOM offspring that were stressed to our prior data from similar offspring obtained using the same breeding scheme, and similarly sacrificed at 14 week of age, but without the postnatal stress. The differences in response to phenylephrine between KOM and KOP (KOM − KOP) were significantly higher at every PE concentration in animals that were not exposed to shaker stress compared to those that were stressed (Figure 8).
Figure 8.
Differences in contractile response to phenylephrine in the carotid artery of maternally- (KOM) and paternally (KOP) derived male heterozygous offspring exposed or not to shaker stress at 14 weeks of age (n=8–12 mice per group).
DISCUSSION
The findings from this study suggest that in this animal model of fetal programming, the adverse intrauterine environment, induced by NOS3 deficiency, affects the postnatal vascular response to stress. This was evident in our in vivo (blood pressure) and in vitro (vascular reactivity) studies in which heterozygous offspring born to mothers lacking functional NOS-3 (KOM) had a distinctly different vascular characteristics compared to those born to wild type mothers (KOP).
KOM mice had worse BP profile compared to KOP before and after introduction of stress, in addition their contractile response to phenylephrine was higher compared to KOP. All of these parameters describe a vascular profile at higher risk for cardiovascular disease and mortality. The higher PP seen in KOM compared to KOP, evident before stress and exacerbated after the introduction of stress, underscores the implications of an adverse uterine environment on adult cardiovascular function. Human data show that pulse pressure in particular is a major determinant of cardiovascular risk,12 and can be regarded as an index of arterial aging. Studies have shown that biological aging (assessed by telomere length) was inversely related to pulse pressure, especially in males.13,14 This inverse association between pulse pressure and longevity was much stronger than the effect of chronological age. Biologic age of individuals with relatively wide pulse pressures is more advanced than their chronological age would indicate and, at least in males, wide pulse pressure denotes a forward resetting of biologic age.15
What the introduction of stress showed mostly was that KOP animals mounted a higher vascular response (compared to KOM) after the introduction of the shaker stress. The differences in SBP and DBP between the two groups were decreased after stress; moreover, the differences in contractile responses to PE were smaller in mice exposed to shaker stress compared to our prior data in non-stressed animals. This possibly means that with stress KOP mice increased their responses to a level close to KOM and/or that KOM mice are at an already maximally stressed vascular profile (due to intrauterine environment) that they cannot mount a higher vascular response. The adverse uterine environment blunts part of the stress-induced autonomic response in KOM offspring. This suggests either a resetting of the autonomic system or a reduced level of vascular responsiveness probably due to structural vascular changes in the KOM offspring, however these speculations need to be further evaluated. The autonomic cardiovascular response to stress has been described in mice, and involves both and receptors activation in addition to the rennin angiotensin system.16,17,18 Moreover, sudden increases in BP caused by shaker or other forms of stress lead to alterations in shear stress across the vessel which can increase nitric oxide production by endothelial cells to compensate for the BP changes.19 At the same time, these BP changes can induce damage in endothelial cells thus reducing their protective mechanism. This highlights the importance of a healthy endothelial cell monolayer in the normal response to stress, and may explain the abnormal findings observed in KOM animals especially that prior work in our lab showed aberrant endothelium-dependent relaxation to acetylcholine in this group similar to knockout animals.10
Stress is believed to be a major risk factor in the development of cardiovascular disease. In mice, many studies showed that stress alters cardiovascular functions and drinking behaviors.11 Stress induced by a shaker system, produced changes in blood pressure variability in mice mediated by increased sympathetic activity and increased baroreflex sensitivity leading to reduction in BP buffering.20,21 BP variability can be viewed as a marker for cardiovascular diseases.22
In this study, we used a shaker system to introduce stress in the conscious unrestrained mouse, and we recorded changes in blood pressure using a telemetry system. This method of stress generation is well characterized and circumvents the major artifacts caused by animal handling, restraining, noise, and pain. The environment in which the experiments are being performed is more stable since this method does not involve switching cages. Moreover, our in vivo data were obtained continuously in the conscious unrestrained offspring residing in their usual environment (i.e. usual cage and room) without the potential drawbacks introduced by animal handling and physical restraining.23
We only used male animals in our experiment because we previously found that male first and second generation offspring mice have increased contractile responses compared to females.10,24 We elected to study the effect of the intrauterine environment on the most severely affected gender i.e. males, and thus eliminate the potential protective effect mediated by estrogen via NO. Estrogen has been found to decrease myointimal smooth muscle cell proliferation25, inhibit TNF-α induced apoptosis in endothelial cells26 and induce NOS-3 in a variety of tissues27 both by increasing mRNA and protein levels and by increasing NOS3 activity.28,29 Other limitations of the study is the fact that we only compared first generation heterozygote KOM and KOP to each others but not to first generation wild type or knockout mice. This was based on our prior observation that heterozygote KOP had similar vascular characteristics as WT mice, and heterozygotes KOM had similar vascular characteristics to KO mice.10
In conclusion, this study demonstrates that an adverse intrauterine environment affects the postnatal vascular responses to stress stimuli, and reinforces the importance and significance of the effects of fetal programming on long term health outcomes.
Footnotes
Presentation Information: Presented in Oral Concurrent IV Session (ID: 199906), and Poster Session II (ID: 199592) at the 29th Annual Meeting of the Society of Maternal-Fetal-Medicine 2009 in San Diego.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13:807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 4.Clark SM, Makhlouf M, Hankins GDV, Anderson GD, Saade GR, Longo M. The effect of the postnatal environment on altered fetal programming of adult vascular function in mice lacking endothelial nitric oxide synthase. Am J Obstet Gynecol. 2007;196:354 e1–354 e7. doi: 10.1016/j.ajog.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Palmer RM, Higgins EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 7.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 8.Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod. 1995;10:2723–30. doi: 10.1093/oxfordjournals.humrep.a135775. [DOI] [PubMed] [Google Scholar]
- 9.Edwards DL, Arora CP, Bui DT, Castro LC. Long-term nitric oxide blockade in the pregnant rat: effects on blood pressure and plasma levels of endothelin-1. Am J Obstet Gynecol. 1996;175:484–8. doi: 10.1016/s0002-9378(96)70166-7. [DOI] [PubMed] [Google Scholar]
- 10.Longo M, Jain V, Vedernikov YP, Bukowski R, Garfield RE, Hankins GD, Anderson GD, Saade GR. Fetal origins of adult vascular dysfunction in mice lacking endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2005;288:1114–21. doi: 10.1152/ajpregu.00367.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bernatova I, Key MP, Lucot JB, Morris M. Circadian differences in stress induced pressor reactivity in mice. Hypertension. 2002;40:768–73. doi: 10.1161/01.hyp.0000036405.27562.02. [DOI] [PubMed] [Google Scholar]
- 12.O’Rourke M, Frohlich ED. Pulse pressure: is this a clinically useful risk factor? Hypertension. 1999;34:372–374. doi: 10.1161/01.hyp.34.3.372. [DOI] [PubMed] [Google Scholar]
- 13.Jeanclos E, Schork NJ, Kyviv KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 15.Aviv A. Hypothesis: pulse pressure and human longevity. Hypertension. 2001;37:1060–1066. doi: 10.1161/01.hyp.37.4.1060. [DOI] [PubMed] [Google Scholar]
- 16.Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2214–R2221. doi: 10.1152/ajpregu.2000.279.6.R2214. [DOI] [PubMed] [Google Scholar]
- 17.Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1394–R1398. doi: 10.1152/ajpregu.00306.2004. [DOI] [PubMed] [Google Scholar]
- 18.Farah VM, Joaquim LF, Morris M. Stress cardiovascular/autonomic interactions in mice. Physiol Behav. 2006;89:569–575. doi: 10.1016/j.physbeh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Stauss HM, Persson PB. Role of nitric oxide in buffering short-term blood pressure fluctuations. News Physiol Sci. 2000;15:229–233. doi: 10.1152/physiologyonline.2000.15.5.229. [DOI] [PubMed] [Google Scholar]
- 20.Farah VM, Joaquim LF, Bernatova I, Morris M. Acute and chronic stress influence blood pressure variability in mice. Physiol Behav. 2004;83:135–142. doi: 10.1016/j.physbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Joaquim LF, Farah VM, Bernatova I, Fazan R, Jr, Grubbs R, Morris M. Enhanced heart rate variability and baroreflex index after stress and cholinesterase inhibition in mice. Am J Physiol Heart Circ Physiol. 2004;287(1):H251–7. doi: 10.1152/ajpheart.01136.2003. [DOI] [PubMed] [Google Scholar]
- 22.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–8. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kramer K, Kinter LB. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics. 2003;13:197–205. doi: 10.1152/physiolgenomics.00164.2002. [DOI] [PubMed] [Google Scholar]
- 24.Costantine MM, Ghulmiyyah LM, Tamayo E, Hankins GDV, Saade GR, Longo M. Transgenerational effect of fetal programming on vascular phenotype and reactivity in endothelial nitric oxide knockout mouse model. Am J Obstet Gynecol. 2008;199:250.e1–250.e7. doi: 10.1016/j.ajog.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhalla RC, Toth KF, Bhatty RA, Thompson LP, Sharma RV. Estrogen reduces proliferation and agonist-induced calcium increase in coronary artery smooth muscle cells. Am J Physiol. 1997;272:1996–2003. doi: 10.1152/ajpheart.1997.272.4.H1996. [DOI] [PubMed] [Google Scholar]
- 26.Spyridopoulos I, Sullivan AB, Kearney M, Isner JM, Losordo DW. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis: estradiol as a survival factor. Circulation. 1997;95:1505–1514. doi: 10.1161/01.cir.95.6.1505. [DOI] [PubMed] [Google Scholar]
- 27.Cid MC, Schnaper W, Kleinman HK. Estrogens and the vascular endothelium. Ann NY Acad Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan E, Gurjar MV, Sharma RV, Bhalla RC. Estrogen receptor-a gene transfer into bovine aortic endothelial cells induces eNOS gene expression and inhibits cell migration. Cardiovascular Research. 1999;43:788–797. doi: 10.1016/s0008-6363(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Yuhanna IS, Galcheva-Garagova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]