Abstract
Background
The goal of HAART is to promote reconstitution of CD4+ T cells and other immune responses. We evaluated the extent and the kinetics of immune reconstitution in HIV-infected children over 144 weeks of successful HAART.
Methods
Thirty-seven children receiving their first HAART regimen had plasma HIV RNA; T cells and subpopulations; T-cell rearrangement excision circles (TREC) DNA; candida, HIVCD4 and HIVCD8 enzyme-linked immunospot measured at regular intervals.
Results
Plasma HIV RNA became undetectable in 81% of patients at 24 weeks and remained undetectable in 77% at 144 weeks. In contrast, CD4+% continuously increased. Distribution of T-cell subpopulations changed rapidly during the first 48 weeks of HAART and more slowly thereafter. At 144 weeks, total, naive and activated CD4+% and naive CD8+% of HIV-infected children were not significantly different from those of healthy age-matched controls, whereas total and activated CD8+% remained elevated. CD4+ and CD8+ TREC content increased only during the first 48 weeks of HAART. They positively correlated with each other and with total CD4+%, naive CD4+% and naive CD8+%. Candida and HIVCD4 enzyme-linked immunospot increased over time reaching peak values at 48 weeks and 144 weeks, respectively. HIVCD8 enzyme-linked immunospot decreased in magnitude over 144 weeks of HAART but retained its breadth. Baseline CD4+% positively correlated with CD4+% and with functional immune reconstitution at week 144, whereas baseline TREC correlated with TREC at week 144.
Conclusion
HIV-infected children acquired normal distribution of CD4+ T cells and other subpopulations and recovered CD4-mediated HIV immunity after 144 weeks of HAART.
Keywords: candida, cell-mediated immunity, children, highly active antiretroviral therapy, HIV, T-cell rearrangement excision circle, T-cell subpopulations
Introduction
HAART significantly decreases and many times suppresses HIV replication, thus promoting recovery of CD4+ T-cell numbers and other immunologic functions [1,2]. HAART, however, is also associated with numerous side effects and antiviral resistance, and, therefore, the current recommendations are to use HAART sparingly. Initiation of HAART is guided by CD4+ T-cell numbers in HIV-infected adults [3]. In children, both the optimal time to initiate HAART and the best laboratory assay to determine such a time still need to be defined [4,5].
Several immunologic parameters reflecting critical functions of the immune system maybe used to assess and/ or predict immune recovery or preservation in response to HAART. The interest in the effect of HAART on the thymic output, which is commonly measured by the abundance of T-cell rearrangement excision circles (TREC) [6,7], derives from the assumption that it contributes to the expansion of the CD4+ T cells [8,9]. Conversely, T-cell activation is considered a critical contributor to CD4+ T-cell consumption during HIV infection [10].
Recovery of immune functions in response to HAART varies with different pathogens [11,12]. Candida-specific immunity is one of the earliest to recover, which is consistent with the disappearance of clinical signs in response to HAART [12,13]. HIV-specific CD8-cell-mediated responses tend to decline with HAARTwhereas CD4-cell-mediated responses have infrequently been demonstrated in chronic HIV infection [14-16]. Both CD4+ and CD8+ T-cell-mediated anti-HIV responses have been associated with control of viral replication in different stages of HIV infection [17-19]. HIV-specific CD8+ T cells inhibit HIV replication in vitro [20-24]. HIV-specific CD4+ T-cell immune responses are regularly demonstrated in long-term non-progressors and have been associated with protection against disease progression [25,26]. Furthermore, individuals who demonstrate CD4+ T-cell-mediated anti-HIV responses during acute retroviral infection have a good long-term prognosis with respect to disease progression [27].
In this study, we examined the changes in TREC, T-cell subpopulations and functional cell-mediated immunity in children who started their first HAART regimen. We evaluated correlates of these immunologic parameters with control of viral replication, increase of CD4+ T cells and recovery of T-cell function.
Patients and methods
Study design
The study, approved by local institutional review boards (IRB), enrolled 3-21-year-old HIV-1 infected children and adolescents into two cohorts: 3-12 and 13-21 years of age. All patients were infected perinatally. The children were either antiretroviral therapy naive or had limited exposure (≤56 days of perinatal prophylaxis or <7 days of cumulative antiretroviral treatment). Patients were required to have plasma HIV-1 RNA of at least 5000 copies/ml at entry. All children received emtricitabine, didanosine and efavirenz once daily [28]. Children discontinued study participation if they developed severe toxicity or virologic rebound defined by plasma HIV RNA of at least 1000 copies/ml on two consecutive measurements. Immunologic assays were performed at weeks 0, 24, 48, 144 or end of study if different from 144 weeks.
TREC assay
TREC were measured using a real-time PCR amplification and laser detection (Taqman) assay. CD4+ and CD8+ T cells from ethylene diamine tetraacetic acid-anticoagulated blood were purified using Rosette-Sep technique (StemCell Technologies, Vancouver, British Columbia, Canada). DNA, extracted from 50 000 CD4+ or CD8+ cells using Qiagen (Hilden, Germany) blood columns, was amplified with a PCR primer pair specific for TREC alongside serial dilutions from 20 to 2 000 000 copies of a TREC standard [6] and negative controls using a Taqman 3700 apparatus (PE Biosystems, Foster City, California, USA). The TREC copy number in each sample was calculated by interpolation on the standard curve, and median results were reported as TREC/million peripheral blood mononuclear cell (PBMC).
Enzyme-linked immunospot (ELISPOT) assays were performed as previously described [14] using candida antigen (Greer), aldithriol-inactivated HIV-1 antigens [29] (which measured predominantly CD4+ T-cell-mediated responses), and HIV-1 Gag, Pol, Nef and Env peptide pools [National Institute of Health (NIH) reagent repository], which measured predominantly CD8+ T-cell responses. The peptide pools consisted of 15-mer overlapping by 11 at final concentrations of 2 μg/ml. In order to accommodate all the peptides, we used two pools each for Gag and Pol and single pools for Nef and Env. Results were expressed as spot forming centers (SFC)/1 × 106 PBMC of antigen-stimulated wells after subtraction of SFC in unstimulated wells. Positive results were defined by at least 20 SFC/1 × 106 PBMC for candida or inactivated HIV virion and at least 100 SFC/1 × 106 PBMC for HIV peptide-stimulated wells after subtraction of background, provided there was a at least two fold increase in SFC in antigen-stimulated wells compared with background.
T-cell-immunophenotyping was performed as per the pediatric and adult AIDS Clinical Trials Group consensus protocol (http://pactg.s-3.com/immlab.htm) using fluorescently labeled anti-CD4, CD8, CD45RA, CD62L, CD28, CD95, CD38 and HLADR monoclonal antibodies (Becton Dickinson, Franklin Lakes, New Jersey, USA). Results are presented primarily as percentage, because absolute numbers vary with age in the pediatric population.
Statistical analyses
Comparison of immunological responses (T-cell distribution and functional immune responses) from time on study to baseline and comparison of the two age cohorts for different responses were analyzed using the Wilcoxon signed-rank test. Normative data from HIV-uninfected children and phenotypic distribution of CD4+ and CD8+ T cells from the P1021 study population were analyzed by t-tests and stratified t-tests. Linear regression was used to investigate correlations of TREC data with T-cell responses.
Results
Baseline characteristics
The study enrolled 37 children, including 17 girls; five white non-Hispanic, 23 black non-Hispanic and nine Hispanic participants; with a median age of 10.5 years including 21 children less than 12 years of age. Baseline CD4+ T cells and plasma HIV RNA values were 17% (range, 1-40%) and 4.7 log10 copies/ml (range, 2.6-6.4 log10 copies/ml), respectively.
Changes in HIV RNA and CD4+% in response to HAART
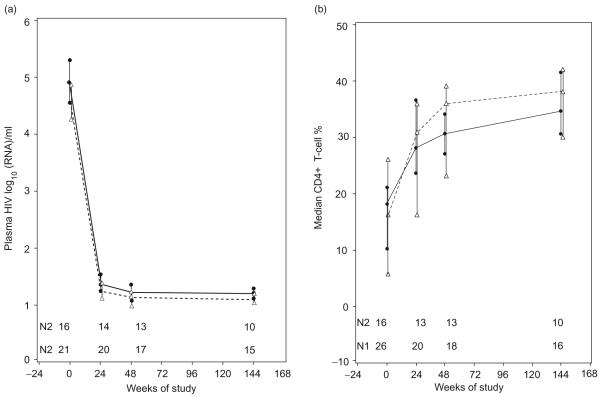
The plasma HIV RNA rapidly decreased and became undetectable (<50 copies/ml) in 81% of the patients at week 24 (Fig. 1a). At week 144, a similar proportion of patients, 77%, had undetectable plasma HIV RNA.
Fig. 1. Kinetics of plasma HIV RNA levels and CD4+% in HIV-infected children on their first HAART regimen.
(a) HIV RNA and (b) CD4+%. Data represent medians, upper and lower quartiles at each time point by age group. The continuous line indicates data derived from 21 children aged 3-12 years and the interrupted line from 16 children aged 13-21 years. N1 indicates the number of patients in the younger group that contribute data at each time point and N2 in the older group.
The CD4+% significantly increased at each visit compared with the immediately preceding one (Fig. 1b). The CD4+ cell numbers increased proportionally to the CD4+% from a median of 310 cells/μl at baseline to 703 cells/μl at week 144 of HAART. CD4+ cell numbers vary with the age of children, whereas CD4+% does not. For this reason, the CD4+% was used as the main parameter in correlation analyses. The CD4+% at baseline was inversely correlated with the plasma HIV RNA (P=0.02) but not at subsequent time points. At all time points during treatment, the CD4+% increased with higher baseline CD4+%(P≤0.02), whereas the time on study to CD4+ percentage of at least 25 decreased with higher baseline CD4+% (P=0.001). Patients with higher baseline CD4+% also tended to increase their CD4+% by at least 5% more rapidly (P=0.07). There were no appreciable differences in virologic or immunologic responses between the two age groups at any time point.
Changes of T-cell subpopulations in response to HAART
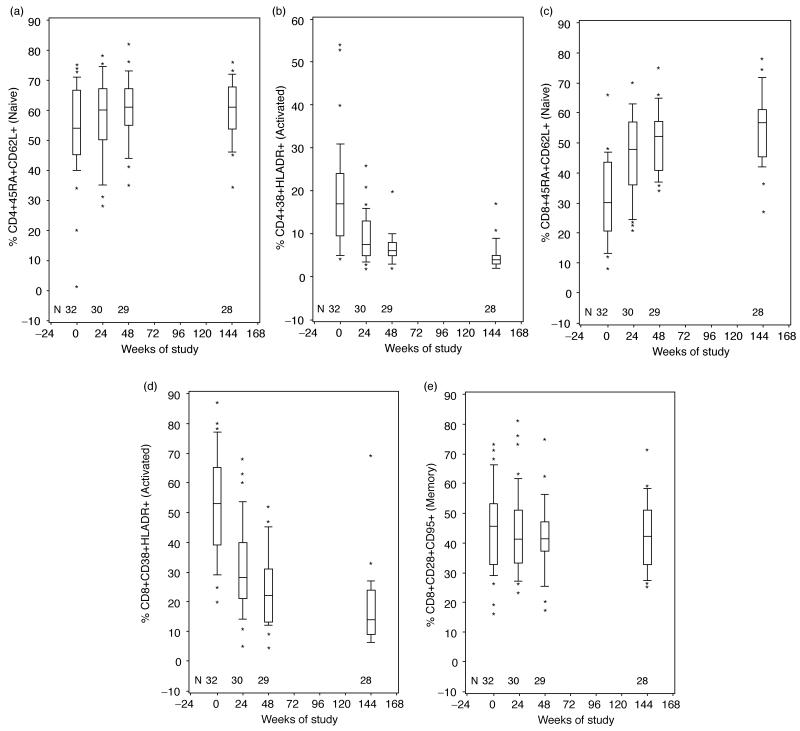
The studies of the T-cell phenotypic distribution showed that naive CD4+CD45RA+CD62L+% increased during the first 48 weeks of HAART (P=0.03) and remained stable thereafter (Fig. 2a). This was confirmed by analysis of the CD4+CD28+CD95-% over time, which showed similar kinetics (not depicted). The memory CD4+CD45RA-% decreased during the first 48 weeks of HAART (P=0.02, not depicted) but not thereafter. There were no significant changes over time of HAART in the central memory CD4+CD28+CD95+% (Fig. 2e). Activated CD4+CD38+HLADR+% decreased during the first 48 weeks of HAART (P<0.0001) but not thereafter (Fig. 2b). Because the CD4+ T-cell number dramatically increased with HAART, the number of all CD4+ T-cell subpopulations also increased with the exception of the CD4+CD38+HLADR+ cells, whose absolute number did not change appreciably between baseline and week 144. There were no appreciable differences between the two age cohorts with respect to changes in CD4+ T-cell subpopulations over time.
Fig. 2. Kinetics of lymphocyte subpopulations in HIV-infected children receiving their first HAART regimen.
Boxes represent upper and lower quartiles with the median as an internal line; whiskers show 95% upper and lower boundaries; and asterisks indicate outliers. N indicates the number of patients contributing data at each time point. (a) Naive CD4+CD45RA+CD62L+%; (b) activated CD4+CD38+HLADR+%; (c) naive CD8+CD45RA+CD62L+%; (d) activated CD8+CD38+HLADR %; (e) memory CD4+CD28+CD95+% over time of HAART.
In contrast to CD4+ T-cell changes, which occurred mostly during the first year of HAART, CD8+ T cells and subpopulations were continuously remodeled during the entire 144 weeks of observation. Naive CD8+CD45RA+CD62L+% (Fig. 2c) and CD8+CD28+CD95-% (not depicted) increased from baseline to week 144 of HAART (P<0.001), whereas memory CD8+CD45RA- or 62L-% continuously decreased (P<0.001; not depicted). Activated CD8+CD38+HLADR+ (Fig. 2d) continuously decreased over the entire period of observation (P<0.001). The absolute cell numbers of the CD8+ T-cell subpopulations mirrored the changes in the corresponding percentages. There were no appreciable differences between the two age cohorts with respect to the phenotypic changes of CD8+ T cells in response to HAART.
To determine the extent to which HAART improved the distribution of T-cell subpopulations, we compared selective CD4+ and CD8+ T-cell subpopulations of our study participants with normative data from HIV-uninfected children [30]. The age-adjusted comparison showed that before HAART, HIV-infected children had on the average two fold lower CD4+% and two fold higher CD8+% compared with uninfected controls (Table 1). Naive, memory and activated CD4+ and CD8+ T-cell subpopulations significantly differed in untreated HIV-infected children compared with uninfected controls. At week 144 of HAART, total, naive and activated CD4+% of HIV-infected children were not appreciably different from those of uninfected controls. CD4+ memory percentage decreased over 144 weeks of HAART in HIV-infected children but remained significantly higher than those of uninfected controls. The total and activated CD8+% remained significantly elevated in HAART recipients compared with healthy controls but the CD8+ naive and memory percentage normalized. These data indicate that after 144 weeks of HAART, HIV-infected children acquired a normal distribution of most CD4+ T-cell subpopulations, while retaining signs of chronic infection such as high total and activated CD8+% and memory CD4+%.
Table 1. Distribution of T-cell phenotypes of HIV-infected children approaches normal standards after 144 weeks of HAART.
| Phenotype | Controla | Week 0b | P valuec | Week 144b | P valued |
|---|---|---|---|---|---|
| CD4+ total | 34 ± 15e | 17 ± 10 | <0.0001 | 36 ± 7 | 0.60 |
| CD4+CD28+CD95- naive | 56 ± 15 | 46 ± 22 | <0.0001 | 55 ± 12 | 0.38 |
| CD4+CD28+CD95+ memory | 39 ± 14 | 45 ± 15 | 0.01 | 43 ± 12 | 0.05 |
| CD4+CD38+HLADR+ activated | 4 ± 4 | 19 ± 13 | <0.0001 | 5 ± 3 | 0.66 |
| CD8+ total | 22 ± 10 | 50 ± 13 | <0.0001 | 31 ± 8 | <0.0001 |
| CD8+CD45RA+CD62L+ naive | 60 ± 15 | 31 ± 14 | <0.0001 | 55 ± 12 | 0.22 |
| CD8+CD45RA-CD62L+ memory | 42 ± 14 | 69 ± 14 | <0.0001 | 45 ± 12 | 0.20 |
| CD8+CD38+HLADR+ activated | 12 ± 11 | 53 ± 18 | <0.0001 | 17 ± 13 | 0.01 |
Measures in healthy children.
Measures in HIV-infected children at weeks 0 and 144 of HAART.
Age-adjusted comparison of week 0 measures in HIV-infected children against uninfected controls.
Age-adjusted comparison of week 144 measures in HIV-infected children against uninfected controls.
Numbers represent means ± SD%.
Changes of the CD4+ and CD8+ T-cell rearrangement excision circle content in response to HAART
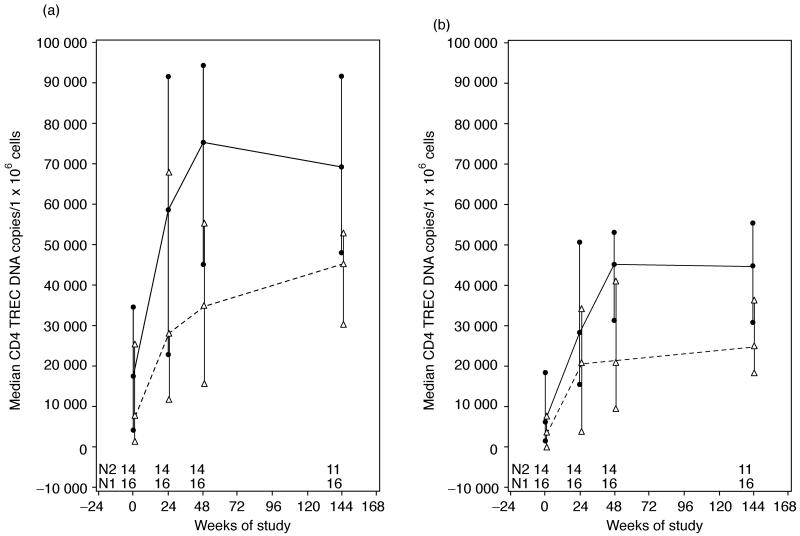
We analyzed the kinetics of DNA TREC copies/CD4+ or CD8+ T cells in our two age groups (Fig. 3a and b). There was a rapid at least four fold increase in the CD4+ and CD8+ TREC content from baseline to week 48 (P<0.0001 for both), after which changes occurred in small increments that did not reach statistical significance. The CD4+ and CD8+ TREC increases were parallel and highly associated with each other at all time points (r2≤0.84; P<0.001). Higher age was a significant determinant of lower CD4+ and CD8+ TREC levels at all time points (P<0.001). CD4+ TREC values positively correlated with total and naive CD4+% at all time points (r2≤0.51; P<0.001). There was a negative correlation of CD8+ TREC values with CD8+% (r2≤0.1; P<0.0001) at all time points, though in the first 24 weeks of therapy, naive CD8+CD45RA+CD62L+% marginally increased with higher CD8+ TREC numbers (P=0.06). Both CD4+ and CD8+ TREC values at week 144 were higher in patients with higher baseline CD4+ and CD8+ TREC values, respectively. The effect of the baseline TREC level on the week 144 TREC level was independent of age.
Fig. 3. Kinetics of CD4+ and CD8+ T-cell rearrangement excision circle content in HIV-infected children on their first HAART regimen.
(a) CD4+TREC; (b) CD8+TREC DNA contents per 106 CD4+ and CD8+ cells, respectively. The continuous line indicates data derived from 21 children aged 3-12 years and the interrupted line from 16 children aged 13-21 years. N1 indicates the number of patients in the younger group that contribute data at each time point and N2 in the older group. TREC, T-cell rearrangement excision circle.
Changes in functional immune responses during HAART
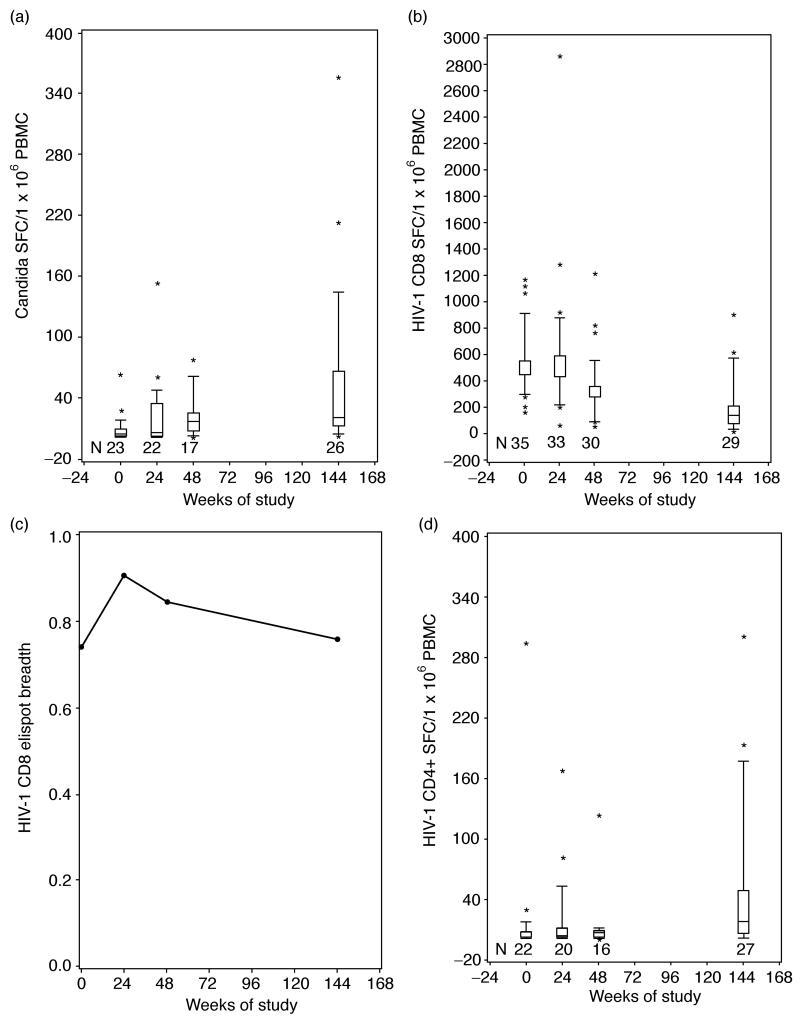
Non-specific changes in CD4+ T-cell-mediated immunity were measured by ELISPOT responses to Candida albicans antigen. The proportion of patients with detectable candida-ELISPOT increased from 61% at baseline to 96% at week 144. Reconstitution of candida-ELISPOT responses peaked at week 48 and remained stable thereafter (Fig. 4a). There were no appreciable differences in candida-ELISPOT values between the two age cohorts at any time points. Correlation analyses were used to identify determining factors of functional immune reconstitution. Candida-ELISPOT values at week 144 were positively associated with higher baseline and week 144 CD4+% (P=0.02 at both time points) and with higher week 144 CD4+CD28+CD95+% (P=0.01).
Fig. 4. Functional immune reconstitution in HIV-infected children receiving their first HAART regimen.
Boxes represent upper and lower quartiles with the median as an internal line; whiskers show 95% upper and lower boundaries; and asterisks indicate outliers. N indicates the number of patients contributing data at each time point. (a) The time course of candida-specific ELISPOT responses; (b) CD8-mediated HIV-specific ELISPOT responses after stimulation with peptide pools derived from Gag, Pol, Nef and Env; (c) the breadth of CD8-mediated HIV-specific ELISPOT responses expressed as a fraction of the number of peptide pools that elicited any response divided by the total number of pools tested; (d) CD4-mediated HIV-specific responses after stimulation with inactivated whole HIV virion. N indicates the number of patients contributing data at each time point. ELISPOT, enzyme-linked immunospot; PBMC, peripheral blood mononuclear cells; SFC, spot forming centers.
CD8+ T-cell-mediated HIV-specific immunity (HIVCD8) was measured by ELISPOTresponses to Gag, Pol, Env and Nef 15-mer peptides overlapping by 11 amino acids (Fig. 4b and c). HIVCD8 ELISPOT values continuously decreased from week 24 to 144 (P<0.003 at all time points compared with the immediately preceding one). However, the breadth of the HIVCD8 ELISPOT, measured by the number of peptide pools that elicited any response divided by the total number of pools tested, did not significantly change on HAART (Fig. 4c). After 144 weeks of HAART, all patients had measurable HIVCD8 ELISPOT at least to one of the peptide pools. Correlation analyses showed no significant association of the HIVCD8 ELSIPOT values with plasma HIV RNA. At week 48 of HAART, the decrease of HIVCD8 ELISPOT values positively correlated with the decrease of CD8+% (P=0.009).
HIV-specific CD4+ T-cell-mediated responses (HIVCD4) were measured by ELISPOT using an inactivated whole virion preparation [29]. The number of patients with detectable HIVCD4 ELISPOT responses increased from 50% at baseline to 89% after 144 weeks of HAART. HIVCD4 ELISPOT took longer to reconstitute and were significantly different from baseline only at 144 weeks of HAART (Fig. 4d). There were no appreciable differences in HIVCD4 ELISPOT values between the two age cohorts at any time points. Correlation analyses showed positive associations between HIVCD4 and candida-ELISPOT values at all time points (P<0.001). Similar to candida-specific ELISPOT, week 144 HIVCD4 ELISPOT values increased with higher central memory CD4+CD28+CD95+% (P=0.01).
Correlations of HIV replication and T-cell activation with immune reconstitution
At baseline, high levels of plasma HIV RNA copies/ml correlated with lower CD4+% T cells (P=0.02). Detectable plasma HIV RNA at week 24 was associated with lower CD4+ and CD8+ TREC content (P≤0.002) at week 48 of HAART. Activated CD4+CD38+HLADR+% tended to increase with higher plasma HIV RNA at baseline and at 48 weeks of HAART (P=0.07). No other appreciable correlations were observed between immune reconstitution and HIV replication.
During the first year of HAART, higher percentage of activated CD4+CD38+HLADR+ was significantly associated with immune dysfunction including lower CD4+% (P<0.001) and lower CD4+ TREC content (P≤0.009). The association of activated CD8+CD38+HLADR+% with immune dysfunction was apparent only at 144 weeks of HAART, including lower CD4+% (P=0.02) and lower candida-ELISPOT and HIVCD4 ELISPOT values (P=0.03 for both).
Discussion
Our data demonstrate that HIV-infected children undergo progressive immune reconstitution in response to HAART that could potentially lead to normalization of immune parameters. Previous studies showed immunologic improvement in HIV-infected children and adults during the first year of HAART [31-33] and continuous increase of CD4+ T cells over 6 years of therapy in children and adults who maintained undetectable plasma HIV RNA [34-36]. Here, we extend these observations by showing that not only CD4+ T cells, but also functional and phenotypic immune measures continue to improve in HIV-infected children over 3 years of effective HAART. A unique feature of immune reconstitution in our study was that after 3 years of HAART, the CD4+% of HIV-infected children was similar to those of healthy age-matched controls. Furthermore, the reconstitution of CD4+ T cells did not differ appreciably between the two age cohorts of 3-6 and 7-21 years enrolled in this study.
It has been long recognized that HIV infection alters the distribution of T-cell phenotypes, which may be partially reversed by HAART [31,33,37,38]. However, this study is the first one to demonstrate complete normalization of T-cell subpopulations including naive and activated CD4+ and naive CD8 T cells. The activated and total CD8+% remained elevated, which may be due to persistent low-level viremia that can be demonstrated even in patients with plasma HIV RNA less than 50 copies/ml [39]. However, as changes in the total and subpopulations of CD8+% were still actively occurring at week 144 of HAART, further improvement, leading perhaps to normal CD8+% after more than 3 years of HAART, could not be ruled out.
The robust reconstitution of T cells and their subpopulations demonstrated in this study may derive from the large thymic reserve associated with the relatively young age of our study participants. We assessed thymic responses to HAART by the TREC content of CD4+ and CD8+ T cells. The TREC content steadily increased in both age cohorts but was consistently higher in the younger group, indicating that the thymus, whose activity increases with younger age, was the main contributor to the TREC rebound. An alternative explanation ascribing the TREC increase to HAART-associated decrease of CD4+ T-cell proliferation [40] is less likely, because it does not explain the significant increase of TREC content with age (when all other responses to HAART did not differ between age groups) nor the significant correlation between CD4+ and CD8+ TREC content increases. The association of CD4+ with CD8+ TREC increases in response to HAART suggests that HIV infection inhibits thymic activity at early stages of T-cell ontogeny, before CD4/CD8 differentiation [41].
While the de-novo generation of CD4+ and CD8+ T cells continuously increased during the first year of HAART, this translated into an increase of CD4+ but not of CD8+ T cells. This finding illustrates that the number of circulating CD4+ and CD8+ T cells is controlled at multiple levels, such that when HAART suppresses viral replication withdrawing the antigenic stimulus for cytotoxic T-cell lymphocyte (CTL) proliferation, HIV-specific CD8+ T cells decrease leading to the contraction of the entire CD8+ T-cell compartment. This is further evidenced by the strong association between the decrease of HIVCD8 ELISPOT values and total CD8+% during the first year of HAART. Ultimately, the CD8+ T-cell compartment contracts during HAART, in spite of increased thymic de-novo CD8+ T-cell production.
Functional immune reconstitution is an important goal of HAART, as ultimately the ability of the immune system to protect the host against opportunistic agents is a critical prognostic factor. Previous studies have shown that the reconstitution of pathogen-specific CD4+ T-cell responses differs with the microbial agent, though the mechanism that underlies this difference is not well understood [12,42,43]. Here, we confirmed rapid reconstitution of Candida and delayed reconstitution of HIV-specific CD4+ T-cell responses. However, Candida and HIVCD4 values positively correlated with each other and with total and memory CD4+% and negatively correlated with activated CD8+%, suggesting that functional immune reconstitution has multiple common features.
CD4+ T-cell-mediated HIV-specific immunity is a hall-mark of long-term non-progression of the infection, but it is unclear whether these responses contribute to the control of viral replication or denote immune preservation [44]. Our study was not designed to address this question, and the analysis of HIVCD4 ELISPOT was exploratory. However, our findings, together with previous ones [14], support pursuing this question in future studies. Finding a positive association between control of viral replication and CD4+ T-cell-mediated responses to HIV may change the currently accepted paradigm that HAART has a deleterious effect on HIV-specific immune defenses.
The concept that HAART decreases HIV-specific immunity stems from the observation that HAART is associated with a decrease in HIV-specific CD8+ CTL [45,46]. These cells have been shown to limit HIV replication in vitro [22-24,47]. The role of CTL in the control of in-vivo retroviral infection was demonstrated in animal models [21,24,48]. In humans, this subject remains controversial particularly with respect to the relative importance of the magnitude against breadth of the CTL response [19,26,49,50]. Our data confirm previous reports that effective HAART is associated with a decrease in the magnitude of HIVCD8 ELISPOT. However, HAART did not affect the breadth of HIVCD8 ELISPOT.
A goal of this study was to increase our understanding of the differential contributions of HIV replication against immune activation to CD4+ T-cell dysfunction. We found significant negative correlations of plasma HIV RNA with CD4+% and thymic output before and during the first year of HAART. This coincided with rapid changes in the CD4+ T-cell numbers and phenotypes. Activated CD4+% not only negatively correlated with CD4+% and thymic output in the first year of HAART, but also tended to positively correlate with HIV plasma RNA over the same period, complicating the understanding of their role in immune suppression. Activated CD8+% did not correlate with plasma HIV RNA but negatively correlated with lower CD4+% and function at week 144 of HAART, when most patients had undetectable viral replication. The model that emerges is that immune recovery of HIV-infected children is a biphasic process, including an early rapid phase in which the decay of viral replication is associated with recovery of thymic activity and repopulation of the T-cell compartment and with recovery of functional responses to mitogens and some antigens. A second phase of immune recovery begins or becomes evident after 6 months to 1 year of HAART, when viremia is stable, but low. During this phase, gains in CD4+ T-cell numbers and function and redistribution of CD4+ T-cell subpopulations continue at a slower rate and negatively correlate with CD8+ T-cell activation. Further studies are needed to determine whether the mechanism that underlies the maintenance of CD8+ T-cell activation after 3 years of HAART is the viral replication, albeit at low levels, and to identify the mediators of the immune suppression after prolonged HAART.
In conclusion, this study showed a robust immune reconstitution in HIV-infected children in response to 3 years of effective HAART, that could be best predicted by the baseline immunologic characteristics of the patients. Baseline CD4+% predicted the recovery of CD4+ T-cell numbers and function in response to HAART, which is in accordance with previous reports [36]. We also showed that thymic output in response to HAART increased with higher thymic function at the initiation of therapy. Although HAART has potential side effects and poses significant adherence problems, its initiation in early stages of HIV infection has clear advantages with respect to immune reconstitution.
Acknowledgement
Part of this work was presented at the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, California, 25-28 Feb 2007.
This study was supported by grant number U01AI068632 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS, contracts NO1-HD-3-3345 from National Institute of Child Health and Human Development and N01-HD-3-3162 (A.W.) and grants AI069441, AI36211 and RR0188 (W.T.S.). Antiretrovirals were provided by Gilead Sciences, Inc., and Bristol-Myers Squibb Company.
A.W. designed the study, analyzed the results and wrote the manuscript; R.D. designed the study, analyzed the results, wrote sections of the manuscript; P.B. performed the statistical analysis and wrote sections of the manuscript; C.H. performed the statistical analysis and wrote sections of the manuscript; J.P.-B. standardized and executed immunological assays and wrote sections of the manuscript; J.K. coordinated the study; H.G. coordinated the laboratory portion of the study; W.T.S. designed and analyzed the immunological studies of uninfected children; M.R. designed and analyzed the study; R.M. designed and analyzed the study. All authors reviewed and gave input into the manuscript preparation.
We thank Anna Vazquez for support with manuscript preparation. We thank the children and their families for their participation in this research and the personnel at the following institutions involved in the conduct of PACTG P1021: St. Jude Children’s Research Hospital, Memphis (Katherine Knapp, MD; Jill Utech, RN, MSN; Sandra Jones, RN, MSN), Chicago Children’s Memorial Hospital (Pediatric) (James McCauley, MD; Maureen Haak, RN), UCSD Mother, Child and Adolescent HIV Program (Rolando Viani, MD, MTP; Anita Darcey, RN, PN), Duke University (Pediatrics) (Carole Mathison,; Yong II Choi, RN; Jean Hurwitz, RPh.; Juliana Simonetti, RN), Harlem Hospital (Maxine Frere, RN; Susan Champion, MD), SUNY Upstate Medical University (Leonard B. Weiner, MD; Kathie A. Contello, NP, MSN; Wendy Holz, MS, PNP; Maureen Famiglietti, BSN, CCRP), University of Miami (Pediatric) (Gwendolyn B. Scott, MD; Charles D. Mitchell, MD; Liset Taybo, MD; Sylvia Willumsen, RN; grant number 5UO1 AI 27560-18), University of Florida (Ayesha Mirza, MD; Ana Alvarez, MD; Saniyyah Mahmoudi, ARNP, MSN; Kathy Thoma, MA), Health Science Center Children’s Hospital, University of Colorado, Denver (Mark Abzug, MD; Emily Barr, CPNP, CNM; Suzanne Paul, RN, CFNP; grant number 5 M01 RR00069, General Clinical Research Centers Program, National Center for Research Resources, NIH). Children’s Hospital of Boston (Sandra Burchett, MD; Catherine Kneut, PNP), Baylor, Texas Children’s Hospital (Nancy Calles, RN; Chivon Jackson, RN; Mary E. Paul, MD), UCSF, Moffitt Hospital (Pediatric) (Diane W. Wara, MD; Deborah Trevithick, RN MS: grant number: 5 M01 RR-01271 [Pediatric Clinical Research Center, University of California San Francisco funded by National Center for Research Resources (NCRR), 5 M01 RR-01271, U.S. Public Health Service]. New York University School of Medicine (Nagamah Deygoo; William Borkowsky, MD; Sulachni Chandwani, MD; Siham Akleh, RN), San Juan City Hospital (Eleanor Jiménez, MD; Midnela Acevedo, MD; Isis Moraima Burgos, RN; Lizbeth Fábregas), University of Puerto Rico, U. Children’s Hospital AIDS (Irma L. Febo Rodriguez, MD; Ruth E. Santos Otero, RN, MPH; Maritza Cruz; Lisette Lugo, MD), University of Massachusetts Medical School (Katherine Luzuriaga, MD).
Footnotes
There was no conflict of interest.
References
- 1.Gortmaker SL, Hughes M, Cervia J, Brady M, Johnson GM, Seage GR, 3rd, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 2.Nesheim SR, Kapogiannis BG, Soe MM, Sullivan KM, Abrams E, Farley J, et al. Trends in opportunistic infections in the pre and posthighly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986-2004. Pediatrics. 2007;120:100–109. doi: 10.1542/peds.2006-2052. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. 2006:1–113. http://www.aidsinfo.nih.gov/
- 4.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children Aa. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2006:1–126. http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 5.Dunn D. Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–1611. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 6.Douek DC, Betts MR, Hill BJ, Little SJ, Lempicki R, Metcalf JA, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 7.Diaz M, Douek DC, Valdez H, Hill BJ, Peterson D, Sanne I, et al. T cells containing T cell receptor excision circles are inversely related to HIV replication and are selectively and rapidly released into circulation with antiretroviral treatment. AIDS. 2003;17:1145–1149. doi: 10.1097/00002030-200305230-00005. [DOI] [PubMed] [Google Scholar]
- 8.Chavan S, Bennuri B, Kharbanda M, Chandrasekaran A, Bakshi S, Pahwa S. Evaluation of T cell receptor gene rearrangement excision circles after antiretroviral therapy in children infected with human immunodeficiency virus. J Infect Dis. 2001;183:1445–1454. doi: 10.1086/320197. [DOI] [PubMed] [Google Scholar]
- 9.De Rossi A, Walker AS, Klein N, De Forni D, King D, Gibb DM. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. J Infect Dis. 2002;186:312–320. doi: 10.1086/341657. [DOI] [PubMed] [Google Scholar]
- 10.Finzi D, Plaeger SF, Dieffenbach CW. Defective virus drives human immunodeficiency virus infection, persistence, and pathogenesis. Clin Vaccine Immunol. 2006;13:715–721. doi: 10.1128/CVI.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourtis AP, Bansil P, Posner SF, Johnson C, Jamieson DJ. Trends in hospitalizations of HIV-infected children and adolescents in the United States: analysis of data from the 1994-2003 nationwide inpatient sample. Pediatrics. 2007;120:e236–e243. doi: 10.1542/peds.2006-3268. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A, Pahwa S, Oyomopito R, Carey VJ, Zimmer B, Mofenson L, et al. Antimicrobial-specific cell-mediated immune reconstitution in children with advanced human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:107–114. doi: 10.1086/420931. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg A, Pott GB. Immunity to human immunodeficiency virus (HIV) in children with chronic HIV infection receiving highly active antiretroviral therapy. Clin Diagn Lab Immunol. 2003;10:821–825. doi: 10.1128/CDLI.10.5.821-825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chougnet C, Jankelevich S, Fowke K, Liewehr D, Steinberg SM, Mueller BU, et al. Long-term protease inhibitor-containing therapy results in limited improvement in T cell function but not restoration of interleukin-12 production in pediatric patients with AIDS. J Infect Dis. 2001;184:201–205. doi: 10.1086/322006. [DOI] [PubMed] [Google Scholar]
- 16.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 17.Alexander L, Cuchura L, Simpson BJ, Andiman WA. Virologic and host characteristics of human immunodeficiency virus type 1-infected pediatric long term survivors. Pediatr Infect Dis J. 2006;25:135–141. doi: 10.1097/01.inf.0000199299.00345.83. [DOI] [PubMed] [Google Scholar]
- 18.Pontesilli O, Klein MR, Kerkhof-Garde SR, Pakker NG, de Wolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 19.Frater AJ, Brown H, Oxenius A, Gunthard HF, Hirschel B, Robinson N, et al. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J Virol. 2007;81:6742–6751. doi: 10.1128/JVI.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 21.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, et al. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 25.Greenough TC, Brettler DB, Kirchhoff F, Alexander L, Desrosiers RC, O’Brien SJ, et al. Long-term nonprogressive infection with human immunodeficiency virus type 1 in a hemophilia cohort. J Infect Dis. 1999;180:1790–1802. doi: 10.1086/315128. [DOI] [PubMed] [Google Scholar]
- 26.Martinez V, Costagliola D, Bonduelle O, N’go N, Schnuriger A, Theodorou I, et al. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J Infect Dis. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 27.Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix ML, Deveau C, et al. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42:709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- 28.McKinney RE, Jr, Rodman J, Hu C, Britto P, Hughes M, Smith ME, et al. Long-term safety and efficacy of a once-daily regimen of emtricitabine, didanosine, and efavirenz in HIV-infected, therapy-naive children and adolescents: Pediatric AIDS Clinical Trials Group Protocol P1021. Pediatrics. 2007;120:e416–e423. doi: 10.1542/peds.2006-0925. [DOI] [PubMed] [Google Scholar]
- 29.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Borkowsky W, Stanley K, Douglas SD, Lee S, Wiznia A, Pelton S, et al. Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced human immunodeficiency virus-infected children. J Infect Dis. 2000;182:96–103. doi: 10.1086/315672. [DOI] [PubMed] [Google Scholar]
- 32.Resino S, Galan I, Perez A, Leon JA, Seoane E, Gurbindo D, Munoz-Fernandez MA. HIV-infected children with moderate/severe immune-suppression: changes in the immune system after highly active antiretroviral therapy. Clin Exp Immunol. 2004;137:570–577. doi: 10.1111/j.1365-2249.2004.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenblatt HM, Stanley KE, Song LY, Johnson GM, Wiznia AA, Nachman SA, Krogstad PA. Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis. 2005;192:445–455. doi: 10.1086/431597. [DOI] [PubMed] [Google Scholar]
- 34.Landay A, da Silva BA, King MS, Albrecht M, Benson C, Eron J, et al. Evidence of ongoing immune reconstitution in subjects with sustained viral suppression following 6 years of lopinavirritonavir treatment. Clin Infect Dis. 2007;44:749–754. doi: 10.1086/511681. [DOI] [PubMed] [Google Scholar]
- 35.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 36.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 37.Valdez H, Connick E, Smith KY, Lederman MM, Bosch RJ, Kim RS, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 38.Goicoechea M, Smith DM, Liu L, May S, Tenorio AR, Ignacio CC, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 39.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 41.Bhandoola A, Sambandam A. From stem cell to T cell: one route or many? Nat Rev Immunol. 2006;6:117–126. doi: 10.1038/nri1778. [DOI] [PubMed] [Google Scholar]
- 42.Connick E, Lederman MM, Kotzin BL, Spritzler J, Kuritzkes DR, St Clair M, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–363. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 43.Havlir DV, Schrier RD, Torriani FJ, Chervenak K, Hwang JY, Boom WH. Effect of potent antiretroviral therapy on immune responses to Mycobacterium avium in human immunodeficiency virus-infected subjects. J Infect Dis. 2000;182:1658–1663. doi: 10.1086/317620. [DOI] [PubMed] [Google Scholar]
- 44.Jansen CA, De Cuyper IM, Steingrover R, Jurriaans S, Sankatsing SU, Prins JM, et al. Analysis of the effect of highly active antiretroviral therapy during acute HIV-1 infection on HIV-specific CD4 T cell functions. AIDS. 2005;19:1145–1154. doi: 10.1097/01.aids.0000176214.17990.94. [DOI] [PubMed] [Google Scholar]
- 45.Sester U, Sester M, Kohler H, Pees HW, Gartner BC, Wain-Hobson S, et al. Maintenance of HIV-specific central and effector memory CD4 and CD8 T cells requires antigen persistence. AIDS Res Hum Retroviruses. 2007;23:549–553. doi: 10.1089/aid.2006.0234. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel HM, DeFalcon E, Ogg GS, Larsson M, Beadle TJ, Tao P, et al. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J Infect Dis. 1999;180:359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara M, Takiguchi M. HIV-1-specific CTLs effectively suppress replication of HIV-1 in HIV-1-infected macrophages. Blood. 2007;109:4832–4838. doi: 10.1182/blood-2006-07-037481. [DOI] [PubMed] [Google Scholar]
- 48.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geldmacher C, Currier JR, Herrmann E, Haule A, Kuta E, McCutchan F, et al. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J Virol. 2007;81:2440–2448. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chouquet C, Autran B, Gomard E, Bouley JM, Calvez V, Katlama C, et al. Correlation between breadth of memory HIV-specific cytotoxic T cells, viral load and disease progression in HIV infection. AIDS. 2002;16:2399–2407. doi: 10.1097/00002030-200212060-00004. [DOI] [PubMed] [Google Scholar]