Abstract
Our previous study showed poor mechanical durability and nano-sized heterogeneities in cross-linked dentin adhesives cured in the presence of water. To further explore the relationship between nano-scale heterogeneities and the long-term mechanical properties of dentin adhesives, the properties of model dentin adhesives polymerized using hydrophilic photoinitiators were compared with those of adhesives polymerized using hydrophobic camphorquinone-based photoinitiators. There was a continuous decline of mechanical properties for the specimens cured in the presence of water during 3 months aqueous storage, especially for the specimens that contained hydrophobic photoinitiators. The multi-component systems containing hydrophilic photoinitiators were shown to produce superior model dental adhesives when these materials are cured in the presence of water.
Keywords: hydrophilic/hydrophobic photoinitiator, dentin adhesive, durability, mechanical property, nanoheterogeneity
Introduction
The long-term success of clinical composite restorations depends in part upon complete and appropriate polymerization. Efficient photoinitiators have been regarded as a basic requirement for complete polymerization, especially for deep cavities. With the hydrophilic and ionic resin monomers used in the simplified total-etch and self-etch adhesives, water may be incompletely removed and remain trapped as “bound” and “free” water at the interface with the dentin substrate 1–5. The presence of water leads to phase separation 6 and the resin will thus contain both hydrophilic and hydrophobic domains during photopolymerization. The common hydrophobic initiators, (e.g., camphorquinone, CQ), and co-initiators (e.g., ethyl-4-(dimethylamino)benzoate, EDMAB), are likely to be distributed preferentially to the hydrophobic domains, impeding the overall polymerization of the adhesive 7. In contrast, some common methacrylate monomers used in dentin adhesives are relatively hydrophilic (e.g., 2-hydroxyethyl methacrylate, HEMA) and are likely to be distributed into the aqueous domains. It is doubtful whether CQ can polymerize such water-soluble monomers in an aqueous solution, and whether CQ can sufficiently initiate the polymerization of hydrophilic monomers that have diffused into the hydrated dentin substrate 8. Therefore, it is of current interest to develop a visible light photoinitiator system for dental resins that will be effective when adhesive may undergo phase separation with both hydrophobic/hydrophilic domains.
Photoinitiated radical polymerization may be initiated by both cleavage (type I) and H-abstraction type (type II) initiators 9. The need for visible light radical photoinitiators has been addressed primarily by using bimolecular initiator systems containing two components. In these systems, active radicals are generally produced via electron transfer and subsequent proton transfer from an amine to a photoexcited molecule 10. The history of hydrophilic photoinitiators dates back to the 19th century 11. Several water-compatible photoinitiators such as benzophenone derivatives and thioxanthone derivatives are known, but their performance is far from ideal, especially for light curing in the visible range. Among them, 3-(3,4-dimethyl-9-oxo-9H-thioxanthen-2-yloxy)-2-hydroxypropyl] trimethyl ammonium chloride (QTX) has been applied in the printing industry and can initiate the polymerization of water-soluble monomers in an aqueous solution on exposure to light 12. Hayakawa et al. reported on the efficacy of QTX-containing dentin primers or hydrophilic bonding agents for the adhesion of composite resin to dentin 13,14, and found that self-etching primers containing QTX could improve the bond strength when used in combination with commercially available bonding agents 15. Kikutake-Sugiyama developed a one-step bonding system using QTX16. The self-etching primer adhesive consisted of 10-methacryloyloxydecyl dihydrogen phosphate, HEMA, QTX, and a tertiary amine, and produced high bond strengths to both enamel and dentin. These studies were mainly focused on the bond strength of composite resin to enamel or dentin 17–19. Few studies of hydrophilic initiators have addressed polymerization kinetics, adhesive resin micro-structure or long-term mechanical stability. Since these properties are critical to the in vivo performance of the dentin adhesive, understanding the relationship of formulation, structure and long-term mechanical properties is of vital importance.
In the last decade, three-component photoinitiator systems have emerged as an improvement over two-component electron transfer initiator systems. Like the two component systems, the three-component initiators include a light absorbing moiety and an electron donor that is almost always an amine. The third component is usually a water soluble iodonium salt 20,21. It serves both to regenerate the photosensitizer by replacing inactive terminating radicals with active phenyl initiating radicals, and also to generate additional active phenyl radicals. Thus three-component systems are flexible, faster, more efficient, and more sensitive than their two-component counterparts in terms of cure rate and polymerization degree 20,21. However, there are few studies in which QTX has been included in a multi-component system.
Previous studies by our group showed the presence of nano-sized heterogeneities in cross-linked dentin adhesives cured in the presence of water. These heterogeneities were detected by Tapping Mode Atomic Force Microscopy (TM-AFM) and Scanning Electron Microscopy (SEM) 22,23, and were associated with poor mechanical durability in these model adhesives 24. In the studies reported here, we have characterized the polymerization behavior and tensile properties of model bisGMA-based adhesives cured in the presence of water and using both hydrophobic and hydrophilic photoinitiator systems. The study explores the effects of additional water-compatible photoinitiators on the mechanical properties of model dentin adhesives, and tests the null hypothesis that the photopolymerization and mechanical properties are unaffected by photoinitiator hydrophobicity.
Materials and Methods
Composition of the model adhesives used in this study
The model adhesives consisted of HEMA (Acros Organics, NJ, USA) and 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]-propane (bisGMA, Polysciences Inc., Washington, PA, USA) with a mass ratio of 45/55 (HEMA/bisGMA). Model adhesives were photopolymerized in the presence of 8.3 mass% water to simulate the wet environment of the oral cavity. The water content was controlled to maintain visually homogenous specimens prior to photopolymerization, which simulated the homogeneous adhesives at the threshold of water absorption 23. This means these formulations present one solution phase prior to photopolymerization based upon visual examination. The model resins were generally obtained using a two or three-component initiator system that contained a photosensitizer, an amine as the co-initiator and an iodonium salt as the third component. The following photoinitiators (all from Aldrich, Milwaukee, WI, USA) were selectively used in the study: camphorquinone (CQ) as a hydrophobic photosensitizer, 3-(3,4-dimethyl-9-oxo-9H-thioxanthen-2-yloxy)-2-hydroxypropyl] trimethyl ammonium chloride (QTX) as a hydrophilic photosensitizer, ethyl-4-(dimethylamino)benzoate (EDMAB) as a hydrophobic co-initiator, 2-(dimethylamino) ethyl methacrylate (DMAEMA) as a hydrophilic co-initiator, and diphenyliodonium hexafluorophosphate (DPIHP) as the iodonium salt (hydrophilic). The amounts of photosensitizer, co-initiator amine and iodonium salt were fixed at 0.5 mol%, 0.5 mol% and 1.0 mass%, respectively, with respect to the total amount of monomer 25. Five-minute sonication (Brason ultrasonic cleaner B-22–4, Fisher Scientific, USA) and 2-day shaking (Orbit Shaker 3520, Lab-Line Instruments Inc., Melrose Park, IL, USA) were required to yield well-mixed resin solutions. All the materials in this study were used as received. The two-component CQ/EDMAB was used as a control initiator system, presenting a very hydrophobic case. The other initiator systems were prepared by varying the hydrophobicity and hydrophilicity of the initiator components, as indicated by their solubilities in water and/or mixtures of monomers. The relative solubility of the photoinitiator components was determined by adding photoinitiator to the medium, sonicating the mixture, and inspecting it visually. The solubility values were reported in milligrams of photoinitiator component per gram of medium.
Polymerization and Degree of Conversion (DC) measurements
The model adhesives were light-cured for 40 s using a dental curing light (UltraLume® LED5, Ultradent, South Jordan, UT, USA) operated at 550 mW/cm2. The photo-polymerization of the model adhesives during irradiation was monitored in situ using a Perkin-Elmer Spectrum One Fourier transform infrared spectrophotometer (FTIR) with a resolution of 4 cm−1 in the ATR sampling mode. A novel time-based spectrum collector (PerkinElmer™ Spectrum TimeBase) was also used to offer continuous and automatic collection of IR spectra of adhesives during polymerization 26. One drop of adhesive solution was placed on the horizontal face of the internal reflectance crystal where total internal reflection occurs. The Zinc Selenide (ZnSe) crystal with a transmission range of 4000~650cm−1 was used in this experiment. The change in the ratio of band intensities measured at 1637 cm−1 (C=C) to that at 1608 cm−1 (phenyl) (i.e., the “intensity band ratio”) was monitored during polymerization 26. The degree of conversion (DC) was calculated using the following equation based on the time-dependent decrease in the absorption intensity band ratios before and after light curing:
Mechanical Testing
The model adhesives were injected into glass tubing (Fiber Optic Center Inc., Vitrocom hollow square capillaries, 1.00 mm square I.D., 0.200 mm wall thickness, borosilicate glass) using a micropipette (Centaur, Labsciences, Inc.) and light-cured. After 24 h the rectangular beam specimens (1×1×11 mm) were pushed out with a small needle point. The mechanical properties were determined using a SSTM-5000 mechanical tester (United Calibration Corporation, CA, USA) with a 150 lb load cell. The tensile properties were determined for all samples after dry storage at room temperature (24 ± 2 °C), or after aqueous storage in distilled deionized water. Specimens were tightly and fully attached to the upper and lower grips using cyanoacrylate cement (Zapit, Dental Ventures of America, Corona, CA, USA) and were loaded at a cross-head speed of 0.5mm/min. The elastic modulus (E, GPa) was measured as the slope of the linear portion of the stress-strain curve between 5% and 15% strain for all samples. Specimen toughness (T, MN/m2) was calculated as the area under the stress-strain curves. At least 24 specimens were prepared for each formulation; these specimens were randomly distributed into three groups, i.e. for 1 day, 30 days and 90 days aqueous storage. The specimens were carefully evaluated for defects under an optical microscope, and those with visible defects were discarded. Eight specimens were tested for each time point. For all experimental groups, the differences between modulus or toughness values were evaluated using one-way analysis of variance (ANOVA), together with Tukey’s test at α=0.05 to identify significant differences in the means. Both toughness and modulus values at each time point were analyzed using separate one-way ANOVA to determine if there was a statistical difference as a function of resin for each storage time point.
Results
The approximate solubilities of the photoinitiator components used in this study are listed in Table 1. As expected, the hydrophobic photoinitiators, CQ and EDMAB, have lower solubility in water than the more hydrophilic photoinitiators such as QTX, DMAEMA and DPIHP. When the solubility is measured in 80:20 mixtures of HEMA and water, both the absolute and relative solubilities differ from those in water (Table 1). With the exception of QTX, absolute solubilities of the photoinitiators in HEMA/water are greater than in water alone. The hydrophobic photoinitiators CQ and EDMAB show approximately 10-fold greater solubilities in HEMA/water mixtures than in water, a reflection of the more hydrophobic environment. Solubilities of hydrophobic photoinitiators CQ and EDMAB are greatest in the relatively hydrophobic bisGMA/HEMA mixtures, as expected, while the cationic QTX shows low solubility in this medium (Table 1). Interestingly, the solubilities of DMAEMA and DPIHP are relatively unaffected by this change in medium hydrophobicity (Table 1).
Table 1.
Comparison of photoinitiator solubility in water and/or monomers.
| Chemicals | Abbr. | Water- solubility (mg/g) |
Solubility in HEMA/Water (80/20) |
Solubility in BisGMA/HEMA (60/40) |
|---|---|---|---|---|
| Photosensitizer | ||||
| Camphorquinone | CQ | ~0.7 mg/g | ~8 mg/g | > 10 mg/g |
| Cationic thioxanthen | QTX | > 10 mg/g | ~3 mg/g | < 0.5 mg/g |
| Coinitiator | ||||
| 2-(dimethylamino) ethyl methacrylate | DMAEMA | > 10 mg/g | > 20 mg/g | > 10 mg/g |
| Ethyl-4- (dimethylamino) benzoate | EDMAB | < 0.5 mg/g | ~5 mg/g | > 10 mg/g |
| Iodonium Salt | ||||
| Diphenyliodonium hexafluorophosphate | DPIHP | ~5 mg/g | > 10 mg/g | ~5 mg/g |
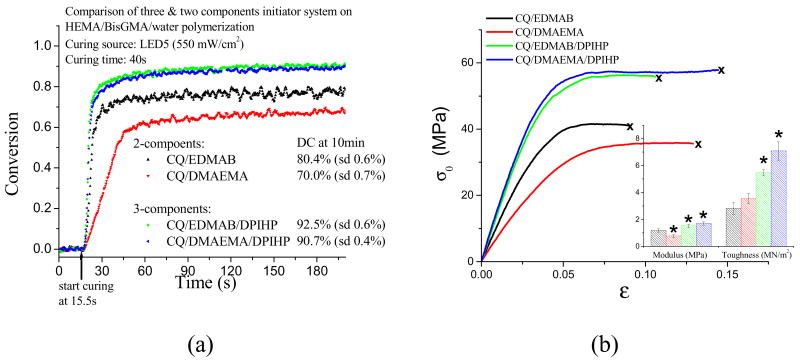
Adding the iodonium salt DPIHP to either the CQ/EDMAB or the CQ/DMAEMA photoinitiator system produced dramatic improvements in polymerization conversion and mechanical properties when cured in the presence of water (Fig. 1). The addition of DHIHP to resins cured with the hydrophobic CQ/EDMAB photoinitiator system caused a significant increase in the ultimate degree of conversion, from 80.4% to 92.5% (Fig. 1a, p < 0.05). Similarly, the addition of DHIHP to resins cured with the CQ/DMAEMA system, containing a hydrophobic initiator (CQ) and a soluble co-initiator (DMAEMA), showed a significant increase in the ultimate degree of conversion from 70.0 % to 90.7% (Fig. 1a, p < 0.05). The resins cured with CQ/DMAEMA also showed a marked increase in the initial rate of polymerization with the addition of DHIHP (Fig. 1a). These improvements in polymerization conversion were paralleled by improved mechanical properties (Fig. 1b). Adhesives cured with photoinitiators containing DHIHP showed significantly greater modulus and toughness values than those without DHIHP (Fig. 1b, p< 0.05).
Fig. 1.
Comparison of two and three component initiator system on photo-polymerization (a) and tensile properties of model adhesives (b). ( * = significantly different from the property of the adhesives prepared with CQ/EDMAB at α=0.05)
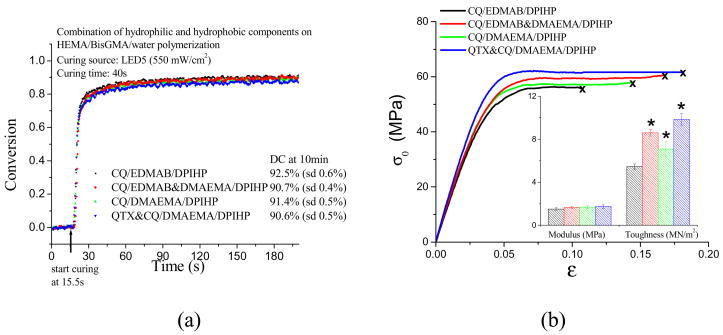
The polymerization conversion and tensile properties for resins cured with DPIHP-containing photoinitiator systems in the presence of water are shown in Figure 2. The photoinitiator systems differ in their expected hydrophobicity (Table 1), with hydrophobicity decreasing in the order CQ+EDMAB+DPIHP > CQ+EDMAB&DMAEMA+DPIHP > CQ+DMAEMA+DPIHP > CQ&QTX+DMAEMA+DPIHP. Differences in DC among the four photoinitiators are modest, particularly when the resins are cured in the presence of water (Fig. 2a). However, the specimens cured with more hydrophilic initiator components show improved modulus and toughness values relative to the CQ/EDMAB control (Fig. 2b & Table 2) (p< 0.05).
Fig. 2.
Effect of additional hydrophilic component on photopolymerization (a) and tensile properties (b) of model adhesives.( * = significantly different from that with sample A prepared with initiators CQ/EDMAB/DPIHP at α = 0.05)
Table 2.
Degree of conversion values and tensile properties of model adhesive resins cured in the presence of 8.3 wt% water.
| Sample ID | Initiators Composition | Modulus of elasticity (GPa) | Toughness (MN/m2) | Degree of Conversion (%) |
|---|---|---|---|---|
| Control | CQ/EDMAB | 1.19 (0.12) | 2.8 (0.4) | 80.4 (0.9) |
| A | CQ/EDMAB/DPIHP | 1.52 (0.11)* | 5.5 (0.2)* | 92.5 (1.3)* |
| B | CQ/DMAEMA/DPIHP | 1.70 (0.12)* | 7.1 (0.7)* | 90.7 (1.8)* |
| C | CQ/EDMAB&DMAEMA/DPIHP | 1.67 (0.09)* | 8.6 (0.3)* | 91.4 (1.5)* |
| D | QTX&CQ/DMAEMA/DPIHP | 1.76 (0.16)* | 9.8 (0.5)* | 90.6 (2.2)* |
= significantly different from control sample prepared with CQ/EDMAB at α = 0.05) The mechanical properties of model adhesives were determined at 1 day storage-in-water.
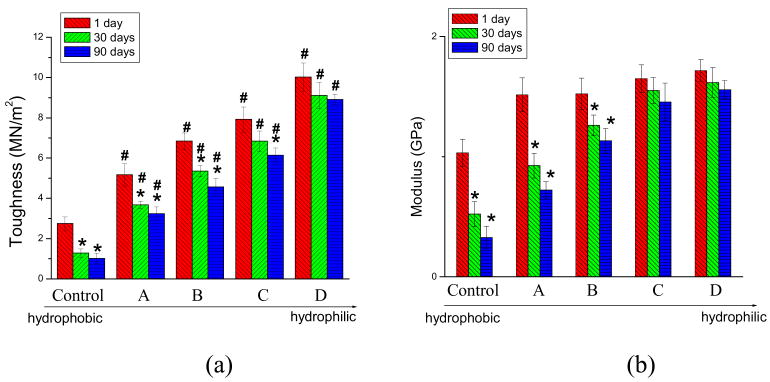
The tensile properties of rectangular beams prepared with different photoinitiator systems and cured in the presence of 8.3 mass% water, were determined after 1, 30 and 90 days of aqueous storage (Fig. 3). There is a continuous decline in tensile properties during the storage period for most of the model adhesive specimens cured in the presence of water. For example, use of the hydrophobic photoinitiator systems (Table 2, CQ/EDMAB, control or CQ/EDMAB/DPIHP, sample A) corresponded to a decline in mechanical properties after 90 days aqueous storage. However, the modulus and toughness values of resins containing more hydrophilic photoinitiator components did not decrease significantly relative to the 1-day values, especially for the adhesive formulations that contained combined hydrophilic/hydrophobic photoinitiator systems (i.e., Samples C and D, Table 2). Thirty days of aqueous storage did not significantly reduce the mechanical properties of these two model adhesives (p>0.05). The combination of hydrophilic/hydrophobic photosensitizer (QTX&CQ/DMAEMA/DPIHP; Sample D, Table 2) even maintained the mechanical properties of the model adhesives without significant change after 90 days aqueous storage (p>0.05). Compared with the control adhesive (cured with CQ/EDMAB; Control, Table 2), the mechanical properties of these optimized formulations were significantly improved at each time point (Fig. 3, p< 0.05).
Fig. 3.
Effect of three months aqueous storage on the mechanical properties (a) toughness; (b) modulus of elasticity of model adhesives cured in the presence of water; n = 8 at each time point. ( * = significantly different from the mechanical property of the same resin at t = 1d, α = 0.05; # = significantly different from the mechanical property of control resin at the same time point, α = 0.05)
Discussion
Among the commercially available dental adhesives, the most widely used are adhesives based on cross-linked glassy polymers prepared from a combination of hydrophobic resin monomers (e.g., bisGMA) and hydrophilic resin monomers (e.g., HEMA) 27. The conventional photoinitiator systems, such as those containing camphorquinone (CQ) combined with a co-initiator aromatic amine (EDMAB or DHEPT), tend to be relatively hydrophobic and show efficient photoreactivity in systems with low water content. Ideally, all solvents and water should be completely eliminated from the adhesive before light-curing, as they may have an adverse effect on polymerization 28. However, as water evaporates from the adhesive, the monomer-to-water ratio increases, and this increased monomer/water ratio impedes the complete removal of water via evaporation 4,29. It is likely that residual water will be trapped within the adhesive resin upon curing and this may compromise the overall bonding and the mechanical properties of the cured resin 5,30. However, little is known of the role of water in more hydrophilic methacrylate-based adhesives which include hydrophilic and/or ionic resin monomers to enable them to bond to intrinsically wet dentin substrates, although it is reasonable to suspect that the hydrophobic photoinitiators result in poor efficiency in such an environment.
In the studies reported here, model resins with the formulation HEMA 45/bisGMA 55 and 8.3 mass% water were selected to simulate homogeneous adhesives which confront the threshold of water/monomer (liquid/liquid) phase separation. These formulations present one solution phase prior to photopolymerization based upon visual examination. Although the water concentration (8.3 mass%) is below that required for visible macro-phase separation, the polymerized resins show nanoheterogeneity in the copolymer network 22,23. Studies of these initially homogeneous formulations facilitate the understanding of the effect of hydrophilic/hydrophobic photoinitiators on the bulk properties of adhesives, such as polymerization behavior, mechanical properties and thermal properties.
The solubility results (Table 1) suggest that the hydrophobic initiators CQ and EDMAB would distribute primarily to the hydrophobic monomer phase, because they show 10-fold greater solubilities in BisGMA/HEMA than in water. It must be noted that the distribution of the initiators in the different phases would make the real solubility different from the case of a simple solubility test. The solubility of the iodonium salt DHIHP is relatively unaffected by the changes in medium hydrophobicity; this component is expected to distribute approximately equally in both hydrophobic and hydrophilic phases. Including DHIHP as a third component in the photoinitiator systems was associated with dramatic improvements in the mechanical properties (Fig. 1b), probably due to increased polymerization conversion and/or the polymerization rate (Fig. 1a). The iodonium salt, which is an electron acceptor, serves both to regenerate the photosensitizer molecules (e.g., CQ) by replacing inactive terminating radicals with active phenyl initiating radicals, and also to generate additional active phenyl radicals 20,21. These characteristics potentially contribute to the observation in this study that DPIHP is an efficient, water compatible initiating component. However, it should be emphasized that the iodonium salt is inactive without the existence of photosensitizer.
Further modification in the photoinitiator-system was based on the addition of hydrophilic components, such as the photosensitizer QTX and the co-initiator DMAEMA. The solubilities of DMAEMA are similar in the hydrophilic medium and hydrophobic monomer mixtures, while the cationic QTX shows low solubility in the hydrophobic medium (Table 1). Including both CQ and QTX in the photoinitiator is expected to allow a photosensitizer to be present in both the hydrophobic and hydrophilic phases. Among Type II photoinitiators, thioxanthone derivatives 31 in conjunction with tertiary amines are efficient photoinitiators with absorption characteristics that compare favorably with benzophenones. The photosensitizer QTX is more hydrophilic and developed by introducing ionic substitutes in the skeleton of the corresponding thioxanthone. Thioxanthone is also one of the most widely used bimolecular photoinitiators in vinyl polymerizations and its photoinitiating activity can be promoted by the presence of a tertiary amine 32–35. The thioxanthone ketyl radical has been detected through the transient appearance of an absorption peak at ~450 nm, with the peak position dependent on the medium and the substituents on the aromatic ring 36.
The four formulations (Samples A-D) made with different photoinitiator systems were selected to provide differences in hydrophilicity. The design of fixing each component concentration in the initiators is based on similar photoefficiency, i.e. two photosensitizers (QTX and CQ) two amine (EDMAB, DMAEMA) are similar in terms of polymerizing the resin exposed to the LED5 curing light (unpublished data). The very close conversion-time curves (Fig. 2a) suggest that there are minimal differences in overall photo-reactivity and average degree of polymerization for formulations prepared with hydrophobic or hydrophilic photoinitiators. However, differences in the mechanical property measurements of hydrated specimens suggest that there are differences in polymer structure despite the similar degree of conversion (Fig. 2b). Thus, conversion data cannot fully represent the material properties, even for resin formulations with identical monomer compositions, a finding consistent with a previous report from our group 25. The hypothesis is rejected, i.e., the photopolymerization and mechanical properties are unaffected by photoinitiator hydrophobicity. Polymers differing in linearity and therefore having different crosslink densities may have similar conversion values. In the present study, incorporating water-compatible photoinitiators in the model adhesive may have enhanced the uniformity of the spatial distribution of the initiator under conditions of phase separation at the micro- or nano-level.
After three months of storage, model adhesives prepared using only hydrophobic photoinitiators showed poor mechanical durability (Fig. 3, Control). Adding the iodonium salt DHIHP improved mechanical properties on storage, this was particularly apparent in systems that contained mixtures of hydrophobic and hydrophilic co-initiators (Fig. 3, C) or hydrophobic and hydrophilic photosensitizers (Fig. 3, D). This suggests that even in heterogeneous adhesives showing micro-level phase separation, the different phases can be polymerized effectively if active radicals are produced by both hydrophobic and hydrophilic initiators. Thus, when designing a cross-linked polymer for a specific application, it is important to understand the network formation and the resulting material properties as each application has specific material requirements. The material properties, such as the molecular weight between cross-links, swelling, and diffusion of a solute within its mesh, are all determined by the extent of cross-linking in the network. Moreover, the degree of heterogeneity acting as a microstructure distribution may have important effects on material properties. Designing initiator systems to perform in this heterogeneous environment may improve the mechanical performance of dentin adhesives, as the results presented here indicate.
Conclusions
For model adhesive resins photopolymerized in the presence of water, the inclusion of an iodonium salt (DHIHP) accelerated the polymerization and improved the polymer mechanical properties both immediately following polymerization and for up to three months of aqueous storage. The specimens cured with more hydrophilic initiator components show improved toughness values and maintained the mechanical properties without significant change relative to the hydrophobic control. The addition of a hydrophilic component to photoinitiator systems may reduce the detrimental effects of nano-scale phase separation on the performance of these adhesives by promoting polymerization of both hydrophilic and hydrophobic domains.
Acknowledgments
This work was supported by grant R01DE14392 (PI: P. Spencer), from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reis AF, Giannini M, Pereira PN. Long-term TEM analysis of the nanoleakage patterns in resin-dentin interfaces produced by different bonding strategies. Dent Mater. 2007;23(9):1164–72. doi: 10.1016/j.dental.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Tay FR, Gwinnett AJ, Pang KM, Wei SHY. An optical, micromorphological study of surface moisture in the total etched resin-dentin interface. Am J Dent. 1996;9:43–48. [PubMed] [Google Scholar]
- 3.Tay FR, Gwinnett AJ, Wei SHY. Micromophological sepctrum from overdrying to overwetting acid-conditioned dentin in water-free, acetone-based, single-bottle primer/adhesives. Dent Mater. 1996;12:236–244. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 4.Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26(34):6863–72. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZX, Huang C, Zheng TL, Wang S, Cheng XR. Effects of residual water on microtensile bond strength of one-bottle dentin adhesive systems with different solvent bases. Chin Med J (Engl) 2005;118(19):1623–8. [PubMed] [Google Scholar]
- 6.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62(3):447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Spencer P, Yao X, Ye Q. Effect of Co-Initiator and Water on the Photoreactivity and Photopolymerization of HEMA/Camphoroquinone-Based Reactant Mixtures. J Biomed Mater Res. 2006;78A(3):580–587. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanuma K. Studies on the development of hydrophilic photocurable dentin bonding agents -The effect of QTX-DMAEMA system. J Jpn Conserv Dent. 1993;36:315–330. [Google Scholar]
- 9.Pappas SP. UV curing science and technology. Norwalk, CT: Technology marketing corp; 1978. [Google Scholar]
- 10.Fouassier JP. Photoinitiation, photopolymerization, and photocuring. Cincinati, OH: Hanser-Gardner; 1995. [Google Scholar]
- 11.Green WA. Water soluble photoinitiators: a review. European coatings journal. 1994;5:274–291. [Google Scholar]
- 12.Davis MJ, Gawne G, Green PN, Green WA. The synthesis and properties of a novel series of water soluble thioxanthone photoinitiators. Polymers Paint Colous Journal. 1985;176:536–545. [Google Scholar]
- 13.Hayakawa T, Horie K. Effect of water-soluble photoinitiator on the adhesion between composite and tooth substrate. Dent Mater. 1992;8(6):351–3. doi: 10.1016/0109-5641(92)90017-7. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T, Nemoto K, Horie K. Adhesion of composite to polished dentin retaining its smear layer. Dent Mater. 1995;11(3):218–22. doi: 10.1016/0109-5641(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa T, Kikutake K, Nemoto K. Influence of self-etching primer treatment on the adhesion of resin composite to polished dentin and enamel. Dent Mater. 1998;14(2):99–105. doi: 10.1016/s0109-5641(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 16.Kikutake-Sugiyama K. Development of one step adhesive system using photo curing self-etching primer. J Jpn Dent Mater. 1999;18:151–169. [Google Scholar]
- 17.Nakanuma K, Hayakawa T, Tomita T, Yamazaki M. Effect of the application of dentin primers and a dentin bonding agent on the adhesion between the resin-modified glass-ionomer cement and dentin. Dental Materials. 1998;14(4):281–286. doi: 10.1016/s0109-5641(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa T, Kikutake-Sugiyama K, Nemoto K. Efficacy of water-soluble photoinitiator on the adhesion of composite resin to bovine teeth in all-in-one bonding system. Dent Mater J. 2005;24(2):213–8. doi: 10.4012/dmj.24.213. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa T, Kikutake-Sugiyama K, Fukushima T, Nemoto K. Development of self-etching primer adhesive in all-in-one bonding system. Dental Materials Journal. 2005;24(2):251–256. doi: 10.4012/dmj.24.251. [DOI] [PubMed] [Google Scholar]
- 20.Fouassier JP, Ruhlmann D, Graff B, Takimoto Y, Kawabata M, Harada M. A New 3-Component System in Visible Laser-Light Photoinduced Polymerization. Journal of Imaging Science and Technology. 1993;37(2):208–210. [Google Scholar]
- 21.Padon KS, Scrantion AB. In recent research developments in polymer science. Trivandrum, India: 1999. pp. 369–385. [Google Scholar]
- 22.Ye Q, Spencer P, Wang Y. Nanoscale patterning in crosslinked methacrylate copolymer networks: an atomic force microscopy study. J Appl Polym Sci. 2007;106:3843–3851. doi: 10.1002/app.27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Q, Wang Y, Spencer P. NanoPhase Separation in Polymers Exposed to Simulated Oral Environment. J Biomed Mater Res Appl Biomater. 2008;(special issue) doi: 10.1002/jbm.b.31047. early view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. Effect of Nanophase Separation on Dentin Adhesive Durability. J Dent Res. 2008 doi: 10.1177/154405910808700911. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res A. 2007;80(2):342–50. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res Appl Biomater. 2007;80B:440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes MFEJS, Perdigao J. Effects of adhesive composition on microtensile bond strength to human dentin. Am J Dent. 2001;14:340–343. [PubMed] [Google Scholar]
- 28.Jacobsen T, Soderholm K-J. Some Effects of Water on Dentin Bonding. Dental Materials. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 29.Pereira PNR, Okuda M, Nakajima M, Sano H, Tagami J, Pashley DH. Relationship between bond strengths and nanoleakage: evaluation of a new assessment method. Am J Dent. 2001;14:100–104. [PubMed] [Google Scholar]
- 30.Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 31.Aydin M, Arsu N, Yagci Y. One-component bimolecular photoinitiating systems, 2 - Thioxanthone acetic acid derivatives as photoinitiators for free radical polymerization. Macromolecular Rapid Communications. 2003;24(12):718–723. [Google Scholar]
- 32.Corrales T, Peinado C, Catalina F, Neumann MG, Allen NS, Rufs AM, Encinas MV. Photopolymerization of methyl methacrylate initiated by thioxanthone derivatives: photoinitiation mechanism. Polymer. 2000;41(26):9103–9109. [Google Scholar]
- 33.Valderas C, Bertolotti S, Previtali CM, Encinas MV. Influence of the amine structure on the polymerization of methyl methacrylate photoinitiated by aromatic ketone/amine. Journal of Polymer Science Part a-Polymer Chemistry. 2002;40(16):2888–2893. [Google Scholar]
- 34.Cokbaglan L, Arsu N, Yagci Y, Jockusch S, Turro NJ. 2-mereaptothioxanthone as a novel photoinitiator for free radical polymerization. Macromolecules. 2003;36(8):2649–2653. [Google Scholar]
- 35.Yang JW, Zeng ZH, Chen YL. Amine-linked thioxanthones as water-compatible photoinitiators. Journal of Polymer Science Part a-Polymer Chemistry. 1998;36(14):2563–2570. [Google Scholar]
- 36.Ferreira GC, Schmitt CC, Neumann MG. Dependence of the thioxanthone triplet-triplet absorption spectrum with solvent polarity and aromatic ring substitution. Journal of the Brazilian Chemical Society. 2006;17(5):905–909. [Google Scholar]