Abstract
Objective
A monkey model of the menopausal transition (perimenopause) would facilitate efforts to understand better the effect of hormonal fluctuations during this life phase on the initiation of chronic diseases associated with the postmenopausal years. Antimüllerian hormone (AMH) is a promising marker of ovarian reserve (primordial follicle number) in women. Here we describe the relationship between AMH and ovarian reserve in cynomolgus monkeys (Macaca fascicularis) estimated to be 12-15 years of age (∼36-45 years in women).
Design and Results
The results of daily vaginal swabbing (to detect menses) and thrice weekly blood sampling for 12 weeks indicate that AMH is relatively stable across the menstrual cycle (intraclass correlation ∼0.80), with a slight, though significant (p < 0.02), reduction (∼1.4 fold) on days 2-5 post-ovulation. Substantial inter-individual variation in AMH concentrations were observed between monkeys, with values ranging from 4.46±0.17 to 18.80±0.71ng/ml (±SE). AMH concentrations were reduced ∼63% after the removal of one ovary (7.6±0.77 vs. 2.75±0.37ng/ml, p<0.001, n=19), and were below the level of detection following removal of both ovaries (5.8±0.42ng/ml to <0.05ng/ml, p<0.001, n=84), suggesting that the ovary is likely either the major or the sole source of AMH in the monkey. Finally, we examined the association between AMH and primordial, primary and secondary follicles in 29 monkeys, and found significant associations with all follicle types (r=0.78, r=0.66, r=0.80, p<0.01 respectively).
Conclusion
The relationship between AMH and ovarian reserve in the monkey is similar to that of women, suggesting that monkeys may be a useful model for studying hormonal fluctuations across the menopausal transition.
Keywords: AMH, ovarian reserve, monkey
Introduction
Nonhuman primates, especially macaque monkeys, have long served as a model for understanding both the reproductive biology of women and the health of women in relation to reproductive stage and hormone exposure. Because natural menopause occurs in numerous monkey species (including rhesus and cynomolgus macaques [Macaca mulatta and M. fascicularis]) and results in hormonal profiles resembling those of women, it seems reasonable to extend biomedical investigations in monkeys to the perimenopausal transition and menopause.1,2 However, studies focused on the hormonal disruption and presumed increased vulnerability to chronic disease characteristic of the perimenopausal transition and early menopause have heretofore been limited by the inability to assess ovarian reserve noninvasively and thereby identify potential subjects for further investigation.3
Serum antimüllerian hormone (AMH) is increasingly prominent as a noninvasive marker of ovarian reserve in women and might by extension be used similarly in monkeys. AMH is produced by granulosa cells of small growing (preantral and small antral) follicles in the ovary of rodents, nonhuman and human primates.4-7 Women experience declining AMH values as they transition to menopause, suggesting that AMH is a reliable marker of ovarian reserve (primordial follicle number) and that it might eventually be used to predict the onset of menopause.8-12 Finally, AMH concentrations fall to undetectable levels in naturally and surgically postmenopausal women.13,14 In this report we describe for the first time the relationship among AMH concentrations, menstrual cycle stage, and follicle numbers in cynomolgus monkeys.
Materials and Methods
Animals
Three studies are described, all of which made use of animals assigned to the control groups of ongoing studies: 1) Characterization of AMH across the menstrual cycle. Used here were 12 Indonesian origin female monkeys, average age ∼12-15 years (determined by radiographic evidence of complete epiphyseal closure at the distal radius, ulna and the proximal tibia), which equates to ∼36-45 years in women. Monkeys were housed in groups of four and underwent daily vaginal swabbing for 12 weeks to detect menstrual bleeding. Additionally, blood samples were collected three times per week (without anesthesia) for measurement of AMH, estradiol (E2), progesterone (P4) and follicle stimulating hormone (FSH); 2) Determination of the source of AMH production in the monkey. Here, AMH was measured in Chinese origin monkeys (∼7yrs of age, equivalent to 21 years in women) before and after unilateral ovariectomy (n=19) and in Indonesian origin monkeys (12-15 years of age) before and after complete ovariectomy (n=84); and 3) Determination of the association between AMH and follicle number. Indonesian monkeys age 12-15 years (n=29) that had been consuming an isoflavone-free, human like diet for 3 years15 were ovariectomized and their ovaries fixed for histological evaluation. AMH was measured from samples taken at the time of ovariectomy. All animal manipulations were approved by the Wake Forest University Animal Care and Use Committee and were conducted in accordance with state and federal laws and following the guidelines of the US Department of Health and Human Services.
Hormone Assays
Serum AMH was measured using ELISA (DSL, Texas) and the coefficients of variation (CV) were: Intra-assay CV: 4.53% at 13.62ng/ml; interassay CV: 19.75% at 0.23ng/ml, 11.42% at 9.61ng/ml, and 11.27% at1.89ng/ml. It should be noted that two commercial assays are available to measure AMH, and a recent study that compared them in women found that AMH concentration was ∼4.6 fold lower in samples assayed with the DSL ELISA than in those measured with the ultra sensitive Immunotech-Coulter assay (Marseilles, France).16 Estradiol17 and progesterone18 were measured using modifications of commercially available kits from Diagnostic Products Corporation (Los Angeles CA). Intra-assay and interassay CVs were <4% and 10%, respectively. FSH was measured using radioimmunoassay using macaque primary antibodies (Wisconsin National Primate Research Center) and intra-assay CV was 3.49% and the inter-assay CV was 9.33%.
Ovarian Follicle Counts
Both ovaries were removed, trimmed of fat, weighed and fixed in Bouin’s solution (75ml picric acid solution (1.3%), 25ml of formaldehyde (37%), and 5ml glacial acetic acid) for 24 hours and then transferred to 70% ethanol. One ovary from each monkey was transferred to the University of Arizona for follicle counting (PBH, PJC) using methods published previously.19 Briefly, the entire ovary was sectioned serially (4 to5μm) and stained with hematoxylin and eosin. Follicles were classified as primordial (oocytes surrounded by a single layer of flattened granulosa cells), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), and secondary (oocytes surrounded by two or more layers of cuboidal granulosa cells) and counted in every 100th section to avoid double counting of small preantral follicles. Only follicles with an oocyte nucleus were counted. Data are recorded as total follicles counted per ovary.
Statistical Analysis
In Experiment 1, we employed a general linear mixed effect model to assess the concentration of AMH across the menstrual cycles. Least square means were reported graphically for the different time points. Tukey’s pairwise comparison procedure was used to identify where the significant differences exist across the menstrual cycles and the intraclass correlation was reported. In Experiment 2, AMH concentration before and after removal of ovaries was reported as means ± SE. Paired t-tests were used to determine significance of differences in mean AMH prior to and following the removal of ovaries. In Experiment 3, we used Pearson correlation coefficients and linear regression models to examine the associations between follicle types and AMH. The outcome variables (the primordial, primary, and secondary follicle counts) were square root transformed to achieve better normality and equality of variance assumptions. The regression coefficients and the corresponding 95% confidence intervals are reported. All analyses were performed using SAS v9.1.3 (SAS Institute, Cary, NC). Significance was assigned at p<0.05.
Results
AMH across the menstrual cycle
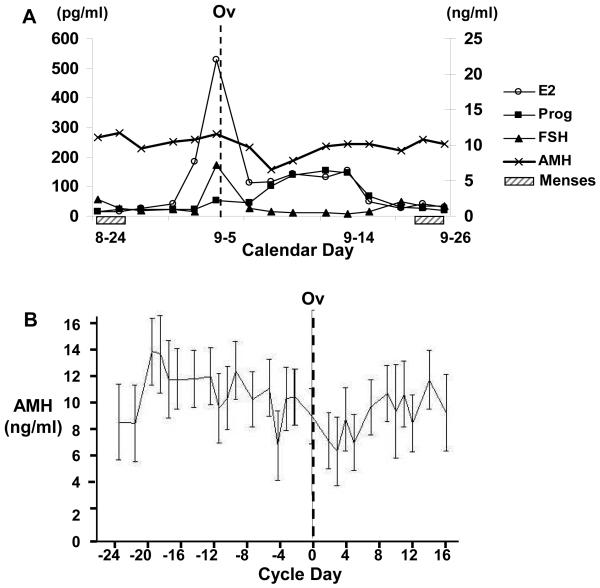
As shown in Figure 1A, AMH is relatively stable across the menstrual cycle (intra-class correlation is ∼0.8) of cynomolgus monkeys, especially in comparison to the large variation apparent in E2, P4 and FSH concentrations. Nonetheless, analysis of AMH concentration across 18 menstrual cycles (from 12 monkeys), revealed that there is slight (∼1.4 fold) though significant variation, with the lowest concentrations of AMH occurring 2-5 days after ovulation (p<0.02 for difference in AMH concentration between days -19 and days 2 and 5, relative to ovulation) (Figure 1B). In addition, there was substantial inter-individual variation in AMH among the 12 monkeys studied, with the mean AMH per monkey (across a mean of 1.75 cycles) ranging from 4.46±0.17 to 18.80±0.71 ng/ml. Among the 12 monkeys, mean estradiol concentrations ranged from 2.86-475 pg/ml (mid cycle peak), progesterone ranged from 0.54-11.58 ng/ml and FSH from 0.17-4.56 ng/ml.
Figure1.
Panel A. Representative graph of serum estradiol (E2, Left axis), antimüllerian hormone (AMH), progesterone (P4) and follicle stimulating hormone (FSH) concentrations (Right axis) across an ovulatory menstrual cycle of a cynomolgus monkey. Monkeys were swabbed daily to detect menses (dashed box) and blood was collected 3 times/wk (M,W,F) for 12 weeks for AMH measurement. Panel B. Concentration of AMH prior to and following ovulation (ov) in twelve cynomolgus monkeys of estimated age 12-15 years. A total of 18 ovulatory cycles were included in the analysis and data are shown as mean ± SE for each cycle day relative to the day of ovulation (determined by FSH peak).
Source of AMH
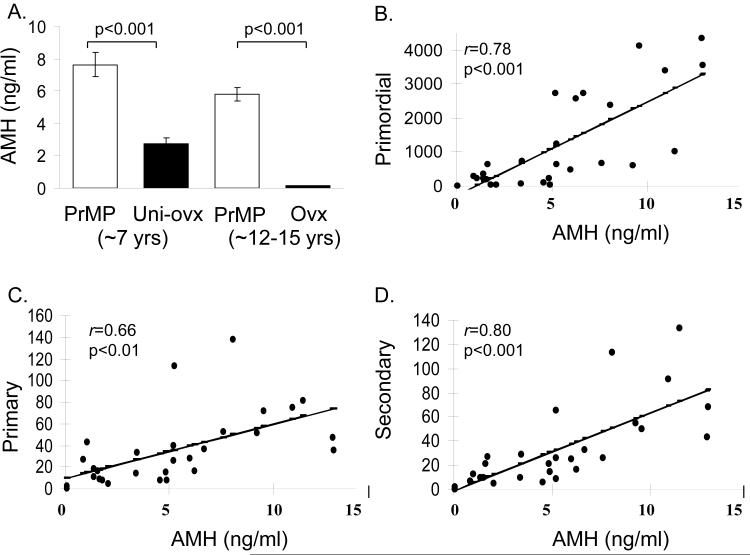
The source of AMH production in premenopausal monkeys was determined by comparing mean AMH concentrations in premenopausal monkeys (age 7 years) prior to and following removal of one ovary (7.6±0.77 vs. 2.75±0.37ng/ml, p<0.001) and premenopausal monkeys (age 12-15 years) prior to and following removal of both ovaries (5.8±0.42 ng/ml to <0.05ng/ml, p<0.001). Figure 2A shows that removal of one ovary results in a ∼63% reduction in AMH, while AMH was undetectable following bilateral ovariectomy.
Figure 2.
Panel A. Antimüllerian hormone (AMH) concentrations in cynomolgus monkeys ∼7 years of age while premenopausal (PrMP, n=19) and after removal of one ovary (Uni-Ovx, n=19); and cynomolgus monkeys ∼12-15 years of age while premenopausal (PrMP, n=84) and after complete ovariectomy (OVX, n=84). AMH was below the level of detection of the assay in OVX monkeys. Data are expressed as mean±SE. Panels B-D. Association between serum antimüllerian hormone (AMH) concentrations and primordial (B), primary (C) and secondary (D) follicles in cynomolgus monkeys age 12-15 years (n=29). Pearson correlation coefficients (r) are reported for each association.
AMH and follicle number
The relationship between AMH and primordial, primary and secondary follicles in the monkey ovary was examined. Significant correlations were seen between AMH and primordial (r=0.78, p<0.001), primary (r=0.66, p=0.001) and secondary follicles (r=0.80, p<0.001) (Figure 2 B, C, D). The regression (beta) coefficients for the association between primordial, primary and secondary follicles and AMH were also significant (p<0.001) and were as follows respectively: 4.16 (CI -2.85-5.48), 0.47 (CI-0.25-0.68) and 0.57 (CI-0.39-0.74).
Discussion
The data presented here suggest that, in monkeys, AMH is relatively stable across the menstrual cycle; the ovary is most likely the main source of AMH; and the relationship between AMH and ovarian reserve is similar to the positive association observed in women.
Analysis of 18 ovulatory cycles indicated that AMH was relatively stable across the menstrual cycle in monkeys, especially in comparison to the large (up to 50 fold) variation apparent in E2, P4 and FSH concentrations. Slight but significant intracycle variation (∼1.4 fold) was observed, however, with the lowest values occurring in the early luteal phase. Several studies of women (age 24-38) have reported that cyclic variations in AMH do not occur,20-23 although intracycle AMH variations of 14-17% were observed, presumably due to continuous, non-cyclic growth of follicles. In contrast, a peri-ovulatory rise in AMH has been reported in two studies.24,25 In one of those studies, young women (age=23 years, n=36) were sampled intensively across the menstrual cycle and a 25% intracycle variability in AMH was observed, with maximal concentrations in the late follicular phase and declines in the early luteal phase.25 It is important to note that, while significant statistically, the absolute changes in AMH were small. Furthermore, although our data in monkeys, and studies of women, suggest there may be some variation in AMH across the menstrual cycle, this should be interpreted within the context of the extremely large cyclic variations in E2 and FSH. Thus, for purposes of determination of ovarian reserve in middle aged women, and monkeys, AMH can be sampled without reference to cycle day.
Our finding that AMH concentrations are reduced by just over 50% following unilateral ovariectomy and further reduced below the level of detection following removal of both ovaries suggests that, as among women, the ovary is most likely either the major or the sole source of AMH in the monkey. This finding is supported by studies in women showing that AMH is not detectable in both naturally and surgically postmenopausal women.13,14
In women, the reproductive events leading to the perimenopausal transition and culminating in menopause are the direct consequence of declining numbers of ovarian primordial follicles.26 The age at which follicular depletion occurs can vary from ∼40 to 60 years and presumably reflects the variation in rate of follicle loss.27 Similarly, monkeys experience a decline in primordial follicle counts with age and are essentially devoid of follicles at menopause.28 Our data suggest that, as in women, AMH is highly correlated with follicle number in monkeys, indicating that AMH could be used to identify monkeys with low ovarian reserve. In support of our findings in cynomolgus monkeys is a recent study of rhesus macaques, in which older monkeys (∼24yr) that were still cycling regularly could be classified as perimenopausal according to their low AMH and high FSH concentrations.29 Follicle counts were not reported in that study, but our data would suggest that those monkeys had decreased numbers of primordial, primary and secondary follicles and would soon become menopausal.
Finally, we report significant interindividual variation in AMH in monkeys and this is similar to what has been reported in women.20-25 Our data relating AMH to follicle number suggest that this variation is due to variation in follicle number among the monkeys, which in turn may be related to the variation in age. However, since the age of the monkeys is estimated using dentition and physeal closure we cannot test this hypothesis using statistical means, and future investigations of AMH concentrations across a wider age range than was available in this opportunistic assessment are needed.
Conclusion
The relationship between AMH and ovarian reserve in monkeys is nearly identical to that of women. This finding, along with the many other reproductive similarities between monkeys and women (similar menstrual cycle patterns and hormonal fluctuations, endometrial sloughing etc.) make this species (and likely its close relatives) a potentially useful model for studying the effect of hormonal fluctuations across the menopausal transition on disease processes later in life.
Acknowledgments
This work was supported in part by NIH grants R01 HL079421 (JRK) from the National Heart, Lung, and Blood Institute and R24 RR022191 (JRK) from the National Center for Research Resources. This work was also supported by grant R01 AG 021948 from the National Institute of Aging (PBH) and Center Grant ES06694 (PBH).
Abbreviations
- AMH
antimüllerian hormone
- E2
estradiol
- P4
Progesterone
- FSH
follicle stimulating hormone
Footnotes
The authors have no financial disclosures to report.
Contributor Information
Susan E. Appt, Wake Forest University Primate Center and Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC.
Thomas B. Clarkson, Wake Forest University Primate Center and Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC.
Haiying Chen, Wake Forest University Public Health Sciences, Section on Biostatistics, Winston-Salem, NC.
Michael R. Adams, Wake Forest University Primate Center and Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC.
Patricia J. Christian, The University of Arizona, Department of Physiology, Tucson, AZ.
Patricia B. Hoyer, The University of Arizona, Department of Physiology, Tucson, AZ.
Mark E. Wilson, Yerkes National Primate Research Center, Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA.
Jay R. Kaplan, Wake Forest University Primate Center and Department of Pathology/Comparative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC.
References
- 1.Kavanagh K, Williams JK, Wagner JD. Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol. 2005;34:171–177. doi: 10.1111/j.1600-0684.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- 2.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- 3.Derby CA, FitzGerald G, Lasser NL, Pasternak RC. Application of national screening criteria for blood pressure and cholesterol to perimenopausal women: prevalence of hypertension and hypercholesterolemia in the Study of Women’s Health across the Nation. Prev Cardiol. 2006;9:150–159. doi: 10.1111/j.1520-037x.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- 4.Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction. 2006;132:443–453. doi: 10.1530/rep.1.01178. [DOI] [PubMed] [Google Scholar]
- 5.Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Thomas FH, Telfer EE, Fraser HM. Expression of anti-müllerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–2281. doi: 10.1210/en.2006-1501. [DOI] [PubMed] [Google Scholar]
- 7.Sahambi SK, Visser JA, Themmen AP, Mayer LP, Devine PJ. Correlation of serum anti-Müllerian hormone with accelerated follicle loss following 4-vinylcyclohexene diepoxide-induced follicle loss in mice. Reprod Toxicol. 2008 Jul 25; doi: 10.1016/j.reprotox.2008.07.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 9.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 10.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 11.Robertson DM, Hale GE, Fraser IS, Hughes CL, Burger HG. A proposed classification system for menstrual cycles in the menopause transition based on changes in serum hormone profiles. Menopause. 2008 Sep 4; doi: 10.1097/gme.0b013e3181735687. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Marca A, De Leo V, Giulini S, et al. Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Investig. 2005;12:545–548. doi: 10.1016/j.jsgi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13:559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 15.Walker SE, Register TC, Appt SE, et al. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15(5):950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fréour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;384:174–175. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Pazol K, Kaplan JR, Abbott D, Appt SE, Wilson ME. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta. 2004;340:117–126. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin reuptake transporter promoter polymorphism (5HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- 19.Pederson T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 20.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 21.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 22.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22(7):1837–1840. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 24.Cook CL, Siow Y, Taylor S, Fallat ME. Serum müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73:859–861. doi: 10.1016/s0015-0282(99)00639-1. [DOI] [PubMed] [Google Scholar]
- 25.Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89:927–933. doi: 10.1016/j.fertnstert.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 26.Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163(12):43–48. doi: 10.1016/s0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 27.Te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 28.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75(4):539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]