CONSPECTUS
Molecular imaging has evolved over the past several years into an important tool for diagnosing, understanding, and the monitoring of disease. Molecular imaging has distinguished itself as an interdisciplinary field with contributions from the areas of chemistry, biology, physics and medicine. The cross-disciplinary impetus has led to significant achievements in the development of more sensitive imaging instruments, robust and safer radiopharmaceuticals, thereby providing more choices to fit the personalized medical needs. Molecular imaging is making steadfast progress in the field of cancer research among others. Cancer is a challenging disease, characterized by heterogeneity, uncontrolled cell division, and the ability of cancer cells to invade other tissues. Molecular imaging is addressing these challenges by aggressively identifying and studying key cancer-specific biomarkers such as growth factor receptors, protein kinases, cell adhesion molecules, proteases, as well as biological processes such as hypoxia, apoptosis, and angiogenesis.
Positron emission tomography (PET) has become a widely used diagnostic molecular imaging tool by clinicians in the United States. Small animal PET systems that can image rodents and generate reconstructed images in a non-invasive manner with a resolution as low as 1 mm have been developed and used frequently, which in turn facilitate radiopharmaceutical development and drug discovery. Currently, [18F]-labeled 2-fluorodeoxyglucose (FDG) is the only PET radiotracer used for routine clinical evaluation, primarily for oncological imaging.
There is now increasing interest in non-traditional positron-emitting radionuclides, particularly those of the transition metals for imaging with PET because of increased production and availability. Copper based radionuclides are currently being extensively evaluated as they offer a varying range of half-lives and positron energies. For example, the half-life (12.7 h) and decay properties (β+, 0.653 MeV [17.8 %]; β−, 0.579 MeV [38.4 %]) of 64Cu make it an ideal radioisotope for PET imaging (β+) and radiotherapy (β−). In addition, the well established coordination chemistry of copper allows for its reaction with a wide variety of chelator systems that can potentially be linked to antibodies, proteins, peptides and other biologically relevant molecules. New chelators with greater in vivo stability, such as the cross bridged (CB) versions of tetraazamacrocyclic TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid), are now available. Finally, one of the major aspects of successful imaging is the identification and characterization of a relevant disease biomarker at the cellular and sub-cellular level, and a targeting moiety highly specific for the target. This account will discuss specific examples of PET imaging of some of the key cancer biomarkers such as epidermal growth-factor receptor (EGFR), somatostatin receptors (SSRs), and integrin alpha v beta 3 (αvβ3) with new and improved 64Cu based radiopharmaceuticals. 
Molecular Imaging with Positron Emission Tomography (PET)
PET requires the injection of molecules labeled with radionuclides (radiopharmaceuticals) into the subject for obtaining an image. The amount of material that is injected into the subject is extremely small (at the level of nmol to pmol), and causes minimal pharmacological effect. In this regard, PET enables the imaging and monitoring of disease in a non-invasive manner. PET has become a widely used diagnostic imaging tool by clinicians in the United States with 1.52 million PET and PET/CT imaging procedures performed in 2008, compared to 1.46 million scans performed in 2007 (http://www.molecularimaging.net/index.php?option=com_articles&view=article&id=16290). Although there have been thousands of radiopharmaceuticals developed for potential use in a clinical imaging setting, at present, [18F]-labeled 2-fluorodeoxyglucose (FDG) is the only PET radiotracer used for routine clinical evaluation, primarily for oncological imaging. The underlying mechanism for generating a PET image is depicted in Figure 1.
Figure 1.
Schematic of imaging with positron emission tomography (PET). The positron travels away from its origin and then collides with a negatively charged electron in tissue, producing annihilation radiation of two 511 keV photons approximately 180 degrees apart. These coincident emissions are detected by the PET scanner.
Non-traditional positron-emitting radionuclides, particularly those of the transition metals, have gained considerable interest for imaging with PET because of increased production and availability. Significant research effort has been devoted to the 64Cu because of its longer half-life (12.7 h) and decay properties (β+, 0.653 MeV [17.8 %]; β−, 0.579 MeV [38.4 %]). In addition, the well-established coordination chemistry of copper allows for its reaction with a wide variety of chelator systems that can potentially be linked to antibodies, proteins, peptides and other biologically relevant molecules. This Account will discuss the chemistry of developing 64Cu radiopharmaceuticals and their applications in the molecular imaging of cancer.
Coordination Chemistry of Copper
The aqueous coordination chemistry of copper is limited to its three accessible oxidation states (I-III).1,2 The lowest oxidation state, Cu(I) has a diamagnetic d10 configuration and forms complexes without any crystal-field stabilization energy. Complexes of this type are readily prepared using relatively soft polarizable ligands like thioethers, phosphines, nitriles, isonitriles, iodide, cyanide and thiolates; however, Cu(I) complexes are typically not used for in vivo imaging due to their lability. Copper (II) is a d9 metal of borderline softness which favors amines, imines, and bidentate ligands like bipyridine to form square planar, distorted square planar, trigonal pyramidal, square pyramidal, as well as distorted octahedral geometries. Jahn-Teller distortions in six-coordinate Cu(II) complexes are often observed as an axial elongation or a tetragonal compression. Due to the presence of some crystal-field stabilization energy, Cu(II) is generally less labile toward ligand exchange as compared to Cu(I) and in our opinion is the best candidate for incorporation into radiopharmaceuticals. A third oxidation state Cu(III) is relatively rare and difficult to attain without the use of strong π-donating ligands.
Production of 64Cu
Copper-64 can be effectively produced by both reactor-based and accelerator-based methods. One method of 64Cu production is the 64Zn(n,p)64Cu reaction in a nuclear reactor.3 Most reactor-produced radionuclides are produced using thermal neutron reactions, or (n,γ) reactions, where the thermal neutron is of relatively low energy, and the target material is of the same element as the product radionuclide. For producing high-specific activity 64Cu, fast neutrons are used to bombard a natural Zn target in a (n,p) reaction. This method enabled the production of high specific activity 64Cu at the Missouri University Research Reactor (MURR) in amounts averaging 9.25 GBq (250 mCi).3 Unfortunately, one of the byproducts of producing 64Cu with a natural Zn target was 65Zn (T1.2 = 245 d), which limits the practicality of production by this method.
The production of no-carrier-added 64Cu via the 64Ni(p,n)64Cu reaction on a biomedical cyclotron was proposed by Szelecsenyi et al.4 In this study, small irradiations were performed demonstrating the feasibility of 64Cu production by this method.4 Subsequent studies by McCarthy et al. were performed, and this method is now used to provide 64Cu to researchers throughout the United States.5
Chelating Ligands for 64Cu
High specific activity 64Cu with a chelator that forms highly stable complexes in vivo is critical for achieving high uptake of 64Cu in the target tissue while minimizing non-target tissue uptake. Ligands that can form 64Cu complexes with high kinetic inertness to Cu(II) decomplexation (proton-assisted as well as transchelation or transmetallation) are ideal, since this is more significant than thermodynamic stability after the 64Cu complex is injected into a living organism.6,7 Reduction of Cu(II) to Cu(I) and subsequent Cu(I) loss may also be a pathway for loss of 64Cu, and resistance of the 64Cu complex to Cu(II)/Cu(I) reduction as well as reversibility can also be important.8 Rapid complexation kinetics are also essential to allow for the facile formation of the 64Cu complex. Finally, chelators also must be designed with available functional groups that allow them to be covalently linked to targeting peptides, proteins, and antibodies.
Chelators Based on Cyclam and Cyclen Backbones
The most widely used chelators for attaching 64Cu to biological molecules are tetraazamacrocyclic ligands with pendant arms that utilize both the macrocyclic and chelate effects to enhance stability. By far the most extensively used class of chelators for 64Cu has been the macrocyclic polyaminocarboxylates shown in Figure 2. These systems have been thoroughly investigated, and in vitro and in vivo testing have shown them to be superior to acyclic chelating agents for 64Cu.6 This enhanced stability is most likely due to the greater geometrical constraint incorporated into the macrocyclic ligand that enhances the kinetic inertness and thermodynamic stability of their 64Cu complexes.9,10 Two of the most important chelators studied were DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid). While DOTA has been used as a BFC (bifunctional chelator) for 64Cu, its ability to bind many different metal ions, and its decreased stability compared to TETA make it less than ideal.11-13 The tetraazamacrocyclic ligand TETA therefore, has been extensively used as a chelator for 64Cu, and successful derivatization of this ligand has allowed researchers to conjugate it to antibodies, proteins, and peptides.14-16
Figure 2.
Macrocyclic chelators that have been investigated for chelating copper radionuclides.
Although 64Cu-TETA complexes are more stable than 64Cu-DOTA and 64Cu-labeled complexes of acyclic ligands, their instability in vivo has been well documented. Bass et al. demonstrated that when 64Cu-TETA-octreotide (OC) was injected into normal Sprague-Dawley rats, nearly 70% of the 64Cu from 64Cu-TETA-OC was transchelated to a 35 kDa species believed to be superoxide dismutase (SOD) in the liver 20 h post-injection.17 These results are supported by the observations of Boswell et al.7
Despite the considerable efforts made by researchers to use tetraaza-tetracarboxylate macrocyclic ligands as effective BFCs for 64Cu, it is evident that the in vivo instability of these 64Cu complexes emphasizes the need for more inert 64Cu chelators. With this goal, new ligand systems, including those based upon the cross-bridged (CB) tetraazamacrocycles have been developed to complex 64Cu more stably.
The Cross-bridged Tetraamine Ligands
This class of chelators was first conceived of and synthesized by Weisman, Wong and coworkers in the 1990's,18,19 and they were originally designed to complex metal cations like Li+, Cu2+, and Zn2+ within their clamshell-like clefts. Numerous copper complexes of these and related ligands have since been prepared and studied by the Wong and Weisman labs as well as other research groups.20-22 With the available structural data, the expected cis-folded coordination geometry of these chelators has been confirmed in all cases. Attachment of two carboxymethyl pendant arms to CB-cyclam to give CB-TE2A (4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane) further ensures complete envelopment of a six-coordinate Cu(II) as shown in Figure 2.
While the measurement of stability constants of Cu(II)-CB complexes has been limited by the proton-sponge nature of these chelators, available data for Cu(II)-CB-cyclam (log Kf = 27.1) revealed very similar values to non-bridged Cu(II)-cyclam (log Kf = 27.2) and related complexes.23 On the other hand, their kinetic inertness, especially in aqueous solution, has been shown to be truly exceptional.8,24 Proton-assisted decomplexation is one indicator of solution inertness. Under pseudo-first order conditions of high acid concentration (e.g. 5 M HCl), decomplexation half-lives can provide a comparative gauge. Remarkable resistance of Cu-CB complexes toward such processes has recently been demonstrated.8,24 As shown in Table 1, Cu-CB-cyclam is almost an order of magnitude more inert than Cu (II)-cyclam in 5M HCl at 90°C, while Cu(II)-CB-TE2A is 4 orders of magnitude more inert. Impressively, the latter complex resists acid decomplexation even better than the fully-encapsulated sarcophagine complex Cu(II)-diamsar (3,6,10,13,16,19-hexaazabicyclo[6.6.6]eicosane-1,8-diamine). It was confirmed that both the cross-bridged cyclam backbone as well as presence of two enveloping carboxymethyl arms are required for this unusual kinetic inertness.
Table 1.
Pseudo-first order half-lives for acid-decomplexation and reduction potentials of Cu(II) complexes (all values are from Heroux et al.24 unless otherwise noted).
| Chelator | 5 M HCl 90°C | 12 M HCl 90°C | Ered (V) vs Ag/AgCl |
|---|---|---|---|
| DOTA | <3 min | <3 min | −0.94 (irrev.)a |
| cyclam | <3 min | <3 min | −0.68 (quasi-rev) |
| TETA | 4.5(5) min | <3 min | −1.18 (irrev.) |
| CB-cyclam | 11.7(1) min | <3 min | −0.52 (quasi-rev) |
| CB-TE2A | 154(6) h | 1.6(2) h | −1.08 (quasi-rev.) |
| CB-DO2A | < 3 min | n.d. | −0.92 (irrev.) |
| CB-TEAMA | n.d. | n.d. | −0.96 (quasi-rev.) |
| diamsar | 40(1) h | < 3 min | −1.1 (irrev) |
reference 7
Biological stability of 64Cu-labeled cross-bridged complexes, including CB-cyclam, 64CuCB-TE2A and CB-DO2A (10-bis(carboxymethyl)-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane), has been investigated.7,23 The biodistribution of these 64Cu complexes in female Sprague Dawley rats were highly dependent upon the chelator. Based on the rapid clearance from blood, liver and kidney, 64Cu-CB-TE2A was thought to be the most stable (Figure 3).23 Follow-up metabolism studies of 64Cu-CB-TE2A and 64Cu-CB-DO2A compared to 64Cu-DOTA and 64Cu-TETA demonstrated the robust stability of 64Cu-CB-TE2A in vivo, with low amounts of transchelation to liver and blood proteins.7
Figure 3.
Biodistribution data of selected 64Cu-labeled cyclam and bridged cyclam analogs at 24 h p.i. (post-injection) in normal rats. Adapted from references.7,23,45
Selection of Biological Targets of Disease for Molecular Imaging
The selection of right target/biomarker has been greatly enhanced by the improved fundamental understanding of various aspects of cellular functioning. The target could be a surface protein/integrin or an intracellular component such as DNA, mRNA or cytoplasmic protein (Figure 4). Surface proteins are easier to target, as the targeting ligand does not have to gain access to the inside the diseased cell. Another key factor in target selection is the number of target sites in the diseased cell. Choosing an abundant target will make imaging more feasible.
Figure 4.
Schematic of a tumor cell showing the various intracellular, cell surface and extracellular targets available for molecular imaging.
Imaging markers, such as a labeled antibody, peptide or peptidomimetic, can be used to reach the target to serve an imaging or therapeutic purpose. In the case of a surface protein target, the targeting unit should be able to bind competitively to the target with an affinity comparable to or better than the natural binding ligand, thereby inhibiting the binding of the activating (natural) ligand to the receptor. Some of the cell surface proteins that our laboratory has investigated include: epidermal growth-factor receptor (EGFR), somatostatin receptors (SSRs), and integrin alpha v beta 3 (αvβ3). Select studies on some of these targets are covered in the following sections.
Targeting Somatostatin Receptors (SSRs)
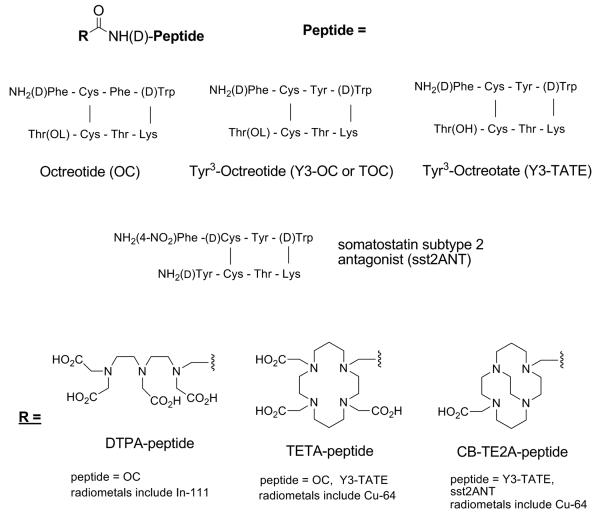
Somatostatin is a 14 amino acid peptide which is involved in the regulation and release of a number of hormones, and SSRs are present in many different normal organ systems such as the central nervous system (CNS), the gastrointestinal tract, and the exocrine and endocrine pancreas. Several human tumors of the neuroendocrine system, CNS, breast and lung are SSR positive making it a viable disease target. An eight amino acid analog of somatostatin, octreotide (OC) has a longer biological half-life and is shown to be several times more effective than somatostatin in the suppression of growth hormone secretion in animals.25 Somatostatin analogs that have been conjugated with various metal chelators and labeled with 111In or 64Cu for evaluating somatostatin receptor positive tumors in rodent models and humans are represented in Figure 5.
Figure 5.
Somatostatin analogs that have been conjugated with various metal chelators and labeled with 111In or 64Cu for evaluating somatostatin receptor positive tumors in rodent models and humans.
In one of earlier studies with SSRs, we conducted in vitro and in vivo evaluation of 64Cu-labeled OC conjugates.15 OC was conjugated with TETA for labeling with 64Cu and this agent was compared with 111In-DTPA-D-Phe1-OC (111In-DTPA-OC; Octreoscan®), a SPECT imaging agent approved for routine clinical use as a diagnostic agent for neuroendocrine cancer in the US and Europe.26 64Cu-TETA-OC was evaluated as a PET imaging agent in humans (8 subjects) and compared to 111In-DTPA-OC with gamma scintigraphy and SPECT imaging.27 64Cu-TETA-OC and PET imaged more tumors in two patients compared to 111In-DTPA-OC and SPECT, and in one patient 111In-DTPA-OC and SPECT weakly imaged a lung lesion that was not detected with 64Cu-TETA-OC. Overall, 64Cu-TETA-OC and PET showed greater sensitivity for imaging neuroendocrine tumors, in part due to the greater sensitivity of PET compared to SPECT.
In vitro and in vivo evaluation of a second generation somatostatin analog, 64Cu-TETA-Y3-TATE (Y3-TATE: tyrosine-3-octreotate) were conducted. Y3-TATE differs from OC in that tyrosine (Tyr) replaces phenylalanine (Phe) in the 3-position, and the C-terminal threonine (Thr) is an acid rather than an alcohol, and has shown improved targeting of somatostatin-rich tissues.28,29 64Cu-TETA-Y3-TATE had high binding affinity to somatostatin in receptor-positive CA20948 rat pancreatic tumor cell membranes. In vivo, in CA20948 and AR42J rat pancreatic tumor models, 64Cu-TETA-Y3-TATE had twice as much uptake as 64Cu-TETA-OC. This new reagent demonstrated superior potential as a radiopharmaceutical for imaging and therapy of SSR-positive tissues.
From a synthetic point of view, it was a worthwhile prospect to evaluate which chemical modification in the structure led to the increased uptake in somatostatin-rich tissues in vivo: was it due to the C-terminal carboxylate or the substitution of Tyr (Y) for Phe (F) at the 3-position? These issues were addressed in a study where four 64Cu-labeled somatostatin analogues were compared in vitro and in a tumor-bearing rat model.28 The effect of single modifications to the OC peptide on target tissue uptake was investigated. In particular, two new modifications of the OC peptide were synthesized: one with the substitution of the Tyr for Phe in the 3-position (Y3-OC), and other with replacing the C-terminus from an alcohol to a carboxylic acid (TATE). Both of these peptides were conjugated with TETA for labeling with 64Cu. The in vitro and in vivo behavior of these two peptides was compared with 64Cu-TETA-OC and 64Cu-TETA-Y3-TATE. Receptor binding studies on CA20948 cell membranes showed highest affinities for TATE derivatives, 64Cu-TETA-Y3-TATE (0.308 ± 0.0375 nM) and 64Cu-TETA-TATE (0.297 ± 0.0005 nM), and lower affinities for 64Cu-TETA-OC (0.498 ± 0.039 nM) and 64Cu-TETA-Y3-OC (0.397 ± 0.0206 nM), suggesting that the C-terminal modification may contribute more to high-affinity receptor binding than the substitution at the 3-position. Similar trends were observed in cell uptake studies done with AR42J rat pancreatic tumor cells. These structure–activity relationship trends were not demonstrated in biodistributions in CA20948 tumor-bearing rats, but 64Cu-TETA-Y3-TATE exhibited tumor uptake 1.75-3.5 times higher than the other derivatives at 4 h post-injection (p.i.). These results reinforce the choice of 64Cu-TETA-Y3-TATE as the better PET imaging and targeted radiotherapy agent.
After demonstrating the superiority of CB-TE2A compared to TETA for stably chelating 64Cu in vivo,7 we set out to attach CB-TE2A to the well-characterized tumor-targeting peptide, Y3-TATE. CB-TE2A was conjugated to the somatostatin analogue Y3-TATE and directly compared to the 64Cu-TETA-Y3-TATE conjugate.30 64Cu-CB-TE2A-Y3-TATE was radiolabeled in high radiochemical purity with specific activities of 1.3-5.1 mCi/μg of peptide at 95 °C and pH 8.0.31 Biodistribution studies using AR42J tumors implanted in male Lewis rats revealed that this complex had greater affinity for somatostatin-positive tissues compared to the TETA conjugate. Accumulation of 64Cu-CB-TE2A-Y3-TATE was lower at all time points in blood and liver, and less accumulation was observed in the kidney at earlier time points when compared to 64Cu-TETA-Y3-TATE. For example, the tumor to blood ratio at 4 hours for 64Cu-CB-TE2A-Y3-TATE was 156 ± 55; for 64Cu-TETA-Y3-TATE the tumor to blood ratio was 8.2 ± 1.6 (P < 0.001). These data suggest that the 64Cu-CB-TE2A-Y3-TATE is more resistant to transchelation than the TETA analogue.
The majority of somatostatin analogs that have been evaluated for PET and SPECT imaging are somatostatin agonists, and as such, they are internalized into cells via receptor-mediated endocytosis and mimic the behavior of somatostatin itself. The pervasive belief has been that greater cellular internalization of a radiolabeled somatostatin analog in vitro is a predictor of improved tumor uptake in vivo. This has been demonstrated by the group at Rotterdam for 111In-labeled somatostatin analogs32,33 as well as by our group.28,30 In 2006, Ginj et al. showed that an 111In-labeled somatostatin receptor type 2 (SSTr2) antagonist, sst2-ANT, had improved uptake compared to 111In-DTPA-Y3-TATE34 in mice bearing SSTr2-transfected HEK cell tumors. The authors showed that sst2-ANT was not internalized in the HEK cells, and demonstrated classical antagonist behavior. We recently compared 64Cu-CBTE2A-sst2-ANT with 64Cu-CB-TE2A-Y3-TATE in AR42J tumor-bearing rats.35 In our hands, 64Cu-CB-TE2A-sst2-ANT showed low levels of internalization in AR42J cells, and similar uptake to 64Cu-CB-TE2A-Y3-TATE in vivo at early time points. An interesting characteristic of the SSTr2 antagonist is that it appears to bind to ∼15-fold higher number of receptors than the agonist (23,000 vs 1551 fmol/mg protein), but with ∼17-fold decreased affinity (26 nM vs 1.5 nM). However, 64Cu-CB-TE2A-sst2-ANT showed longer retention in the AR42J tumor, resulting in improved tumor:blood (72) and tumor:muscle (93) ratios at 24 h p.i. compared to 64Cu-CB-TE2A-Y3-TATE (tumor:blood: 20 and tumor:muscle: 45).35
Targeting Epidermal Growth Factor Receptor (EGFR)
The epidermal growth factor family of membrane receptors is one of the most relevant targets in the tyrosine kinase family.36 EGFR expression is increased in many human tumors such as breast cancer, squamous-cell carcinoma of the head and neck, and prostate cancer. Activation of EGFR contributes to several tumorigenic mechanisms, and in many tumors, EGFR expression may act as a prognostic indicator, predicting poor survival and/or more advanced disease stage.36 At present, monoclonal antibodies (mAbs), which block the binding of EGF to the extracellular ligand-binding domain of the receptor and small tyrosine kinase inhibitors, have shown promise from a therapeutic standpoint. Cetuximab (C225, Erbitux®) was the first mAb targeted against the EGFR approved by the Food and Drug administration for the treatment of patients with EGFR-expressing metastatic colorectal carcinoma. Cetuximab binds to the extracellular domain of EGFR with a KD of 1nM, similar to that of the natural ligand, EGF.37 We evaluated 64Cu-DOTA-cetuximab as a PET agent to image EGFRs in tumors.38 For the cell binding affinity evaluation, highly EGFR-expressing human epithelial carcinoma A431 and low expressing MDA-MB-435 melanoma cells were used. An equilibrium dissociation constant (KD) of 0.28 nM was obtained with the A431 cells, which was comparable with the literature reported values of unlabeled cetuximab with A431 cells.37 The in vivo evaluation of 64Cu-DOTA-cetuximab was performed in A431 and MDAMB-435 tumor-bearing mice. Both biodistribution and microPET data showed higher uptake in the EGFR positive A431 tumor than in the EGFR negative MDA-MB-435 tumor. Also, there was a reduced tumor uptake in A431 mice who received a blocking dose of unlabeled cetuximab, demonstrating the specificity of 64Cu-DOTA-cetuximab for the high-EGFR-expressing A431 tumor (Figure 6).
Figure 6.
(A) Projection microPET images of A431 tumor- bearing nude mice after 20 and 46 hours post-administration of 64Cu-DOTA-cetuximab, with and without an injected blocking dose 20 hours prior to the imaging dose (5.6 MBq, 6 g, left; 5.6 MBq, 1 mg of cetuximab, right). (B) Coronal microPET images of 64Cu-DOTA-cetuximab in A431 (epidermal growth-factor receptor [EGFR]-positive) and MDA-MB-435 (EGFR-negative) tumor-bearing mice after 19 and 48 hours postadministration of 64Cu-DOTA-cetuximab. (C) MicroPET/computed tomography co-registration images of 64Cu-DOTA-cetuximab in a mouse bearing both A431 and MDA-MB-435 tumors (arrow) at 24 hours postinjection. Reprinted by permission of the Mary Ann Liebert, Inc., publishers from Reference 38.
The use of DOTA as the chelator with 64Cu is admittedly less than ideal. For this reason, metabolism experiments were performed to determine the extent of 64Cu transchelation to blood, liver, and tumor proteins in A431 tumor-bearing mice. The results showed minimal metabolism of 64Cu-DOTA-cetuximab in the blood out to 24 hours p.i. Liver metabolism studies demonstrated the transchelation of 64Cu to three proteins, two of which were shown by size-exclusion chromatography to be superoxide dismutase and metallothionein. The third metabolite was believed to be a protein aggregate. We are currently working on the development of CB macrocycles that can be labeled at lower temperatures (<43 °C) and will be compatible with labeling of monoclonal antibodies, as we believe this will significantly improve retention of the agents in the liver, as well as possibly enhance tumor targeting.
64Cu-DOTA-cetuximab's potential to measure EGFR concentration was evaluated by PET imaging in cervical cancer tumors.39 Over-expression of EGFR has been found in over 70% of carcinomas of the cervix. In this study, 64Cu-DOTA-cetuximab was used to correlate EGFR densities on the surface of 5 different cervical cancer lines with the EGFR messenger RNA (mRNA) expression. Based on the cellular data, microPET imaging was performed on the tumor bearing mice using the highest-expressing cervical cancer cell line, CaSki. For the in vitro analysis, five cervical cancer cell lines were selected after a screen of twenty-three human cervical cancer lines based on their level of EGFR gene expression by gene expression microarray analysis. The five human tumor cell lines ranged in EGFR expression with the following order: CaSki (high), ME-180 and DcTc2 4510 (both midrange), HeLa (low), and C-33A (negative). The cell-surface EGFR expression was evaluated by doing saturation binding assays at 4 °C, and the results paralleled the levels of EGFR expression determined by microarray analysis. In vivo, the biodistribution and small-animal PET studies with 64Cu-DOTA-cetuximab in CaSki human tumor-bearing nude mice showed relatively high tumor uptake at 24 h after injection (13.2% of injected activity per gram), with significant retention of radioactivity in blood and liver as well. Overall, the study evaluated 64Cu-DOTA-cetuximab as a biomarker of EGFR expression levels, and a potential PET agent for patient selection and therapeutic monitoring.
Targeting integrin Alpha v Beta 3 (αvβ3)
The integrin αvβ3 is a well known cell surface disease biomarker that is up-regulated in tumor angiogenesis, metastasis, inflammation, certain cardiovascular abnormalities, and bone resorption.40 There are many studies in the literature discussing PET imaging of αvβ3 expression in tumor angiogenesis using arginine-glycine-aspartic acid (RGD) peptides.41 As an alternative to imaging angiogenesis, we investigated imaging the up-regulation of αvβ3 in osteoclasts, which are present in high numbers in osteolytic bone metastases. Osteoclasts are known to have a high expression of αvβ3 integrin compared to other normal cell types.42 We proposed 64Cu-CB-TE2A-c(RGDyK) for detecting osteoclasts in osteolytic bone lesions.43 Selective targeting of 64Cu-CB-TE2A-c(RGDyK) was demonstrated ex vivo in cell studies using αvβ5-positive bone marrow macrophages (BMM) and αvβ3-positive osteoclasts, with a maximal 2.6-fold increase in osteoclast uptake, compared with BMM.
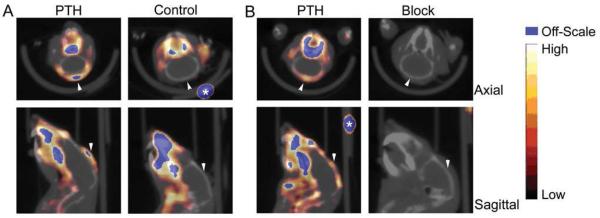
Since αvβ3 is also expressed on many tumor cells, the osteoclast targeting by 64Cu-CB-TE2A-c(RGDyK) was validated in vivo in the absence of tumor cells by using a model of pharmacologically induced osteolysis. Parathyroid hormone (PTH) induces osteoclast-mediated osteolysis when serially injected subcutaneously at the calvarium.44 This pharmacologic model of PTH-induced osteolysis allowed investigation of osteoclast-mediated bone uptake independent of tumor cells. Increased uptake at the site of induced osteolysis was clearly visible on small animal PET as shown in Figure 7. In addition, the biodistribution studies demonstrated a significant increase in calvarium uptake in PTH-treated mice relative to controls. These results suggested the application of 64Cu-CB-TE2Ac(RGDyK) for imaging increased numbers of osteoclasts in osteolytic bone diseases. Efforts are ongoing in our lab to design improved targeting agents, both peptide-based and macromolecular, that target αvβ3 for imaging tumor angiogenesis as well as increased numbers of osteoclasts in diseases that include osteolytic bone metastases.
Figure 7.
Small-animal PET/CT of PTH-treated mice. Calvarium uptake of 64Cu-CB-TE2A-c(RGDyK) was higher in PTH-treated mice (7.4 MBq [199 μCi],115 ng, SUV = 0.53) than in control mice (7.7 MBq [209 μCi], 121 ng, SUV = 0.22) (A). In PTH-treated mice, uptake was reduced in all tissues, including calvarium, after injection of c(RGDyK) (PTH [left]: 159 μCi, 84 ng, SUV = 0.33; block [right]: 164 μCi, 87 ng, SUV = 0.18) (B). Arrowheads indicate calvarium of each animal. Fiducials (*) are indicated. Reprinted by permission of the Society of Nuclear Medicine from Reference 43.
Conclusions
Copper-64 based radiopharmaceuticals have significant potential as oncological PET imaging agents. The longer half-life and ease of production of 64Cu by both reactor and accelerator based methods gives it a logistical and economical advantage over many positron emitting radionuclides. The development of new BFC systems that form stable complexes with high kinetic inertness to Cu(II) decomplexation has proven valuable for producing favorable in vivo pharmacokinetic profile of peptide conjugates as demonstrated by the robust stability of 64Cu-CB-TE2A over traditionally used chelators such as DOTA and TETA. A better understanding of molecular markers has resulted in targeted imaging of tumors. The progress made at the fundamental level by using small animal PET systems will encourage the translation of 64Cu-based imaging radiopharmaceuticals into humans.
Acknowledgements
The authors gratefully acknowledge NIH grants R01 CA64475, R01 CA093375, R21 CA098698 and U01 HL080729-01.
Biographies
Monica Shokeen is an Instructor in Radiology at Washington University School of Medicine. She received a B.S. (Honors) degree in Chemistry from the University of Delhi-New Delhi, India in 1997, a M.B.A. degree from the Kurukshetra University, India in 1999, and a Ph.D. in Inorganic Chemistry in 2006 from Washington University in St. Louis. Dr. Shokeen's research involves evaluation of small molecule and macromolecular agents for molecular imaging and therapy of cardiovascular disease and cancer. She is also involved in imaging sciences based curriculum design and teaching.
Carolyn J. Anderson is a Professor of Radiology, Biochemistry & Molecular Biophysics and Chemistry at Washington University in St. Louis. She received a B.S. degree in Chemistry from the University of Wisconsin-Superior in 1985 and a Ph.D. in Inorganic Chemistry from Florida State University in 1990. From 1990-1992 she was a postdoctoral research associate at the Mallinckrodt Institute of Radiology. In 1993 she was promoted to Assistant Professor of Radiology, and in 2007 she became full Professor of Radiology and Chemistry, followed by a joint appointment in Biochemistry in 2008. Prof. Anderson's research interests include the chelation chemistry of metal radionuclides and their attachment to tumor-targeting biomolecules for PET imaging of cancer and cancer metastasis.
References
- 1.Linder MC. Biochemistry of Copper. Vol. 10. Plenum Press; New York: 1991. [Google Scholar]
- 2.Frieden E. Perspectives on copper biochemistry. Clin Physiol Biochem. 1986;4:11–9. [PubMed] [Google Scholar]
- 3.Zinn KR, Chaudhuri TR, Cheng TP, Morris JS, Meyer WA. Production of No-Carrier-Added Cu-64 from Zinc Metal Irradiated under Boron Shielding. Cancer. 1994;73:774–778. doi: 10.1002/1097-0142(19940201)73:3+<774::aid-cncr2820731305>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Szelecsenyi F, Blessing G, Qaim SM. Excitation function of proton induced nuclear reactions on enriched 61Ni and 64Ni: possibility of production of no-carrier-added 61Cu and 64Cu at a small cyclotron. Appl Radiat Isot. 1993;44:575–580. [Google Scholar]
- 5.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 6.Cole WC, DeNardo SJ, Meares CF, McCall MJ, DeNardo GL, Epstein AL, O'Brien HA, Moi MK. Serum Stability of 67Cu Chelates: Comparison with 111In and 57Co. Nucl Med Biol. 1986;13:363–368. doi: 10.1016/0883-2897(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 7.Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–74. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 8.Woodin KS, Heroux KJ, Boswell CA, Wong EH, Weisman GR, Niu W, Tomellini SA, Anderson CJ, Zakharov LN, Rheingold AL. Kinetic inertness and electrochemical behavior of Cu(II) tetraazamacrocyclic complexes: possible implications for in vivo stability. Eur J Inorg Chem. 2005;23:4829–4833. [Google Scholar]
- 9.Busch DH. The Compleat Coordination Chemistry. One Practitioner's Perspective. Chem Rev. 1993;93:847–860. [Google Scholar]
- 10.Riesen A, Zehnder M, Kaden TA. Structure of the barium salt of a Cu2+ complex with a tetraaza macrocyclic tetraacetate. Acta Crystallogr. 1988;C44:1740–1742. [Google Scholar]
- 11.Wu Y, Zhang X, Xiong Z, Chen Z, Fisher DR, Liu S, Gambhir SS, Chen X. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 12.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of alpha-MSH. J Med Chem. 2005;48:2985–92. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, Bading JR, Laug WE, Conti PS. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression. J Nucl Med. 2004;45:1776–83. [PubMed] [Google Scholar]
- 14.Moi MK, Meares CF, McCall MJ, Cole WC, DeNardo SJ. Copper chelates as probes of biological systems: stable copper complexes with a macrocyclic bifunctional chelating agent. Anal Biochem. 1985;148:249–53. doi: 10.1016/0003-2697(85)90653-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson CJ, Pajeau TS, Edwards WB, Sherman EL, Rogers BE, Welch MJ. In vitro and in vivo evaluation of copper-64-octreotide conjugates. J Nucl Med. 1995;36:2315–25. [PubMed] [Google Scholar]
- 16.Anderson CJ, Jones LA, Bass LA, Sherman EL, McCarthy DW, Cutler PD, Lanahan MV, Cristel ME, Lewis JS, Schwarz SW. Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor-bearing rats. J Nucl Med. 1998;39:1944–51. [PubMed] [Google Scholar]
- 17.Bass LA, Wang M, Welch MJ, Anderson CJ. In vivo Transchelation of Copper-64 from TETA-octreotide to Superoxide Dismutase in Rat Liver. Bioconjug Chem. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 18.Weisman GR, Rogers ME, Wong EH, Jasinski JP, Paight ES. Cross-Bridged Cyclam. Protonation and Li+ Complexation in a Diamond-Lattice Cleft. J Am Chem Soc. 1990;112:8604–8605. [Google Scholar]
- 19.Weisman GR, Wong EH, Hill DC, Rogers ME, Reed DP, Calabrese JC. Synthesis and transition-metal complexes of new cross-bridged tetraamine ligands. Chem Commun (Camb) 1996:947–948. [Google Scholar]
- 20.Hubin TJ, McCormick JM, Collinson SR, Alcock NW, Busch DH. Ultra rigid cross-bridged tetraazamacrocycles as ligands - the challenge and the solution. Chem Commun (Camb) 1998:1675–1676. [Google Scholar]
- 21.Hubin TJ, Alcock NW, Busch DH. The square-pyramidal PdII complex of a cross-bridged tetraazamacrocycle. Acta Crystallogr. 1999;C55:1404–1406. [Google Scholar]
- 22.Wong EH, Weisman GR, Hill DC, Reed DP, Rogers ME, Condon JP, Fagan MA, Calabrese JC, Lam K-C, Guzei IA, Rheingold AL. Synthesis and Characterization of Cross-Bridged Cyclams and Pendant-Armed Derivatives and Structural Studies of their Copper(II) Complexes. J Am Chem Soc. 2000;122:10561–10572. [Google Scholar]
- 23.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J Med Chem. 2002;45:469–477. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 24.Heroux KJ, Woodin KS, Tranchemontagne DJ, Widger PC, Southwick E, Wong EH, Weisman GR, Tomellini SA, Wadas TJ, Anderson CJ, Kassel S, Golen JA, Rheingold AL. The long and short of it: the influence of Ncarboxyethyl versusN-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles. Dalton Trans. 2007:2150–62. doi: 10.1039/b702938a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless J. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31:1133–40. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 26.Krenning EP, Bakker WH, Kooij PP, Breeman WA, Oei HY, de Jong M, Reubi JC, Visser TJ, Bruns C, Kwekkeboom DJ, et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med. 1992;33:652–8. [PubMed] [Google Scholar]
- 27.Anderson CJ, Dehdashti F, Cutler PD, Schwarz SW, Laforest R, Bass LA, Lewis JS, McCarthy DW. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–21. [PubMed] [Google Scholar]
- 28.Lewis JS, Lewis MR, Srinivasan A, Schmidt MA, Wang J, Anderson CJ. Comparison of four 64Cu-labeled somatostatin analogues in vitro and in a tumor-bearing rat model: evaluation of new derivatives for positron emission tomography imaging and targeted radiotherapy. J Med Chem. 1999;42:1341–7. doi: 10.1021/jm980602h. [DOI] [PubMed] [Google Scholar]
- 29.de Jong M, Breeman WA, Bakker WH, Kooij PP, Bernard BF, Hofland LJ, Visser TJ, Srinivasan A, Schmidt MA, Erion JL, Bugaj JE, Macke HR, Krenning EP. Comparison of (111)In-labeled somatostatin analogues for tumor scintigraphy and radionuclide therapy. Cancer Res. 1998;58:437–41. [PubMed] [Google Scholar]
- 30.Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, Anderson CJ. Preparation and biological evaluation of copper-64-labeled tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res. 2004;10:8674–82. doi: 10.1158/1078-0432.CCR-04-1084. [DOI] [PubMed] [Google Scholar]
- 31.Wadas TJ, Anderson CJ. Radiolabeling of TETA- and CB-TE2A-conjugated peptides with copper-64. Nat Protoc. 2006;1:3062–8. doi: 10.1038/nprot.2006.431. [DOI] [PubMed] [Google Scholar]
- 32.de Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, Visser TJ, Jermann E, Behe M, Powell P, Macke HR. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0,d-Phe1,Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med. 1997;24:368–71. doi: 10.1007/BF00881807. [DOI] [PubMed] [Google Scholar]
- 33.de Jong M, Bernard BF, de Bruin E, van Gameren A, Bakker WH, Visser TJ, Maecke HR, Krenning EP. Internalization of radiolabelled [DTPA0]octreotide and [DOTA0, Tyr3]octreotide: peptides for somatostatin receptor-targeted scintigraphy and radionuclide therapy. Nucl Med Comm. 1998;19:283–288. doi: 10.1097/00006231-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Macke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–41. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadas TJ, Eiblmaier M, Zheleznyak A, Sherman CD, Ferdani R, Liang K, Achilefu S, Anderson CJ. Preparation and Biological Evaluation of 64Cu-CB-TE2A-sst2-ANT, a Somatostatin Antagonist for PET Imaging of Somatostatin Receptor-Positive Tumors. J Nucl Med. 2008;49:1819–27. doi: 10.2967/jnumed.108.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laskin JJ, Sandler AB. Epidermal growth factor receptors: a promising target in solid tumors. Cancer Treat Rev. 2004;30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Fan Z, Masui H, Altas I, Mendelsohn J. Blockage of epidermal growth factor receptor function by bivalent and monovalent fragments of C225 anti-epidermal growth factor receptor monoclonal antiobodies. Cancer Res. 1993;53:4322–4328. [PubMed] [Google Scholar]
- 38.Li WP, Meyer LA, Capretto DA, Sherman CD, Anderson CJ. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother Radiopharm. 2008;23:158–71. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- 39.Eiblmaier M, Meyer LA, Watson MA, Fracasso PM, Pike LJ, Anderson CJ. Correlating EGFR expression with receptor-binding properties and internalization of 64Cu-DOTA-cetuximab in 5 cervical cancer cell lines. J Nucl Med. 2008;49:1472–9. doi: 10.2967/jnumed.108.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 41.Haubner R, Decristoforo C. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front Biosci. 2009;14:872–86. doi: 10.2741/3283. [DOI] [PubMed] [Google Scholar]
- 42.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–5. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 43.Sprague JE, Kitaura H, Zou W, Ye Y, Achilefu S, Weilbaecher KN, Teitelbaum SL, Anderson CJ. Noninvasive imaging of osteoclasts in parathyroid hormone-induced osteolysis using a 64Cu-labeled RGD peptide. J Nucl Med. 2007;48:311–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Yates AJ, Gutierrez GE, Smolens P, Travis PS, Katz MS, Aufdemorte TB, Boyce BF, Hymer TK, Poser JW, Mundy GR. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J Clin Invest. 1988;81:932–8. doi: 10.1172/JCI113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones-Wilson TM, Deal KA, Anderson CJ, McCarthy DW, Kovacs Z, Motekaitis RJ, Sherry AD, Martell AE, Welch MJ. The in vivo Behavior of Copper-64-Labeled Azamacrocyclic Compounds. Nucl Med Biol. 1998;25:523–530. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]