Abstract
PURPOSE
Medical therapies for treatment of peripheral artery disease (PAD) are limited. Ginkgo biloba has been reported to increase maximal and pain-free walking distance among patients with (PAD); however, the evidence is inconsistent. The objective of this study was to compare the effects of 300 mg/d of Ginkgo biloba (EGb 761) vs. placebo on treadmill walking time and related cardiovascular measures among subjects with PAD.
METHODS
A double-blind, placebo-controlled, parallel design trial with a 4 month duration was employed. Subjects were 62 adults, age 70 ± 8 years (mean, SD), with claudication symptoms of PAD. The primary study outcomes were maximal and pain free walking time on a treadmill. Secondary outcomes included flow mediated vasodilation (FMVD), a measure of antioxidant status as assessed by determining antibody levels to epitopes of oxidized LDL, and questionnaires addressing walking impairment and quality of life.
RESULTS
Maximal treadmill walking time increased by 20±80s and 91±242s in the placebo and EGb 761 groups, respectively (P=0.12). Pain-free walking time increased by 15±31s and 21±43s, respectively (P=0.28). No significant differences were detected between groups for any of the secondary outcomes.
CONCLUSIONS
In older adults with PAD, Ginkgo biloba produced a modest but insignificant increase in maximal treadmill walking time and flow-mediated vasodilation. These data do not support the use of Ginkgo biloba as an effective therapy for PAD, although a longer duration of use should be considered in any future trials.
Keywords: Ginkgo biloba, peripheral artery disease, claudication, treadmill time
The dietary supplement Ginkgo biloba is among the top selling botanicals in the US and Europe.1 It is most commonly used in the treatment of various forms of dementia including Alzheimer disease and for peripheral claudication.2-4 Numerous extracts of Ginkgo biloba are commercially available and all contain 2 principal components, flavonoids and terpenes, both of which include multiple forms. A widely used and well-standardized Ginkgo biloba extract (EGb 761) contains 24% flavonoids and 6% terpenes.5 These compounds, either alone or in combination, have been reported to have effects on electrochemical, physiologic, neurologic, and vascular systems in humans and animals.2,6 In 2000, in a meta-analysis of 8 double-blind, randomized trials of ginkgo biloba, Pittler and co-authors reported a significant, although “modest”, increase in both pain-free and maximal walking distance in patients with intermittent claudication.3 Potential mechanisms of action include vasodilatory effects mediated through either endothelial or smooth muscle cell interaction,5,7 or inhibition of platelet aggregation,8-11 the latter of which has been reported to be both complex and controversial.12-15 The objective of this study was to determine the potential effect of 300 mg/d of EGb 761 vs. a placebo on “pain-free” and “maximal” treadmill walking distance among patients with PAD. An additional purpose was to seek evidence for the mechanisms of action of this compound.
METHODS
The study was a randomized, double-blind, placebo-controlled, 4-month clinical trial using a parallel design. Adults (n=62) were randomly assigned to receive either EGb 761 or placebo. The enrollment period was October 2002 to August 2004. Data were collected at baseline and 4 months later (mean ± sd of 119 ± 11 days). Randomization was performed by generating random numbers assigned to treatment and control (Excel, Microsoft Office 2000) and assigning each new participant the next number in sequence. Subjects, study staff, and laboratory technicians were blinded to treatment assignments until the conclusion of the trial.
Subjects
Subjects were recruited from the local community through advertisements in local media. Telephone screening was used to identify those who experienced leg pain during walking that was characteristic of the intermittent claudication symptoms of PAD. Medical history, medication use, and a Doppler ankle-brachial index (ABI) were obtained and treadmill testing was conducted during a screening clinic visit. A second treadmill test was conducted within a 3-week window (10 ± 8 days, mean ± sd ) for those who remained eligible. Men and non-pregnant women were invited to enroll if they were over 18 years of age, had a resting ABI < 0.90, and were able to walk on a standardized treadmill at 2 mph and 10% grade for at least 1 minute and for no more than 10 minutes, with at least a 25% drop in ABI within 1 minute after the treadmill test, and with a variability between the 2 treadmill tests of ≤ 25%.16 Given that erroneously high ABI values can be a result of calcified leg arteries that are non-compressible, especially in diabetics,17 all ABI values were allowed for diabetics in this study, as long as the other criteria were met. Exclusion criteria were major surgery or chronic disease in the last 3 months, such as aortic or lower extremity arterial surgery, myocardial infarction, active cancer, uncontrolled hypertension, or other contraindications to treadmill testing. Use of pentoxifylline, carnitine, arginine, prostacyclins, dietary antioxidant supplements (other than those in standard multivitamin/multimineral preparations), or supplement products containing ginkgo biloba were not allowed for at least 1 month prior to screening and during the study. This investigation was reviewed and approved by the Stanford University Human Subjects Committee. Subjects signed an informed consent form before enrollment and the study followed the Declaration of Helsinki guidelines.18
Intervention
Study tablets of the Ginkgo product contained 60 mg of EGb 761 standardized to 24% Ginkgo flavone glycosides and 6% terpene lactones. The EGb 761 was obtained from the Dr. Willmar Schwabe company of Karlsruhe, Germany. Both the EGb 761 and the placebo were tableted in the United States by the Nature’s Way company. EGb 761 is sold in the United States as Ginkgold® manufactured by Nature’s Way. The placebo was composed of dextrose-based tablets with similar excipients. All subjects were instructed to avoid all non-study sources of ginkgo biloba and to consume 5 study tablets daily; 3 with breakfast and 2 with dinner. Adherence to study tablet consumption was assessed by counting remaining tablets in returned study bottles.
Data collection methods
Ankle Brachial Index (ABI) was performed after subjects rested for at least 10 minutes in a supine position. Systolic blood pressure was measured using a handheld Doppler (Nicolet Vascular Elite 100; Madison, WI) with a 5-Mhz probe, in both right and left dorsalis pedis and posterior tibial, and brachial arteries, unless contraindicated. The ankle/arm ratios were calculated using the highest ankle pressure for each leg, divided by the higher of the 2 brachial pressure measurements. The leg with the lowest ABI was used to determine eligibility and for analyses.16
Treadmill testing was performed in accordance with American College of Sports Medicine guidelines19,20 to assess pain, discomfort, fatigue, cramping or tightness in legs associated with walking. A registered nurse certified in advanced cardiac life support supervised the treadmill testing. Subjects walked on a treadmill moving at 2 mph at a 10% grade, without holding onto the handrails (they were allowed to touch the handrail for very brief moments to maintain balance), until they could no longer continue.21 Subjects rated their leg pain using a scale of 0 to 4 (0=no pain, 1=onset, 2=moderate, 3=substantial, 4=maximum) in response to regular prompts ie, every 30 seconds, from the attending nurse. Treadmill testing was performed twice at the beginning and twice at the end of the study with replicate measures taken on separate days, optimally and usually within a 2-week window with a few exceptions (8.7 ± 7.9 days apart, mean ± SD). The 2 baseline and the 2 end-study walking times were averaged.
Flow mediated vasodilation (FMVD)
At baseline and end-study the FMVD of the brachial artery was measured by ultrasound (Image Point Hx, Hewlett Packard Company, Palo Alto, CA) using a 7.5MHz linear-array transducer. Briefly, ultrasound was used to assess the change in the brachial artery diameter after 5 minutes of occlusion using a pneumatic cuff. The response of the vessel wall was expressed as the percent change in diameter from pre-occlusion to 1 minute after the cuff deflation.22 Subjects were instructed to follow a nitrate-free diet for at least 24 hours preceding the assessment and were given nitrate-free water to drink during that time period.
Antibodies to epitopes of oxidized LDL
Fasting (≥12 h) blood samples were collected by venipuncture at baseline and end-study. Chemiluminescent enzyme-linked immunosorbent assay (ELISA) was used to assess different oxidized low density lipoprotein (OxLDL) markers. The amount of oxidized phospholipid (OxPL) per apolipoprotein B-100 (apoB) particle was determined using the monoclonal antibody E06, which binds to the phosphocholine head group of oxidized phospholipids but not native phospholipids. Monoclonal antibody MB47 was used to specifically bind apoB and determine total apoB particles so an equal number of particles are distributed in each well for the ELISA. Other markers of oxidized low density lipoprotein were examined using monoclonal autoantibodies IgG and IgM. Binding capability to IgG and IgM were tested with antigens representing different epitopes of OxLDL: IC of apoB-100, MDA-LDL and Cu-LDL. All data were recorded in relative light units (RLU). These assessments were intended to evaluate a possible antioxidant effect of the Ginkgo biloba extract as a plausible explanatory mechanism for the expected beneficial effect on treadmill walking time.23-25
Walking Impairment Questionnaire (WIQ)
The WIQ assesses self-reported degree of difficulty in 4 categories: 1) walking impairment severity, 2) walking distance, 3) walking speed, and 4) stair climbing. The first category is primarily descriptive; summary scores are calculated for the latter 3 categories. The questionnaire is specifically designed for use in persons with intermittent claudication26 and its reliability and validity in this population have been previously reported.27, 28
Quality of Life
Health-related quality of life was assessed using the Medical Outcomes Study short form 36-item self administered questionnaire (MOS SF-36). The SF-36 yields 8 domain scores including physical functioning (PF), role physical (RP), role emotional (RE), bodily pain (BP), general health perceptions (GH), vitality (VT), social function (SF) and mental health (MH), as well as a Physical Component Summary (PCS) and a Mental Component Summary (MCS).29 The SF-36 has been standardized, validated and used successfully in a variety of patient populations, including older adults with peripheral vascular disease.30,31
Statistical analysis
The primary hypothesis was that maximum walking time (Absolute Claudication Time, ACT) on the treadmill would increase significantly for those subjects receiving the Ginkgo vs. Placebo after 4 months. Sample size (n=30 for each group) was estimated to allow ≥80% power to detect a mean difference from control of 30% for the treatment group, assuming an estimated standard deviation of 0.35 for the natural logarithm of the ratio of ACTEnd-Study/ACTBaseline and a 2-sided α of 0.05. Statistical tests were performed using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina). Descriptive statistics using means, standard deviations, and frequencies were determined for the baseline characteristics of study subjects. Four-month changes in maximum walking time (ln ACTEnd-Study/ACTBaseline) and onset of pain (ln ICTEnd-Study/ICTBaseline; Initial Claudication Time) were compared by treatment group using an analysis of covariance model with the baseline value included as a covariate. The average of the 2 baseline measurements was used as the baseline value and the average of the two 4-month measurements was used as the final value. The primary analyses followed an intent-to-treat approach and baseline values were carried forward for any subjects with missing or incomplete end-study treadmill data. Gender and diabetic status were considered as additional covariates in the models, but their contribution did not achieve statistical significance. All statistical tests were 2-tailed, and the significance level was 0.05.
RESULTS
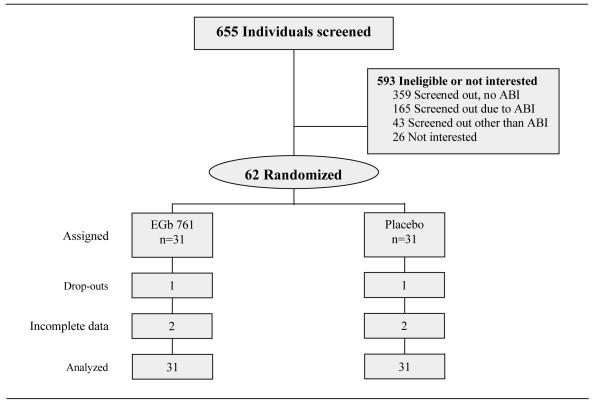
Of the 62 subjects that were randomized, 2 withdrew before study completion for personal reasons, 1 from each treatment arm (Figure 1). End-of-study data were unavailable for 4 subjects, 2 from each treatment arm, who experienced recurrent health problems that prevented them from completing the treadmill assessment eg, chest pain, shortness of breath, ST segment depression. A total of 56 subjects finished the protocol with complete data collection. Baseline demographics and characteristics of the 62 subjects are presented in Table 1.
Figure 1.
Flowchart of subject screening, enrollment, withdrawal, and number included in intention-to-treat analyses.
TABLE 1.
BASELINE DEMOGRAPHICS AND CHARACTERISTICS
| Placebo | EGb 761 | |
|---|---|---|
| (n=31) | (n=31) | |
| Age (years, mean ± SD) | 69 ± 8 | 70 ± 8 |
| Gender (men/women) | 13 / 18 | 24 / 7 |
| Ethnicity (%Caucasian) | 84% | 81% |
| Diabetic [n (%)] | 18 (58% ) | 13 (42%) |
| Current Smokers [n (%)] | 4 (12% ) | 6 (18% ) |
| Medications | ||
| Antihypertensives[n (%)] | 23 (74%) | 23 (74%) |
| Lipid lowering [n (%)] | 17 (55%) | 21 (68%) |
| Aspirin [n (%)] | 20 (65%) | 17 (55%) |
| Hypoglycemic [n (%)] | 18 (58%) | 11 (35%) |
| Clopidogrel, warfarin, or cilostazol[n (%)] | 3 (10%) | 4 (13%) |
| Baseline Treadmill Walking Times (seconds, mean ± SD) | ||
| Onset of pain | 66.7 ± 42.1 | 67.0 ± 29.7 |
| Maximal pain | 205.6 ± 126.0 |
226.6 ± 126.8 |
EGb 761 indicates Ginkgo baloba
The number of medications subjects were taking at baseline was reported to range from none to as many as 24 (5.7 ± 3.7; mean ± sd). The 4 most common classes of medications being taken by participants were antihypertensives, lipid lowering, hypoglycemic agents, and aspirin. The majority of participants were taking more than 1 of these classes of medications (Table 1). A small number of participants were taking anti-platelet or anti-coagulant medications eg, clopidogrel, warfarin, or cilostazol. Medication use did not differ by treatment group, either for the major classes of medications indicated above, or for any particular subclasses of medications eg, beta blockers, ACE inhibitors. Participants were instructed to inform the staff of any changes in medication use during their 4-month participation in the study protocol; none were reported. A total of 10 participants reported being current cigarette smokers at baseline and there were no reported changes in smoking status during the study.
Intervention adherence
As determined by tablet counting, overall adherence was 94% and 92% in placebo and Ginkgo groups, respectively.
Primary outcomes: Treadmill walking times
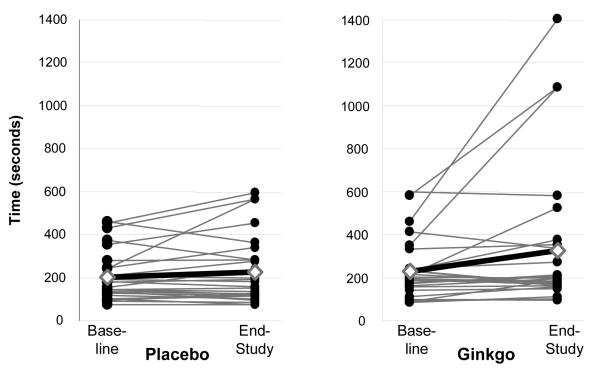
The time to onset of pain at 4 months increased slightly relative to baseline for both groups, 15 ± 31s and 21 ± 43s for the placebo and Ginkgo groups, respectively. The between group difference was not statistically significant, P=0.28. As presented in Figure 2, the average increase in walking time to maximum pain was ∼40% for the Ginkgo group relative to an average increase of ∼10% for the Placebo group. However, the group differences did not achieve statistical significance (P=0.12) (Figure 2).
Figure 2.
Maximal walking time changes for Ginkgo and placebo groups. Mean change represented by  . For the placebo group (n=31), the change after 4 months was a 20 ± 80s increase. For the Ginkgo group (n=31), the change after 4 months was a 91 ± 242s increase. The difference between groups did not achieve statistical significance P=0.12 (intention-to-treat, baseline carried forward for missing values of 3 participants in each group).
. For the placebo group (n=31), the change after 4 months was a 20 ± 80s increase. For the Ginkgo group (n=31), the change after 4 months was a 91 ± 242s increase. The difference between groups did not achieve statistical significance P=0.12 (intention-to-treat, baseline carried forward for missing values of 3 participants in each group).
Secondary and exploratory outcomes
Flow mediated vasodilation changes from baseline to end-study were assessed for both treatment and control groups by determining the pre to post occlusion percent diameter changes at both time points. The baseline to end-study changes favored the Ginkgo group in absolute change, but the difference between groups did not achieve statistical significance, P=0.24 (Table 2). Data were missing in both groups for individuals with possible Reynaud’s disease (n=2 in Ginkgo and n=3 in placebo group), or due to compromised ultrasound readings (n=3 for Ginkgo, and n=3 in placebo group).
TABLE 2.
SECONDARY AND EXPLORATORY OUTCOMES
| Placebo (n=26 to 27*) |
Ginkgo (n=26 to 30*) |
||||
|---|---|---|---|---|---|
| Study outcome | Baseline | End-study | Baseline | End-study | P-valuea |
| FMVDb (%change) | 3.8 ± 9.8 | 2.0 ± 7.8 | 3.4 ± 8.4 | 5.2 ± 7.5 | 0.24 |
|
Antibodies to epitopes of oxidized LDL (RLU)c |
|||||
| E06 | 10210 ± 11167 |
9768 ± 10431 |
8316 ± 10819 |
8314 ± 11726 |
0.49 |
| IgG IC ApoB100 | 2973 ± 1375 |
2844 ± 1327 |
2581 ± 972 | 2538 ± 947 | 0.51 |
| IgM IC ApoB100 | 1836 ± 1055 |
1770 ± 1052 |
2464 ± 1282 |
2348 ± 1282 |
0.55 |
| IgG MDA LDL | 2845 ± 1936 |
2575 ± 1599 |
3661 ± 3410 |
3431 ± 3146 |
0.83 |
| IgM MDA LDL | 12259 ± 6421 |
12158 ± 6133 |
13883 ± 8041 |
13599 ± 8757 |
0.74 |
| IgG Cu LDL | 1040 ± 726 | 1011 ± 554 | 1415 ± 1337 |
1352 ± 1317 |
0.62 |
| IgM Cu LDL | 3925 ± 2219 |
4152 ± 2525 |
5898 ± 7141 |
6033 ± 7491 |
0.74 |
|
Walking Impairment Questionnaired |
|||||
| Distance | 0.53 ± 0.32 | 0.54 ± 0.33 | 0.45 ± 0.25 | 0.52 ± 0.32 | 0.39 |
| Speed | 0.42 ± 0.22 | 0.50 ± 0.26 | 0.44 ± 0.21 | 0.50 ± 0.23 | 0.91 |
| Stairs | 0.51 ± 0.27 | 0.49 ± 0.31 | 0.51 ± 0.25 | 0.52 ± 0.29 | 0.74 |
|
Quality of Life (SF-36)e |
|||||
| Physical component | 39.2 ± 5.7 | 38.6 ± 5.3 | 38.0 ± 5.1 | 38.3 ± 4.7 | 0.40 |
| Mental component | 49.7 ± 12.1 | 49.8 ± 12.5 | 47.0 ± 13.1 | 50.3 ± 10.6 | 0.20 |
LDL indicates low density lipoprotein; (SF-36), Medical Outcomes Study short form 36-item self administered questionnaire
t-test
FMVD, flow mediated vasodilation (n=26 placebo, n=26 Ginkgo)
Antibodies to epitopes of oxidized LDL (RLU, relative light units from chemiluminescence assessments using ELISA, chemiluminescent enzyme-linked immunosorbent assay) (n=26 placebo, n=29 Ginkgo)
Walking Impairment Questionnaire (n=26 placebo, n=30 Ginkgo)
Quality of Life (SF-36) (n=27 placebo, n=30 Ginkgo)
Possible antioxidant effects of the Ginkgo were investigated using assays involving antibodies to epitopes of oxidized LDL. Plasma samples for both baseline and end-study were available for 26 placebo participants and 29 Ginkgo participants. For the 7 different assays used, there were no observed differences between treatment groups (all P-values ≥0.49) (Table 2). The validated Walking Impairment Questionnaire was used to collect self-reported data on practical aspects of daily walking activities and to complement the treadmill walking data. None of the between group differences in pre-post changes for the three summary components of the walking impairment questionnaire were statistically significant (all P-values ≥0.39) (Table 2).
Quality of life data were collected using the standardized MOS SF-36 questionnaire. The observed between-group differences in pre-post changes for the 2 main summary scores of the quality of life questionnaire, the physical and mental component scores, were negligible and not statistically significant. Among the 8 individual domain scores that contribute to the physical and mental component scores, statistical significance was observed for the “role physical” domain. The domain scores are bounded by 0 and 100, and the pre-post difference score is bounded by -100 and 100. The average “role physical” scores for the 2 treatment groups at baseline were 32 ± 38 and 16 ± 37 for the Placebo and Ginkgo groups, respectively. The pre-post changes in scores for this domain were -6 ± 35 and 17 ± 29 for the Placebo and Ginkgo groups, respectively. When analyzed using a simple t-test the between group difference reached an observed P=0.009. Given the baseline differences in scoring, the data were re-analyzed using a General Linear Model with baseline scores included as a covariate to the statistical model, which reduced the significance level to P=0.03. This analysis was not adjusted for the multiple testing done of the secondary and exploratory analyses.
DISCUSSION
The objective of this study was to determine if an extract of Ginkgo biloba (EGb 761) would lengthen the amount of time adults with peripheral artery disease were capable of walking on a treadmill. A moderately large but non-significant increase in maximal walking time and a negligible increase in pain-free walking time were observed for the Ginkgo group relative to the placebo group after 4 months of treatment. Similar to the results for maximal walking time, a modest but non-significant increase in flow mediated vasodilation was observed for the Ginkgo group compared to placebo. There was no impact of treatment on antibodies to epitopes of oxidized LDL.
In a meta-analysis of 8 studies using Ginkgo biloba for patients with peripheral artery disease, 6 of the 7 studies reporting data on maximal walking time observed significant differences that favored the active Ginkgo treatment.3 The range of net improvement, relative to the placebo group, varied from 36-189 meters. In the current study, the net difference in pre-post walking time improvement for the Ginkgo group was equivalent to 87 meters, which in absolute numbers was of greater magnitude than that of 3 of the 6 studies from the meta-analysis that reported statistically significant improvements. The sample sizes of the studies included in the meta-analysis ranged from 20-111 with a mean of 52, placing the current study with 62 patients as one of the larger studies in this set. Six of the studies in the meta-analysis had a duration of 24 weeks. The only study that did not report a significant improvement in walking times for the Ginkgo group had a 12-week duration. The current study had a 16-week duration. It is possible that a longer duration in the current study would have yielded a larger effect. Overall, despite the lack of significant difference achieved for the Ginkgo group in the current study, our findings suggest a trend that is relatively consistent with the small benefits for ginkgo reported in the meta- analysis for maximal treadmill walking time.
One potential moderator of effect in the current study was the baseline walking time. A primary inclusion/exclusion criterion was a baseline maximal walking time of at least 1 minute but not more than 10 minutes (average of 2 assessments). A small number of participants, 6 in the Ginkgo group and 5 in the placebo group, had baseline maximal walking times at the upper end of the allowable range, between 5 and 10 minutes. Among these individuals, 3 of the 6 in the Ginkgo group experienced more than a doubling of their maximal walking time. In contrast, none of the 5 participants in the placebo group with a 5-10 minute baseline walking time experienced dramatic improvements. In a statistical model that included baseline walking time as a potential effect moderator, this factor did not achieve statistical significance. The opportunity to detect a significant moderating or threshold effect was likely limited by the small numbers of study participants in the upper half of the range of baseline walking time. However, it is plausible that individuals with more advanced peripheral artery disease and shorter maximal walking times would be less responsive to therapy. In future studies it may be important to determine if there is a threshold of walking time or of the progression of PAD below which the opportunity for benefit from Ginkgo therapy is substantially diminished.
There are very few available treatments for the intermittent claudication of patients with PAD. Two pharmacological therapies that have received FDA approval are pentoxifylline (Trental) and cilostazol (Pletal). One trial contrasting Pentoxifylline with Ginkgo reported similar, but modest effects on claudication for both therapies, relative to placebo.32 Of the 2 FDA approved medicines, cilostazol is reported to be the more effective of the two.33, 34 We are not aware of any head-to-head comparisons of Cilostazol and Ginkgo. However, neither of the FDA approved medications is currently as effective as exercise therapy. A review of available data on exercise for intermittent claudication identified 10 published trials that met specific inclusion/exclusion criteria related to study quality and data presentation.35 Overall improvement in treadmill walking time was 150% (range 74-230%). At this time, exercise is considered the most effective therapy for PAD.
A secondary goal of the study was to seek mechanisms for any beneficial effects. Although the lack of significance for the main endpoint of increased walking duration in this study provides little reason to explore possible mechanisms, the data collected to examine this question may be of relevance to other studies with other endpoints and health outcomes. Recent as well as earlier studies have reported in vitro and in vivo effects of Ginkgo biloba on nitric oxide production consistent with vasodilatory effects36,37 and antioxidant effects.38,39 We did not observe significant effects for either FMVD or in levels of antibodies to epitopes of oxidized LDL in the current human trial.
There were several strengths and limitations for the current study. The randomized, double-blind, placebo controlled design of the trial was a strength. Other strengths included a high retention rate, good adherence to study tablets, and the use of a well-characterized and standardized Ginkgo product. A limitation may have been the 4-month duration; a greater effect may have been detectable with a longer treatment period. Finally, although not necessarily a limitation, it was notable that the participants in this study suffered from a number of comorbidities as evidenced by their extensive use of prescribed medications. Given the range and frequency of their comorbid conditions, this may have limited the opportunity to demonstrate the effect of Ginkgo supplementation on the outcome of treadmill walking duration.
Currently there are limited pharmacological therapies for the millions of individuals who suffer from the intermittent claudication of PAD. Identifying and testing potential new therapies is of great clinical importance.40 Although previous studies and a meta-analysis have suggested Ginkgo biloba may be a useful herbal therapy for this condition, the results observed in the current trial provide little evidence for effectiveness. Exercise remains the most effective therapy, which typically implies walking activities. However, given that it is walking that brings on the intermittent claudication, this can be a difficult therapy to adhere to for individuals with PAD. Therapies that produce even small benefits in increasing pain-free and maximal walking time could provide helpful adjuvant therapy by making it easier to participate in walking activities. At this time, the emphasis of therapeutic strategies should continue to focus on increasing the frequency of, and time spent, walking, and on any adjuvant therapies that can help to promote this increased exercise.
ACKNOWLEDGEMENTS
We gratefully acknowledge the work of Dr. Deepali Lal in conducting the flow-mediated vasodilation tests, Elton Chan for his help reviewing the literature for the manuscript, and the participants without whom this investigation would not have been possible.
Grant Support: This investigation was supported by NIH grant R01 AT00204
Footnotes
Condensed abstract A standardized extract of Ginkgo biloba (EGb 761) vs. placebo was tested among older adults with PAD. Ginkgo biloba produced a modest but insignificant increase in maximal treadmill walking time and flow-mediated vasodilation. These data do not support the use of Ginkgo biloba as an effective therapy for PAD.
REFERENCES
- 1.Kelly JP, Kaufman DW, Kelley K, Rosenberg L, Anderson TE, Mitchell AA. Recent trends in use of herbal and other natural products. Arch Intern Med. 2005;165:281–286. doi: 10.1001/archinte.165.3.281. [DOI] [PubMed] [Google Scholar]
- 2.Diamond BJ, Shiflett SC, Feiwel N, et al. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668–678. doi: 10.1016/s0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 3.Pittler MH, Ernst E. Ginkgo biloba extract for the treatment of intermittent claudication: a meta-analysis of randomized trials. Am J Med. 2000;108:276–281. doi: 10.1016/s0002-9343(99)00454-4. [DOI] [PubMed] [Google Scholar]
- 4.Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician. 2003;68:923–926. [PubMed] [Google Scholar]
- 5.Mehlsen J, Drabaek H, Wiinberg N, Winther K. Effects of a Ginkgo biloba extract on forearm haemodynamics in healthy volunteers. Clin Physiol Funct Imaging. 2002;22:375–378. doi: 10.1046/j.1475-097x.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Liebgott T, Miollan M, Berchadsky Y, Drieu K, Culcasi M, Pietri S. Complementary cardioprotective effects of flavonoid metabolites and terpenoid constituents of Ginkgo biloba extract (EGb 761) during ischemia and reperfusion. Basic Res Cardiol. 2000;95:368–377. doi: 10.1007/s003950070035. [DOI] [PubMed] [Google Scholar]
- 7.Kubota Y, Tanaka N, Umegaki K, et al. Ginkgo biloba extract-induced relaxation of rat aorta is associated with increase in endothelial intracellular calcium level. Life Sci. 2001;69:2327–2336. doi: 10.1016/s0024-3205(01)01303-0. [DOI] [PubMed] [Google Scholar]
- 8.Akiba S, Kawauchi T, Oka T, Hashizume T, Sato T. Inhibitory effect of the leaf extract of Ginkgo biloba L. on oxidative stress-induced platelet aggregation. Biochem Mol Biol Int. 1998;46:1243–1248. doi: 10.1080/15216549800204812. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF, Dent G, McCusker M, Guinot P, Page CP, Barnes PJ. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet. 1987;1(8527):248–251. doi: 10.1016/s0140-6736(87)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Guinot P, Caffrey E, Lambe R, Darragh A. Tanakan inhibits platelet-activating-factor-induced platelet aggregation in healthy male volunteers. Haemostasis. 1989;19:219–223. doi: 10.1159/000215920. [DOI] [PubMed] [Google Scholar]
- 11.Kudolo GB, Dorsey S, Blodgett J. Effect of the ingestion of Ginkgo biloba extract on platelet aggregation and urinary prostanoid excretion in healthy and Type 2 diabetic subjects. Thromb Res. 2002;108(23):151–160. doi: 10.1016/s0049-3848(02)00394-8. [DOI] [PubMed] [Google Scholar]
- 12.Bal Dit Sollier C, Caplain H, Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clin Lab Haematol. 2003;25:251–253. doi: 10.1046/j.1365-2257.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 13.Braquet P. Cedemin, a Ginkgo biloba extract, should not be considered as a PAF antagonist. Am J Gastroenterol. 1993;88:2138. [PubMed] [Google Scholar]
- 14.Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine. 2005;12(12):10–16. doi: 10.1016/j.phymed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kohler S, Funk P, Kieser M. Influence of a 7-day treatment with Ginkgo biloba special extract EGb 761 on bleeding time and coagulation: a randomized, placebo-controlled, double-blind study in healthy volunteers. Blood Coagul Fibrinolysis. 2004;15:303–309. doi: 10.1097/00001721-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D, Bakal CW, Beatty PT, et al. Position statement on the use of the ankle brachial index in the evaluation of patients with peripheral vascular disease. A consensus statement developed by the Standards Division of the Society of Interventional Radiology. J Vasc Interv Radiol. 2003;14(9 Pt 2):S389. doi: 10.1097/01.rvi.0000094611.61428.3b. [DOI] [PubMed] [Google Scholar]
- 17.Mohler ER., 3rd Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association declaration of Helsinki Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 19.American College of Sports Medicine . Guidelines for exercise testing and prescription. 6th ed Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 20.American College of Sports Medicine . Resource manual for guidelines for prescription testing and prescription. 4th ed Lippincott, Williams and Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- 21.Gardner AW, Skinner JS, Smith LK. Effects of handrail support on claudication and hemodynamic responses to single-stage and progressive treadmill protocols in peripheral vascular occlusive disease. Am J Cardiol. 1991;68:99–105. doi: 10.1016/0002-9149(91)90719-2. [DOI] [PubMed] [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Rodenburg J, Vissers MN, Wiegman A, et al. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J Am Coll Cardiol. 2006;47:1803–1810. doi: 10.1016/j.jacc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 24.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 25.Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–1412. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 26.Regensteiner JG, Steiner JF, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 27.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–1081. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 28.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–115. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 30.Izquierdo-Porrera AM, Gardner AW, Bradham DD, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. J Vasc Surg. 2005;41:625–630. doi: 10.1016/j.jvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 31.McDermott MM, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–181. doi: 10.1046/j.1525-1497.1999.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohmer D, Kalinski S, Michaelis P, Szogy A. Behandlung der PAVK mit Ginkgo-biloba-extrakt (GBE) oder Pentoxifyllin. Herz Kreislauf. 1988;20:5–8. [Google Scholar]
- 33.Hiatt WR. The US experience with cilostazol in treating intermittent claudication. Atheroscler Suppl. 2005;6:21–31. doi: 10.1016/j.atherosclerosissup.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Falcone RA, Hirsch AT, Regensteiner JG, et al. Peripheral arterial disease rehabilitation: a review. J Cardiopulm Rehabil. 2003;23:170–175. doi: 10.1097/00008483-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;(2):CD000990. doi: 10.1002/14651858.CD000990. [DOI] [PubMed] [Google Scholar]
- 36.Auguet M, Delaflotte S, Chabrier PE, Braquet P. Ginkgo biloba extract (EGb 761) and the regulation of vascular tone. Vol 3. Elsevier; Paris: 1994. [Google Scholar]
- 37.Koltermann A, Hartkorn A, Koch E, Furst R, Vollmar AM, Zahler S. Ginkgo biloba extract EGb((R)) 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715–1722. doi: 10.1007/s00018-007-7085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem Pharmacol. 1995;49:1649–1655. doi: 10.1016/0006-2952(95)00089-i. [DOI] [PubMed] [Google Scholar]
- 39.Sener G, Sehirli O, Tozan A, Velioglu-Ovunc A, Gedik N, Omurtag GZ. Ginkgo biloba extract protects against mercury(II)-induced oxidative tissue damage in rats. Food Chem Toxicol. 2007;45:543–550. doi: 10.1016/j.fct.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Hackam DG, Goodman SG, Anand SS. Management of risk in peripheral artery disease: recent therapeutic advances. Am Heart J. 2005;150:35–40. doi: 10.1016/j.ahj.2005.01.008. [DOI] [PubMed] [Google Scholar]