Abstract
BACKGROUND
Similar to volatile anesthetics, the anesthetic noble gas xenon protects the heart from ischemia/reperfusion injury, but the mechanisms responsible for this phenomenon are not fully understood. We tested the hypothesis that xenon-induced cardioprotection is mediated by prosurvival signaling kinases that target mitochondria.
METHODS
Male Wistar rats instrumented for hemodynamic measurements were subjected to a 30 min left anterior descending coronary artery occlusion and 2 h reperfusion. Rats were randomly assigned to receive 70% nitrogen/30% oxygen (control) or three 5-min cycles of 70% xenon/30% oxygen interspersed with the oxygen/nitrogen mixture administered for 5 min followed by a 15 min memory period. Myocardial infarct size was measured using triphenyltetrazolium staining. Additional hearts from control and xenon-pretreated rats were excised for Western blotting of Akt and glycogen synthase kinase 3 β (GSK-3β) phosphorylation and isolation of mitochondria. Mitochondrial oxygen consumption before and after hypoxia/reoxygenation and mitochondrial permeability transition pore opening were determined.
RESULTS
Xenon significantly (P < 0.05) reduced myocardial infarct size compared with control (32 ± 4 and 59% ± 4% of the left ventricular area at risk; mean ± sd) and enhanced phosphorylation of Akt and GSK-3β. Xenon pretreatment preserved state 3 respiration of isolated mitochondria compared with the results obtained in the absence of the gas. The Ca2+ concentration required to induce mitochondrial membrane depolarization was larger in the presence compared with the absence of xenon pretreatment (78 ± 17 and 56 ± 17 μM, respectively). The phosphoinositol-3-kinase-kinase inhibitor wortmannin blocked the effect of xenon on infarct size and respiration.
CONCLUSIONS
These results indicate that xenon preconditioning reduces myocardial infarct size, phosphorylates Akt, and GSK-3β, preserves mitochondrial function, and inhibits Ca2+-induced mitochondrial permeability transition pore opening. These data suggest that xenon-induced cardioprotection occurs because of activation of prosurvival signaling that targets mitochondria and renders them less vulnerable to ischemia-reperfusion injury.
Brief exposure to a volatile anesthetic before prolonged coronary artery occlusion and reperfusion protects the myocardium from irreversible ischemic injury termed “anesthetic preconditioning,” APC.1-3 Brief exposure to the anesthetic noble gas xenon also causes cardio-4 and neuroprotection.5 The underlying mechanisms responsible for xenon-induced cardioprotection have been incompletely characterized. Xenon stimulated several important intracellular prosurvival signaling kinases implicated in cardioprotection, including protein kinase C (PKC)-ε,4,6 p38 mitogen-activated protein kinase (MAPK),4 MAPK-activated protein kinase-2,7 heat-shock protein 27,7 and extracellular signal-regulated kinases 1/2.8
The mitochondrial permeability transition pore (mPTP) seems to be an important end-effector of endogenous cardioprotective signaling. The mPTP plays an essential role in necrosis and apoptosis after prolonged ischemia and reperfusion.9 Feng et al.10 reported that phosphorylation of Akt (protein kinase B) and glycogen synthase kinase 3 β (GSK-3β) by isoflurane prevented mPTP opening and thereby inhibited cell death in ischemia/reperfusion injury. Prevention of mPTP opening has been shown to limit mitochondrial depolarization, preserve mitochondrial function, and prevent the initiation of cell death. Bioenergetics of isolated cardiac mitochondria obtained from isoflurane-preconditioned rats was better preserved after hypoxia compared with those that had not been exposed to the volatile anesthetic.11 In the current investigation, we tested the hypothesis that xenon produces cardioprotection in rat myocardium by phosphorylating Akt and GSK-3β, preventing mPTP opening, and protecting mitochondrial bioenergetics.
METHODS
All experiments conducted in the current investigation were approved by the Animal Use and Care Committee of the Medical College of Wisconsin, Milwaukee, Wisconsin. Furthermore, all experiments conformed to the Guide for Care and Use of Laboratory Animals published by the United States National Institutes of Health.
General Preparation and Experimental Protocol
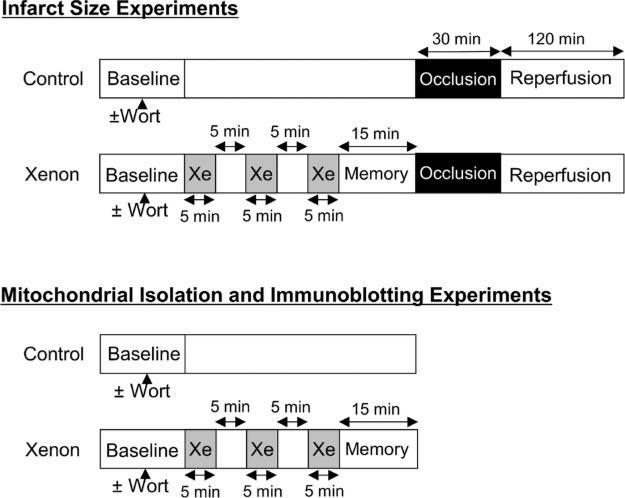
Male Wistar rats (weight, 280−340 g) were anesthetized with sodium thiobutabarbital (Inactin, 150 mg/kg administered intraperitoneally). A tracheostomy was performed and the trachea was cannulated. The lungs of each rat were ventilated with a 70% nitrogen/30% oxygen mixture. Heparin-filled catheters were inserted into the right jugular vein and the right carotid artery for fluid administration and measurement of arterial blood pressure, respectively. A left thoracotomy was performed. The proximal left anterior descending coronary artery (LAD) was identified, and a polypropylene ligature was placed around this vessel. The ends of the suture were threaded through a propylene tube to form a snare. Coronary artery occlusion was produced by clamping the snare and was verified by the presence of epicardial cyanosis in the ischemic zone. After 30 min occlusion, reperfusion was achieved by loosening the snare and confirmed by observing an epicardial hyperemic response. Hemodynamic data were continuously recorded using a computer interfaced with an analog-to-digital converter. All rats were subjected to a 30 min LAD occlusion and 2 h reperfusion. Rats were randomly assigned to 4 groups. One group of rats received no interventions before LAD occlusion and reperfusion. A second group of rats received 3 cycles of 70% xenon/30% oxygen administered for 5 min periods interspersed with 5 min intervals 70% nitrogen/30% oxygen following by a final 15 min interval (memory period in order to allow complete washout of xenon) of 70% nitrogen/30% oxygen before LAD occlusion and reperfusion (Fig. 1). Two additional experimental groups received the phosphoinositol-3-kinase (PI3K) inhibitor wortmannin (0.6 mg/kg) in the absence and presence of xenon after the animals were anesthetized. The drug vehicle alone (dimethyl sulfoxide 0.08 mL/kg) was also tested and did not exhibit any significant effect.
Figure 1.
Schematic illustration of the experimental protocols used in the current investigation. Xe = xenon; Wort = wortmannin.
Measurement of Myocardial Infarct Size
Myocardial infarct size was determined as previously described.12 Briefly, after 120 min reperfusion, the proximal LAD was reoccluded, and patent blue dye was injected IV. The heart was excised, and the left ventricle (LV) was cut into 5 or 6 cross-sectional pieces. The LV area at risk for infarction was obtained by removing normal LV area (blue-stained). The area at risk was incubated at 37°C for 15 min in 1% 2,3,5-triphenyltetrazolium chloride in 0.1 M phosphate buffer adjusted to pH 7.4. After overnight storage in 10% formaldehyde, the infarcted (unstained) tissue was carefully separated from the area at risk and weighed. Infarct size was expressed as a percentage of the LV area at risk.
Preparation of Cardiac Mitochondria
Mitochondria were isolated according to the previously reported procedure.11 Rat hearts, as described above, with or without xenon pretreatment and with and without wortmannin were quickly excised (see experimental protocols in Fig. 1). The atrium and right ventricle were removed, and LV was minced into 1.0 mm pieces in isolation buffer (50 mM sucrose, 200 mM mannitol, 5 mM KH2PO4, 1 mM ethylene glycol-bis(β-aminoethylether)-N,N,N’,N’-tetraacetic acid [EGTA], 5 mM 3-(N-morpholino)propanesulfonic acid, and 0.1% bovine serum albumin [BSA], pH 7.3). The ventricular myocardium was homogenized twice for 5 s with T 25 disperser (IKA-Werke, Staufen, Germany), and the homogenate was centrifuged at 800g. The supernatant was saved, the pellet re-homogenized for 5 s, and the homogenate centrifuged at 800g. This second supernatant was combined with the saved first supernatant, and the pellet was homogenized with Potter-Elvehjem grinder (Weaton, Millville, NJ) in BSA-free isolation buffer containing 4.8 IU/mL protease VIII (Sigma-Aldrich, St. Louis, MO). The combined supernatant and homogenized pellet were centrifuged at 6000g, and the resulting pellets were purified in isolation buffer by homogenization with Potter-Elvehjem grinder and differential centrifugation at 800 and 6000g. The mitochondrial pellet was resuspended in isolation buffer without EGTA, stored on ice and used for experiments within 4 h. Protein concentration was determined by the modified Lowry assay kit (Bio-Rad, Hercules, CA). All procedures were performed on ice.
Measurement of Mitochondrial Oxygen Consumption
Mitochondrial oxygen consumption was measured with an oxygen electrode (Hansatech Instruments, Norfolk, UK) at 30°C in mitochondrial respiration buffer (130 mM KCl, 5 mM KH2PO4, 20 mM 3-(N-morpholino) propanesulfonic acid, 2.5 mM EGTA, 1 μM Na4P2O7, and 0.1% BSA, pH 7.4) containing mitochondria at a final concentration of 1 mg protein/mL. State 2 respiration was initiated with 5 mM pyruvate and 5 mM malate as substrates. The adenosine diphosphate (ADP)-stimulated respiration (state 3) was measured in the presence of 250 μM ADP, and the ADP-independent respiration (state 4) was monitored after all ADP was consumed. Hypoxia was reached within 5 min as mitochondria consumed all available oxygen in the chamber. Throughout the hypoxic interval (20 min), the oxygen concentration within the closed chamber containing the mitochondrial suspension was zero. After the hypoxic interval, the chamber containing mitochondrial suspension was opened to room air to initiate a 10 min reoxygenation period. Oxygen consumption in the chamber was measured again as described above. The respiratory control ratio (RCR), defined as the ratio of oxygen consumption during state 3 to state 4 (a measure for the coupling of adenosine triphosphate synthesis to respiration), was calculated to evaluate the degree of mitochondrial damage before and after exposure to 15 min hypoxia and 10 min reoxygenation.
Measurement of Ca2+-Induced mPTP Opening
Opening of the mPTP was assessed by measurement of isolated mitochondrial membrane potential (Δϕm) with fluorescent indicator rhodamine 123 (Invitrogen, Carlsbad, CA). Mitochondria at a final concentration of 1 mg protein/mL were added into buffer containing 220 mM sucrose, 10 mM KH2PO4, 10 mM HEPES, 50 nM Rhodamine 123, and 5 mM pyruvate and malate. The fluorescence was recorded using a spectrofluorometer (Photon Technology International, Birmingham, NJ) at excitation and emission wavelengths of 503 nm and 527 nm, respectively. After an equilibration period during state 2 respiration, 5 μM aliquots of CaCl2 were sequentially added repeatedly at 60 s intervals until Δϕm was dissipated, indicated by an increase in fluorescence. Specificity for mPTP opening was tested by repeating the experiment in the presence of the mPTP inhibitor cyclosporine A (1 μM).13 The amount of Ca2+ required to induce Δϕm dissipation was used as the variable to evaluate the effect of xenon preconditioning on mPTP opening.
Western Blotting
Western blotting was performed as described previously.14 LV tissue samples were collected from the control and xenon-treated rats after a 15 min memory period (see experimental protocols, Fig. 1), immediately frozen in liquid nitrogen, and stored at −80°C. Tissue was homogenized with T 25 disperser in ice-cold lysis buffer containing 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Nonidet P40, 2.5 mM Na4P2O7, 1 mM Na3VO4, 1 tablet/10 mL protease inhibitor cocktail “complete mini” (Roche, Indianapolis, IN), and 20 mM Tris/HCl, pH 7.4. The homogenate was centrifuged at 10,000g for 15 min at 4°C and the protein concentration was determined in the supernatant by the modified Lowry assay kit. Equivalent amounts of protein (50 μg) samples were mixed with loading buffer and heated at 95°C. All samples were separated on a 4%−15% polyacrylamide gel (Bio-Rad) and electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). After blocking with 5% milk in Tris-buffered saline containing 0.1% Tween-20 and 50 mM NaF, nitrocellulose membranes were incubated overnight at 4°C in 0.1% Tween-20 containing 5% milk and a 1:1000 dilution of monoclonal antibody against Ser 473 of phospho-Akt or Ser 9 of phospo-GSK-3β (Cell Signaling Technology, Beverly, MA). Membranes were washed 3 times with 0.1% Tween-20 for 5 min and incubated with a 1:10,000 dilution of horseradish peroxidase-labeled antimouse immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA) in 0.1% Tween-20 containing 5% milk. Bound antibody was detected by enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ) on radiographic film. Ponceau 0.1% (Sigma-Aldrich, St. Lois, MO) staining of nitrocellulose membranes was used to verify equal protein loading. To determine total Akt and GSK-3β, the membrane was stripped with restore stripping buffer (Pierce, Rockford, IL) and re-probed with polyclonal goat Akt or GSK-3β antibody (Santa Cruz Biotechnology). Quantitative analysis of the band densities was performed using AlphaImager 2000 software (Alpha Innotech Corporation, San Leandro, CA). Band densities obtained from phosphorylated proteins were normalized against the concentrations of total Akt or GSK-3β.
Statistical Analysis
The effect of xenon preconditioning on hemodynamics and mitochondrial bioenergetics were assessed using one-way analysis of variance with Scheffé post hoc test. Comparisons of myocardial infarct size, Akt and GSK-3β phosphorylations and total amount of calcium to open mPTP among experimental groups were made using Student's t-test for unpaired data. Changes were considered statistically significant when P < 0.05. All data are expressed as mean ± sd (SD), with “n” representing the number of experiments.
RESULTS
No significant (P < 0.05) differences in baseline hemodynamics were observed among groups (Table 1). A decrease in heart rate was observed in rats receiving xenon during LAD occlusion compared with those that did not. Coronary artery occlusion and reperfusion decreased heart rate, mean arterial blood pressure, and rate-pressure product in all groups. Heart rate was slower in xenon-pretreated compared with control rats 60 and 120 min after reperfusion. No differences in mean arterial blood pressure were observed among groups. Rate-pressure product was also lower in rats receiving xenon compared with control 60 min after reperfusion. Body weight, LV mass, area at risk weight, and the ratio of area at risk to LV mass were similar among groups (data not shown). Xenon preconditioning reduced myocardial infarct size compared with control (32 ± 4 and 59% ± 4% of the LV area at the risk, respectively; n = 6 per group), and this decrease was reversed by PI3K inhibitor wortmannin (58% ± 5%). Wortmannin alone did not have any significant effect on the infarct size (60% ± 6%).
Table 1.
Hemodynamics During Infarct Size Experiments
| Occlusion | Reperfusion |
|||
|---|---|---|---|---|
| Baseline | 30 min | 60 min | 120 min | |
| HR (bpm) | ||||
| Control | 389 ± 30 | 370 ± 26 | 350 ± 17* | 344 ± 18* |
| Xenon | 354 ± 34 | 325 ± 26† | 286 ± 13*† | 293 ± 23*† |
| Control + Wort | 370 ± 28 | 362 ± 28 | 323 ± 20* | 334 ± 18* |
| Xenon + Wort | 368 ± 43 | 328 ± 14† | 279 ± 28*† | 303 ± 28*† |
| MAP (mm Hg) | ||||
| Control | 113 ± 22 | 104 ± 24 | 82 ± 26* | 72 ± 17* |
| Xenon | 121 ± 30 | 113 ± 10 | 81 ± 4* | 74 ± 10* |
| Control + Wort | 115 ± 17 | 113 ± 13 | 86 ± 18* | 76 ± 8* |
| Xenon + Wort | 120 ± 11 | 121 ± 21 | 87 ± 13* | 82 ± 20* |
| RPP (mm · Hg min−1 · 10−3) | ||||
| Control | 52.9 ± 12.8 | 45.0 ± 11.7 | 35.6 ± 6.8* | 31.5 ± 7.0* |
| Xenon | 50.5 ± 10.4 | 41.9 ± 4.1 | 28.4 ± 2.6*† | 28.2 ± 2.6* |
| Control + Wort | 52.6 ± 10.2 | 40.4 ± 8.0 | 32.1 ± 8.3* | 27.8 ± 6.0* |
| Xenon + Wort | 51.3 ± 9.2 | 35.6 ± 12.4 | 28.7 ± 6.7* | 28.8 ± 7.9* |
Data are mean ± SD; n = 6 per group.
HR = heart rate; MAP = mean arterial blood pressure; RPP = rate pressure product; Wort = Wortmannin.
Significantly (P < 0.05) different from baseline.
Significantly (P < 0.05) different from control.
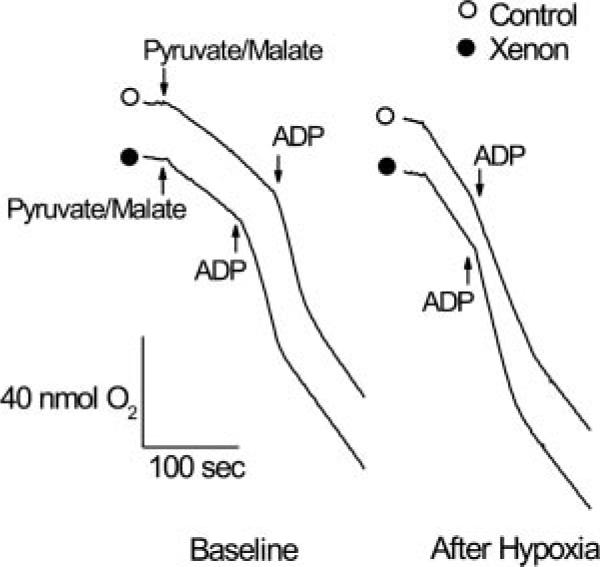
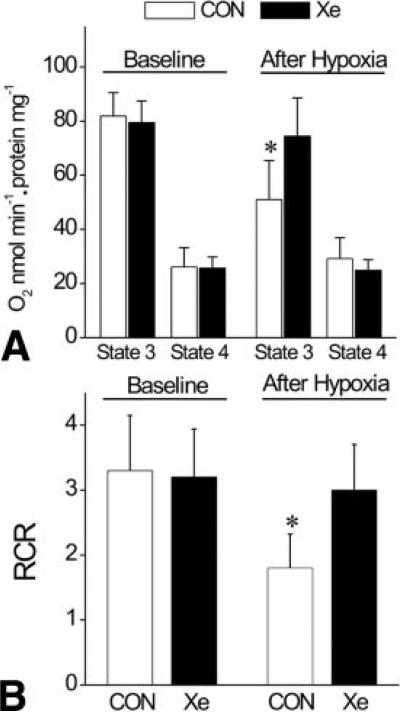
Typical chart recordings of oxygen consumption in isolated rat mitochondria before and after hypoxia/reoxygenation are depicted in Figure 2. After hypoxic stress, the ADP-stimulated oxygen consumption and ADP-independent oxygen consumption (state 3 and 4 respiration, respectively) in isolated mitochondria were preserved in xenon-pretreated (solid circle) compared to control rats (open circle). Distinct respiration states (indicating coupled mitochondria) were also maintained after xenon preconditioning compared with control. Alteration in the rate of state 3 and state 4 respiration and RCR before (baseline) and after hypoxic stress are illustrated in Figure 3. Under baseline conditions, no differences in state 3 and state 4 respiration and RCR were observed in isolated mitochondria among groups (n = 7 each). After exposure to hypoxia/reoxygenation, state 3 respiration in control mitochondria decelerated (82 ± 9 to 51 ± 15 nmol O2 · min−1 · mg protein−1, before and after hypoxia, respectively), producing a lower RCR (3.3 ± 0.9 and 1.8 ± 0.5, before and after hypoxia, respectively). In contrast, state 3 respiration and RCR in mitochondria isolated from xenon-preconditioned rats were similar before (79 ± 8 nmol O2 · min−1 · mg protein−1 and 3.2 ± 0.8, respectively) compared with after exposure (77 ± 14 nmol O2 · min−1 · mg protein−1 and 3.1 ± 0.7, respectively) to hypoxia. The presence of wortmannin during xenon exposure prevented the xenon-induced preservation of RCR (1.4 ± 0.5, not shown). These data suggest that xenon preconditioning protects mitochondria against hypoxia/reoxygenation injury.
Figure 2.
Typical chart recordings showing respiration under baseline conditions and after hypoxic stress in mitochondria isolated from control and xenon-preconditioned rats. Oxygen consumption was initiated by addition of pyruvate/malate, accelerated by addition of adenosine diphosphate (ADP) (state 3 respiration), and decelerated after all ADP was consumed (state 4 respiration). After hypoxic stress, state 3 and state 4 respiration were preserved in xenon-preconditioned mitochondria. Open circle = control; Solid circle = xenon preconditioning.
Figure 3.
Effect of xenon preconditioning on isolated mitochondrial bioenergetics. Under baseline conditions, no difference was detected in state 3 and 4 respiration of control and xenon-preconditioned mitochondria. After exposure to hypoxic stress, state 3 respiration of mitochondria from control rats was significantly decreased (Panel A). Respiratory control ratio (RCR) of mitochondria from xenon-preconditioned rats was preserved after hypoxic stress, in contrast to the findings in the absence of xenon (Panel B). CON = control; Xe = xenon preconditioning (n = 7 per group). Values are mean ± sd. *Significantly (P < 0.05) different from baseline.
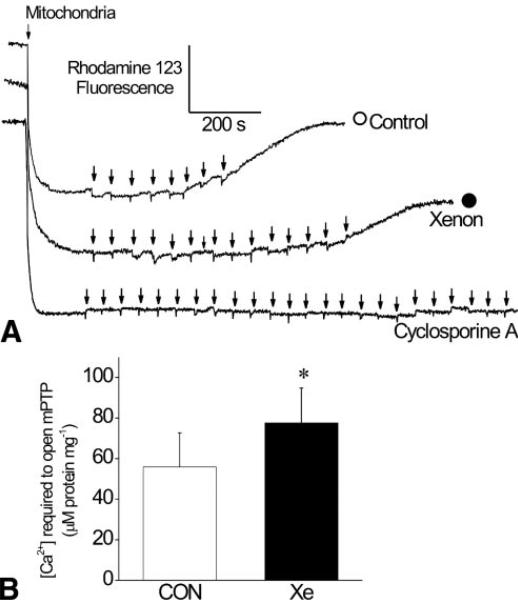
Typical changes in Δϕm after sequential aliquots of CaCl2 (5 μM, arrow) in control (open circle) and xenon-preconditioned (solid circle) rats and in the presence of cyclosporine A are illustrated in Figure 4A. Ca2+ is initially taken up into the mitochondrial matrix and Δϕm remains unaffected. Excess of Ca2+ loading produces sudden Δϕm depolarization, indicating the release of Ca2+ into the medium as a result of mPTP opening. More Ca2+ was required to initiate Δϕm depolarization in xenon-preconditioned compared with control mitochondria. In the presence of cyclosporine A, Δϕm dissipation was not detectable, verifying that depolarization was due to mPTP opening. The Ca2+ concentration required to induce Δϕm depolarization was larger in xenon-preconditioned compared with control mitochondria (Fig. 4B; 78 ± 17 and 56 ± 17 μM, respectively; n = 6 per group).
Figure 4.
Effect of xenon preconditioning on isolated mitochondrial permeability transition pore (mPTP) opening. A typical chart recording showing mitochondrial membrane potential at state 2 with increasing calcium concentration ([Ca2+]) is illustrated in Panel A. The arrows denote the addition of Ca2+ in 5 μM increments. Depolarization was detectable by opening mPTP and releasing Ca2+ from mitochondrial through the mPTP with sequential Ca2+ loading. Xenon preconditioning delayed depolarization, indicating inhibition of mPTP opening. Open circle = control; Solid circle = xenon preconditioning. Depolarization was not observed in the presence of mPTP inhibitor cyclosporine A. Histograms summarizing the results are depicted in Panel B. CON = control; Xe = xenon preconditioning (n = 6 per group). Values are mean ± sd. *Significantly (P < 0.05) different from CON.
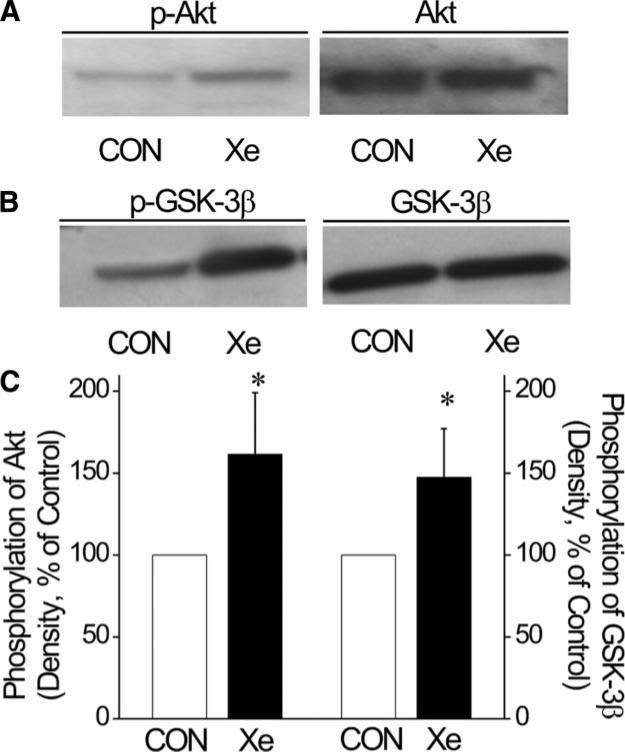
The effect of xenon preconditioning on phosphorylation of Akt (n = 4) and GSK-3β (n = 4) are illustrated in Figure 5. Total Akt and GSK-3β expression were comparable among groups. Xenon preconditioning increased the phosphorylation of Akt at Ser 473 (Fig. 5A) and GSK-3β at Ser 9 (Fig. 5B). Densities of p-Akt and p-GSK-3β relative to total Akt and GSK-3β were greater in xenon-preconditioned compared with control myocardium (Fig. 5C).
Figure 5.
Xenon increased phosphorylation of Akt and glycogen synthase kinase 3 β (GSK-3β) in rat ventricular myocardium. Western blots are shown using antiserum against phosphorylated Akt at Ser 473 (p-Akt) and total Akt (Panel A), and against phosphorylated GSK-3β at Ser 9 (p-GSK-3β) and total GSK-3β (Panel B). Histograms depict the relative density of p-Akt and p-GSK-3β normalized to the total Akt and GSK-3β content, respectively (Panel C). CON = control; Xe = xenon preconditioning (n = 4 per group). *Significantly (P < 0.05) different from CON.
DISCUSSION
The current results confirm previous findings that brief exposure to 70% xenon before coronary artery occlusion and reperfusion reduces myocardial infarct size in rats in vivo.4,6,8 In the present study, cardioprotection by xenon against ischemia-induced damage was obtained at the nonanesthetic dose of 70%. The minimum alveolar concentration value for xenon in rats is significantly higher compared to humans and has been reported to be 1.61 atmosphere15 compared to about 70% in humans.16 Thus, if anesthetic efficacy is used as a measure, and if xenon at 1.61 atm in rats is equivalent to xenon at 70% in humans, then 70% xenon in rats would correspond to about 30% in humans. Xenon is essentially chemically inert, but induced dipoles in its large electron field allow xenon to interact with amphiphilic domains of proteins and membrane.17 The precise mechanism by which this interaction produces selective biological effects remains unknown. Xenon produces minimal hemodynamic effects and may be especially suitable as an inhaled anesthetic for patients with cardiac risk factors undergoing surgery.18-20 Xenon and volatile anesthetics exert very similar actions on cardiac and neuronal signal transaction. The current results extend this observation by implicating prosurvival signaling kinases (Akt, GSK-3β) in xenon preconditioning that have been shown to mediate cardioprotection by volatile anesthetics.1-3
Mitochondria play an essential role in the development of ischemia and reperfusion injury, but also in the protection against it. Recently, we reported that isoflurane preconditioning elicits partial mitochondrial uncoupling, an action that preserves cell viability during the conditions of metabolic challenge.11 The current results are supported, to some degree, by these previous findings. However, in the current study the RCR (a measure of mitochondrial coupling) under baseline condition was not altered in isolated ventricular mitochondria obtained from xenon-preconditioned rats. Since mitochondrial uncoupling was not observed in xenon-preconditioned mitochondria, this phenomenon may not be required for protection of mitochondria, as xenon exposure still preserved mitochondrial function after hypoxia and reoxygenation. Opening of mPTP contributes to cell death after ischemia/reperfusion injury.21-22 Ischemic and anesthetic pre- and postconditioning have been shown to inhibit mPTP opening.23-25 The current results indicate brief, intermittent xenon exposure attenuated Ca2+-induced mPTP opening in vitro, as demonstrated by larger amounts of Ca2+ required for dissipating Δϕm. Collectively, these data suggest that cardioprotection by xenon occurs as a consequence of a mechanism inherent to the mitochondria, independent of the cytosolic environment.
The current findings strongly suggest that xenon preconditioning phosphorylates two key proteins known to modulate the transition state of mPTP. Activation of several endogenous cardioprotective signaling pathways has been shown to be essential for xenon-induced cardioprotection.4,6-8 Interestingly, other noble gases without anesthetic properties have also recently been demonstrated to produce cardioprotection by activating prosurvival signaling kinases and inhibiting mPTP opening in rabbits.26 The exact manner in which xenon and other anesthetic stimuli activate prosurvival signaling pathways remains unknown. The involvement of G protein-coupled receptors has been discussed27 as well as reactive oxygen species (ROS) derived from mitochondria.28 The downstream targets of many of these enzymes have yet to be fully characterized. Nevertheless, the participation of several prosurvival kinases in protection of the myocardium against ischemia has been well described. Activation of PI3K, which is blocked by wortmannin, leads to sequential phosphorylation and thereby activation of phosphoinositide-dependent kinase −1 and Akt.29 PI3K and phosphoinositide-dependent kinase −1may also activate PKC.30 Activated Akt stimulates the activity of several antiapoptotic proteins, such as Bcl-2, endothelial nitric oxide synthase and Mdm2,30 and also inhibits GSK 3β. GSK-3β mediates the convergence of intracellular prosurvival signaling kinases, including Akt, mammalian target of rapamycin, 70 kDa ribosomal s6 kinase, PKC and protein kinase.31 The activity of GSK-3β is inhibited by phosphorylation through activated Akt,32 and inhibition of GSK-3β prevents opening of mPTP.33 The precise mechanism by which phosphorylation of GSK-3β deactivates the enzyme and prevents mPTP opening during reperfusion remains unclear. Nevertheless, our results demonstrating that xenon preconditioning increases phosphorylation of Akt and GSK-3β may represent a link between the activation of these enzymes and protection of mitochondrial function by rendering mPTP less susceptible to Ca2+-induced opening. Our findings, that PI3K-inhibitor wortmannin not only blocked reduction of infarct size but also reversed conservation of mitochondrial RCR, are in agreement with this assumption.
Xenon has been shown to exert substantial organo-protective properties in the brain and in the heart.34 To our knowledge the protective of effect of xenon on other organs has not been studied yet. Considering some of the mechanistic similarities with ischemic preconditioning, it is feasible to assume that other organs, such as the kidney, may also be protected by xenon from ischemic injury. However, it seems that some of the xenon-induced effects are organ-specific, for example the antagonism of aspartate receptors35 in neurons. Thus, ultimately only future experiments will be able to answer the question whether other organs besides brain and heart are protected by xenon.
Xenon-induced, volatile anesthetic-induced and ischemic preconditioning have all been shown to produce a strong cardioprotective effect in the rat heart in vivo, and overall, the mechanistic similarities of those protective strategies are striking. Particularly, prosurvival signaling pathways are shared by all of these strategies, including the involvement of PKC isoforms and downstream kinases, such as p38 and ERK1/2 MAPK. Nevertheless, little is known specifically about the mechanism of xenon preconditioning, and some differences have been pointed out. For example, c-Jun N-terminal kinase MAPK activation has been shown to play a role in ischemic preconditioning,36 but not in xenon-induced preconditioning,8 and its role in APC has not been studied. A microarray study revealed differences between APC versus ischemic preconditioning in transcripts predominantly related to biosynthesis and apoptosis where ischemic preconditioning elicited a postischemic gene expression profile closer to unprotected myocardium than preconditioning with a volatile anesthetic.37 However, a similar study has not been performed for xenon-induced preconditioning. In our study, we confirmed the participation of signaling kinases similarly to ischemic and APC, but also found subtle differences in the effect of the preconditioning agent on mitochondrial function as described above. This is interesting since a direct effect of the preconditioning agent on mitochondria has been suggested to be involved in the triggering of prosurvival pathways, for example through ROS.38 Therefore, future studies will be needed to further investigate the direct effect of xenon on mitochondrial bioenergetics, including ROS production. However, mitochondrial function remained preserved after ischemic stress on mitochondria isolated from xenon-preconditioned rats, similarly as previously reported for isoflurane-induced preconditioning. Thus, in summary, xenon-, anesthetic- and ischemic-induced preconditioning certainly share mechanistic similarities, in particular in regard to signaling pathways, but they also exhibit potentially significant differences in the triggering phase.
The current results should be interpreted within the constraints of several possible limitations. Isolated mitochondria used for assessing mitochondrial function before and after hypoxia and reoxygenation injury constitute an artificial system deprived of a normal cellular environment that most certainly influences their function under control conditions. Nevertheless, this model allowed us to address the question of whether the signaling pathways induce a “protective” memory effect within mitochondria. The experiments were conducted in barbiturate-anesthetized, acutely instrumented rats. Whether similar results also occur in other animal species or humans is unknown. A xenon dose-response relationship was not examined with the current investigation. Whether longer periods of xenon exposure produce relatively larger reductions in infarct size, phosphorylation of Akt or GSK-3β, or protection against Ca2+-induced mPTP opening will require additional study to ascertain. We did not measure further variables of myocardial function, such as left ventricular developed pressure and left ventricular end-diastolic pressure or cardiac output. In order to explain the observation that, in spite of decreased infarct size in the xenon group, we did not find differences in mean arterial blood pressure between control and xenon groups, we speculate that a decrease in cardiac output was paralleled by an increase in systemic vascular resistance in the controls. Sympathetic nerve activity is activated to compensate for an attenuation of contractility, leading to tachycardia and increasing afterload. Myocardial infarct size is determined primarily by the size of the area at risk and the extent of coronary collateral perfusion. The area at risk expressed as a percentage of total LV mass was similar between groups in the current investigation, and coronary collateral blood flow is minimal in rats.39 Thus, differences in collateral perfusion among groups probably did not account for the observed results in rats, but coronary collateral blood flow was not specifically measured. Coronary venous oxygen tension was not directly measured nor was myocardial oxygen consumption calculated. Rate-pressure product, an index of myocardial oxygen consumption, was similar among groups during coronary occlusion, suggesting that the ischemic burden was not responsible for differences in infarct size in rats pretreated with xenon compared with those that did not receive the noble gas. We used a 30 min LAD occlusion to produce myocardial infarction in rats. Whether xenon produces cardioprotection after more prolonged periods of ischemia is unknown.
In summary, the current results indicate that xenon preconditioning reduces myocardial infarct size, phosphorylating Akt and GSK-3β, inhibiting Ca2+-induced opening of mPTP and preserving mitochondrial function. The data suggest that xenon-induced cardioprotection occurs as a consequence of activation of prosurvival signaling that targets mitochondria and renders them less vulnerable to ischemia-reperfusion injury.
ACKNOWLEDGMENTS
The authors thank Ms. Chiaki Kwok, MS and David A. Schwabe, BSEE (Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, WI) for technical assistance and Shoichi Uezono, MD (Professor, Department of Anesthesiology, Jikei University School of Medicine, Tokyo, Japan) for helpful discussions.
Supported, in part, by National Institutes of Health (Bethesda, MD) grants HL34708 and GM066730 (to Z.J.B.), Gemi Fund (Lindigo, Sweden) (to M.B.), and funds from the Department of Anesthesiology, Medical College of Wisconsin.
REFERENCES
- 1.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of KATP channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Novalija E, Fujita S, Kampine JP, Stowe DF. Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric oxide release in isolated hearts. Anesthesiology. 1999;91:701–12. doi: 10.1097/00000542-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Toller WG, Gross ER, Kersten JR, Pagel PS, Gross GJ, Warltier DC. Sarcolemmal and mitochondrial adenosine triphosphate-dependent potassium channels: mechanism of desflurane-induced cardioprotection. Anesthesiology. 2000;92:1731–9. doi: 10.1097/00000542-200006000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Weber NC, Toma O, Wolter JI, Obal D, Mullenheim J, Preckel B, Schlack W. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-∈ and p38 MAPK. Br J Pharmacol. 2005;144:123–32. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm S, Ma D, Maze M, Franks NP. Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology. 2002;96:1485–91. doi: 10.1097/00000542-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Weber NC, Toma O, Damla H, Wolter JI, Schlack W, Preckel B. Upstream signaling of protein kinase C-∈ in xenon-induced pharmacological preconditioning. Implication of mitochondrial adenosine triphosphate dependent potassium channels and phosphatidylinositol-dependent kinase-1. Eur J Pharmacol. 2006;539:1–9. doi: 10.1016/j.ejphar.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Weber NC, Toma O, Wolter JI, Wirthle NM, Schlack W, Preckel B. Mechanisms of xenon- and isoflurane-induced preconditioning - a potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway. Br J Pharmacol. 2005;146:445–55. doi: 10.1038/sj.bjp.0706324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber NC, Stursberg J, Wirthle NM, Toma O, Schlack W, Preckel B. Xenon preconditioning differently regulates p44/42 MAPK (ERK 1/2) and p46/54 MAPK (JNK 1/2 and 3) in vivo. Br J Anaesth. 2006;97:298–306. doi: 10.1093/bja/ael153. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–42. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology. 2005;103:987–95. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning un-couples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–C1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 12.Warltier DC, Zyvoloski MG, Gross GJ, Hardman HF, Brooks HL. Determination of experimental myocardial infarct size. J Pharmacol Methods. 1981;6:199–210. doi: 10.1016/0160-5402(81)90109-1. [DOI] [PubMed] [Google Scholar]
- 13.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–60. [PMC free article] [PubMed] [Google Scholar]
- 14.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–9. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Koblin DD, Fang Z, Eger EI, 2nd, Laster MJ, Gong D, Ionescu P, Halsey MJ, Trudell JR. Minimum alveolar concentrations of noble gases, nitrogen, and sulfur hexafluoride in rats: helium and neon as nonimmobilizers (nonanesthetics). Anesth Analg. 1998;87:419–24. doi: 10.1097/00000539-199808000-00035. [DOI] [PubMed] [Google Scholar]
- 16.Nakata Y, Goto T, Ishiguro Y, Terui K, Kawakami H, Santo M, Niimi Y, Morita S. Minimum alveolar concentration (MAC) of xenon with sevoflurane in humans. Anesthesiology. 2001;94:611–4. doi: 10.1097/00000542-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Tang P. Amphiphilic sites for general anesthetic action? Evidence from 129Xe-{1H} intermolecular nuclear overhauser effects. Biochim Biophys Acta. 1997;1323:154–62. doi: 10.1016/s0005-2736(96)00184-8. [DOI] [PubMed] [Google Scholar]
- 18.Preckel B, Ebel D, Mullenheim J, Frassdorf J, Thamer V, Schlack W. The direct myocardial effects of xenon in the dog heart in vivo. Anesth Analg. 2002;94:545–51. doi: 10.1097/00000539-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Preckel B, Schlack W, Heibel T, Rutten H. Xenon produces minimal haemodynamic effects in rabbits with chronically compromised left ventricular function. Br J Anaesth. 2002;88:264–9. doi: 10.1093/bja/88.2.264. [DOI] [PubMed] [Google Scholar]
- 20.Stowe DF, Rehmert GC, Kwok WM, Weigt HU, Georgieff M, Bosnjak ZJ. Xenon does not alter cardiac function or major cation currents in isolated guinea pig hearts or myocytes. Anesthesiology. 2000;92:516–22. doi: 10.1097/00000542-200002000-00035. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–9. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–24. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–43. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 25.Piriou V, Chiari P, Gateau-Roesch O, Argaud L, Muntean D, Salles D, Loufouat J, Gueugniaud PY, Lehot JJ, Ovize M. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004;100:581–8. doi: 10.1097/00000542-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, Warltier DC, Pratt PF., Jr Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–9. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 27.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 29.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 30.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–15. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 31.Uchiyama T, Englelman RM, Maulik N, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–9. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 32.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 34.Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology. 2006;105:187–97. doi: 10.1097/00000542-200607000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Nagele P, Metz LB, Crowder CM. Xenon acts by inhibition of non-N-methyl-D-aspartate receptor-mediated glutamatergic neurotransmission in Caenorhabditis elegans. Anesthesiology. 2005;103:508–13. doi: 10.1097/00000542-200509000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- 37.da Silva R, Lucchinetti E, Pasch T, Schaub MC, Zaugg M. Ischemic but not pharmacological preconditioning elicits a gene expression profile similar to unprotected myocardium. Physiol Genomics. 2004;20:117–30. doi: 10.1152/physiolgenomics.00166.2004. [DOI] [PubMed] [Google Scholar]
- 38.Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–69. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- 39.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21:737–46. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]