Abstract
Background
Mannose-binding lectin (MBL) is part of the lectin pathway of complement activation against various pathogens; however, its role in innate immune responses against HIV-1 infection in children is unknown.
Objective
This study evaluated the effects of mannose-binding lectin-2 (MBL2) alleles on HIV-1 disease progression and central nervous system (CNS) impairment in children.
Methods
A cohort of 1037 HIV-1-infected children enrolled in Pediatrics AIDS Clinical Trial Group protocols P152 and P300 before the availability of effective antiretroviral therapy was genotyped for MBL2 and evaluated for disease progression.
Results
Children with the homozygous variant MBL2-O/O genotype were more likely to experience rapid disease progression and CNS impairment than those with the wild-type AA genotype. The effects were predominantly observed in children younger than 2 years. In unadjusted Cox proportional hazards models, children younger than 2 years with MBL2-O/O experienced more rapid disease progression (O/O vs AA: relative hazard [RH], 1.54; 95% CI, 1.07-2.22; P = .02; O/O vs A/O: RH, 2.28; 95% CI, 1.09-4.79; P = .029). Similarly, children with MBL2-O/O were more likely to experience rapid progression to CNS impairment (O/O vs A/A: RH, 2.78; 95% CI, 1.06-2.69, P = .027; O/O vs A/O: RH, 1.69; 95% CI, 1.07-7.21; P = .035). The effects remained significant after adjustment for CD4+ lymphocyte count, plasma HIV-1 RNA, and other genotypes.
Conclusions
MBL2-O/O genotypes, which result in lower expression of MBL, are associated with more rapid HIV-1-related disease progression, including CNS impairment, predominantly in children younger than 2 years. These data suggest that MBL2 variants are associated with altered HIV-1 disease progression, particularly in young children.
Keywords: Mannose binding lectin-2, polymorphisms, HIV-1 disease, central nervous system impairment, children
Innate immunity comprises antigen-nonspecific defense mechanisms that a host uses immediately or within hours after exposure to a broad spectrum of antigens to eliminate microbes and prevent infection. Unlike adaptive immunity, innate immunity is designed to recognize a few highly conserved structures present in many different microorganisms. Mannose-binding lectin protein (MBL), also called mannose-binding protein, encoded by the mannose binding lectin-2 (MBL2) gene, is an important determinant of the innate immune response during an infection.1-4 MBL is an acute-phase protein that is synthesized by the liver and released into the blood-stream, where it binds to the mannose residues present on some bacteria, yeast, viruses, and parasites. Binding activates the lectin complement pathway and production of C3b protein through MBL-associated serine proteases,5 which results in opsonization of pathogens, chemotaxis, activation of leukocytes, and direct killing of pathogens.
The MBL2 gene encodes 32-kd subunits that oligomerize to form 96-kd MBL structural units or “monomers.” The “monomers” then further associate to form high-molecular-weight MBL oligomers.4 Only the high-molecular-weight oligomer structure is capable of activating complement. MBL2 variants at the following nucleotide positions affect MBL levels: 2 single nucleotide polymorphisms at promoter positions -550-G/C (H/L variant) and -221-G/C (X/Y variant), one in the 5′ untranslated region +4-C/T (P/Q variant) and 3 genetic variants at codons 52, 54, and 57 in exon 1 at nucleotide positions 223-C/T (Arg52Cys, A/D allele), 230-G/A (Gly54Asp, A/B allele), and 239-G/A (Gly57Glu, A/C allele), respectively. MBL2 exon 1 variants result in single amino acid changes affecting oligomerization of MBL. Homozygous wild-type (A/A) sera contain predominantly fully functional MBL, whereas homozygous mutant sera (any combination of B, C, or D alleles) contain mostly low-molecular-weight MBL. Sera containing heterozygous variant alleles (A/O, O = B, C, or D) contain both high-molecular-weight and low-molecular-weight MBL, with the ratio determined by the promoter type on the normal haplotype (A allele).
MBL deficiency was initially recognized as an opsonic defect in children with frequent unexplained infections and has been linked to increased severity and incidence of complications for several inherited immunodeficiency and autoimmune diseases.6-11 MBL deficiency has also been associated with increased HIV-1 vertical transmission.12 Recently, an age-dependent association of MBL2 variants on the susceptibility to acute respiratory tract infection and meningococcal disease was reported.8,13
The current study examined the distribution of MBL2 genotypes/haplotypes and their effect on HIV-1-related disease progression and central nervous system (CNS) impairment in children. We hypothesized that the presence of genetic variants of MBL2 resulting in production of lower or nonfunctional protein would be associated with susceptibility to HIV infection and disease progression in children. We further hypothesized that younger children with MBL2 variants would experience more rapid HIV-1 disease than older children. Our findings support an age-dependent association of MBL2 variants with HIV-1-related disease in children.
METHODS
Patient populations
Two randomized, double-blind, multicenter Pediatric AIDS Clinical Trial Group (PACTG) protocols, P15214 and P30015, for seroprevalent children with symptomatic HIV-1 infection were designed to compare the effects of mono-therapy with either zidovudine or didanosine and combination therapy with zidovudine plus didanosine and to compare the effects of zidovudine plus lamivudine with didanosine monotherapy and zidovudine plus didanosine combination therapy, respectively. Children from both the studies, PACTG 152 (n = 448) and PACTG 300 (n = 589), were screened for MBL2 exon 1, untranslated region, and promoter region polymorphisms, retrospectively.
Details of characteristics of children, eligibility criteria, study end points, disease progression, and neuropsychological tests used have been described earlier.14-16 The neuropsychological and neurodevelopmental tests administered were the Bayley Scales of Infant Development (3-30 months old), the McCarthy Scales of Children’s Abilities (31 months to 6 years old), and the Wechsler Intelligence Scale for Children: Revised (WISC-R, for 6-15 years, 11 months old) or the Wechsler Adult Intelligence Scale: Revised (WAIS-R; for >16 years of age). The Mental Developmental Index of Bayley scales, the General Cognitive Index of the McCarthy scales, and the full-scale IQ of the WISC-R or WAIS-R were used to assess cognitive function. The standard score for each test was 100, and the SD was 16 points for the Bayley Mental Developmental Index and the McCarthy General Cognitive Index and 15 points for the WISC-R and WAIS-R. Children were considered to have significant developmental delay if they had a standardized score of 70 or less or (almost equal to) 2 SDs less than the normal test score of 100. Informed consent was obtained from study participants. This study followed the human experimentation guidelines of the US Department of Health and Human Services and the University of California, San Diego review board.
Genotyping
MBL2 genotyping was done by means of real-time PCR with melting curve analysis (LightCycler; Roche, Indianapolis, Ind), as described earlier.17 The studied genotypes included 2 single nucleotide polymorphisms at promoter positions -550-G/C (H/L variant) and -221-G/C (X/Y variant), one in the 5′ untranslated region +4-C/T (P/Q variant) and 3 genetic variants at codons 52, 54, and 57 in exon 1 at nucleotide positions 223-C/T (Arg52Cys, A/D allele), 230-G/A (Gly54Asp, A/B allele), and 239-G/A (Gly57Glu, A/C allele), respectively.
Statistical analyses
The primary end points for the analyses were either time to progression to first clinical HIV-1-related disease end point or death, which constituted the progression-free survival (PFS). The disease progression included weight-growth failure, ≥2 opportunistic infections, malignancy, and/or a new abnormality of the CNS (eg, neurologic deterioration, a decrease in neurocognitive test scores, and/or brain growth failure). The CNS impairment end point, a subset of PFS, was defined as time from entry on the study to the deterioration in brain growth, psychologic function, and/or neurologic status.14-16 Table I provides a summary of end points by age group. The cross-tabulations of MBL2 genotypes/haplotypes by race/ethnicity and age groups were used to evaluate the genotype and allele frequencies.
TABLE I.
Characteristics of children by age groups
| Total (n = 1037) | ≥2 y (n = 559) | <2 y (n = 478) | P value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 469 (45%) | 249 (45%) | 220 (46%) | .6614 |
| Female | 568 (55%) | 310 (55%) | 258 (54%) | |
| Race/ethnicity | ||||
| Black | 625 (60%) | 332 (59%) | 293 (61%) | .4025 |
| Hispanic | 271 (26%) | 155 (28%) | 116 (24%) | |
| White | 141 (14%) | 72 (13%) | 69 (14%) | |
| Follow-up time | ||||
| <12 mo | 300 (29%) | 149 (27%) | 151 (32%) | .3084 |
| ≥12-23 mo | 450 (43%) | 254 (45%) | 196 (41%) | |
| >23-35 mo | 197 (19%) | 109 (19%) | 88 (18%) | |
| >35 mo | 90 (9%) | 47 (8%) | 43 (9%) | |
| PFS events | ||||
| No | 811 (78%) | 484 (87%) | 327 (68%) | <.0001 |
| Yes | 226 (22%) | 75 (13%) | 151 (32%) | |
| Disease progression end point | ||||
| No event | 811 (78%) | 484 (87%) | 327 (68%) | |
| death | 25 (2%) | 1 (0%) | 24 (5%) | |
| ≥2 Opportunistic infections | 7 (1%) | 4 (1%) | 3 (1%) | |
| Weight growth failure | 75 (7%) | 41 (7%) | 34 (7%) | |
| Malignancy | 1 (0%) | 0 (0%) | 1 (0%) | |
| CDC clinical disease category C | 12 (1%) | 4 (1%) | 8 (2%) | |
| All CNS disease progression events: | 106 (10%) | 25 (4%) | 81 (17%) | |
| a. Deterioration of brain growth | 65 (6%) | 21 (4%) | 44 (9%) | |
| b. Deterioration of neurocognitive function | 12 (1%) | 1 (0%) | 11 (2%) | |
| c. Deterioration of neurologic status | 29 (3%) | 3 (1%) | 26 (5%) | |
| Baseline characteristics by age groups | ||||
| Baseline CD4 (%) | ||||
| N | 1,029 | 557 | 472 | <.001 |
| Median | 24 | 22.45 | 26.98 | |
| Baseline CD4 count/mm3 | ||||
| N | 1,029 | 557 | 472 | NA* |
| Median | 774.98 | 596 | 1269.50 | |
| Baseline log10 RNA copies/mL | ||||
| N | 861 | 467 | 394 | <.001 |
| Median | 5.12 | 4.73 | 5.64 | |
| Baseline cognitive score | ||||
| N | 979 | 525 | 454 | .274 |
| Median | 83 | 83 | 83 | |
| Baseline WAZ score | ||||
| N | 1037 | 559 | 478 | <.001 |
| Median | -0.69 | -0.29 | -1.11 | |
Not calculated because younger children generally have higher absolute CD4 counts than older children.
Analyses of time to PFS and CNS impairment end points were performed by using Kaplan-Meier methods, and proportional hazards models were used to investigate the effects of genotype variants on PFS and CNS end points in univariate and multivariate analyses that included baseline covariates (CD4+ lymphocyte count, HIV-1 RNA, treatments, race, and sex), as well as other host genotypes previously found to alter HIV-related disease. We also controlled for potential effects of treatment and treatment failure by including the change in CD4+ lymphocyte counts and plasma HIV-1 viral load during follow-up as time-varying covariates in the Cox proportional hazard model. Based on the hypothesis that younger children with MBL2 variants might have a different experience with regard to disease progression from the older children and because the median age of the children in this cohort was 2.3 years, we performed subgroup analysis using age less than 2 years and ≥2 years as the cutoff points to provide a balanced number of children in each age group. The interaction term between age and MBL2 genotype was also included in the model. Although the results were as hypothesized, with the effects of genotype on outcome most evident within the younger age group, the interaction term between age groups and MBL2 genotypes that were included in the model was not statistically significant. The log-rank test was used for comparisons of survival curves for different genotypes. All P values are 2-sided.
RESULTS
Characteristics of children
Of the 1037 children included in this analysis, 568 (55%) were girls and 469 (45%) were boys. The children evaluated were 60.3% non-Hispanic black, 26.1% Hispanic, and 13.6% non-Hispanic white. Ages ranged from 42 days to 18 years (<2 years, 46%; >2-5 years, 26%; 5-10 years, 19%; and >10 years, 9%). The median age was 2.3 years (10th and 90th percentiles, 0.45 and 9.5 years). Of the 1037 children, 300 (29%) were followed for less than 12 months, 450 (43%) for 12 to 23 months, 197 (19%) for 24 to 35 months, and 90 (9%) for more than 35 months. A total of 226 (22%), of whom 151 (67%) were less than 2 years old, were identified as having HIV-1-related disease progression during the course of the 2 studies. Characteristics of the children by 2 age groups have been summarized in Table I.
Distribution of MBL2 genotypes and haplotypes
MBL2-O allele frequency was 0.27 in non-Hispanic blacks, 0.25 in Hispanics, and 0.21 in non-Hispanic whites. The prevalence of the homozygous wild type (A/A) was similar across all races/ethnicities (see Table E1 in the Online Repository at www.jacionline.org). The heterozygous A/B genotype was less common and the heterozygous A/C genotype was more frequent in the non-Hispanic blacks when compared with either Hispanics or non-Hispanic whites (P < .001). The non-Hispanic black children had the highest C allele frequency (non-Hispanic blacks, 0.20; Hispanics, 0.07; and non-Hispanic whites, 0.05). Of note, the homozygous mutant C/C genotype was found exclusively in the non-Hispanic blacks. The incidence of L/L and Q/Q genotypes was more frequent in the non-Hispanic blacks. The P/P genotype was less common in the non-Hispanic blacks compared with Hispanics or non-Hispanic whites (P < .001). The homozygous Y/Y genotype was the most common, whereas X/X and X/Y genotypes were less frequent in all races/ethnicities. Haplotype LYPA was more common in non-Hispanic blacks compared with Hispanics or non-Hispanic whites, whereas haplotype HYPA was less common in non-Hispanic blacks. The distribution of MBL2 variants did not differ significantly by age groups of less than 2 or more than 2 years (see Table E2 in the Online Repository at www.jacionline.org).
TABLE E1.
Distribution of MBL2 genotypes and haplotypes by race/ethnicity
| Race/ethnicity |
|||||
|---|---|---|---|---|---|
| Total (n = 1037) | Black (n = 625) | Hispanic (n = 271) | White (n = 141) | P value | |
| Exon 1 genotype | |||||
| A/A | 570 (55%) | 335 (54%) | 148 (55%) | 87 (62%) | <.0001 |
| A/B | 137 (13%) | 45 (7%) | 62 (23%) | 30 (21%) | |
| A/C | 223 (22%) | 182 (29%) | 33 (12%) | 8 (6%) | |
| A/D | 45 (4%) | 18 (3%) | 16 (6%) | 11 (8%) | |
| O/O | 62 (6%) | 45 (7%) | 12 (4%) | 5 (4%) | |
| Promoter region genotype | |||||
| Y/Y | 762 (73%) | 472 (76%) | 192 (71%) | 98 (70%) | .4474 |
| X/Y | 248 (24%) | 139 (22%) | 71 (26%) | 38 (27%) | |
| X/X | 27 (3%) | 14 (2%) | 8 (3%) | 5 (4%) | |
| Promoter region genotype | |||||
| H/H | 60 (6%) | 20 (3%) | 24 (9%) | 16 (11%) | <.0001 |
| H/L | 359 (35%) | 171 (27%) | 124 (46%) | 64 (45%) | |
| L/L | 618 (60%) | 434 (69%) | 123 (45%) | 61 (43%) | |
| Untranslated region genotype | |||||
| P/P | 362 (35%) | 159 (25%) | 139 (51%) | 64 (45%) | <.0001 |
| P/Q | 492 (47%) | 317 (51%) | 110 (41%) | 65 (46%) | |
| Q/Q | 183 (18%) | 149 (24%) | 22 (8%) | 12 (9%) | |
| Haplotypes | |||||
| HYPA | 74 (7%) | 25 (4%) | 30 (11%) | 19 (13%) | <.0001 |
| HXPA | 328 (32%) | 156 (25%) | 112 (41%) | 60 (43%) | |
| LYPA | 199 (19%) | 127 (20%) | 48 (18%) | 24 (17%) | |
| LXPA | 221 (21%) | 153 (24%) | 47 (17%) | 21 (15%) | |
| Others | 215 (21%) | 164 (26%) | 34 (13%) | 17 (12%) | |
TABLE E2.
Distribution of MBL2 genotypes and haplotypes by age
| Age groups |
||||
|---|---|---|---|---|
| Total (n = 1037) | ≥2 y (n = 559) | <2 y (n = 478) | P value | |
| Exon 1 genotype | ||||
| O/O | 34 (3%) | 19 (3%) | 15 (3%) | .9650 |
| A/O | 405 (39%) | 219 (39%) | 186 (39%) | |
| A/A | 598 (58%) | 321 (57%) | 277 (58%) | |
| Promoter region genotype | ||||
| Y/Y | 762 (73%) | 416 (74%) | 346 (72%) | .0394 |
| X/Y | 248 (24%) | 135 (24%) | 113 (24%) | |
| X/X | 27 (3%) | 8 (1%) | 19 (4%) | |
| Promoter region genotype | ||||
| H/H | 60 (6%) | 35 (6%) | 25 (5%) | .6483 |
| H/L | 359 (35%) | 188 (34%) | 171 (36%) | |
| L/L | 618 (60%) | 336 (60%) | 282 (59%) | |
| Untranslated region genotype | ||||
| P/P | 362 (35%) | 184 (33%) | 178 (37%) | 0.2501 |
| P/Q | 492 (47%) | 278 (50%) | 214 (45%) | |
| Q/Q | 183 (18%) | 97 (17%) | 86 (18%) | |
Associations between disease status at study entry and MBL2 polymorphisms
At study entry, none of the MBL2 genotypes or haplotypes was significantly associated with CD4+ lymphocyte count, CD4+ lymphocyte percentage, plasma HIV-1 RNA, weight for age z score (WAZ), and sex and cognitive score (data not shown). Additionally, there were differences in baseline characteristics in children younger than 2 years compared with those in children ≥2 years (Table I). Of note, the children younger than 2 years had statistically significant lower baseline WAZ scores.
MBL2 genotypes and HIV-1-related disease progression and CNS impairment in all children
In the whole cohort the children with the MBL2 variant genotypes showed trends toward more rapid disease progression; the results, however, were not statistically significant (Table II). These associations followed the same trends in multivariate analyses, controlling for baseline CD4+ lymphocytes, log10 HIV-1 RNA, treatments, race, sex, or other genotypes (CCR5-wt/Δ32, CCR5-59029, CX3CR1-249-V/I, -280-T/M, or stromal cell-derived factor-1). However, children with the homozygous variant MBL2-O/O genotype experienced more rapid CNS impairment than those with the wild-type A/A genotype (relative hazard [RH]O/O vs A/A, 1.46; 95% CI, 0.98-2.16; P = .054) and those with the heterozygous A/O genotype (RHO/O vs A/O, 2.24; 95% CI, 1.00-5.01; P = .045; see Table III). In addition, children with the O/O genotype showed more rapid CNS impairment than those with either the A/A or A/O genotypes (RHO/O vs (A/O+A/A), 2.15; 95% CI, 1.00-4.64; P = .045). To address the issue of potential bias by race/ethnicity, we controlled for race/ethnicity in a multivariate model to assess the association between disease progression/CNS end points and the MBL2-O/O genotype. The baseline WAZ score was also controlled for the multivariate analyses. The results showed that the significant association of the MBL2-O/O group and CNS disease progression remained. No other MBL2 variants in the promoter or untranslated regions affected HIV-1 disease in children.
TABLE II.
Association of MBL2 genotypes on progression-free survival in children: Whole cohort
| MBL2 genotypes compared | RH (95% CI), P value |
|---|---|
| O/O vs A/O* | 1.45 (0.78-2.72), .24 |
| Adjusted† | 1.90 (0.94-3.85), .07 |
| Adjusted‡ | 1.49 (0.78-2.84), .22 |
| Adjusted§ | 1.46 (0.77-2.76), .25 |
| O/O vs A/A* | 1.29 (0.95-1.76), .10 |
| Adjusted† | 1.37 (0.97-1.94), .07 |
| Adjusted‡ | 1.22 (0.89-1.67), .21 |
| Adjusted§ | 1.34 (0.97-1.84), .078 |
| O/O vs A/O+A/A* | 1.57 (0.86-2.88), .14 |
| Adjusted† | 1.91 (0.97-3.76), .06 |
| Adjusted‡ | 1.49 (0.80-2.75), .20 |
| Adjusted§ | 1.62 (0.87-3.00), .13 |
Unadjusted.
Adjusted for CD4+ lymphocyte count, HIV-1 RNA, treatments, race, and sex.
Adjusted for CCR5-wt/Δ32, -59029-G/A, CX3CR1-249-V/I, -280-T/M, and SDF-1-180-G/A.
Adjusted for MBL2-H/L, MBL2-P/Q, and MBL2-X/Y.
TABLE III.
Association of MBL2 genotypes on CNS progression-free survival in children: Whole cohort
| MBL2 genotypes compared | RH (95% CI), P value |
|---|---|
| O/O vs A/O* | 2.24 (1.00-5.01), .045 |
| Adjusted† | 2.77 (1.13-6.79), .03 |
| Adjusted‡ | 2.51 (1.09-5.81), .03 |
| Adjusted§ | 2.37 (1.02-5.50), .04 |
| O/O vs A/A* | 1.46 (0.98-2.16), .054 |
| Adjusted† | 1.63 (1.06-2.51), .03 |
| Adjusted‡ | 1.38 (0.92-2.06), .12 |
| Adjusted§ | 1.58 (1.04-2.41), .032 |
| O/O vs A/O+A/A* | 2.15 (1.00-4.64), .04 |
| Adjusted† | 2.74 (1.19-6.33), .02 |
| Adjusted‡ | 2.04 (0.94-4.46), .07 |
| Adjusted§ | 2.44 (1.10-5.39), .027 |
Unadjusted.
Adjusted for CD4+ lymphocyte count, HIV-1 RNA, treatments, race, and sex.
Adjusted for CCR5-wt/Δ32, -59029-G/A, CX3CR1-249-V/I, -280-T/M, and SDF-1-180-G/A.
Adjusted for MBL2-H/L, MBL2-P/Q, and MBL2-X/Y.
MBL2 haplotypes and HIV-1-related disease progression and CNS impairment in all children
MBL2 haplotypes were defined as described earlier (eg, the MBL2*HYPA haplotype has -550C>G, -221C>G, 4T>C, 223T>C, 230A>G, and 239A>G variants).18 For combined A/O and H/L genotypes, children with combined heterozygous and homozygous variants (AHOL+OLOL) showed trends toward more rapid disease progression compared with those with the wild-type AHAH haplotype (P = .084). Furthermore, the combined AHOL+OLOL haplotype was associated with more rapid CNS disease progression than was seen in those with the wild-type AHAH haplotype (P = .023). Children with the LYPA haplotype exhibited a higher RH for CNS impairment compared with the combined group with LYPA or LXPO haplotypes (RH, 3.39; P = .053). There were no other significant associations of haplotypes with HIV-1 disease in children.
HIV-1 disease progression and CNS impairment for children younger than 2 versus ≥ 2 years with MBL2 variants
Children younger than 2 years have immature adaptive immune responses and are known to rely heavily on innate immunity. We examined the association of MBL2 variants on HIV-1-related disease for children younger than 2 and ≥2 years (Tables IV and V and Figs 1 and 2).
TABLE IV.
Association of MBL2 genotypes on progression-free survival in children: Age (≥2 vs <2 y)
| MBL2 genotypes Compared | Age ≥2 y: RH (95% CI), P value | Age <2 y: RH (95% CI), P value |
|---|---|---|
| O/O vs A/O* | 0.83 (0.25-2.70), .75 | 2.28 (1.09, 4.79) p=0.029 |
| Adjusted† | 3.73 (0.71-19.6), .12 | 2.21 (0.94-5.15), .067 |
| Adjusted‡ | 0.91 (0.26-3.16), .88 | 2.07 (0.95-4.49), .066 |
| Adjusted§ | 0.88 (0.26-2.95), .84 | 2.27 (1.05-4.88), .036 |
| O/O vs A/A* | 1.05 (0.58-1.90), .87 | 1.54 (1.07-2.22), .02 |
| Adjusted† | 1.00 (0.46-2.17), .99 | 1.38 (0.92-2.08), .12 |
| Adjusted‡ | 1.02 (0.56-1.88), .94 | 1.53 (1.04-2.24), .03 |
| Adjusted§ | 1.11 (0.60-2.03), .74 | 1.53 (1.04-2.25), .029 |
| O/O vs A/O+A/A* | 0.97 (0.30-3.07), .95 | 2.33 (1.14-4.76), .02 |
| Adjusted† | 1.55 (0.35-6.83), .56 | 2.14 (0.98-4.69), .058 |
| Adjusted‡ | 0.87 (0.27-2.87), .82 | 2.19 (1.05-4.55), .036 |
| Adjusted§ | 1.04 (0.32-3.35), .95 | 2.33 (1.12-4.83), .024 |
Unadjusted.
Adjusted for CD4+ lymphocyte count, HIV-1 RNA, treatments, race, and sex.
Adjusted for CCR5-wt/Δ32, -59029-G/A, CX3CR1-249-V/I, -280-T/M, and SDF-1-180-G/A.
Adjusted for MBL2-H/L, MBL2-P/Q, and MBL2-X/Y.
TABLE V.
Association of MBL2 genotypes on CNS progression-free survival in children: Age (≥2 vs <2 y)
| MBL2 genotypes compared | Age ≥2 y: RH (95% CI), P value | Age <2 y: RH (95% CI), P value |
|---|---|---|
| O/O vs A/O* | 1.93 (0.41-9.01), .40 | 2.78 (1.07-7.21), .035 |
| Adjusted† | 4.72 (0.43-52.1), .20 | 3.56 (1.24-10.3), .019 |
| Adjusted‡ | 3.71 (0.67-20.4),.13 | 3.08 (1.11-8.54), .031 |
| Adjusted§ | 2.25 (0.44-11.6), .33 | 3.11 (1.14-8.47), .027 |
| O/O vs A/A* | 1.33 (0.63-2.81), .45 | 1.69 (1.06-2.69), .027 |
| Adjusted† | 1.00 (0.35-2.86), 1.00 | 1.75 (1.06-2.90), .028 |
| Adjusted‡ | 1.46 (0.68-3.13), .33 | 1.65 (1.01-2.69), .045 |
| Adjusted§ | 1.85 (0.82-4.15), .14 | 1.67 (1.02-2.73), .042 |
| O/O vs A/O+A/A* | 1.78 (0.42-7.62), .44 | 2.82 (1.14-7.00), .03 |
| Adjusted† | 1.30 (0.17-10.28), .80 | 3.38 (1.31-8.74), .012 |
| Adjusted‡ | 2.25 (0.50-10.1), .29 | 2.81 (1.10-7.16), .031 |
| Adjusted§ | 2.64 (0.58-12.0), .21 | 3.05 (1.19-7.77), .020 |
Unadjusted.
Adjusted for CD4+ lymphocyte count, HIV-1 RNA, treatments, race, and sex.
Adjusted for CCR5-wt/Δ32, -59029-G/A, CX3CR1-249-V/I, -280-T/M, and SDF-1-180-G/A.
Adjusted for MBL2-H/L, MBL2-P/Q, and MBL2-X/Y.
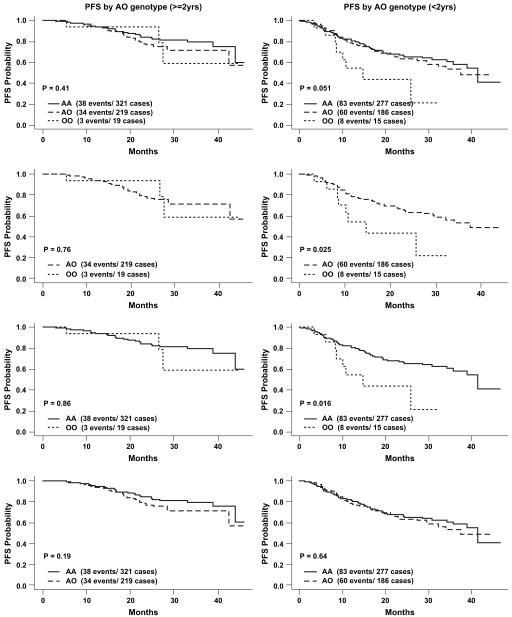
FIG 1.
Association of MBL2 exon 1 genotypes on PFS by age (≥2 vs <2 years).
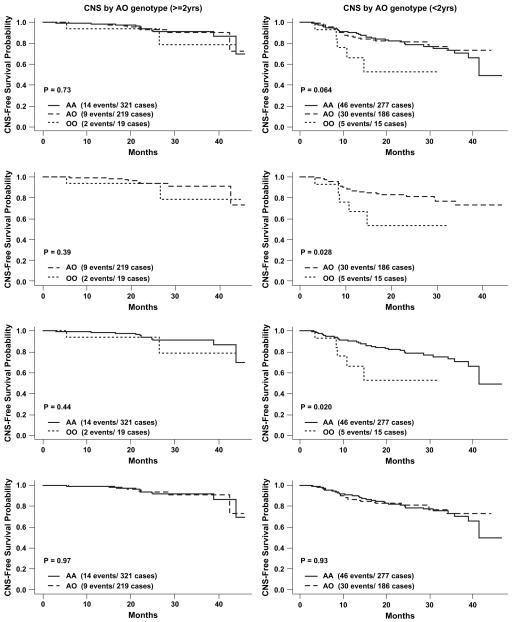
FIG 2.
Association of MBL2 exon 1 genotypes on CNS PFS by age (≥2 vs <2 years).
For the analyses of time to disease progression comparing MBL2 genotypes, children younger than 2 years experienced more rapid disease progression (O/O vs A/O: RH, 2.28; 95% CI, 1.09-4.79; P = .029; O/O vs A/A: RH, 1.54; 95% CI, 1.07-2.22; P = .02; O/O vs A/O+A/A: RH, 2.33; 95% CI, 1.14-4.76; P = .02; Table IV). However, no statistically significant associations were observed in the analyses for children ≥2 years (O/O vs A/O: RH, 0.83; 95% CI, 0.25-2.70; P = .75; O/O vs A/A: RH, 1.05; 95% CI, 0.58-1.90; P = .87; O/O vs A/O+A/A: RH, 0.97; 95% CI, 0.30-3.07; P = .95; Table IV).
The analyses of time to CNS impairment comparing MBL2 genotypes yielded similar results. Children younger than 2 years with O/O genotypes versus A/O genotypes demonstrated more rapid development of cognitive impairment (RHOO vs AO, 2.78; 95% CI, 1.07-7.21; P = .035), whereas this association was again not significant for children ≥2 years (RHOO vs OA, 1.93; 95% CI, 0.41-9.01; P = .40). Similarly, for a comparison between O/O and A/A genotypes, children younger than 2 years experienced more rapid CNS impairment (RHOO vs AA, 1.69; 95% CI, 1.06-2.69; P = .027), whereas children ≥2 years did not (RHOO vs AA, 1.33; 95% CI, 0.63-2.81; P = .45). A comparison between O/O and A/O+A/A genotypes for the children younger than 2 years also showed a difference in CNS impairment (O/O vs A/O+A/A: RH, 2.82; 95% CI, 1.14-7.00; P = .03). The comparison for the children ≥2 years did not show significant effects (see Table V). The associations of MBL2 genotypes and disease progression or CNS impairment when comparing MBL2-O/O versus A/O, A/A, or A/O+A/A remained significant in children younger than 2 years after controlling for the effects of H/L, P/Q, and X/Y alleles. Additionally, children younger than 2 years with P/Q or Q/Q genotypes underwent more rapid CNS impairment compared with those with the wild-type P/P genotype (RHP/Q vs P/P, 1.73; 95% CI, 1.03-2.91; P = .037; RHQ/Q vs P/P, 1.40; 95% CI, 1.01-1.93; P = 0.042).
DISCUSSION
To our knowledge, this is the first comprehensive study of the distribution of MBL2 alleles and their association with HIV-1 disease in children. The homozygous variant MBL2-O/O and L/L promoter genotype, associated with production of nonfunctional MBL, were more common in non-Hispanic black children compared with Hispanic and non-Hispanic white children, suggesting this population might have even a higher risk of HIV-1 infection. The low frequency of the X/X promoter genotype, associated with low MBL expression, suggests that there might be a related altered susceptibility to infection in the US population, regardless of race/ethnicity. It is important to note, however, that we found no differences in the MBL2 polymorphisms studied based on race/ethnicity on disease progression or development of CNS impairment. We found common MBL2 haplotypes in the studied cohort; however, an earlier reported rare HXPA haplotype was also observed.19 At baseline, no effects of MBL2 polymorphisms on HIV-1 disease were observed. The hypothesis that reduced MBL expression and function can compromise immune responses is also supported by the data demonstrating that decreased expression of MBL on lymphocytes is associated with disease progression in HIV-infected adults.20
The presence of the MBL2-O/O genotype, which results in lower expression of MBL protein and impaired innate immunity, was associated with more rapid development of HIV-1-related CNS impairment in children. Similar to earlier studies of the association of MBL2 in HIV-1-infected adults,12,20,21 we observed an overall trend toward a more rapid disease progression in children with variant MBL2 genotypes; however, these results were not statistically significant for the whole cohort. Most strikingly, we observed significant associations between MBL2 variants and disease progression, including cognitive impairment in children younger than 2 years but little evidence of such an effect in older children. Thus older children did not behave as did adults in their susceptibility to more frequent infections. This finding in children older than 2 years likely reflects the high susceptibility that children have to infectious complications associated with advanced HIV-1 disease or AIDS when effective antiretroviral therapy is unavailable. Similar to our study in HIV-1-infected younger children, others have also reported age-related association of MBL2 variants with acute respiratory tract infections and meningococcal disease.8,13 Our results demonstrate that children younger than 2 years who have MBL2 genetic variants that result in lower MBL levels have a significantly greater risk for disease progression and CNS impairment. The findings in children younger than 2 years support a major role of MBL in the immune defense of young children when the adaptive immune system is still immature and passively acquired maternal antibodies are exhausted.22 The presence of an MBL2-O/O variant allele in an HIV-1-infected child suggests a higher risk of disease progression and CNS impairment and could be useful as an indicator of antiretroviral therapy in a child not receiving treatment.
The MBL carbohydrate recognition domain has been shown to interact with the HIV-1 gp120/gp41 complex,23 and dendritic cell-specific intercellular adhesion molecule-grabbing noninte-grin-mediated transinfection of T cells is inhibited by MBL.24 The presence of the MBL2-O/O genotype, which results in lower expression of MBL protein and impaired innate immunity, might lead to impaired viral recognition and increased infection and inflammation associated with HIV-1-related CNS impairment in children. Additionally, modulatory effects of MBL on cytokine responses have been reported.25 Enhanced TNF-α responses were observed when PBMCs from healthy control subjects and HIV-infected patients were stimulated with MBL and costimulated with HIV-1 gp120 or mannan. Thus the presence of a variant allele causing lower expression and function of MBL protein might potentially impair both innate and adaptive immune responses during HIV-1 neuropathogenesis. Deficiency of MBL related to impaired innate and adaptive immune responses could be an important determinant of susceptibility to neurologic diseases. Lower levels of MBL have been reported previously to be associated with meningococcal disease26,27 and Alzheimer’s disease.28
Although the role of MBL2 genetic variants in susceptibility of several diseases is well established, a recent evolutionary analysis of MBL2 alleles suggested that the patterns of MBL2 variation were compatible with neutral evolution, as opposed to negative, positive, or balanced natural selection.29 Accordingly, the high worldwide frequencies of MBL2 alleles associated with the production of little or no protein appear to have evolved from human migration and genetic drift and not survival pressure.
To our knowledge, this is the first study to examine the effect of genetic variants of MBL2 on CNS impairment in HIV-1-infected children. When compared with adults, children have more rapid HIV-1 disease progression, higher absolute numbers of CD4+ lymphocytes, higher viral loads, and more neurocognitive impairment. Thus the effect of MBL2 polymorphisms on HIV-1 infection in children could be fundamentally different from that seen in adults. Although our cohort was a seroprevalent population, the timing of infection for this perinatally infected cohort is known, it does not suffer from widely disparate timing and routes of infection that might be present in adult cohorts, and antiretroviral therapy was well documented. Children in our cohort received 1 or 2 nucleoside reverse transcriptase inhibitors (NRTIs), which were standardized for each participant. Additionally, the high number of clinical end points suggests that the effects of mono-NRTI or dual-NRTI therapy were limited. We also extended the analysis of the associations of MBL2 and disease progression to evaluate whether the associations varied between the study treatments by including interaction terms in the model. However, no significant variations were observed.
In summary, our findings indicate that the presence of MBL2 variants is associated with more rapid HIV-1 disease progression and CNS impairment in children in an age-dependent manner, with the greatest effect observed in children younger than 2 years.
Acknowledgments
Supported by the Pediatric AIDS Clinical Trials Group and the International Maternal Perinatal Adolescent AIDS Clinical Trials (IMPAACT) Network AI068632 and AI-36214 (Virology Core, University of California, San Diego, Center for AIDS Research), and Rest Haven, San Diego, Calif.
Abbreviations used
- CNS
Central nervous system
- MBL
Mannose-binding lectin
- MBL2
Mannose binding lectin-2
- NRTI
Nucleoside reverse transcriptase inhibitor
- PACTG
Pediatric AIDS Clinical Trial Group
- PFS
Progression-free survival
- RH
Relative hazard
- WAIS-R
Wechsler Adult Intelligence Scale: Revised
- WISC-R
Wechsler Intelligence Scale for Children: Revised
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Presented in part at the XIVth Conference on Retroviruses and Opportunistic Infections, Los Angeles, Calif, February 25-28, 2007.
Clinical implications: HIV-1-infected children who have MBL2 genetic variants that result in lower MBL levels have a significantly greater risk for HIV-related complications, including cognitive impairment, particularly young children.
REFERENCES
- 1.Garred P, Larsen F, Madsen HO, Koch C. MBL deficiency-revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 2.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiology. 1998;199:327–39. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick DC. Mannan-binding lectin: clinical significance and applications. Biochim Biophys Acta. 2002;1572:401–13. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 4.Dommett RM, Klein N, Turner MW. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 6.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;334:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 7.Valdimarsson H, Stefansson M, Vikingsdottir T, Arason GJ, Koch C, Thiel S, et al. Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBL-deficient humans. Scand J Immunol. 1998;48:116–23. doi: 10.1046/j.1365-3083.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Koch A, Melbye M, Sorensen P, Homoe P, Madsen HO, Molbak K, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 9.Kakkanaiah VN, Shen GQ, Ojo-Amaize EA, Peter JB. Association of Low Concentrations of Serum Mannose-Binding Protein with Recurrent Infections in Adults. Clin Diagn Lab Immunol. 1998;5:319–21. doi: 10.1128/cdli.5.3.319-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidler KJ, Wilson P, Davies JC, Turner MW, Peters MJ, Klein NJ. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Med. 2004;30:1438–45. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 11.Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496–505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 12.Boniotto M, Crovella S, Pirulli D, Scarlatti G, Spano A, Vatta L, et al. Polymorphisms in the MBL2 promoter correlated with risk of HIV-1 vertical transmission and AIDS progression. Genes Immun. 2000;1:346–8. doi: 10.1038/sj.gene.6363685. [DOI] [PubMed] [Google Scholar]
- 13.Faber J, Schuessler T, Finn A, Murdoch C, Zenz W, Habermehl P, et al. Age-dependent association of human mannose-binding lectin mutations with susceptibility to invasive meningococcal disease in childhood. Pediatr Infect Dis J. 2007;26:243–6. doi: 10.1097/01.inf.0000256751.76218.7c. [DOI] [PubMed] [Google Scholar]
- 14.Englund JA, Baker CJ, Raskino C, McKinney RE, Petrie B, Fowler MG, et al. AIDS Clinical Trials Group (ACTG) Study 152 Team Zidovudine, didanosine, or both as the initial treatment for symptomatic HIV-infected children. N Engl J Med. 1997;336:1704–12. doi: 10.1056/NEJM199706123362403. [DOI] [PubMed] [Google Scholar]
- 15.McKinney RE, Jr, Johnson GM, Stanley K, Yong FH, Keller A, O’Donnell KJ, et al. The Pediatric AIDS Clinical Trials Group Protocol 300 Study Team A randomized study of combined zidovudine-lamivudine versus didanosine monotherapy in children with symptomatic therapy-naive HIV-1 infection. J Pediatr. 1998;133:500–8. doi: 10.1016/s0022-3476(98)70057-5. [DOI] [PubMed] [Google Scholar]
- 16.Singh KK, Barroga CF, Hughes MD, Chen J, Raskino C, McKinney RE, et al. Genetic influence of CCR5, CCR2 and SDF-1 variants on human immunodeficiency virus (HIV-1) related disease progression and neurological impairment in children with symptomatic HIV-1 infection. J Infect Dis. 2003;188:1461–72. doi: 10.1086/379038. [DOI] [PubMed] [Google Scholar]
- 17.Steffensen R, Hoffmann K, Varming K. Rapid genotyping of MBL2 gene mutations using real-time PCR with fluorescent hybridisation probes. J Immunol Methods. 2003;278:191–9. doi: 10.1016/s0022-1759(03)00190-x. [DOI] [PubMed] [Google Scholar]
- 18.Boldt AB, Petzl-Erler ML. A new strategy for mannose-binding lectin gene haplotyping. Hum Mutat. 2002;19:296–306. doi: 10.1002/humu.10051. [DOI] [PubMed] [Google Scholar]
- 19.Boldt AB, Culpi L, Tsuneto LT, de Souza IR, Kun JF, Petzl-Erler ML. Diversity of the MBL2 gene in various Brazilian populations and the case of selection at the mannose-binding lectin locus. Hum Immunol. 2006;67:722–34. doi: 10.1016/j.humimm.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 21.Dzwonek A, Novelli V, Bajaj-Elliott M, Turner M, Clapson M, Klein N. Mannose-binding lectin in susceptibility and progression of HIV-1 infection in children. Antivir Ther. 2006;11:499–505. [PubMed] [Google Scholar]
- 22.Turner MW, Hamvas RM. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–22. [PubMed] [Google Scholar]
- 23.Ji X, Gewurz H, Spear GT. Mannose binding lectin (MBL) and HIV. Mol Immunol. 2005;42:145–52. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Spear GT, Zariffard MR, Xin J, Saifuddin M. Inhibition of DC-SIGN-mediated trans infection of T cells by mannose-binding lectin. Immunology. 2003;110:80–5. doi: 10.1046/j.1365-2567.2003.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heggelund L, Mollnes TE, Ueland T, Christophersen B, Aukrust P, Froland SS. Mannose-binding lectin in HIV infection: relation to disease progression and highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;32:354–61. doi: 10.1097/00126334-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Bax WA, Cluysenaer OJ, Bartelink AK, Aerts PC, Ezekowitz RA, van Dijk H. Association of familial deficiency of mannose-binding lectin and meningococcal disease. Lancet. 1999;354:1094–5. doi: 10.1016/s0140-6736(99)02563-5. [DOI] [PubMed] [Google Scholar]
- 27.Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M. Association of variants of the gene for mannose binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–53. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 28.Lanzrein AS, Jobst KA, Thiel S, Jensenius JC, Sim RB, Perry VH, et al. Mannan-binding lectin in human serum, cerebrospinal fluid and brain tissue and its role in Alzheimer’s disease. Clin Neurosci. 1998;9:1491–5. doi: 10.1097/00001756-199805110-00045. [DOI] [PubMed] [Google Scholar]
- 29.Verdu P, Barreiro LB, Patin E, Gessain A, Cassar O, Kidd JR, et al. Evolutionary insights into the high worldwide prevalence of MBL2 deficiency alleles. Hum Mol Genet. 2006;15:2650–8. doi: 10.1093/hmg/ddl193. [DOI] [PubMed] [Google Scholar]