Abstract
The skeletal muscle contractile machine is fueled by both calcium and ATP. Calcium ions activate the contractile machinery by binding to troponin C and relieving troponin-tropomyosin inhibition of actinomyosin interaction. ATP binding to myosin during the contractile cycle results in myosin detachment from actin and energy liberated from subsequent ATP hydrolysis is then used to drive the next contractile cycle. ATP is also used to lower myoplasmic calcium levels during muscle relaxation. Thus, muscle contractility is intimately linked to the proper control of both sarcomeric Ca2+ delivery/removal and ATP generation/utilization. In skeletal muscle, the sarcoplasmic reticulum (SR) is the primary regulator of calcium storage, release, and reuptake while glycolysis and the mitochondria are responsible for cellular ATP production. However, the SR and mitochondrial function in muscle are not independent as calcium uptake into the mitochondria increases ATP production by stimulating oxidative phosphorylation and mitochondrial ATP production and production/detoxification of reactive oxygen and nitrogen species (ROS/RNS), in turn modulates SR calcium release and reuptake. Close spatial Ca2+/ATP/ROS/RNS communication between the SR and mitochondria is facilitated by the structural attachment of mitochondria to the calcium release unit (CRU) by 10 nm long electron dense “tethers.” The resultant anchoring of mitochondria to the CRU provides a structural basis for maintaining bi-directional SR-mitochondrial “through-space” communication during vigorous contraction. This review will consider the degree to which this structural link enables “privileged” or “microdomain” communication between the SR and mitochondria in skeletal muscle.
Keywords: Calcium, mitochondria, sarcoplasmic reticulum, triad, skeletal muscle, muscle disease
INTRODUCTION
The primary role of muscle is to produce force in order to do work. In skeletal muscle, this function is achieved through the establishment of complex mechanisms designed to ensure: 1) rapid delivery of an excitation stimulus simultaneously throughout the entire muscle fiber, 2) conversion of this electrical impulse into a myoplasmic Ca2+ transient (Ca2+), and 3) efficient delivery/removal/availability of Ca2+ and ATP, which are required for activation of the contractile filaments of the sarcomere.
Near simultaneous and uniform delivery of an action potential impulse throughout the mammalian skeletal muscle fiber is accomplished through the use of rapid, sodium-based action potentials that propagate both longitudinally across the surface membrane and transversely through invaginations of the sarcolemma (T-tubules) located at the A-I band junction on either side of the sarcomere (Franzini-Armstrong and Protasi, 1997). The T-tubule membrane and adjacent sarcoplasmic reticulum terminal cisternae on either side form the triad or calcium release unit (CRU), which converts the electrical impulse of the action potential into a chemical signal (a Ca2+ transient) during a process termed excitation-contraction (EC) coupling (Melzer et al., 1995). Ca2+ released from the CRU during EC coupling activates ATP-dependent crossbridge cycling within the sarcomere, the fundamental unit of muscle contraction. Finally, efficient delivery of Ca2+ and ATP molecules to the sarcomere is mediated by an intimate structural and functional interaction between the CRU and mitochondria. This bi-compartment interaction ensures efficient Ca2+ storage/release/reuptake and ATP availability during muscle contraction (Rossi et al., 2009). This review focuses on recent work detailing the structural and functional aspects of this bi-directional SR-mitochondrial communication in skeletal muscle.
ESTABLISHMENT OF SR-MITOCHONDRIAL CONNECTIVITY
Ogata and Yamasaki used electron microscopy to provide the first detailed description of mitochondrial localization in adult mammalian skeletal muscle (Ogata and Yamasaki, 1985). These studies revealed that mitochondria are located within the I-band, adjacent to the CRU on either side of the Z-line. Precise localization of mitochondria adjacent to the CRU at the A-I band junction was subsequently confirmed in non-fixed rat skeletal and cardiac muscle (Vendelin et al., 2005). Juxtapositioning of mitochondria adjacent to sites of Ca2+ release that occur during EC coupling provides a structural basis for potential local or privileged communication between these two organelles (Rossi et al., 2009). In addition, clusters of mitochondria were also observed directly under the sarcolemma and occasionally in longitudinal columns between the myofibrils of red (oxidative) skeletal muscle (Ogata et al., 1985).
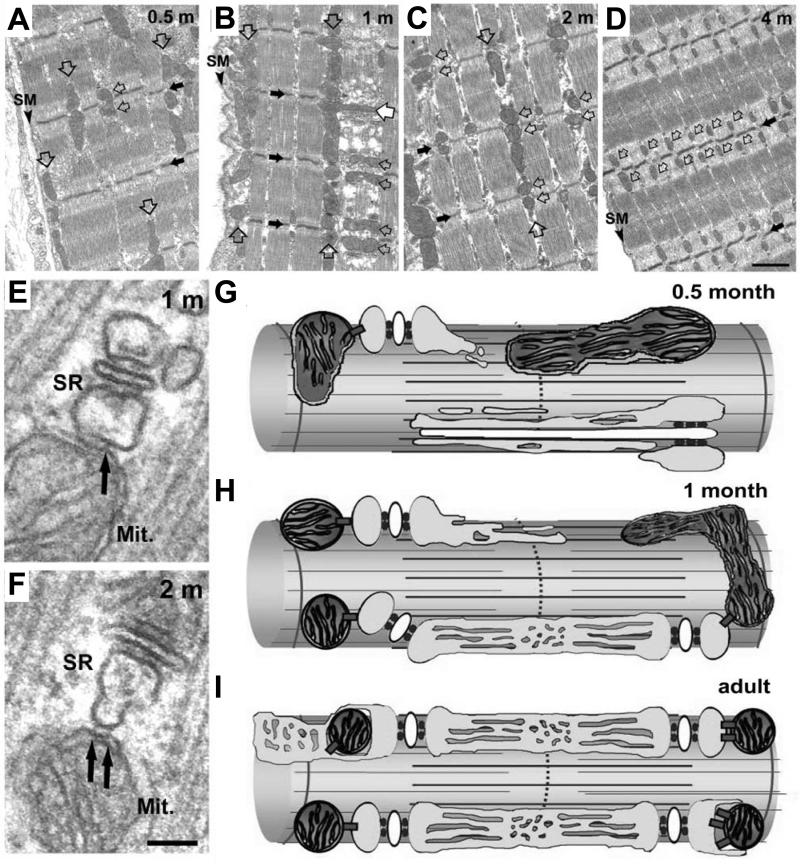
While the formation and localization of CRUs at the A-I band junction in mammalian skeletal muscle has been carefully detailed by Franzini-Armstrong and colleagues (Franzini-Armstrong and Jorgensen, 1994; Takekura et al., 2001), much less information is available with regard to the time course and mechanisms that control mitochondrial localization to the CRU. Recently, Boncampagni et al (2009) used a combination of confocal microscopy and electron microscopy/tomography to characterize mitochondrial subcellular localization in mouse fast twitch skeletal muscle throughout postnatal development (Boncompagni et al., 2009). This study found that early in development mitochondria are located primarily in longitudinal clusters either under the sarcolemma or in between the myofibrils (Fig. 1A). As postnatal development progresses, mitochondria move from these longitudinal clusters into their final position adjacent to the CRU, on either side of the Z-line (Fig.1B-D). Importantly, small (∼10 nm) electron dense bridges, termed tethers, were found to connect the outer mitochondrial membrane to the CRU on the side opposite to that containing the ryanodine receptor (RyR) Ca2+ release channels (Fig. 1E and F). As a result, the mitochondrial Ca2+ uptake mechanisms (see below) are located no closer than ∼130 nm from the point source of junctional RyR-mediated Ca2+ release (Boncompagni et al., 2009).
Figure 1. Change in mitochondrial positioning during postnatal development.
A-D) Representative electron micrographs depicting mitochondrial positioning in mouse FDB muscle fibers at 0.5 (A), 1 (B), 2 (C), and 4 (D) months after birth. Triads, small black arrows; mitochondria, open arrows; Z-line, large black arrows; SM, surface membrane. E and F) Representative high resolution electron micrographs showing mitochondrial association with the SR in mouse FDB muscle 1 (E) and 2 (F) months after birth. Small electron dense tethers (black arrows) bridge individual mitochondria (Mit.) to the SR. (G-I) Schematic representation of the parallel movement of the mitochondria and the CRU to the A-I band junction at 0.5 months after birth (G), 1 month after birth (H), and in adult skeletal muscle (I). Figure adapted with permission from Boncompagni, Rossi, Micaroni, Beznoussenko, Polishchuk, Dirksen, and Protasi, Mitochondria are Linked to Calcium Stores in Striated Muscle by Developmentally Regulated Tethering Structures, Molecular Biology Cell, 20(3):1058−1067, 2009.
Interestingly, triad maturation follows a similar postnatal time course in that T-tubules are initially positioned in a longitudinal orientation near the sarcolemma early in development and then only later establish the classic transverse orientation observed in adult skeletal muscle (Takekura et al., 2001). During this critical developmental time period, tether length remains constant while parallel increases are observed in: 1) the number of triads and mitochondria, 2) the frequency of mitochondria positioned adjacent to the CRU, and 3) the number of electron dense tethering elements observed per cross-sectional area (Boncompagni et al., 2009). These results indicate triad formation and mitochondrial association with the CRU via tethering elements occur in parallel during postnatal development (Fig. 1G).
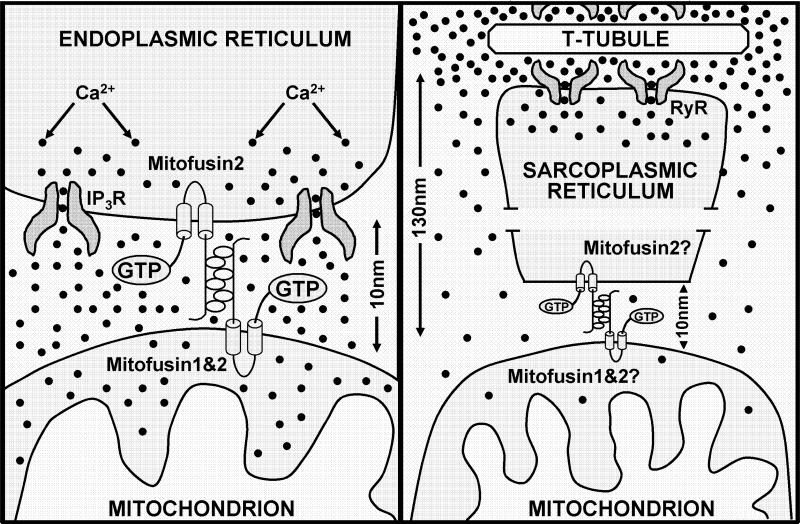
The molecular identity of the SR-mitochondrial tethering element in skeletal muscle has yet to be determined. However, mitofusin 2, a dynamin-like GTPase required for mitochondrial fusion, is one likely candidate as de Brito and Scorrano (2008) recently found that mitofusin 2 tethers mitochondria to the endoplasmic reticulum in mouse embryonic fibroblasts and HeLa cells (Figure 2A). Whatever their molecular identity, the SR-mitochondrial tethers in skeletal muscle act to establish and maintain SR/mitochondrial association during postnatal maturation, ensure mitochondria remain associated with the CRU during cell shortening, and thus, provide a structural framework for bi-directional SR-mitochondrial signalling in adult striated muscle (Figure 2B). This anchoring-mechanism is likely to be applicable to a wide variety of cells as similar structures have also been observed between ER/SR and mitochondrial elements in cardiac myocytes (Boncompagni et al., 2009), liver cells (Csordas et al., 2006), mouse embryonic fibroblasts and HeLa cells (de Brito et al., 2008).
Figure 2. Schematic representation of the dependence of microdomain Ca2+ signaling on mitochondrial location with respect to the site of Ca2+ release.
(Left) Model of ER-mitochondrial localization and degree of microdomain Ca2+ signaling during IP3-mediated Ca2+ release (Merkwirth and Langer, 2008). When the mitochondrion is located only 10 nm from the point source of Ca2+ release, significant microdomain Ca2+ signaling occurs and mitochondrial Ca2+ uptake is driven by a large but rapidly dissipating local Ca2+ signal. Figure adapted from Cell, 135(7), Merkwirth and Langer, Mitofusin 2 builds a bridge between ER and mitochondria, 1165−1167, 2008, with permission from Elsevier. (Right) Model of SR-mitochondrial localization and degree of microdomain Ca2+ signaling during RyR1-mediated dependent Ca2+ release in adult mammalian skeletal muscle. Since CRU-associated mitochondria are located 130 nm from the point source of Ca2+ release (Boncompagni et al., 2009), limited microdomain signaling occurs and mitochondrial Ca2+ uptake is driven largely by the global myoplasmic Ca2+ signal.
EXCITATION-METABOLISM COUPLING
The formation of a highly stable association between mitochondria and SR during postnatal development (Fig. 1) provides the structural framework for potential “local” or “privileged” communication between these two organelles (Franzini-Armstrong, 2007). Indeed, I-band delimited mitochondria are essentially immotile and their attachment to the CRU is strong enough to sustain significant swelling induced by hypertonic shock (Boncompagni et al., 2009). As a result of being anchored to the CRU, mitochondria are exposed to calcium released from nearby junctional RyR Ca2+ release channels activated during EC coupling. As detailed below, the relative change in free Ca2+ within the mitochondrial matrix that occurs during Ca2+ release from the CRU will depend upon: 1. the amount, affinity, and kinetics of the mitochondrial Ca2+ uptake/removal mechanisms, 2. the spatiotemporal Ca2+ concentration profile around the mitochondrion, 3. the driving force for mitochondrial Ca2+ uptake, and 4. the buffering power of the mitochondrial matrix.
Mitochondria possess the machinery required to transport Ca2+ into and out of the matrix. A large electrochemical gradient (>−180 mV) across the mitochondrial inner membrane provides a substantial driving force for mitochondrial Ca2+ entry that can occur through several mechanisms including: 1) a ruthenium red sensitive uniporter (Gunter and Gunter, 1994), 2) a rapid mode Ca2+ transport mechanism (RaM) (Buntinas et al., 2001), and 3) a mitochondrial ryanodine receptor (Beutner et al., 2001). Because of the low affinity of the uniporter for Ca2+ (half-maximal transport at 30 μM Ca2+) (Scarpa and Graziotti, 1973), the physiological relevance of mitochondrial Ca2+ uptake has been questioned. However, direct measurements of mitochondrial Ca2+ using targeted Ca2+ probes have demonstrated that mitochondria sequester Ca2+ during physiological Ca2+ oscillations in fibroblasts, endothelial/epithelial cells, cardiac myocytes, neurons, and pancreatic acinar cells (Duchen, 1999).
Significant mitochondrial Ca2+ uptake in skeletal muscle following RyR-mediated Ca2+ release has been reported in both skeletal myotubes (Robert et al., 2001)and intact mouse skeletal muscle (Rudolf et al., 2004). In addition, mitochondrial Ca2+ uptake occurs in slow and fast-twitch skeletal muscle fibers during both electrical stimulation and caffeine exposure, and this uptake persists in a subpopulation of these mitochondria even following buffering of the global myoplasmic Ca2+ transient with BAPTA (Shkryl and Shirokova, 2006). These results are consistent with a privileged “Ca2+ tunneling” from the SR to the mitochondria. On the other hand, mitochondrial Ca2+ uptake during repetitive tetanic stimulation was absent in fast twitch glycolytic mouse toe muscle fibers (Lannergren et al., 2001), but was observed in both slow-twitch soleus and fast-twitch EDL muscle fibers (Bruton et al., 2003).
Efficient mitochondrial Ca2+ uptake via low affinity Ca2+ transport processes can occur if the mitochondrial Ca2+ uptake mechanisms are localized close to sites of Ca2+ release, termed “Ca2+ microdomains” (Figure 2A). Within these Ca2+ microdomains, even low-affinity Ca2+ uptake mechanisms can be activated since free Ca2+ levels reach many tens of micromolar. However, Ca2+ microdomains dissipate rapidly by virtue of Ca2+ diffusion and binding to buffers. Simulation studies assuming a point source of Ca2+ release in an isotropic medium indicate that high Ca2+ microdomains in muscle indeed dissipate markedly only a few tens of nanometers away from the point source of release and decay >90% about 100 nm away (Stern, 1992; Pape et al., 1995). As electron microscopy measurements in FDB fibers indicate that the outer mitochondrial membrane is no closer than 130 nm from the site of RyR-mediated Ca2+ release (Boncompagni et al., 2009), microdomain Ca2+ signaling between sites of RyR-mediated Ca2+ release and the Ca2+ uptake mechanisms in the mitochondrial inner membrane is likely to be minimal (Figure 2B), consistent with the results of Lannergren et al. (2001).
Ca2+ efflux from the mitochondrial matrix involves electroneutral Na/Ca and H/Ca exchange mechanisms (Gunter and Gunter, 1994). In addition, high levels of mitochondrial Ca2+ may induce Ca2+ efflux through opening of the mitochondrial permeability transition pore (Balaban, 2002; Brookes et al., 2004). When mitochondrial Ca2+ efflux mechanisms operate slower than the influx pathways, mitochondria serve as Ca2+ integrators during repetitive excitation. However, in other cases mitochondria are able to readily track rapid cytoplasmic Ca2+ oscillations with remarkable fidelity (Robert et al., 2001; Rudolf et al., 2004).
Mitochondrial ATP production can be stimulated by an increase in Ca2+ in the mitochondrial matrix. This stimulation results from Ca2+ activating three mitochondrial dehydrogenases responsible for NADH production (pyruvate, NAD-isocitrate, and 2-oxoglutarate dehydrogenases). In addition, mitochondrial Ca2+ also stimulates the ATP synthetic capacity of the F1F0-ATPase (Balaban, 2002; Brookes et al., 2004). As a result, mitochondrial Ca2+ uptake during skeletal muscle contraction could potentially serve to stimulate aerobic ATP production needed to keep pace with increased ATP consumption associated with cross bridge cycling and SERCA-mediated Ca2+ reuptake (Figure 3 – “orthograde” SR-mitochondrial signaling). As noted above, the relative degree of mitochondrial Ca2+ uptake during muscle contraction remains unclear and may differ depending on species and muscle type. Nevertheless, given the low level of Ca2+ buffering in the mitochondrial matrix (e.g. compared to the SR), even minimal Ca2+ uptake could be sufficient to increase free Ca2+ to levels needed to stimulate mitochondrial respiration and ATP production. Thus, it will be important for future work to carefully compare changes in mitochondrial Ca2+ and aerobic ATP production during muscle stimulation. Such experiments are likely to reveal that the efficiency of excitation-coupled ATP production differs significantly depending on species, developmental stage, and muscle type (e.g. slow-twitch oxidative, fast-twitch oxidative, and fast-twitch glycolytic) studied.
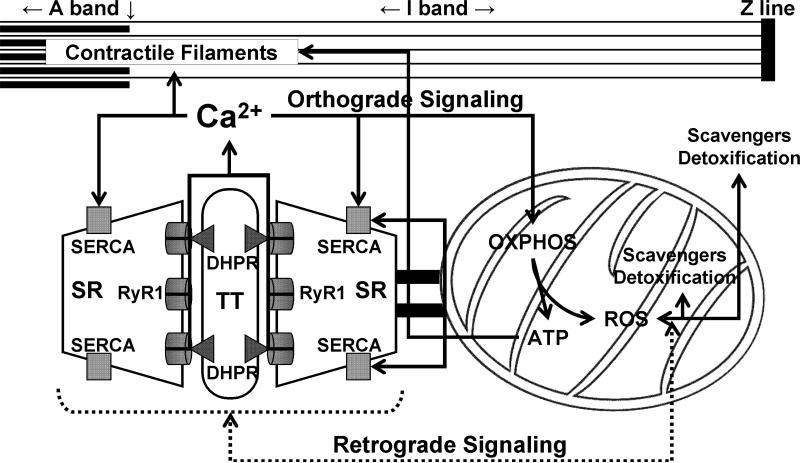
Figure 3. Bidirectional SR-mitochondrial signaling in skeletal muscle.
Ca2+ release during excitation-contraction (EC) coupling stimulates mitochondrial Ca2+ uptake and subsequent ATP production (Orthograde SR-mitochondrial signaling, solid lines). Mitochondrial-mediated Ca2+ spark suppression (Retrograde mitochondrial-SR signaling, broken lines) involves mitochondrial ROS scavenging and detoxification maintaining proper redox balance of the adjacent and tethered CRU. OXPHOS, oxidative phosphorylation; SR, sarcoplasmic reticulum; RyR1, type 1 ryanodine receptor; DHPR, dihydropyridine receptor; TT, transverse tubule; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase. Figure modified with permission from Rossi, Boncampagni, and Dirksen, Sarcoplasmic Reticulum-Mitochondrial Symbiosis: Bidirectional Signaling in Skeletal Muscle, Exercise and Sports Science Reviews, 37(1):29−35, 2009.
MITOCHONDRIAL IMPACT ON MYOPLASMIC CALCIUM SIGNALING
While mitochondrial Ca2+ uptake following SR release in muscle may stimulate respiratory ATP production via excitation-metabolism coupling, SR Ca2+ signaling is also markedly influenced by mitochondrial function. First of all, mitochondrial ATP production is used to drive both crossbridge cycling during muscle contraction and SR Ca2+ ATPase-mediated myoplasmic Ca2+ removal during relaxation. In fact, up to 80% of ATP consumed during muscle contraction is used to fuel SR Ca2+ removal during contractile relaxation (Allen et al., 2008). The impact of mitochondrial ATP production is particularly important during sustained activity in when glycolytic reserves become depleted. In addition, mitochondria have been shown to track SR Ca2+ release and modify the myoplasmic free Ca2+ in frog skeletal muscle (Lannergren et al., 2001). Finally, energized or respiring mitochondria have also been shown to contribute to the inhibition of Ca2+-induced-Ca2+-release (CICR) in skeletal muscle, and thus, suppression of local SR Ca2+ release events (termed Ca2+ sparks) (Isaeva and Shirokova, 2003). As a result, local SR-mitochondrial signaling is bi-directional; SR Ca2+ release stimulates mitochondrial Ca2+ uptake and ATP production and mitochondrial activity modifies the release process and shapes the myoplasmic Ca2+ signal (Figure 3).
Local Ca2+ release events, termed Ca2+ sparks, were first observed in cardiac muscle and represent all-or-none elementary events of RyR-mediated Ca2+ release. While similar spontaneous events are also observed in amphibian skeletal muscle (Tsugorka et al., 1995), Ca2+ sparks are absent in resting mammalian muscle in spite of the fact both the skeletal (RyR1) and cardiac (RyR2) RyR channel isoforms exhibit a bell-shaped Ca2+ dependence (i.e. activation at low Ca2+ and inhibition at high Ca2+) needed to support all-or-none CICR (Franzini-Armstrong and Protasi, 1997). Several “Ca2+ spark suppression” mechanisms have been proposed to account for the absence of Ca2+ sparks in mammalian skeletal muscle. First of all, RyR1 channel exhibit a significantly lower sensitivity for Ca2+ activation than that of RyR2 (Franzini-Armstrong et al., 1997). Thus, under resting conditions, RyR2 channels in the heart are more likely to be activated by Ca2+ than RyR1 channels in mammalian skeletal muscle. In support of this mechanism, spontaneous Ca2+ sparks readily observed in frog skeletal muscle arise from the parajunctional expression of RyR3 channels, which are present in amphibian, but not mammalian, skeletal muscle (Felder and Franzini-Armstrong, 2002). Moreover, spontaneous Ca2+ sparks are observed in mammalian skeletal muscle following transient ectopic expression of RyR3 channels (Pouvreau et al., 2007). A second mechanism for Ca2+ spark suppression in mammalian skeletal muscle involves a strong inhibition of CICR by mechanical coupling between RyR1 and the dihydropyridine receptor in the transverse tubule (T-tubule) membrane (Shirokova et al., 1998; Zhou et al., 2006). Indeed, Ca2+ sparks in skeletal muscle are observed following degeneration of the T-tubule network in de-differentiating cultured adult skeletal muscle cells (Brown et al., 2007). However, mechanisms other than lower RyR1 Ca2+ sensitivity of activation and DHPR inhibition must contribute to Ca2+ spark suppression in skeletal muscle because Ca2+ sparks are observed in mechanically skinned fibers in which RyR1 Ca2+ sensitivity and DHPR-RyR1 interaction are preserved (Kirsch et al., 2001).
A third mechanism for Ca2+ spark suppression in adult mammalian skeletal muscle involves a retrograde signal from the mitochondria to the adjacent CRU. As noted above, spontaneous Ca2+ sparks are observed in mammalian skeletal muscle following membrane permeabilization (Kirsch et al., 2001). Shirokova and colleagues subsequently concluded that this effect correlates strongly inhibition of mitochondrial function (Isaeva et al., 2003; Isaeva et al., 2005; Martins et al., 2008). Specifically, Ca2+ spark activity during permeabilization develops slower in mitochondrial-enriched oxidative muscle, is reduced by interventions that energize mitochondria and is increased following inhibition of mitochondrial Ca2+ uptake with protonophores and RU360, an inhibitor of the Ca2+ uniporter (Isaeva et al., 2003; Isaeva et al., 2005). In addition, interventions that increase levels of reactive oxygen species (ROS) (e.g. addition of H2O2) augment and addition of ROS scavengers decrease Ca2+ spark onset and frequency (Isaeva et al., 2005; Martins et al., 2008). Together, these results indicate that mitochondrial ROS scavenging and detoxification activity plays a critical role in Ca2+ spark suppression in mammalian skeletal muscle, presumably by maintaining proper redox balance of the adjacent and tethered CRU (Figure 3). Alternatively, Ca2+ spark activity is unmasked by conditions that overwhelm this local redox control mechanism. In interesting prediction from this local control model for Ca2+ spark suppression is that Ca2+ spark activity may be increased under conditions in which mitochondrial tethering to the CRU is reduced (e.g. during early postnatal development; Figure 1) or in muscle diseases in which mitochondrial function is significantly compromised (Wang et al., 2005; Weisleder et al., 2006; Durham et al., 2008). However, future detailed investigations are needed to test the validity of these predictions.
CONCLUSIONS
Muscle contraction requires both Ca2+ and ATP. Ca2+ ions, which are stored and released by the SR, initiate striated muscle contraction by binding to troponin-C and removing troponin-tropomyosin inhibition of actomyosin interaction. In addition, an increase in mitochondrial Ca2+ can also stimulate aerobic ATP production. ATP produced by mitochondria (and glycolysis) is used to drive both crossbridge cycling during muscle contraction and Ca2+ reuptake into the SR during relaxation. Thus, regulation of muscle contraction requires careful dynamic control by the SR and mitochondria of sarcomeric Ca2+ and ATP availability. Tethering of mitochondria to the CRU provides a physical basis for stable, bidirectional crosstalk between these two organelles (Figure 3). Ca2+ released from the SR stimulates ATP production from the adjacent mitochondrion, which is then used to replenish ATP reserves utilized during muscle activity. Mitochondria, in turn, influence muscle Ca2+ signaling by providing ATP required for Ca2+ removal, shaping the myoplasmic transient, and inhibiting uncontrolled CICR and local Ca2+ release through a modulation of the redox environment of the CRU. Thus, bidirectional SR-mitochondrial communication provides a powerful local control mechanism for integrating Ca2+ release/reuptake and ATP utilization during muscle contraction with ATP production and skeletal muscle bioenergetics (Rossi et al., 2009). Defects in this local control mechanism are likely to result in alterations in proper dynamic and use-dependent control of sarcomeric Ca2+ and ATP availability that can contribute to muscle dysfunction during aging, fatigue, and certain forms of muscle disease (Wang et al., 2005; Weisleder et al., 2006; Durham et al., 2008).
ACKNOWLEDGEMENTS
Support was obtained from grant from the National Institutes for Health (AR044657 and AR052354). Thanks also to Drs. Ann Rossi, Simona Boncampagni, and Feliciano Protasi for many thoroughly enlightening scientific discussions.
REFERENCES
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- Boncompagni S, Rossi AE, Micaroni M, Beznoussenko GV, Polishchuk RS, Dirksen RT, Protasi F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brown LD, Rodney GG, Hernandez-Ochoa E, Ward CW, Schneider MF. Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2007;292:C1156–C1166. doi: 10.1152/ajpcell.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Westerblad H, Lannergren J. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions. J. Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim. Biophys. Acta. 2001;1504:248–261. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder E, Franzini-Armstrong C. Type 3 ryanodine receptors of skeletal muscle are segregated in a parajunctional position. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1695–1700. doi: 10.1073/pnas.032657599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. ER-mitochondria communication. How privileged? Physiology(Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Gunter KK, Gunter TE. Transport of calcium by mitochondria. J. Bioenerg. Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J. Physiol. 2003;547:453–462. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J. Physiol. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch WG, Uttenweiler D, Fink RH. Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J. Physiol. 2001;537:379–389. doi: 10.1111/j.1469-7793.2001.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J. Muscle Res. Cell Motil. 2001;22:265–275. doi: 10.1023/a:1012227009544. [DOI] [PubMed] [Google Scholar]
- Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J. Physiol. 2008;586:197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Merkwirth C, Langer T. Mitofusin 2 builds a bridge between ER and mitochondria. Cell. 2008;135:1165–1167. doi: 10.1016/j.cell.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Scanning electron-microscopic studies on the three-dimensional structure of mitochondria in the mammalian red, white and intermediate muscle fibers. Cell Tissue Res. 1985;241:251–256. doi: 10.1007/BF00217168. [DOI] [PubMed] [Google Scholar]
- Pape PC, Jong DS, Chandler WK. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mM EGTA. J. Gen. Physiol. 1995;106:259–336. doi: 10.1085/jgp.106.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S, Royer L, Yi J, Brum G, Meissner G, Rios E, Zhou J. Ca2+ sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5235–5240. doi: 10.1073/pnas.0700748104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J. Biol. Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- Rossi AE, Boncompagni S, Dirksen RT. Sarcoplasmic reticulum-mitochondrial symbiosis: bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009;37:29–35. doi: 10.1097/JES.0b013e3181911fa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A, Graziotti P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J. Gen. Physiol. 1973;62:756–772. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, Garcia J, Rios E. Local calcium release in mammalian skeletal muscle. J. Physiol. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkryl VM, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J. Biol. Chem. 2006;281:1547–1554. doi: 10.1074/jbc.M505024200. [DOI] [PubMed] [Google Scholar]
- Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Takekura H, Flucher BE, Franzini-Armstrong C. Sequential docking, molecular differentiation, and positioning of T-Tubule/SR junctions in developing mouse skeletal muscle. Dev. Biol. 2001;239:204–214. doi: 10.1006/dbio.2001.0437. [DOI] [PubMed] [Google Scholar]
- Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995;269:1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am. J. Physiol. Cell Physiol. 2005;288:C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell. Biol. 2005;7:525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, Parness J, Takeshima H, Ma J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J. Cell Biol. 2006;174:639–645. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J, Rios E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 2006;290:C539–C553. doi: 10.1152/ajpcell.00592.2004. [DOI] [PubMed] [Google Scholar]