Abstract
Background and Purpose
The presence of white matter hyperintensities on brain MRI is common among elderly individuals. Previous research suggests that cardiovascular risk factors are associated with increased white matter hyperintensities. Examining the role of direct physiological measures of vascular function will help to clarify the vascular mechanisms related to white matter hyperintensities. The aim of the present study was to examine the association between endothelial-dependent and endothelial-independent vasodilatation and white matter hyperintensity volume.
Methods
Twenty-five older adults with a range of cardiovascular diseases underwent brain MRI and completed assessments of blood vessel integrity using endothelial-dependent and independent flow-mediated dilation of the brachial artery. A semi-automated pixel-based method was used to quantify total brain volume and white matter hyperintensity volume, with white matter hyperintensity volume corrected for total brain volume. The association between measures of flow-mediated dilation and log-transformed white matter hyperintensities was examined.
Results
Correlation analysis revealed that endothelial-dependent vasodilatation was significantly and inversely associated with white matter hyperintensity volume. In contrast, endothelial-independent vasodilatation was not associated with white matter hyperintensities. Neither endothelial-dependent nor endothelial-independent vasodilatation was associated with total brain volume.
Conclusions
These data provide preliminary evidence that the integrity of the vascular endothelium is associated with white matter hyperintensities in older adults with cardiovascular disease. Impaired vascular function may be one mechanism that contributes to the development of white matter hyperintensities in the brain. Additional longitudinal research combining measures of vessel function, neuroimaging and cognition will be helpful in clarifying this potential mechanism.
Keywords: cardiovascular disease, endothelium, magnetic resonance, white matter disease
Evidence of cerebrovascular disease on neuroimaging is common among elderly individuals,1,2 particularly regions of hyperintense signal in cerebral white matter observed on proton density T2-weighted or fluid-attenuated inversion recovery images. Several studies have reported that increased white matter hyperintensities (WMH) are associated with cognitive impairment, even in the absence of dementia.3-5 Furthermore, WMH have been associated with depression and increased risk for stroke and death.6-8 The presence of traditional cardiovascular risk factors (eg, increasing age, smoking, hypertension) have a well-recognized impact on vascular function and have been associated with increased WMH,9 suggesting that WMH may arise from an underlying vascular cause. Identifying specific vascular abnormalities associated with the presence of WMH will improve our understanding of the underlying physiology involved in the relationship between cardiovascular disease and cerebrovascular injury to the brain, reflected by WMH.
One potentially relevant aspect of vascular function in relation to WMH is the capacity of the cerebral blood vessels to regulate in response to physical and chemical stimuli by adjusting vascular tone and blood flow. Indeed, previous studies have documented a decrease in vasodilatory capacity in the cerebral cortex of individuals with WMH in response to CO2 and acetazolamide challenges to cerebral circulation.10,11 Given the unique anatomical and physiological characteristics of cerebral blood vessels, regulation of vascular tone in the cerebral circulation is complex and is mediated by multiple factors.12 One important influence on the regulation of vascular tone is the release of nitric oxide (NO) from the endothelium; this effect was initially established in the peripheral vasculature,13 although there is increasing evidence that NO plays an important role in cerebral circulation. For example, studies in animal models using inhibitors of NO synthase suggest that NO mediates basal cerebral blood flow and may be involved in the vasodilatory response to hypercapnia,14-18 although findings have been inconsistent within and across species.17,19,20 In vitro examination of human cerebral arteries suggests that NO mediates vessel relaxation21,22 and there is some in vivo evidence of a contribution of NO to cerebrovascular regulation among healthy adults.23,24 Given that impaired NO synthesis and release has been demonstrated in peripheral arteries of patients with risk factors for cerebrovascular disease (eg, hypertension and coronary artery disease25,26) and increasing evidence of a role of NO in cerebrovascular regulation, a logical extension of these findings is to examine whether impairments of endothelial function and NO release affect the cerebrovasculature of individuals with cardiovascular disease.
Endothelial function in humans is commonly assessed by measuring the degree of brachial artery dilatation in response to increased flow after forearm occlusion with a blood pressure cuff.27-29 This measure relies on the fact that an increase in blood flow causes shear stress stimulating vasodilators, resulting in endothelial-dependent dilatation within an intact vessel.30 Flow-mediated dilatation measures in the brachial artery have been linked to vessel function in the coronary artery suggesting that it may be a noninvasive marker of “global” endothelial function.31,32 One previous study examined flow-mediated dilatation in relation to CO2 vasoreactivity in the cerebral circulation;33 however, to our knowledge, no study has examined flow-mediated dilatation in relation to neuroimaging findings among patients with cardiovascular disease.
The purpose of the current study was to examine the association between the integrity of the vascular endothelium and WMH among nondemented older adults with cardiovascular disease. To accomplish our goal, we measured brachial artery blood vessel function using a well-validated technique to assess endothelial-mediated and endothelial-independent vascular responses.31 In addition to completing the blood vessel measures, study participants underwent structural MRI of the brain, from which indices of WMH and total brain volume were quantified. We hypothesized that dysfunction of the vascular endothelium would be associated with greater WMH volume. Furthermore, because the participants were not demented and we anticipated that their total brain volume would be relatively normal, we hypothesized that endothelial function would not be significantly associated with total brain volume.
Materials and Methods
The study was approved by local institutional review boards and written informed consent was obtained from all participants. Twenty-five participants enrolled in an ongoing study examining the effects of cardiovascular disease on brain functioning in the elderly were included in the current analysis. Participants were recruited from cardiac rehabilitation programs, cardiology practices, and advertisements. All participants had a documented history of cardiovascular disease including at least 1 of the following: myocardial infarction, cardiac surgery, heart failure, coronary artery disease, or hypertension.
Demographic and clinical characteristics are presented in Table 1. None of the participants scored within the demented range on the Mini Mental State Exam.34 Cardiovascular risk factors (ie, hypertension, hypercholesterolemia, tobacco use, and diabetes) were coded according to the number of factors present (ie, 0 to 4). Eight of 25 participants (32%) endorsed 1 risk factor, 10 of 25 (40%) endorsed 2, 3 of 25 (12%) endorsed 3, and 2 of 25 (8%) had all 4 factors. Medication information for the sample was as follows: 22 of 25 (88%) participants were currently being treated with antihypertensive medication, 17 of 25 (68%) with aspirin/antithrombotics, 17 of 25 (68%) with lipid-lowering agents, 12 of 25 (48%) with vitamins, 11 of 25 (44%) with gastric acid inhibitors, 3 of 25 (12%) with hypoglycemics, 2 of 25 (8%) with vasodilators, and 2 of 25 (8%) with psychoactive medications.
TABLE 1. Demographic and Clinical Characteristics of Sample.
| Variable | Mean (SD) | No. of Participants |
|---|---|---|
| Age, y | 72.2 (7.7) | |
| Sex, % female | 10/25 (40%) | |
| Mini-Mental State Exam Score | 28.8 (1.1) | |
| Beck Depression Inventory | 4.2 (2.5) | |
| Systolic BP, mm Hg* | 134.4 (19.9) | |
| Diastolic BP, mm Hg* | 68.5 (10.1) | |
| % Change in brachial artery diameter, reactive hyperemia, endothelial-dependent dilatation | 5.99 (4.53) | |
| % Change in brachial artery diameter with nitroglycerin, endothelial-independent dilatation | 15.64 (6.80) | |
| WMH, voxels (WMH/TBVx100) | 0.68 (1.21) | |
| Total brain volume, cm3 | 1226.77 (155.89) | |
| Hypertension | 19/25 (76%) | |
| Hypercholesterolemia | 14/25 (56%) | |
| Diabetes | 4/25 (16%) | |
| History of smoking | 8/25 (32%) | |
| Myocardial infarction | 11/25 (44%) | |
| Coronary artery bypass surgery | 9/25 (36%) | |
| Angioplasty/stents | 2/25 (8%) | |
| Heart failure | 2/25 (8%) |
Blood pressure was collected before vascular assessment.
Exclusion criteria included neurological disease (eg, stroke, traumatic head injury with loss of consciousness >10 minutes, dementia), major psychiatric illness (ie, schizophrenia, bipolar disorder), substance abuse (current abuse or previous hospitalization for abuse), and MRI contraindications. Participants underwent cardiovascular assessment of blood vessel functioning using Doppler ultrasound flow-mediated dilatation and brain MRI on separate visits.
Brachial Artery Flow-Mediated Dilatation
Peripheral vascular autoregulation and associated endothelial function were assessed by flow-mediated brachial artery vasodilatation. Participants fasted and held vasoactive medications (eg, calcium channel blockers, angiotensin-converting enzyme inhibitors), caffeine, and smoking for 6 hours before the vascular assessment. Before initiating vascular interrogation, participants remained supine for 15 minutes in a quiet room. High-frequency B-mode ultrasound was used to visualize the brachial vessel. A Hewlett Packard 5500 ultrasound system, equipped with a linear array vascular (7.5 MHz) transducer was used to acquire 2-dimensional and Doppler flows of each participant’s left arm. Images were obtained in longitudinal orientation ≈5 cm above the antecubital fossa; straight segments were obtained at least 10 mm and were targeted for optimal assessments. Blood pressure was measured with an automated Datascope accutor 3SAT (Paramus, NJ) in the contralateral arm.
To assess endothelial function, hyperemic (flow-mediated) vascular responses were assessed. First, baseline images of brachial artery diameter and blood flow velocity were recorded for 1 minute (sequential images, captured and digitized on each R-wave). Thereafter, a 4-cm cuff positioned on the mid-forearm was inflated to 40 mg above the baseline systolic blood pressure for 5 minutes. The same brachial segment was interrogated for 3 minutes after the cuff was deflated (the period of hyperemic flow). Analyses of the digital images were performed by an investigator who was blinded to subject characteristics. Arterial diameter was determined using a validated software algorithm that automatically calculates the average diameter over the selected segment.8 Flow-mediated vasodilation was calculated as the percentage change in diameter from baseline to the maximum diameter induced by reactive hyperemia.
Ten minutes after the hyperemic brachial assessment was completed, endothelial-independent vascular function was assessed by measuring the same portion of the brachial artery before and 5 minutes after the administration of 0.4 mg sublingual nitroglycerin, a time corresponding to peak vasodilatory responses. Nitroglycerinmediated vasodilation was calculated as the percentage change in arterial diameter from the pre-nitroglycerin baseline to the diameter after nitroglycerin.
Brain MRI and WMH Quantification
The brain MRI and WMH quantification techniques used in the current study have been described elsewhere.35 MRI was obtained using a Siemens Symphony 1.5-T unit. Fluid-attenuated inversion recovery weighted (repetition time/echo time=6000/105) images (5-mm thickness with a 2-mm gap) were obtained for each participant and used to quantify WMHs via a semi-automated threshold technique in ANALYZE (Biomedical Imaging Resource, Mayo Foundation). The total number of voxels representing WMH from each slice was summed to calculate the total WMH for each participant. Total brain volume was calculated using threshold histogram values consistent with brain parenchyma. Total brain volume was used as a correction factor for WMH values (ie, WMH pixel total/TBV×100), and this ratio was used as the primary dependent variable. Given that WMH volume was positively skewed, log transformation was used to normalize this variable. Nonparametric correlations were considered; however, given the relatively normal distribution of the blood vessel functioning measures, transformation of the WMH variable was deemed to be more appropriate.
Data Analysis
Four bivariate correlations were calculated to examine the association between blood vessel functioning and WMH. Correlations were calculated between endothelial-dependent vasodilatation (percent change in brachial artery diameter, reactive hyperemia), endothelial-independent vasodilatation (nitroglycerin-induced percent change in brachial artery diameter), and the 2 MRI variables (ie, log-transformed WMH volume and raw TBV). These correlations were then recalculated as partial correlations adjusting for age and level of cardiovascular risk, coded as described. An α level of 0.05 was retained for all analyses given the exploratory nature of the study and the fact that only 4 main correlations were calculated with a priori hypotheses.
Results
As shown in Table 2, endothelial-dependent flow-mediated dilatation was significantly and inversely associated with WMH (r=-0.63, P<0.01; Figure), but not total brain volume (r=0.26, not significant), suggesting that as endothelial-dependent flow-mediated dilatation decreases, WMH significantly increase. In contrast, endothelial-independent dilatation was not significantly correlated with either WMH volume (r=-0.25, not significant) or total brain volume (r=0.04, not significant). These relationships remained consistent after adjusting for age and level of cardiovascular risk (Table 2).
TABLE 2. Correlations (r) between Flow-Mediated Dilatation and MRI Measures.
| WMH* |
Total Brain Volume |
|||
|---|---|---|---|---|
| Flow-Induced % Δ in Vessel Diameter | Nitroglycerin-Induced % Δ in Vessel Diameter | Flow-Induced % Δ in Vessel Diameter | Nitroglycerin-Induced % Δ in Vessel Diameter | |
| No adjustments | -0.63 (P=0.001) | -0.25 (P=0.26) | 0.26 (P=0.20) | 0.04 (P=0.87) |
| Adjusting for age | -0.53 (P=0.01) | -0.16 (P=0.49) | 0.23 (P=0.32) | -0.01 (P=0.97) |
| Adjusting for age and risk factors | -0.53 (P=0.02) | -0.19 (P=0.42) | 0.21 (P=0.37) | -0.02 (P=0.93) |
WMH values are ratios (WMH/TBVx100) normalized via log transformation.
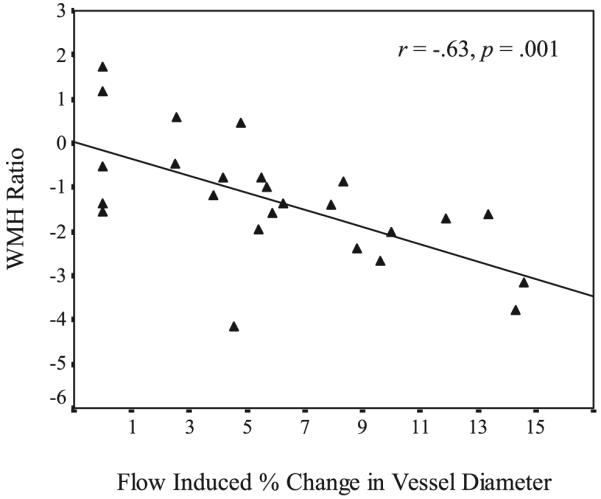
Scatterplot of flow-mediated vessel dilatation and WMH ratio.
Discussion
The present study provides preliminary evidence suggesting that impaired endothelial function is associated with increased cerebral WMH volume in older adults with cardiovascular disease. Endothelial dysfunction, as measured by flow-mediated dilatation of the brachial artery, was significantly associated with greater volume of WMH on brain MRI. Endothelial-independent vasodilatation, measured after administration of nitroglycerin, was not significantly associated with WMH volume. Importantly, the relationship between endothelial function and WMH remained significant after adjusting for the effects of age and level of cardiovascular risk, calculated based on the presence of traditional cardiovascular risk factors (ie, hypertension, diabetes, hypercholesterolemia, and smoking). This suggests that endothelial dysfunction may be associated with WMH independent of these factors. Thus, flow-mediated dilatation may provide an integrated measure of large conduit blood vessel regulation, reflecting the cumulative deleterious effects of multiple risk factors on vascular function.26,27
The current study included a clinically heterogeneous sample with regard to type of cardiovascular disease. Such heterogeneity maximized our ability to examine endothelial function as an integrated measure of vascular health among a clinical sample with a broad range of cardiovascular risk factors known to contribute to endothelial dysfunction. The current study extends previous research that has demonstrated a relationship between cardiovascular risk factors and WMH by examining a more direct physiological measure of blood vessel function, particularly involvement of NO, potentially reflecting the combined effects of various vascular risk factors.9
Our initial evidence of an association between peripheral endothelial function and WMH supports the notion that impaired regulation of vascular tone and the vasodilatory response may be one mechanism underlying the development of WMH in the brain. Previous studies have reported that decreased cerebrovascular dilatory capacity in response to CO210 and acetazolamide11 challenges are associated with increased white matter lesions. For example, Bakker et al examined the association between vasomotor reactivity, using CO2 transcranial Doppler, and white matter lesions on MRI in a population-based cohort and concluded that their data supported the hypothesis that some white matter lesions might be secondary to hemodynamic ischemic injury to the brain.10 Furthermore, neuropathological studies have documented that chronic vascular injury in the blood vessels of the brain leads to atrophic changes immediately surrounding these damaged vessels.36,37 These atrophic spaces become filled with extracellular fluid that then appears as WMH on MRI. Thus, it is possible that chronic impairment of the vasodilatory response may contribute, in part, to the development of WMH over time. Furthermore, the measure that we used to assess endothelial function (ie, flow-mediated dilatation) is thought to be a noninvasive measure of endothelium-derived NO activity.29 This observation, coupled with evidence from studies examining the role of NO in cerebral circulation,14-18 raises the possibility that NO may be one mediator of the relationship.
It is important to note that our sample consisted of a relatively small number of patients and, as a result, the findings should be considered preliminary. However, the correlations were consistent with our expectations and remained significant after adjusting for the effects of age and level of cardiovascular risk, providing some evidence of robust effects despite the small sample size. Given the correlational nature of the study, the observed association does not directly link impaired endothelial function to WMH, because WMH likely result from multiple complex mechanisms. It will be important to examine these associations longitudinally to clarify the potential causal role of endothelial dysfunction in the development of WMH.
The current findings suggest several directions for future studies. First, an important extension of these findings would be to examine relationships among endothelial functioning, WMH, and neuropsychological performance within the same sample of patients. Previous research has demonstrated a relationship between an invasive measure of peripheral endothelial function and global neuropsychological performance.38 The literature on vascular cognitive impairment suggests that the frontal subcortical networks implicated in complex attention, executive functioning, and processing speed are particularly susceptible to ischemic damage resulting from blood flow abnormalities.39,40 Thus, it is reasonable to hypothesize that executive function and processing speed deficits may be observed in relation to endothelial dysfunction and WMH. Second, in future larger studies it would be interesting to examine whether there is a specific quantitative relationship between measures of endothelial function and WMH, such that a certain level of cardiovascular impairment is necessary before there is a significant impact on the brain. Finally, in the current study we included only individuals who had cardiovascular disease. The idea that endothelial function and WMH are associated would be strengthened by research examining the relationship between brachial response and WMH in healthy individuals, also, to determine whether the association differs among elderly people free of cardiovascular risk.
Summary
We observed an inverse association between endothelial dependent flow mediation dilation and WMH volume. Overall, the present findings suggest the possibility that endothelial dysfunction is associated with increased WMH. This observation raises the prospect that impaired regulation of vascular tone and the vasodilatory response may be one mechanism that contributes to the development of WMH in the brain. Although this could not be directly tested given the cross-sectional and correlational nature of the current study, future large-scale prospective studies will be important to extend the current findings and determine the causal nature of this relationship. The current findings also underscore the importance of potential interventions to improve vessel function, given the possible deleterious effects of cerebrovascular changes.
Acknowledgments
The authors thank Marie Gerhard-Herman, MD, and Nicole Wake, BA, for their help measuring the brachial artery responses.
Sources of Funding
This work was supported in part by grant AG017975 (R.A.C.), AG026850 (K.F.H.), MH073416 (D.F.T.), HL074568 (J.G.), AG020649 (D.J.M.), MH065857 (R.P.H.), AG022773, and HD043444 (A.L.J.).
Footnotes
Disclosures
None.
References
- 1.Almkvist O, Wahlund LO, Andersson-Lundman G, Basun H, Backman L. White-matter hyperintensity and neuropsychological functions in dementia and healthy aging. Arch Neurol. 1992;49:626–632. doi: 10.1001/archneur.1992.00530300062011. [DOI] [PubMed] [Google Scholar]
- 2.Soderlund H, Nyberg L, Adolfsson R, Nilsson LG, Launer LJ. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39:1093–1105. doi: 10.1016/s0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- 3.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 4.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 5.Paul RH, Haque O, Gunstad J, Tate DF, Grieve SM, Hoth K, Brickman AM, Cohen R, Lange K, Jefferson AL, MacGregor KL, Gordon E. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Arch Clin Neuropsychol. 2005;20:697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briley DP, Haroon S, Sergent SM, Thomas S. Does leukoaraiosis predict morbidity and mortality? Neurology. 2000;54:90–94. doi: 10.1212/wnl.54.1.90. see comment. [DOI] [PubMed] [Google Scholar]
- 7.Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. see comment. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psych. 2002;72:576–582. doi: 10.1136/jnnp.72.5.576. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 10.Bakker SLM, de Leeuw F-E, de Groot JC, Hofman A, Koudstaal PJ, Breteler MMB. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–583. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- 11.Isaka Y, Okamoto M, Ashida K, Imaizumi M. Decreased cerebrovascular dilatory capacity in subjects with asymptomatic periventricular hyperintensities. Stroke. 1994;25:375–381. doi: 10.1161/01.str.25.2.375. [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM, Heistad DD. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiological Reviews. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Joannides R, Haefeli W, Linder L, Richard V, Bakkali E, Thuillez C, Luscher T. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 14.Bonvento G, Seylaz J, Lacombe P. Widespread attenuation of the cerebrovascular reactivity to hypercapnia following inhibition of nitric oxide synthase in the conscious rat. J Cerebral Blood Flow Metab. 1994;14:699–703. doi: 10.1038/jcbfm.1994.90. [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–552. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- 16.Thompson BG, Pluta RM, Girton ME, Oldfield EH. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84:71–78. doi: 10.3171/jns.1996.84.1.0071. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Pelligrino DA, Paulson OB, Lassen NA. Comparison of the effects of ng-nitro-l-arginine and indomethacin on the hypercapnic cerebral blood flow increase in rats. Brain Res. 1994;641:257–264. doi: 10.1016/0006-8993(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Pelligrino DA, Baughman VL, Koenig HM, Albrecht RF. The role of neuronal nitric oxide synthase in regulation of cerebral blood flow in normocapnia and hypercapnia in rats. J Cerebral Blood Flow Metab. 1995;15:774–778. doi: 10.1038/jcbfm.1995.97. [DOI] [PubMed] [Google Scholar]
- 19.Goadsby PJ. Nitric oxide is not the sole determinant of hypercapnic or metabolically driven vasodilation in the cerebral circulation. J Autonomic Nervous System. 1994;49(Suppl):S67–S72. doi: 10.1016/0165-1838(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.McPherson RW, Kirsch JR, Ghaly RF, Traystman RJ. Effect of nitric oxide synthase inhibition on the cerebral vascular response to hypercapnia in primates. Stroke. 1995;26:682–687. doi: 10.1161/01.str.26.4.682. [DOI] [PubMed] [Google Scholar]
- 21.Aldasoro M, Martinez C, Vila JM, Medina P, Lluch S. Influence of endothelial nitric oxide on adrenergic contractile responses of human cerebral arteries. J Cerebral Blood FlowMetab. 1996;16:623–628. doi: 10.1097/00004647-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Toda N. Mediation by nitric oxide of neurally-induced human cerebral artery relaxation. Experientia. 1993;49:51–53. doi: 10.1007/BF01928789. [DOI] [PubMed] [Google Scholar]
- 23.White RP, Deane C, Vallance P, Markus HS. Nitric oxide synthase inhibition in humans reduces cerebral blood flow but not the hyperemic response to hypercapnia. Stroke. 1998;29:467–472. doi: 10.1161/01.str.29.2.467. [DOI] [PubMed] [Google Scholar]
- 24.White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clinical Science. 2000;99:555–560. [PubMed] [Google Scholar]
- 25.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 26.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 27.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task F Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. erratum appears in J Am Coll Cardiol 2002;39:1082. [DOI] [PubMed] [Google Scholar]
- 28.Kathiresan S, Gona P, Larson MG, Vita JA, Mitchell GF, Tofler GH, Levy D, Newton-Cheh C, Wang TJ, Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–945. doi: 10.1161/CIRCULATIONAHA.105.580233. [DOI] [PubMed] [Google Scholar]
- 29.Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005;99:1233–1234. doi: 10.1152/japplphysiol.00601.2005. see comment. discussion 1237-1238. [DOI] [PubMed] [Google Scholar]
- 30.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vita JA, Keaney JF., Jr. Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. comment. [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. see comment. [DOI] [PubMed] [Google Scholar]
- 33.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity- association with endothelial dysfunction. Am J Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE. “Mini Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Gunstad J, Cohen R, Tate D, Paul R, Poppas A, Hoth K, Macgregor K, Jefferson A. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Pressure. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chimowitz MI, Awad IA, Furlan AJ. Periventricular lesions on MRI. Facts and theories. Stroke. 1989;20:963–967. doi: 10.1161/01.str.20.7.963. [DOI] [PubMed] [Google Scholar]
- 37.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 38.Moser DJ, Hoth KF, Robinson RG, Paulsen JS, Sinkey CA, Benjamin ML, Schultz SK, Haynes WG. Blood vessel function and cognition in elderly patients with atherosclerosis. Stroke. 2004;35:e369–e372. doi: 10.1161/01.STR.0000145050.35039.51. [DOI] [PubMed] [Google Scholar]
- 39.Bowler JV, Steenhuis R, Hachinski V. Conceptual background to vascular cognitive impairment. Alzheimer Dis Assoc Dis. 1999;13(Suppl 3):S30–S37. [PubMed] [Google Scholar]
- 40.Rockwood K. Vascular cognitive impairment and vascular dementia. J Neurol Sci. 2002;203-204:23–27. doi: 10.1016/s0022-510x(02)00255-1. [DOI] [PubMed] [Google Scholar]


