Abstract
Apraxia of speech (AOS) is a motor speech disorder characterized by slow speaking rate, abnormal prosody and distorted sound substitutions, additions, repetitions and prolongations, sometimes accompanied by groping and trial-and error articulatory movements. Although AOS is frequently subsumed under the heading of aphasia, and indeed most often co-occurs with aphasia, it can be the predominant or even the sole manifestation of a degenerative neurologic disease. In this study we determined whether the clinical classifications of aphasia and AOS correlated with pathological diagnoses and specific biochemical and anatomical structural abnormalities. Seventeen cases with initial diagnoses of a degenerative aphasia or AOS were reclassified independently by two speech-language pathologists — blinded to pathologic and biochemical findings - into one of five operationally defined categories of aphasia and AOS. Pathological diagnoses in the 17 cases were progressive supranuclear palsy in six, corticobasal degeneration in five, frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes in five, and Pick’s disease in one. Voxel-based morphometry and SPECT were completed, blinded to the clinical diagnoses, and clinico-imaging and clinico-pathological associations were then sought. Interjudge clinical classification reliability was 87% (κ =0.8) for all evaluations. Eleven cases had evidence of AOS, of which all (100%) had a pathological diagnosis characterized by underlying tau biochemistry, while five of the other six cases without AOS did not have tau biochemistry (p=0.001). A majority of the 17 cases had more than one yearly evaluation, demonstrating the evolution of the speech and language syndromes, as well as motor signs. Voxel-based morphometry revealed the premotor and supplemental motor cortices to be the main cortical regions associated with AOS, while the anterior peri-sylvian region was associated with non-fluent aphasia. Refining the classification of the degenerative aphasias and AOS may be necessary to improve our understanding of the relationships among behavioral, pathological, and imaging correlations.
Keywords: Premotor cortex, supplementary motor cortex, progressive supranuclear palsy, apraxia of speech, aphasia
INTRODUCTION
The term aphasia designates impairment in the primary domains of language (vocabulary, semantics, phonology, syntax, morphology) that may be manifest in spoken and written comprehension and production but cannot be explained by motor, sensory, or generalized cognitive deficits. Aphasia is most commonly encountered in patients with vascular insults but there is now an extensive literature from multiple authors and institutions documenting it as the dominant clinical feature in some people with a neurodegenerative disease. In fact, aphasia is one of the most frequently cited examples of a focal manifestation of asymmetric cortical degeneration (Black, 1996; Caselli, 1996).
While most authors and clinicians agree that aphasia can be the presenting and predominant feature of neurodegenerative disease (often called primary progressive aphasia, or PPA), there is no universal approach to classifying the aphasia. Mesulam, whose case series in 1982 spurred modern attention to PPA, recognizes both agrammatical/nonfluent and fluent presentations, and notes that anomia is a near-universal finding and that semantically-based verbal comprehension deficits can be present within the symptom complex (Mesulam, 1982). He has also observed that patients with PPA rarely fit classical, stroke-based clinicopathologic patterns of aphasia (e.g., Broca’s, Wernicke’s), and that there is no single pathognomonic type of aphasia in PPA (Mesulam, 2001).
More recently, various manifestations of PPA have been considered as major subcategories of frontotemporal lobar degeneration (FTLD) (Kertesz et al., 1994; Neary et al., 1998). In this classification scheme, the designation of progressive non-fluent aphasia (PNFA) is used if the presenting syndrome is dominated and almost exclusively characterized by nonfluent spontaneous speech, and at least one of agrammatism, “phonemic” paraphasic errors, or anomia (Neary et al., 1998). Stuttering and oral apraxia are considered supportive features. The precise behavioral manifestations of the defining clinical characteristics of the syndrome, particularly phonemic paraphasias and stuttering, are not, however, well-specified. The second designation, semantic dementia (SD), is used when spontaneous speech is fluent and lacks specificity, and there is loss of concept knowledge resulting in loss of word meaning, knowledge about objects and facts, and impaired comprehension (Snowden et al., 1989; Hodges et al., 1992). SD and PNFA are considered dichotomous, although it is not uncommon for patients with PNFA to have demonstrable sentence-level comprehension deficits during careful testing. In addition, specific criteria permitting a distinction between “loss” of word meaning and anomia are generally lacking. This scheme apparently collapses all varieties of “fluent” PPA under the heading of SD, even though clinical experience suggests that some patients with PPA who clearly are not nonfluent do not have obvious loss of word meaning on basic clinical testing. An additional subtype that is not generally used as a subcategory of FTLD, called “logopenic progressive aphasia” (Weintraub et al., 1990; Gorno-Tempini et al., 2004a), is characterized by word finding problems and slow speaking rate, relatively preserved syntax and phonological speech output, and impaired syntactic comprehension. It may capture patients who do not fit under the PNFA and SD subcategories. Recent neuroimaging findings provide some support for this subcategory as distinct from PNFA and SD (Gorno-Tempini et al., 2004a).
Further complicating the understanding and categorization of degenerative aphasias is the possible influence of motor speech deficits, namely dysarthria and apraxia of speech (AOS); AOS is also referred to as aphemia, phonetic disintegration, speech apraxia, or oral verbal apraxia. The distinction between dysarthria and aphasia is usually easily made, but that between AOS and aphasia is another matter, for at least two reasons. First, the existence of AOS as a distinct clinical entity, reflecting a deficit in the planning or programming of movements for speech, is not often recognized in the neurologic literature as a problem distinct from aphasia. Second, it is likely that the characteristics of AOS, when recognized, are subsumed under the diagnosis of dysarthria or aphasia. When included as a manifestation of aphasia, the characteristics of AOS are usually embedded within other language signs and symptoms of Broca’s or nonfluent aphasia or, when degenerative disease is the etiology, PNFA. Some have argued that AOS is an integral part of the syndrome of Broca’s aphasia (McNeil and Kent, 1990) and it is well-established that aphasia and AOS very frequently co-occur (McNeil et al., 2000; Duffy, 2005). Terms such as “labored speech,” labored articulation,” “distortion of speech,” and “poorly articulated,” are frequently among the clinical characteristics described for patients with PNFA, but they are not likely to be explained by a language disorder, and are crudely compatible with features associated with AOS (or dysarthria) (Duffy, 2005). The term, “phonemic paraphasias,” as a part of the PNFA syndrome, is likely a misnomer, probably used to refer to phonetic (i.e., motor) rather than phonemic (i.e., linguistic) distortions. True phonemic or phonological errors are not usually distorted and are most commonly associated with fluent aphasias (Goodglass and Kaplan, 1983).
Perhaps more important than conceptual and terminological inconsistencies, AOS has been explicitly recognized as the predominant clinical manifestation in a number of cases with degenerative neurological disease (Nestor et al., 2003; Gorno-Tempini et al., 2004a), sometimes with minimal or no accompanying aphasia (Cohen et al., 1993; Broussolle et al., 1996; Chapman et al., 1997; Didic et al., 1998; Tebartz van Elst et al., 2002; Duffy, in press), and sometimes in cases with clinical diagnoses or pathologically confirmed progressive supranuclear palsy (Boeve et al., 2003a; Josephs et al., 2005) or corticobasal degeneration (Rosenfield et al., 1991; Frattali and Sonies, 2000; Lehman Blake et al., 2003; Gorno-Tempini et al., 2004b; Kertesz et al., 2005). In other cases, its presence has been reported, although not necessarily as the predominant communication disorder (Craenhals et al., 1990; Hart et al., 1997; Gorno-Tempini et al., 2004a). In still other cases classified as PPA or PNFA, descriptions of speech as laborious, lacking in prosody, or severely distorted suggest that AOS may have been a more accurate diagnosis or at least an additional diagnosis (Kartsounis et al., 1991; Greene et al., 1996; Hodges and Patterson, 1996; Turner et al., 1996; Abe et al., 1997). More recently, it has been suggested that some proportion of people with PPA actually have an “aphemic” disorder (Kertesz et al., 2003), and others have noted the importance of distinguishing PPA from “pure progressive dysarthria or phonologic disintegration” (Mesulam, 2001). Some authors consider dysarthria and “buccofacial apraxia” to be “boundary” signs associated with PPA, although ones that generally develop later and are less prominent than the language deficits (Mesulam, 2003). In general, although the explicit recognition of AOS as a clinical problem distinct from aphasia has implications for behavioral management (McNeil and Duffy, 2001; Duffy, 2005), it is uncertain if it has any important implications for localization or clinical or pathologic diagnosis beyond that provided by PPA or FTLD subtypes.
Pathological diagnoses in the degenerative aphasias are heterogenous. A report of eight cases of PNFA demonstrated that argyrophilic, tau positive Pick disease with Pick bodies (PiD) was the most common cause of PNFA, occurring in 75%, while corticobasal degeneration and “dementia lacking distinctive histology” accounted for the other 25% (Hodges et al., 2004). Conversely, PiD has been reported to account for only 16% of cases with SD (Davies et al., 2005) signifying that tau-positive diseases more frequently underlie PNFA, while non tau-positive diseases more frequently underlie SD (Knibb et al., 2006). Others have reported that “nonspecific focal atrophy” or dementia lacking distinctive histology, accounts for most cases of PPA, occurring in up to 60% while argyrophilic, tau positive Pick disease with Pick bodies (PiD) account for approximately 20% of cases (Mesulam, 2001). Cases of PiD and variants of PiD underlying cases of PPA have also been reported (Wechsler et al., 1982; Graff-Radford et al., 1990; Lippa et al., 1991; Lang, 1992; Kertesz et al., 1994) and motor neuron disease (Caselli et al., 1993; Doran et al., 1995; Bak and Hodges, 2001) and Alzheimer’s disease (Greene et al., 1996; Galton et al., 2000; Kertesz et al., 2005; Knibb et al., 2006) have been reported in cases of PNFA and PPA. We also recently described four cases with aphasia, but dominated by AOS, that were found to have atypical progressive supranuclear palsy (PSP) at autopsy (Josephs et al., 2005).
Recent classification of the degenerative diseases, however, takes into account the finding of the presence or absence of abnormally phosphorylated tau in neuronal and glial cells and processes. Therefore, while PSP and PiD are different diseases pathologically, they are both classified as “tauopathies,” similar to another neurodegenerative disease, corticobasal degeneration (CBD). This raises the possibility that recognition of predominant AOS in degenerative disease may not only have implications for pathologic diagnosis, but may also have implications for the prediction of the underlying biochemistry.
The literature reveals differences in approaches to the classification of degenerative aphasias, and inconsistencies in the recognition or accounting for the influences of AOS on clinical disease and pathologic diagnoses. In addition, there is an uncertain or variable relationship between clinical and pathologic diagnoses in patients with degenerative aphasias. The purpose of this study was to determine clinicopathologic correlation in a relatively large autopsy-confirmed series of patients with degenerative aphasia, using operational definitions of aphasia type and AOS. Apraxia of speech was included as an important clinical variable in the study. Clinical experience suggested to us that the identification of a predominant AOS seemed related to specific clinical neurologic diagnoses, and might help predict pathologic diagnoses and even biochemistry. We also set out to determine if the operational criteria would correlate with specific regional head MRI and single photon emission computer tomography (SPECT) abnormalities.
METHOD
Case ascertainment
The Mayo Clinic medical records database was used to identify all cases in which PPA, PNFA, SD, or AOS was considered a diagnostic possibility, by using a textword and diagnostic code search criteria for aphasic dementia, aphasia, apraxia, PPA, PNFA, SD or AOS. A total of 5222 cases were identified. From these 5222, 197 cases had an autopsy examination completed at our institution between 1984 and 2004. The historical medical records of all 197 cases identified were retrospectively reviewed by a behavioral neurologist (KAJ) to (1) abstract demographic data and information regarding additional early and late clinical features, (2) confirm that the clinical histories, especially the temporal profile, met published criteria for a diagnosis of PPA, PNFA, SD or AOS (Neary et al., 1998; Duffy, 1995; Mesulam, 1982) and (3) establish that no other structural abnormalities were present that may have accounted for, or contributed to the syndrome. Therefore, any case in which there were cerebral ischemic or hemorrhagic vascular lesions, tumors or other structural abnormalities, paraneoplastic or any other non-degenerative disease that was felt to be a possible cause of the aphasia or AOS, was excluded from the study.
Seventeen cases met these criteria. Fifteen cases had been diagnosed by a neurologist as PPA and two as aphasic dementia. At the time of the first speech and language evaluation, ten patients had disease duration of less than two years, six patients had disease duration of 2-5 years, and one patient had disease duration of more than 5 years. These 17 cases were further reviewed by an independent behavioral neurologist (DSK).
Classification
Categorization of the language and speech disorders for each patient at each visit was performed by two speech-language pathologists (JRD and EAS) with expertise in acquired neurological speech and language disorders. Their judgments were based on the results of retrospectively reviewed speech-language pathology assessments and audio tapes or video tapes when available. In no case did the speech-language pathologist have access to autopsy results. For those few cases for which there was disagreement about final classification, records were re-reviewed, discussed, and an agreed upon classification made.
Language examination employed a variety of tasks that assessed verbal comprehension and expression, reading, and writing. Tasks most often included several subtests from the Minnesota Test for Differential Diagnosis of Aphasia (Schuell, 1972), the Boston Naming Test (Kaplan et al., 2001), Part V of the Token Test (DeRenzi and Vignolo, 1962), and a letter word fluency task (Wertz et al., 1971). In a few cases the language examination was incomplete. In all cases, quantitative data from these tests were used to estimate severity of aphasia.
The speech sample that permitted diagnoses of AOS and dysarthria was derived from conversation, verbal responses during formal language assessment, and structured tasks for assessing AOS and dysarthria (Wertz et al., 1984; Duffy, 2005). The perceptual characteristics (described below) that helped identify AOS were consistent with current diagnostic criteria (McNeil et al., 1997; McNeil et al., 2000; Duffy, 2005). Severity of abnormal motor speech characteristics was often judged on a 0-4 rating (0 = normal; 4 = severe) of each abnormal characteristic, as well as a rating of speech intelligibility. Comparison of these ratings to the quantitative and qualitative language examination results formed the basis for judgments about which, if any, disorder was the predominant one. All 17 patients had at least one speech and language evaluation, eleven patients had at least two evaluations, two patients had at least three evaluations and one patient had four evaluations. Evaluations were conducted on a yearly basis. Interjudge classification reliability for all speech and language evaluations was 87% (27/31), (κ =0.8) and for the first evaluation, 88% (15/17).
“Operational” Definitions
Progressive Nonfluent Aphasia
Cases were classified as PNFA if the dominant feature during the first few years or at the time of initial evaluation was aphasia in which verbal output characteristics contained evidence of agrammatism or telegraphic speech. Difficulties with verbal and reading comprehension and writing could be present, as could anomia. AOS and dysarthria could also be present, but only if they were less prominent than the overall aphasic language impairment.
Apraxia of Speech
Cases were classified as AOS if apraxia of speech was the sole or dominant feature of the communication disorder during the first few years of the disease course or at the time of initial presentation. Cases were also classified as AOS if the AOS became the prominent disorder over time, with relatively less progression of the aphasia. Dysarthria could also be present and could be more severe, equal in severity, or less severe than AOS. The primary features leading to a diagnosis of AOS included: consonant and vowel distortions; distorted sound substitutions; distorted sound additions; sound prolongations, trial and error attempts to correct articulation; slow overall rate; prolonged and often variable vowel duration and inter-word intervals; segregation of syllables; errors of stress assignment; decreased phonetic accuracy with increased rate (McNeil et al., 2000; Duffy, 2005).
PNFA-AOS
Cases meeting criteria for PNFA but in which AOS was also present and not clearly less severe than the aphasia, or cases meeting criteria for AOS in which aphasia was also present but not clearly less severe than the AOS
Semantic Dementia
Cases were classified as SD if during the first few years, or at the time of initial evaluation, language difficulties were characterized by fluent verbal output (i.e., grossly normal grammar and syntax, normal phase length for the longest utterances, normal prosody) plus evidence of anomia, and evidence of apparent loss of word meaning (e.g., inability to name an object plus inability to recognize the target word when provided). There must also have been impairment or loss of visual object knowledge (visual associative agnosia). AOS must have been absent or less severe than the semantic dementia. Any dysarthria must have been less severe than the aphasia.
Primary Progressive Aphasia, not otherwise specified (PPA-NOS)
Cases were classified as PPA-NOS if there was evidence of language impairment consistent with aphasia, but the profile of impairment did not meet criteria for PNFA or SD. These cases typically had evidence of difficulties in all language modalities but did not have prominent difficulties with grammar or syntax, or clear evidence of loss of word meaning or visual associative agnosia. Some cases with PPA-NOS had slow speech rate, frequent word finding pauses, and syntactically simple but not clearly agrammatic or telegraphic sentence structure (“logopenic” progressive aphasia (Gorno-Tempini et al., 2004a)). Others had more prosodically fluent and syntactically more complex verbal output. AOS and dysarthria could be present but must have been less severe than the aphasia.
The temporal profile for each syndrome must have been one of insidious onset with a progressive course. In all cases there could not have been any significant impairment of episodic memory, visuospatial skills (e.g., dot counting) (Warrington and James, 1991), or visual perceptual impairment (apperceptive agnosia), (e.g., recognition of fragmented drawings of letters) (Warrington and James, 1967), or significant frontal lobe features including apathy, behavioral dyscontrol, or executive disfunction.
MRI
T1-weighted volumetric MRI scans were acquired at 1.5T (22×16.5cm FOV, 25° flip angle, 124 contiguous 1.6mm thick coronal slices). If a patient had more than one MRI then we used the closest scan of adequate quality to the time of first neurological evaluation. Patterns of cerebral atrophy were assessed using the automated and unbiased technique of voxel-based morphometry (VBM) (Ashburner and Friston, 2000). An optimized method of VBM was applied using both customized templates and prior probability maps (Senjem et al., 2005), implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). All patient scans, plus age and gender-matched healthy controls, were registered to the MNI template using a 12dof affine transformation and segmented into grey matter (GM), white matter (WM) and CSF using MNI priors. GM images were normalized to the MNI GM prior using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented using the MNI priors. Average images were created of whole head, GM, WM and CSF, and smoothed using 8mm full-width at half-maximum (FWHM) smoothing kernel. All images were then registered to the customized whole brain template using a 12dof affine transformation and segmented using the customized priors. The GM images were normalized to the custom GM prior using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again using the customized priors. All images were modulated and smoothed with a 10mm FWHM smoothing kernel. Two-sided T-tests were used to assess the patterns of grey matter atrophy in the AOS, PNFA-AOS and PPA-NOS groups compared to the control subjects. Grey matter differences were assessed at an uncorrected statistical threshold (p<0.001), and after correction for multiple comparisons using the false discovery rate (p<0.05).
SPECT
SPECT studies were re-examined and visually assessed for regional abnormalities by a nuclear medicine specialist (MFH), completely blinded to pathology, clinical diagnoses, and the study objectives. For each SPECT scan, focal or asymmetric hypoperfusion in several brain regions was assessed and the findings described. The regions assessed for each hemisphere were frontal lobe (anterior and posterior), temporal lobe (anterior and posterior), parietal lobe (anterior and posterior), basal ganglia, and thalamus, and were compared to the cerebellum.
Pathological re-examination
All cases underwent histological re-examination by two neuropathologists independently (JEP and DWD) and a pathological diagnosis was rendered based upon the most recent accepted pathological consensus criteria for diagnosing the different neurodegenerative diseases (Lowe, 1998; McKhann et al., 2001; Dickson, 2003). Both neuropathologists were blinded to all clinical data.
Pathological methods
All cases had routine stains completed including hematoxylin and eosin, glial fibrillary acid protein and modified Bielschowsky or Bodian silver.
In addition, immunohistochemistry was performed with a battery of antibodies, including markers of glial pathology: glial fibrillary acid protein for astrocytes (clone GA5, 1:1000; BioGenex, San Ramon, CA) and either CD68 (clone PG-M1, 1:1000; DAKO, Carpenteria, CA) or HLA-DR (LN-3, 1:5; ICN, Costa Mesa, CA) for microglia. Neuronal pathology was studied with antibodies to neurofilament protein (NF-L: clone 2F11, 1:75; DAKO, Carpenteria, CA; NF-H: clone SMI-31, 1:2000; Sternberger Monoclonals, Lutherville, MD); ubiquitin (clone Ubi-1 (MAB1510), 1:250; Chemicon, Temecula, CA); alpha-synuclein LB509, 1:200; Zymed, South San Francisco, CA or NACP98, polyclonal antibody, 1:2000; Mayo Clinic Jacksonville), phospho-tau (CP13: gift from Dr. Peter Davis, Albert Einstein College of Medicine, Bronx, NY or clone AT8, 1:1000; Innogenetics, Alpharetta, GA).
Statistical analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 5.1.2; SAS Institute Inc, Cary, NC) with statistical significance set at p < 0.05. Kruskal-Wallis test was used to compare the mean ages of onset and survival times, between the three different clinical groups. Gender ratios were compared using a Chi-squared test. Fisher’s Exact Test was used to compare the association between the presence of AOS and the finding of a tauopathy.
RESULTS
Demographic data, presenting clinical features and the progression of the clinical course are summarized in Tables 1-5. A total of 17 cases were identified. Nine of the 17 cases were female. The mean age of onset, defined as patient’s age at the time of the first noticeable symptom(s), was 63.8 years (standard deviation 8.0 years). Mean disease duration, calculated as the difference between the age at death and age at onset, was 7.8 years (3.1 years).
Table 1.
Demographics and clinical features in nonfluent aphasias and AOS, based on initial patient evaluation
| Case | Sex | Age at onset |
Time from symptom onset to initial evaluation (years) |
Time from symptom onset to death (years) |
Pathologic diagnosis |
|---|---|---|---|---|---|
| AOS | |||||
| 1 | F | 53 | 1 | 8 | PSP |
| 2 | F | 69 | 2.5 | 9 | PSP |
| 3 | F | 79 | 1 | 4 | PSP |
| 4 | F | 69 | 3.5 | 9 | PSP |
| 5 | F | 63 | 7 | 16 | PiD |
| 6 | M | 66 | 0.7 | 7 | CBD |
| 7 | M | 74 | 2 | 8 | PSP |
| PPA-NOS | |||||
| 8 | M | 70 | 1.5 | 8 | PSP |
| 9 | M | 58 | 3 | 12 | CBD |
| 10 | F | 62 | 2 | 10 | FTLD-U |
| 11 | F | 63 | 1 | 6 | FTLD-U |
| 12 | M | 74 | 1.5 | 7 | FTLD-U |
| 13 | F | 58 | 3 | 8 | FTLD-U |
| 14 | M | 55 | 1 | 3 | FTLD-U |
| PNFA-AOS | |||||
| 15 | M | 64 | 1 | 5 | CBD |
| 16 | M | 54 | 2.5 | 7 | CBD |
| 17 | F | 64 | 3 | 5 | CBD |
Table 5.
Demographics based on initial speech and language diagnoses
| AOS | PNFA-AOS | PPA-NOS | |
|---|---|---|---|
| N | 7 | 3 | 7 |
| Gender (M/F) | 2/5 | 2/1 | 4/3 |
| Mean age at onset (SD) |
66.1 (9.9) | 60.7 (5.8) | 62.9 (6.9) |
| Mean disease duration to death |
8.7 (3.6) | 5.7 (1.2) | 7.7 (2.9) |
Clinical features
All cases had been examined by a behavioral neurologist and a speech-language pathologist during the course of their illness. All cases had been given an antemortem clinical diagnosis of PPA (15 cases) or aphasic dementia (2 cases) when first evaluated by the consulting behavioral neurologist. In all cases the temporal profile was insidious in onset and the clinical course progressive. All 17 patients had early presenting symptoms in keeping with aphasia or a motor speech disorder syndrome. In none of the patients was there widespread or significant memory, visuospatial, visual perceptual, praxis, oculomotor or parkinsonian features early in the disease course. However, after at least two years of disease duration, all had progressed to more widespread cognitive impairment, even though the language impairment or AOS always remained more severe than other cognitive impairments. In addition, in some cases motor features also developed late in the disease course (Table 2).
Table 2.
The evolution of motor features in cases of nonfluent aphasias and AOS
| Case | Signs present on initial examination | Documented on subsequent examination | Pathological Diagnosis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Supranuclear gaze palsy |
Limb Apraxia |
Rigidity | Bradykinesia | Supranucle gaze palsy |
Limb Apraxia |
Rigidity | Bradykinesia | ||
| AOS | |||||||||
| 1 | No | No | No | No | No | No | No | Yes* | PSP |
| 2 | No | No | No | No | Yes | Yes** | Yes** | Yes** | PSP |
| 3 | No | Yes* | Yes* | No | Noα | Yes*** | Yes** | Yes** | PSP |
| 4 | No | No | No | No | No | No | No | No | PSP |
| 5 | No | No | No | No | No subsequent examination | PiD | |||
| 6 | No | No | No | No | No | Yes* | No | No | CBD |
| 7 | No | No | No | No | No | Yes* | Yes* | Yes* | PSP |
| PPA-NOS | |||||||||
| 8 | No | No | No | No | No subsequent examination | PSP | |||
| 9 | No | No | No | No | No | No | No | No | CBD |
| 10 | No | No | No | No | No | No | No | No | FTLD-U |
| 11 | No | Yes* | No | No | No | Yes** | Yes* | Yes*** | FTLD-U |
| 12 | No | No | No | No | No | Yes*** | Yes* | Yes* | FTLD-U |
| 13 | No | No | No | No | No | No | No | No | FTLD-U |
| 14 | No | No | No | No | No | No | No | No | FTLD-U |
| PNFA-AOS | |||||||||
| 15 | No | No | No | No | No subsequent examination | CBD | |||
| 16 | No | No | No | No | No | No | No | No | CBD |
| 17 | No | No | No | Yes* | No | Yes* | Yes* | Yes* | CBD |
= Subtle findings only
= The sign was obvious
= The sign was severe
= Note slowing of down gaze velocity was noted
In three patients, there was evidence of subtle motor impairment on initial neurological examination. Two of these had subtle evidence of limb apraxia, of which one also had mild rigidity. The third patient had mild slowing of alternating motor rates. Fourteen of the 17 cases had at least one subsequent yearly examination. In eight of these, there was evidence of motor impairment. One patient developed complete supranuclear gaze palsy. The supranuclear gaze palsy was first documented four years after the initial examination but was absent on prior examinations. In another patient, there was only slowing of vertical saccades, which was present one year after initial examination; no subsequent examination occurred for this patient. Both patients with oculomotor impairment were found to have PSP pathology. Limb apraxia worsened in the two patients in whom the sign was noted on initial examination, and developed subsequently in another five patients. Of the seven cases with limb apraxia three had a pathological diagnosis of PSP and two each CBD and FTLD-U. Rigidity developed in five patients and persisted in one, while bradykinesia subsequently developed in six cases and persisted in one. A mild postural tremor developed in three patients’ only (data not shown). In no patients was postural instability documented, in keeping with an absence of a history of falls.
Language and Speech Classification
Language and speech classifications are summarized Tables 3 and 4. On initial evaluation, seven cases were classified as AOS, seven cases as PPA-NOS and three cases as PNFA-NOS. All cases classified as AOS based on initial evaluation records that had a subsequent evaluation (N=6) retained that diagnosis. Three of the seven cases classified as AOS had no evidence of aphasia on initial examination; three had nonfluent aphasia, and one had evidence of aphasia that was otherwise not classifiable. Of the three cases without aphasia on initial examination, one developed a nonfluent aphasia and the other two equivocal signs of aphasia, one year later. Five of the seven cases in which initial evaluation resulted in a diagnosis of PPA-NOS, had a second evaluation. In two of these the diagnosis of PPA-NOS was retained while a change of diagnosis to PNFA or PNFA-AOS (case 7) occurred in the other three. Only one of the cases of PPA-NOS had any AOS on initial evaluation (case 7). None of the three cases with an initial diagnosis of PNFA-AOS had a second evaluation. When the clinical diagnosis was broken down into one of the three diagnoses rendered at the time of first evaluation, (AOS, PNFA-AOS and PPA-NOS), there was no significant difference between the groups for gender, age at disease onset, or disease duration (table 5).
Table 3.
Summary of primary speech and language characteristics on initial examination, based largely on performance on formal speech-language measures described in text.
| Case | Verbal comprehension** |
Naming | Fluency | Repetition1 | Reading comprehension |
Writing | AOS | AOS characteristics | Non-verbal oral apraxia |
Dysarthria |
|---|---|---|---|---|---|---|---|---|---|---|
| AOS | ||||||||||
| 1 | Normal | Normal | F2 | Normal | Normal | Normal | 1, 1-2 | Prosodic excess; consonant & vowel distortions & prolongations; articulatory groping; slow rate |
No | No |
| 2 | Normal | Normal (but delays) |
NF (1) | 1 | Normal | Normal | 1-2 | Syllable segregation; articulatory distortions; distorted substitutions; sound omissions; irregular articulatory breakdowns; slow rate |
Yes | Noα |
| 3 | 1 | 2 | NF (1) | 1 | Normal | 1 | 2 | Syllable segregation; articulatory distortions & distorted substitutions; increased errors with increasing length; slow rate |
Yes | Spastic |
| 4 | 1,2 | 1,2 | NF (2) | 2 | 1 | 1,2 | 2,3 | Syllable segregation; excess & equal stress; vowel & consonant distortions; distorted sound sequencing errors; slow rate |
Yes | Possible, type undetermined |
| 5 | 0,1 | CNT | Not classifiable3 |
DNT | 1,2 | Impaired4 | 3,4 | Nearly mute; significant struggle to produce even single words |
Yes | No |
| 6 | Normal | Normal | F2 | 0,1 | Normal | Normal | 1 | Not described but AOS unambiguous5 |
Noε | No |
| 7 | 0,1 | Normal | F | Normal | Normal | Normal | 1 | Syllable segregation; reduced pitch & loudness variability; short phrases; distorted substitutions & additions; articulatory distortions; increased errors with increasing length; slow rate |
Equivocalς | Equivocalς hypokinetic |
| PPA-NOS | ||||||||||
| 8 | 0,1 | 0,1 | F2 | 1 | Normal | 1 | 0,1 | Subtle articulatory substitutions; vowel distortions; articulatory sequencing difficulty on multisyllabic words |
Equivocalγ | Noγ |
| 9 | 2 | 1 | Not classifiable2 |
NR | 2 | Impaired4 | None | - | No | No |
| 10 | 2,3 | 2 | F | 2,3 | 2 | 2 | None | _ | No | No |
| 11 | 1 | Normal | F | 1 | 1,2 | 3 | None | _ | No | No |
| 12 | Normal | 1 | F2 | NR | Normal | 1 | None | _ | Noδ | Noδ |
| 13 | 2 | 2 | F | 2 | 2 | 2 | None | _ | No | No |
| 14 | 3,4 | 4 | F | 1,2 | 3 | 3 | None | - | No | No |
| PNFA-AOS | ||||||||||
| 15 | 2 | 2,3 | NF | Normal | Normal | NR | Present but severity not specified |
Articulatory blocks; sound substitutions |
Yes | Hypokinetic |
| 16 | 1 | 2 | NF | 3,4 | 1 | 2 | 2 | Effortful speech production; slow rate; articulatory groping; off-target articulatory errors |
Yes | No |
| 17 | 2 | 2 | NF | 2,3 | 2 | 2 | 2 | Distorted articulatory substitutions, worse with increasing length; reduced pitch & loudness variability; slow rate; sound/syllable repetitions at end of words/sentences |
No | Equivocal hypokinetic |
F = fluent (normal grammar/syntax); NF = nonfluent (agrammatic/telegraphic; severity in parentheses)
= rating based on language characteristics (semantic, grammatic), not motor speech characteristics or verbal retention.
= nonfluent (mild) on second examination
= AOS too severe to assess spoken grammar/syntax.
= impaired but recorded data insufficient to rate severity.
= second examination noted mild, nonfluent aphasia but moderately severe AOS with syllable segregation; poor coordination of respiration and speech; short phrases; distorted sound substitutions and additions which increased with increased rate; intrusive schwa in consonant clusters; occasional syllable repetitions.
demonstrated hypokinetic dysarthria on 4th examination
demonstrated NVOA on 3rd examination
demonstrated NVOA and possible hypokinetic dysarthria on 2nd examination
demonstrated NVOA, and undetermined dysarthria on 2nd examination
demonstrated equivocal NVOA on initial exam and definite NVOA and hypokinetic dysarthria on 2nd exam
DNT = could not test because of severity of AOS
NR = not reported
Ratings: 0 = absent; 1 = mild; 2 = moderate; 3 = marked; 4 = severe
- Rating of verbal comprehension was based on judgment of combined performance on a variety of comprehension tasks, ranging from single word comprehension to complex sentence comprehension (e.g., Token Test). Although it contributed to decisions about the presence and severity of aphasia, it was not used to establish aphasia type.
Table 4.
Evolution of speech and language phenotypes over time
| Case | 1st evaluation |
2nd Evaluation |
3rd evaluation |
4th evaluation |
|---|---|---|---|---|
| AOS | ||||
| 1 | AOS | AOS | ||
| 2 | AOS | AOS | AOS | AOS |
| 3 | AOS | AOS | ||
| 4 | AOS | AOS | ||
| 5 | AOS | |||
| 6 | AOS | AOS | AOS | |
| 7 | AOS | AOS | ||
| PPA-NOS | ||||
| 8 | *PPA-NOS | PNFA-AOS | ||
| 9 | PPA-NOS | PNFA | ||
| 10 | PPA-NOS | PPA-NOS | ||
| 11 | PPA-NOS | PPA-NOS | ||
| 12 | PPA-NOS | PNFA | ||
| 13 | PPA-NOS | |||
| 14 | PPA-NOS | |||
| PNFA-AOS | ||||
| 15 | PNFA-AOS | |||
| 16 | PNFA-AOS | |||
| 17 | PNFA-AOS | |||
AOS had been present on initial evaluation
Eight of the 17 cases had unambiguous or equivocal nonverbal oral apraxia (NVOA); this was evident in five of the seven cases classified as AOS, 2 of the 3 cases classified as PNFA-AOS, but only one of the seven cases classified as PPA-NOS.
Unequivocal or possible dysarthria was identified on initial examination in five cases. Dysarthria type was spastic in one case, hypokinetic in one case, equivocal hypokinetic in two cases, and of indeterminate type in one case. Three of the cases with dysarthria were classified as AOS, and two cases as PNFA-AOS.
MRI
Twelve cases had T1-weighted MRI scans that were available and of sufficient quality for analysis. Six of these 12 had been diagnosed as AOS, two PNFA-AOS, and four PPA-NOS. Of the six with AOS, five had PSP and one CBD on pathology. Of the four cases with PPA-NOS all had FTLD-U pathology. The mean age at time of scan was 72.3 (9.1 years) in AOS, 63.3 (7.1) in PNFA-AOS, and 68.6 (11.9) in PPA-NOS. The mean time from onset to scan was 3.8 years (1.5 years) in AOS, 4.3 (0) in PNFA-AOS, and 6.1 (4.2) in PPA-NOS.
The patients with AOS showed a bilateral pattern of gray matter atrophy predominantly affecting superior premotor cortex spreading to the anterior bank of the precentral gyrus, and supplemental motor area, compared to a group of 12 age and gender-matched healthy controls (uncorrected for multiple comparisons, p<0.001, Figure 1A and B). Gray matter loss was also observed in the posterior middle and inferior frontal gyri, slightly anterior to the premotor cortex, more so on the right, and the bilateral heads of the caudate and right medial globus pallidus (uncorrected, p<0.001, Figure 1B). All these regions remained after the correction for multiple comparisons (corrected, p<0.05). Similarly the PNFA-AOS group showed loss in the superior premotor cortex, although without involvement of the supplemental motor area or left-sided deep nuclei, yet with greater involvement of the posterior inferior frontal lobe than the AOS group (uncorrected, p<0.001, Figure 1A). However it is difficult to draw definite conclusions from such a small group of subjects. No regions survived after the correction for multiple comparisons (p<0.05).
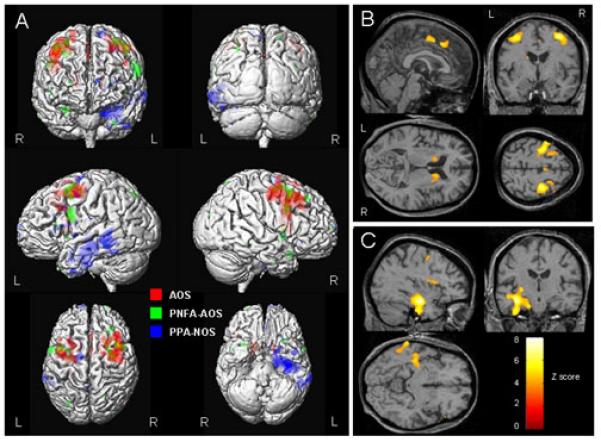
Surface rendering (A) showing regions of gray matter atrophy found in AOS (red), PNFA-AOS (green) and PPA-NOS (blue) groups compared to a group of controls (uncorrected for multiple comparisons, p<0.001). The results have also been overlaid on representative slices from a control, illustrating loss in the superior premotor cortex, supplemental motor area and bilateral heads of the caudate in AOS (B), and the medial temporal and lateral posterior temporal lobe in PPA-NOS (C) (uncorrected, p<0.001).
In contrast, the patients with PPA-NOS showed a pattern of gray matter atrophy predominantly affecting the left temporal lobe, involving the hippocampus, amygdala and perirhinal cortex, and the lateral posterior temporal cortex, particularly the middle temporal gyrus, compared to controls (uncorrected, p<0.001, Figure 1A and C). The frontal lobes also showed some minor involvement. Atrophy of the left hippocampus survived the correction for multiple comparisons (corrected, p<0.05).
SPECT
A SPECT study was completed in five cases. Two had a diagnosis of AOS with PSP pathology, two with PPA-NOS of which one had PSP, the other FTLD-U, and one case of PNFA-AOS with CBD pathology. There was decreased uptake predominantly affecting the posterior frontal and anterior parietal lobes and basal ganglia. The occipital lobes and thalamus were not affected in all five cases.
Pathological diagnoses
Of the 17 cases, six had atypical PSP (Hauw et al., 1998), five had corticobasal degeneration (CBD) (Dickson et al., 2002), five had frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes (FTLD-U) (Lowe and Rossor, 2003; Josephs et al., 2004; Paviour et al., 2004), and one case had PiD with argyrophilic and tau-positive Pick bodies (Dickson, 1998). Detailed gross and histopathological findings, as well as semiquantitative analysis of four of the six cases of atypical PSP were recently published (Josephs et al., 2005). The fifth and sixth cases of atypical PSP had findings similar to the other four including moderate frontal and mild temporal and parietal atrophy. There were globose neurofibrillary tangles in cardinal and brainstem structures including subthalamic nucleus, substantia nigra, putamen and widespread tau-positive tufted astrocytes in superior frontal gyrus and other cortical areas. There was more tau-positive pathology in frontal extramotor and temporal and parietal neocortex than is usually seen in typical PSP. Alzheimer’s disease, Lewy body disease and strokes were not present.
Five of the cases had pathological features consistent with a diagnosis of FTLD-U (Lowe and Rossor, 2003; Josephs et al., 2004; Paviour et al., 2004). In these five patients, there was variable superficial spongiosis affecting the frontal and temporal neocortices. There were also numerous tau and alpha-synuclein negative, but ubiquitin positive inclusions affecting the frontal and temporal neocortices, and the dentate cell layer of the hippocampus. The hypoglossal nucleus and anterior horn cells of the cervical cord (when available) did not show any evidence of motor neuron degeneration. Ubiquitin positive inclusions in frontal and temporal neocortex and hippocampal dentate granular cells ranged from mild to severe.
Five cases had findings consistent with a pathological diagnosis of CBD (Dickson et al., 2002). In these five cases there was moderate to severe neuronal loss and gliosis in the frontal and temporal lobes with mild-moderate neuronal loss and gliosis affecting the parietal lobe. In all five cases there were balloon neurons, significant glial pathology, and threads and astrocytic plaques.
One case had typical features of Pick’s disease (McKhann et al., 2001).
Correlation with pathology
Of the seven patients with an initial diagnosis of AOS, five had a pathological diagnosis of PSP, one CBD, and one PiD (Table 1). Of the seven cases with an initial diagnosis of PPA-NOS, five had pathological features of FTLD-U, one CBD and one PSP. Of the seven cases of PPA-NOS, six did not have any AOS, of which five (83%) had FTLD-U on pathological analysis and the other had CBD. The single case with an initial diagnosis of PPA-NOS that also had an AOS was found to have PSP pathology. All three cases with an initial speech and language diagnosis of PNFA-AOS had CBD pathology. The pathologic diagnosis in the eight cases with NVOA was PSP in five cases, CBD in two cases and PiD in one case. All five cases with a dysarthria also had tau biochemistry; three were found to have PSP and two CBD. Overall, 11 cases had some evidence of an AOS on initial examination and all (100%) were found to have a tauopathy. In contrast, of the six cases that did not have any AOS on initial examination, five (83%) did not have a tauopathy. The association between the presence of AOS and tauopathy was highly significant (p = 0.001).
DISCUSSION
The findings of this study have implications for clinical diagnosis and prediction of pathology and biochemistry in patients with a progressive degenerative aphasia and/or AOS; a number of them relate directly to the presence or absence of AOS.
A total of 11 cases had evidence of AOS, and in seven of these the AOS was the most dominant feature; in three of them there was no evidence of aphasia on initial examination. All 11 of these cases had biochemical evidence of tau deposition accounting for the syndromic presentation. Of the six cases without AOS, however, five did not have tau pathology. Therefore, the presence of AOS, with or without aphasia, suggests the presence of tau biochemistry underlying the syndrome.
For the cases in which AOS was the most dominant feature of the presenting syndrome, PSP was the most common taoupathy. However, when AOS was less than or equal to the aphasia component, as with PNFA-AOS, CBD accounted for most of this syndromic presentation. In contrast, when AOS was absent from the presenting syndrome, tau biochemistry was less likely to account for the syndrome. Of the seven cases initially classified as PPA-NOS, 83% had underlying FTLD-U pathology and absence of tau when AOS was not present. The only case of PPA-NOS with AOS had PSP pathology. Of note, the pathological diagnosis of FTLD-U had originally been considered dementia lacking distinctive histology (Knopman et al., 1990); however, most cases have been reclassified with more recent immunohistochemical techniques (Josephs et al., 2004; Lipton et al., 2004; Kertesz et al., 2005). Therefore, it seems that if we exclude cases with AOS, FTLD-U may be the most common underlying pathology of the pure degenerative aphasias, as suggested by others (Mesulam, 2001).
Another important finding relates to the evolution of the initial syndromic diagnoses. Six of the cases with an initial diagnosis of AOS were seen for a second evaluation, two of which also had a third evaluation and one a fourth. In all six cases the diagnosis remained AOS. In three of these six cases, AOS was an isolated feature on initial evaluation; however, a year later nonfluent aphasia developed in one case, while aphasia was equivocal in another. In the third case, aphasia remained absent when evaluated a second time. Three of the cases with initial diagnosis of PPA-NOS had the diagnosis changed at the second evaluation and in all cases the diagnosis changed to PNFA or PNFA-AOS. Therefore, we suggest that (1) if AOS is the initial diagnosis, AOS will remain the predominant communication disorder throughout the disease course, even though aphasia, if not initially present, may subsequently develop; (2) progression of PPA-NOS may evolve to a syndrome with a non-fluent aphasia (PNFA or PNFA-AOS); (3) if AOS is not found early in course of disease, it is unlikely to develop late, or at least unlikely to become a predominant problem and (4) the speech and language diagnosis, using the method of classification in this study, may not remain uniform throughout the entire disease course.
Of the seven cases with initial diagnosis of PPA-NOS, five had a subsequent evaluation. As stated above, two of these had converted to a diagnosis of PNFA on second evaluation; one had a tauopathy, CBD, and one FTLD-U. A third case of PPA-NOS that also had AOS converted to a diagnosis of PNFA-AOS and was found to have PSP. Of the two cases in which PPA-NOS remained the diagnosis at the second evaluation, both had FTLD-U at pathology. Therefore, it seems to be the case that in the absence of AOS, FTLD-U is the most likely cause of a fluent aphasia, while a taoupathy is at least equally likely if a non-fluent aphasia develops. We did not have any cases of SD in our study. However, FTLD-U has been shown to be the most common pathology underlying SD (Rossor et al., 2000; Davies et al., 2005).
A nonverbal oral apraxia is most commonly associated with the clinical diagnoses of AOS or PNFA-AOS, and pathologic diagnoses of PSP and CBD. Dysarthria was not common at initial evaluation for these cases but, when present, was associated with the clinical diagnoses of AOS or PNFA-AOS, and with pathologic diagnoses of PSP and CBD. All patients with a hypokinetic or unequivocal hypokinetic dysarthria had either PSP or CBD pathology. While these findings are also impressive, further studies are needed that specifically assess whether the presence or absence of NVOA and/or a dysarthria, as well as the type of dysarthria, can further refine the clinicopathological correlates of the degeneration aphasias and AOS.
The pathological diagnoses were heterogenous, with PSP and CBD accounting for over 70% of the cases. This was surprising given the initial presenting symptoms and signs of a non-parkinsonian syndrome. However, as shown in Table 3, many of our patients later developed parkinsonian features, as well as limb apraxia. Unfortunately, these features developed later in the disease course and are, therefore, unlikely to be helpful earlier in the presenting course. Furthermore, none of the features that developed late were specific to any one pathological diagnosis. While the findings of limb apraxia may be suggestive of CBD (Boeve et al., 2003b), it was found in three cases with PSP, two with FTLD-U, as well as two cases with CBD. The findings of limb apraxia in PSP and FTLD-U is not novel and have been previously reported (Leiguarda et al., 1997; Grimes et al., 1999; Pharr et al., 2001; Tsuboi et al., 2005) suggesting it is not specific to CBD. While the presence of supranuclear gaze palsy in one patient and slowing of down gaze eye movements in another may have been suggestive of PSP, neither case would have met the National Institute of Neurological Diseases-Society of Progressive Supranuclear Palsy criteria (Litvan et al., 1996), because in none of our cases was there a history of falls, or evidence of postural instability. However, the development of vertical supranuclear palsy or slowing of vertical saccades, later in the course of an aphasia or AOS syndrome, should suggest PSP pathology. The progression of an aphasic neurodegenerative syndrome into another neurodegenerative syndrome is not uncommon (Gorno-Tempini et al., 2004b; Kertesz et al., 2005). In one recent publication, of twenty-two cases with an initial diagnosis of PPA, twelve (54%) subsequently developed features of a second syndrome, five of which was either PSP-like or CBD-like (Kertesz et al., 2005). Aphasia as a presenting sign or accompanying sign in pathological confirmed CBD is not uncommon (Graham et al., 2003).
The pathological diagnosis of PSP in our cases is also worth mentioning since it was atypical. Unlike in typical PSP where the brunt of the pathology is in the subcortical grey and brainstem nuclei, the distribution of the PSP pathology in our cases was more widespread, affecting cortical regions and more in keeping with atypical PSP (Hauw et al., 1994) as has already been described in detail (Josephs et al., 2005). A recent report of patients with pathologically confirmed PSP separated them into two clinical groups based on presenting features: PSP-parkinsonism for those with a Parkinson’s disease-like phenotype with partial levodopa response, and Richardson’s syndrome for those with a typical PSP presentation with early falls, vertical supranuclear gaze palsy and levodopa resistance (Williams et al., 2005). According to their clinical definitions, however, none of our patients with PSP pathology would have been classified as either PSP-Parkinsonism or Richardson’s Syndrome. Therefore, we suggest that AOS as a presenting sign be recognized as a possible third presentation of PSP; the use of the designation, PSP-AOS, may be of heuristic value in such cases.
Voxel-based morphometry revealed that the premotor and supplementary motor cortices were the regions predominantly associated with AOS. This is not surprising since both the premotor and supplementary motor cortices are important for organizing and planning complex movements including speech and language (Deacon, 1992; Didic et al., 1998). Furthermore there are significant interconnections with the basal ganglia, which were also revealed to be affected in our VBM analysis. The changes noted in the basal ganglia is also not surprising given that four of the five cases with AOS had atypical PSP pathology, and the basal ganglia has been shown to be significantly affected in atypical PSP presenting as AOS (Josephs et al., 2005). While our findings implicate the supplementary and premotor cortices as associated with AOS, other studies have highlighted the insular cortex as the primary region (Dronkers, 1996). These differences are not necessarily divergent but suggest that it is a network of regions rather than a single structure that is responsible for AOS (Deacon, 1992). In a recent case report of longitudinal VBM analysis, the authors show an evolution of regional changes in a patient presenting with aphasia (Gorno-Tempini et al., 2004b). Early in the aphasia syndrome the left insular was affected but later on the premotor regions became involved. It was after the premotor region became involved that the patient developed mild signs of AOS.
The premotor regions were also found to be involved in the patients with PNFA-AOS. However, the PNFA-AOS group appeared to show greater involvement of the posterior inferior frontal lobe than the AOS group. Therefore, when AOS and PNFA are present, but AOS predominates, the regions of greatest atrophy were the superior premotor and supplemental motor areas; however when the non-fluent aphasia was as dominant as the AOS the regions of atrophy spread into the posterior inferior frontal lobe (anterior perisylvian area). These findings suggest that AOS is linked to the premotor and supplemental motor area while non-fluent aphasia is linked to the posterior inferior frontal lobe, although it is difficult to draw definite conclusions with such small numbers in the PNFA-AOS group. Other group studies on nonfluent aphasia have implicated the insular cortex (Nestor et al., 2003), left inferior frontal and anterior insular cortex {Gorno-Tempini, 2004 #29}and left frontotemporal and perisylvian areas (Tyrrell et al., 1990; Caselli and Jack, 1992; Grossman et al., 1996; Abe et al., 1997; Rosen et al., 2002). The difference in the results of these studies and ours further supports the notion that AOS should not simply be lumped with PNFA.
In contrast, the PPA-NOS group showed a pattern of atrophy predominately involving the left posterior temporal lobe. The relative sparing of the anterior temporal lobes clearly differentiates this group from semantic dementia in which the brunt of the atrophy lies in the anterior temporal lobes (Chan et al., 2001; Galton et al., 2001). The pattern of atrophy is more similar to the findings reported in the logopenic variant of aphasia in which the posterior middle temporal gyrus and left hippocampus have been implicated (Gorno-Tempini et al., 2004a). This correlates with the fact that a number of our PPA-NOS cases would have met criteria for logopenic progressive aphasia.
The results of the visual ratings of SPECT were similar to the findings on VBM and included the posterior frontal and basal ganglia regions. However, the superior parietal lobes were also implicated in the AOS group which we speculate may be due to a bias of the visual assessment as the boundary between the posterior frontal and anterior parietal lobe is not well defined. Alternatively, the lack of parietal lobe atrophy on VBM may reflect a large degree of inter-subject variability in this region.
It is always difficult to make significant correlations between early clinical findings and regional histopathological findings since by the time the patient dies, the degenerative process is widespread. However, in atypical PSP presenting with AOS we showed that the pathology tended to shift from more subcortical regions to cortical regions (Josephs et al., 2005).
Although AOS is increasingly recognized as a non-linguistic motor speech problem (i.e., separable from aphasia), many clinicians and investigators do not make an explicit distinction between AOS and PNFA, at least in terms of broad clinical neurologic diagnosis. That is, in many instances AOS is viewed as part of the constellation of characteristics that comprise PNFA. Although all of our subjects with non-fluent aphasia had AOS, our findings do document that AOS can occur in the absence of non-fluent aphasia, at least earlier in the disease course, as occurred in three of our subjects. In such cases, it seems most appropriate to use the designation of AOS from the perspective of clinical accuracy and precision, at least at the points in time when aphasia is not evident. In addition, the fact that the VBM demonstrated a different pattern of atrophy between the AOS and PNFA-AOS groups, and that 5/7 cases of AOS had PSP, while 3/3 cases with PNFA-AOS had CBD, are additional grounds for recognizing a distinction between AOS and PNFA at this time. We acknowledge that most of our cases classified as AOS had or subsequently developed a non-fluent aphasia, suggesting the possibility that eventually all cases of AOS will eventually become aphasic. Additional study is necessary to establish if this is the case and to replicate our basic findings. At this point, however, in our view there are clinical descriptive, diagnostic, and predictive (regarding pathology) reasons for distinguishing between AOS and aphasia. This view is consistent with that of Knibb et al. (2006) who stress that integrating clinical, imaging, and biomarker data has the best chance of predicting pathology in vivo.
There are limitations to our study, including not having any cases diagnosed as SD with a postmortem examination, as well as, the absence of more quantitative data. However, while this latter limitation was due the retrospective nature of our study, we demonstrated a very high kappa score of 0.8, suggesting excellent inter-rate reliability between both speech pathologists. Other limitations acknowledged are the small number of cases within each subclassification, especially with the VBM analysis, and the fact that the operational clinical classifications were applied retrospectively and were based on clinical reports of examinations that were not homogeneous across all cases.
In summary, we have demonstrated that refining the classification of the degenerative aphasias and AOS may improve our understanding of the relationships among behavioral, pathological, and imaging correlations. AOS should not be simply subsumed under the designation of PNFA or, more generally, with primary progressive aphasia, at least when it is the predominant sign. When AOS is present, either as an isolated feature or with aphasia, it suggests underlying tau pathology. If AOS dominates the syndrome, our findings suggest that atypical PSP is the most likely diagnosis, whereas AOS equal to or less than the aphasia suggests CBD as the more likely diagnosis. In pure aphasias, however, (i.e. without AOS or dysarthria), FTLD-U may be the most likely diagnosis, especially if the aphasia remains fluent. Finally, the regions most likely responsible for the AOS syndrome seem to be mainly the premotor and supplementary motor cortices, as well as possibly the left posterior inferior frontal lobe and the anterior superior parietal regions. These suggestions are tentative and represent hypotheses that deserve further testing with much larger numbers of patients.
ACKNOWLEDGEMENT
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786 and R01 AG11378 from the National Institute on Aging, Bethesda MD and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation, U.S.A.
REFERENCES
- Abe K, Ukita H, Yanagihara T. Imaging in primary progressive aphasia. Neuroradiology. 1997;39:556–9. doi: 10.1007/s002340050466. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bak TH, Hodges JR. Motor neurone disease, dementia and aphasia: coincidence, co-occurrence or continuum? J Neurol. 2001;248:260–70. doi: 10.1007/s004150170199. [DOI] [PubMed] [Google Scholar]
- Black SE. Focal cortical atrophy syndromes. Brain Cogn. 1996;31:188–229. doi: 10.1006/brcg.1996.0042. [DOI] [PubMed] [Google Scholar]
- Boeve B, Dickson D, Duffy J, et al. Progressive nonfluent aphasia and subsequent aphasic dementia associated with atypical progressive supranuclear palsy pathology. Eur Neurol. 2003a;49:72–8. doi: 10.1159/000068502. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003b;54(Suppl 5):S15–9. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Broussolle E, Bakchine S, Tommasi M, et al. Slowly progressive anarthria with late anterior opercular syndrome: a variant form of frontal cortical atrophy syndromes. J Neurol Sci. 1996;144:44–58. doi: 10.1016/s0022-510x(96)00096-2. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Jack CR., Jr. Asymmetric cortical degeneration syndromes. A proposed clinical classification. Arch Neurol. 1992;49:770–80. doi: 10.1001/archneur.1992.00530310118022. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33:200–7. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- Caselli RJ. Asymmetric cortical degeneration syndromes. Curr Opin Neurol. 1996;9:276–80. doi: 10.1097/00019052-199608000-00006. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49:433–42. [PubMed] [Google Scholar]
- Chapman SB, Rosenberg RN, Weiner MF, et al. Autosomal dominant progressive syndrome of motor-speech loss without dementia. Neurology. 1997;49:1298–306. doi: 10.1212/wnl.49.5.1298. [DOI] [PubMed] [Google Scholar]
- Cohen L, Benoit N, Van Eeckhout P, et al. Pure progressive aphemia. J Neurol Neurosurg Psychiatry. 1993;56:923–4. doi: 10.1136/jnnp.56.8.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craenhals A, Raison-Van Ruymbeke AM, Rectem D, et al. Is slowly progressive aphasia actually a new clinical entity? Aphasiology. 1990;4:485–509. [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, et al. The pathological basis of semantic dementia. Brain. 2005;128:1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Deacon T. The neuronal circuitry underlying primate calls and human language. In: Wind J, Chiarelli B, Bichakjian B, Nocentini A, editors. Proceedings of NATO Advanced Institute. Kluwer; Amsterdam: 1992. pp. 121–162. [Google Scholar]
- DeRenzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Pick’s disease: a modern approach. Brain Pathol. 1998;8:339–54. doi: 10.1111/j.1750-3639.1998.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press; Basel: 2003. [Google Scholar]
- Didic M, Ceccaldi M, Poncet M. Progressive loss of speech: a neuropsychological profile of premotor dysfunction. Eur Neurol. 1998;39:90–6. doi: 10.1159/000007914. [DOI] [PubMed] [Google Scholar]
- Doran M, Xuereb JH, Hodges JR. Rapidly progressive aphasia with bulbar motor neurone disease: a clinical and neuropsychological study. Behav Neurol. 1995;8:169–180. [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Substrates, differential diagnosis, and management. Mosby; San Louis: 2005. [Google Scholar]
- Duffy JR. Aphasiology. Apraxia of speech in degenerative neurologic disease. In Press. [Google Scholar]
- Frattali CM, Sonies BC. Speech and swallowing disturbances in corticobasal degeneration. In: Litvan I, Goetz CG, Lanf AE, editors. Corticobasal degeneration. Advances in Neurology. Vol 82. Lippincott Williams and Wilkins; Philadelphia: 2000. [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, et al. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–98. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004a;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Murray RC, Rankin KP, et al. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase. 2004b;10:426–36. doi: 10.1080/13554790490894011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Damasio AR, Hyman BT, et al. Progressive aphasia in a patient with Pick’s disease: a neuropsychological, radiologic, and anatomic study. Neurology. 1990;40:620–6. doi: 10.1212/wnl.40.4.620. [DOI] [PubMed] [Google Scholar]
- Graham NL, Bak TH, Hodges JR. Corticobasal degeneration as a cognitive disorder. Mov Disord. 2003;18:1224–32. doi: 10.1002/mds.10536. [DOI] [PubMed] [Google Scholar]
- Greene JD, Patterson K, Xuereb J, et al. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–8. doi: 10.1001/archneur.1996.00550100158027. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Bergeron CB, Lang AE. Motor neuron disease-inclusion dementia presenting as cortical-basal ganglionic degeneration. Mov Disord. 1999;14:674–80. doi: 10.1002/1531-8257(199907)14:4<674::aid-mds1019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted to probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Hart RP, Beach WA, Taylor JR. A case of progressive apraxia of speech and non-fluent aphasia. Aphasiology. 1997;11:73–82. [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–24. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30:369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–96. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Lippincott Williams and Wilkins; Philadelphia: 2001. [Google Scholar]
- Kartsounis LD, Crellin RF, Crewes H, et al. Primary progressive non-fluent aphasia: a case study. Cortex. 1991;27:121–9. doi: 10.1016/s0010-9452(13)80275-4. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Hudson L, Mackenzie IR, et al. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–72. doi: 10.1212/wnl.44.11.2065. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, McCabe P, et al. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–9. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Mastri AR, Frey WH, 2nd, et al. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990;40:251–6. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- Lang AE. Cortical basal ganglionic degeneration presenting with “progressive loss of speech output and orofacial dyspraxia”. J Neurol Neurosurg Psychiatry. 1992;55:1101. doi: 10.1136/jnnp.55.11.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M Lehman, Duffy JR, Boeve BF, et al. Speech and language disorders associated with corticobasal degeneration. J Medical speech-Language Path. 2003;11:131–146. [Google Scholar]
- Leiguarda RC, Pramstaller PP, Merello M, et al. Apraxia in Parkinson’s disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced parkinsonism. Brain. 1997;120(Pt 1):75–90. doi: 10.1093/brain/120.1.75. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Cohen R, Smith TW, et al. Primary progressive aphasia with focal neuronal achromasia. Neurology. 1991;41:882–6. doi: 10.1212/wnl.41.6.882. [DOI] [PubMed] [Google Scholar]
- Lipton AM, White CL, 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–85. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Lowe J. Establishing a pathological diagnosis in degenerative dementias. Brain Pathol. 1998;8:403–6. doi: 10.1111/j.1750-3639.1998.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Rossor MN. Frontotemporal lobar degeneration. In: Dickson DW, editor. Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press; Basel: 2003. pp. 342–348. [Google Scholar]
- McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Kent RD. Motoric characteristics of adult apraxic and aphasic speakers. In: Hammond GR, editor. Cerebral control of speech and limb movements. North Holland; New York: 1990. pp. 349–386. [Google Scholar]
- McNeil MR, Robin DA, Schmidt RA. Apraxia of speech: definition, differentiation, and treatment. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. Thieme; New York: 1997. [Google Scholar]
- McNeil MR, Doyle PJ, Wambaugh J. Apraxia of speech: a treatable disorder of motor planning and programming. In: Nadeau SE, Rothi LJ Gonzalez, Crosson B, editors. Aphasia and language: theory to practice. Guilford Press; New York: 2000. pp. 221–266. [Google Scholar]
- McNeil MR, Duffy JR. Primary Progressive Aphasia. In: Chapey R, editor. Language intervention strategies in adult aphasia. Lippincott Williams and Wilkins; Baltimore: 2001. pp. 472–486. [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, et al. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Paviour DC, Lees AJ, Josephs KA, et al. Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive neuronal changes: broadening the clinical picture to include progressive supranuclear palsy. Brain. 2004;127:2441–51. doi: 10.1093/brain/awh265. [DOI] [PubMed] [Google Scholar]
- Pharr V, Uttl B, Stark M, et al. Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2001;56:957–63. doi: 10.1212/wnl.56.7.957. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Kramer JH, Gorno-Tempini ML, et al. Patterns of cerebral atrophy in primary progressive aphasia. Am J Geriatr Psychiatry. 2002;10:89–97. [PubMed] [Google Scholar]
- Rosenfield DB, Bogatka CJ, Viswanath NS, et al. Speech apraxia in cortical-basal ganglionic degeneration. Ann Neurol. 1991;30:296–297. [Google Scholar]
- Rossor MN, Revesz T, Lantos PL, et al. Semantic dementia with ubiquitin-positive tau-negative inclusion bodies. Brain. 2000;123(Pt 2):267–76. doi: 10.1093/brain/123.2.267. [DOI] [PubMed] [Google Scholar]
- Schuell HM. The minnesota test for differential diagnosis of aphasia. University of Minnesota Press; Minnesota: 1972. [DOI] [PubMed] [Google Scholar]
- Senjem ML, Gunter JL, Shiung MM, et al. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed atrophy. Behav Neurol. 1989;2:167–182. [Google Scholar]
- van Elst LH Tebartz, Juengling FD, Kassubek J, et al. On the role of quantitative brain imaging in the differential diagnosis of speech disorders. Psychiatry Clin Neurosci. 2002;56:111–5. doi: 10.1046/j.1440-1819.2002.00938.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Josephs KA, Boeve BF, et al. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord. 2005;20:982–8. doi: 10.1002/mds.20478. [DOI] [PubMed] [Google Scholar]
- Turner RS, Kenyon LC, Trojanowski JQ, et al. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol. 1996;39:166–73. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- Tyrrell PJ, Warrington EK, Frackowiak RS, et al. Heterogeneity in progressive aphasia due to focal cortical atrophy. A clinical and PET study. Brain. 1990;113(Pt 5):1321–36. doi: 10.1093/brain/113.5.1321. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. Disorders of visual perception in patients with localized cerebral lesions. Neuropsychologia. 1967;5:253–266. [Google Scholar]
- Warrington EK, James M. The visual object and space perception battery. Thames Valley Test Company; Bury St Edmonds: 1991. [Google Scholar]
- Wechsler AF, Verity MA, Rosenschein S, et al. Pick’s disease. A clinical, computed tomographic, and histologic study with golgi impregnation observations. Arch Neurol. 1982;39:287–90. doi: 10.1001/archneur.1982.00510170029008. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–35. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Wertz RT, Keith RL, Custer DD. Normal and aphasic behaviour on a measure of auditory input and a measure of verbal output. Annual Convention of the American Speech and Hearing Association; Chicago, IL. 1971. [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: the disorder and its management. Grune and Stratton; New York: 1984. [Google Scholar]
- Williams DR, de Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005;128:1247–58. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]


