Abstract
Substantial evidence indicates that brain-derived neurotrophic factor (BDNF) plays a crucial role in synaptic plasticity. Long-lasting synaptic plasticity is restricted to active synapses and requires new protein synthesis. Recent work has identified local protein synthesis as an important source for new protein during the expression of enduring synaptic plasticity. This review discusses recent progress in understanding the mechanisms that restrict the action of BDNF to active synapses and by which BDNF mediates chemical and structural modifications of individual synapses, placing an emphasis on the role of local protein synthesis in these processes.
Introduction
Synaptic plasticity is the activity-dependent selective strengthening or weakening of individual synapses so that information can be represented, processed, and stored in complex neural networks. This process most often occurs at excitatory synapses with dendritic spines as the postsynaptic site (Sheng and Hoogenraad, 2007). The enduring form of synaptic plasticity also depends on new protein synthesis and the growth or remodelling of excitatory synapses. Intense investigation has therefore focused on how neurons with thousands of dendritic spines can differentially incorporate new proteins into and modify individual synapses. Activity-dependent local protein synthesis at or near individual spines has emerged as a mechanism for how this type of selective modifications might occur. Although studies conducted as early as 1965 demonstrated that RNA did localize outside of the neuronal soma (Bodian, 1965; Koenig, 1965), it was not until the early 1980s that both mRNAs and polyribosomes were localized to dendritic compartments and to regions beneath individual dendritic spines (Palacios-Pru et al., 1981; Steward and Levy, 1982; Steward and Falk, 1985, 1986). Since then, extensive research has focused on processes by which mRNA might be transported into the dendritic compartment and locally translated there in response to stimulation (Bramham and Wells, 2007).
Brain derived neurotrophic factor (BDNF) is a crucial regulator of activity-dependent synaptic plasticity (Arancio and Chao, 2007; Lu et al., 2008). It belongs to a family of small, closely related, secreted proteins called neurotrophins that also includes nerve growth factor (NGF), neurotrophin-3 (NT3), and neurotrophin-4/5 (NT4/5). Neurotrophins exert their influence on neurons mainly through Trk receptor tyrosine kinases: NGF activates TrkA; BDNF and NT4/5 activate TrkB; and NT3 activates TrkC. In addition, they achieve some biological functions through a common receptor p75NTR (Reichardt, 2006). One recent surprising discovery is that unprocessed neurotrophin precursors, termed proneurotrophins, are secreted and have a unique biological function through the p75NTR/sortilin receptor complex (Lee et al., 2001; Nykjaer et al., 2004). BDNF is widely expressed in both the developing and mature brain (Hofer et al., 1990; Maisonpierre et al., 1990; Phillips et al., 1990). In addition to its role in promoting the proliferation, differentiation, and survival of neurons (Davies, 1994; Bothwell, 1995), BDNF has been shown to be crucial in regulating synaptic activity and plasticity through both functional and structural changes in neurons (Arancio and Chao, 2007; Lu et al., 2008).
This paper outlines the role of BDNF in synaptic plasticity, concentrating on postsynaptic mechanisms in the adult hippocampus. First, we review findings that implicate BDNF in hippocampal synaptic plasticity. Second, we describe specific mechanisms that restrict BDNF to active synapses. Third, we discuss two processes by which localized BDNF may affect synaptic plasticity: phosphorylation of local proteins and induction of local protein synthesis. Finally, we cite recent evidence indicating that BDNF itself can be synthesized in dendrites and propose a model for the action of dendritically synthesized BDNF on functional and structural synaptic plasticity.
BDNF and synaptic plasticity in the mature hippocampus
No paradigm of synaptic plasticity has been better studied than that of long-term potentiation (LTP) in the hippocampus. LTP refers to the relative strengthening of synapses such that subsequent stimuli will more readily produce a postsynaptic response. The majority of fibers in the hippocampus follow a circuit that starts and ends in the entorhinal cortex. Layers II/III of the entorhinal cortex project via the perforant pathway to granule cells of the hippocampal dentate gyrus. The granule cells send axons via mossy fibers to the pyramidal cells of Ammon's horn subfield 3 (CA3), which then project, via Schaffer collaterals, to the pyramidal cells of Ammon's horn subfield 1 (CA1). CA1 pyramidal cells project to deep layers of the entorhinal cortex completing this circuit: entorhinal cortex to granule cells to CA3 pyramidal cells to CA1 pyramidal cells back to entorhinal cortex (Witter et al., 1989). The preponderance of LTP research has focused on the synapses between presynaptic Shaffer collateral axon terminals and postsynaptic CA1 dendrites. By inducing tetanic stimulation in the Shaffer collaterals and recording the amplitude of excitatory postsynaptic potentials (EPSPs) in CA1 neurons, researchers have been able to tease apart important aspects of LTP.
It is generally accepted that LTP can be divided into an early phase (E-LTP) and a late phase (L-LTP). E-LTP lasts between one and two hours and is dependent on the modification and translocation of existing proteins (Malenka and Bear, 2004). L-LTP has been documented to last up to days (Abraham, 2003), and is dependent on de novo protein synthesis (Kelleher et al., 2004). BDNF has been shown to be secreted in an activity-dependent manner from both postsynaptic spines and presynaptic terminals (Hartmann et al., 2001; Kohara et al., 2001; Kojima et al., 2001), and the TrkB receptor has been localized to both pre- and postsynaptic sites (Drake et al., 1999). There is substantial evidence to support a critical role for BDNF in the expression of hippocampal LTP, in both the early and late phases (Figurov et al., 1996; Korte et al., 1996; Patterson et al., 1996; Kang et al., 1997; Pang et al., 2004). Importantly, BDNF may regulate E-LTP and L-LTP through different processes. Evidence suggests that BDNF mediates E-LTP via release of existing presynaptic BDNF pools and subsequent modification of both existing pre- and post-synaptic proteins through the TrkB receptor (Minichiello et al., 1999; Pozzo-Miller et al., 1999; Jovanovic et al., 2000; Xu et al., 2000; Zakharenko et al., 2003; Gartner et al., 2006). By contrast, evidence suggests that the induction of L-LTP requires synthesis and release of proBDNF, extracellular conversion of proBDNF to mature BDNF (Pang et al., 2004), and subsequent BDNF-mediated stimulation of de novo synthesis of proteins crucial for the maintenance of L-LTP (Kang and Schuman, 1996; Yin et al., 2002; Schratt et al., 2004). As LTP-inducing electrical stimulation increases BDNF gene transcription (Patterson et al., 1992; Castren et al., 1993), BDNF released during L-LTP is likely from the increased translation of existing BDNF mRNA as well as newly transcribed BDNF mRNA. It remains unclear whether BDNF is released from presynaptic terminals or postsynaptic sites during the expression of L-LTP.
It has been shown that BDNF transcripts are transported to the dendritic compartment in cultured hippocampal neurons in response to KCl-induced depolarization (Tongiorgi et al., 1997), in the rat hippocampus in response to epileptogenic stimulation (Simonato et al., 2002; Tongiorgi et al., 2004; Chiaruttini et al., 2008), and in the cerebral cortex and hippocampus of resting mice (An et al., 2008). Translation of these dendritically localized BDNF mRNA may serve as the source of BDNF required for the expression of L-LTP. This view would be consistent with the observation that local protein synthesis in dendrites is required for enduring synaptic plasticity (Kang and Schuman, 1996; Martin et al., 1997; Huber et al., 2000; Miller et al., 2002). It is further supported by a recent observation that mice lacking local BDNF synthesis exhibit significant impairment in LTP at the Schaffer collateral-CA1 synapse only 40 min after theta burst stimulation (An et al., 2008). However, it is difficult to conclusively demonstrate the role of local BDNF synthesis in L-LTP using this mouse mutant due to developmental abnormalities in dendritic spines. Future development of inducible up- or down-regulation of local BDNF synthesis will help us uncover distinct roles of BDNF synthesized in the soma or dendrites in the early and late phases of LTP.
Restriction of BDNF action to active synapses
One signature feature of synaptic plasticity is that synaptic strengthening or weakening only occurs at synapses with altered activity. If BDNF is a key regulator of synaptic plasticity, then it is important to understand how BDNF, as a secreted and diffusible molecule, can achieve such spatial specificity. From recent work four mechanisms have emerged that may restrict the action of BDNF to active synapses: (1) local synthesis of BDNF and TrkB in dendrites; (2) activity-dependent release of BDNF from pre- and postsynaptic sites; (3) activity-dependent insertion of TrkB into the plasma membrane; and, (4) cleavage of proBDNF to mature BDNF. These four mechanisms likely work in tandem to ensure the specificity of BDNF action.
As discussed earlier, BDNF mRNA has been found to localize in neuronal dendrites. TrkB mRNA is also transported to dendrites of cultured hippocampal neurons in response to depolarization (Tongiorgi et al., 1997). Some of the effect of activity on dendritic trafficking of BDNF and TrkB mRNAs appears to result from activity-induced BDNF release, because pulse application of BDNF to cultured neurons increased accumulation of BDNF and TrkB mRNAs in dendrites via a phosphatidylinositol-3 kinase (PI3K) dependent pathway (Righi et al., 2000). It is generally believed that dendritically localized mRNAs remain dormant until stimulation (Bramham and Wells, 2007). Thus translation of mRNAs for BDNF and TrkB in response to activity would restrict newly synthesized BDNF and TrkB to activated postsynaptic sites.
A wide range of studies have provided evidence that BDNF is released both pre- and postsynaptically in an activity-dependent manner (Lessmann et al., 2003; Lu, 2003). Recent advancements in our understanding of activity-regulated BDNF release have mainly come from imaging of green fluorescent protein (GFP)-tagged BDNF (BDNF-GFP) in cultured neurons. In cultured cortical neurons BDNF-GFP was targeted into secretogranin II positive dendritic secretory granules of the regulated pathway in the vicinity of synaptic junctions (Haubensak et al., 1998), suggesting that BDNF may be secreted through the postsynaptic membrane in an activity-dependent fashion. Indeed, BDNF was released postsynaptically in response to network activity, as revealed by time-lapsed imaging of BDNF-GFP in hippocampal neurons (Kuczewski et al., 2008). LTP-inducing high-frequency stimulation was found to elicit release of BDNF from secretory granules localized at glutamatergic synapses, and activity-induced BDNF release at the postsynaptic site was dependent on calcium influx and activation of ionotropic glutamate receptors (Hartmann et al., 2001). In cultured hippocampal neurons, network activity also caused BDNF-GFP to be transported to and released from the presynaptic site, and be transferred to the postsynaptic site (Kohara et al., 2001; Kojima et al., 2001). Furthermore, BDNF is a “sticky” molecule likely due to its abundance of positively charged moieties (pI=9.6), so that locally released BDNF should remain in the vicinity of where it is released from, and not spread to distant non-activated synapses.
Activity-dependent surface expression of TrkB further restricts the action of BDNF to stimulated synapses. The concept that surface expression of TrkB is regulated emerged from work examining the survival of retinal ganglion cells (RGCs) in culture. Meyer-Franke found that the survival of cultured postnatal RGCs was low when treated with BDNF unless their intracellular cAMP was increased pharmacologically or their activity was enhanced by KCl-induced depolarization or activation of glutamate receptors (Meyer-Franke et al., 1995). It turned out that few TrkB receptors were localized to the plasma membrane in cultured RGCs, although high levels of TrkB were present intracellularly. Depolarization or cAMP elevation greatly increased surface TrkB levels within minutes by stimulating translocation of intracellularly stored TrkB to the plasma membrane (Meyer-Franke et al., 1998). If activity-dependent TrkB surface expression occurs at postsynaptic sites, the insertion of TrkB into the plasma membrane can strengthen the response to BDNF at a single dendritic spine while leaving nearby synapses unchanged. Du et al demonstrated that there was a rapid (<30 min), and therefore not protein synthesis dependent, membrane insertion of the TrkB receptor in response to high frequency stimulation in cultured hippocampal neurons (Du et al., 2000). This membrane insertion of the TrkB receptor was shown to depend on calcium influx and Ca2+/calmodulin-dependent kinase II (CaMKII). Fluorescent immunocytochemistry revealed that TrkB receptors were preferentially moved from intracellular pools to the plasma membrane of dendrites (Du et al., 2000). Interestingly, it was reported that within 15 seconds of BDNF application, TrkB receptors were up-regulated at the plasma membrane of hippocampal neurons (Haapasalo et al., 2002). This observation suggests that increased TrkB surface expression in response to cellular stimulation is at least in part mediated by activity-dependent BDNF release.
The cleavage of proBDNF to mature BDNF also appears to play a role in the restriction of BDNF to activated synapses. It has only recently been discovered that proBDNF has a unique biological function through the p75NTR/sortilin receptor complex (Lee et al., 2001; Nykjaer et al., 2004). Evidence suggests that proBDNF may play a role in the regulation of hippocampal synaptic plasticity by facilitating long-term depression (LTD) (Woo et al., 2005), and in peripheral neurons by facilitating apoptosis (Teng et al., 2005). It has been postulated that proBDNF is converted to mature BDNF intracellularly by pro-protein convertase 1/3 in secretory granules and by furin in trans-Golgi networks (Seidah et al., 1996; Mowla et al., 2001). However, extracellularly, plasmin and matrix metalloprotease-7 appear to mediate the conversion of proBDNF to mature BDNF (Lee et al., 2001). In tissue, plasmin is mainly present as an inactive precursor form, plasminogen, which is converted into plasmin by tissue plasminogen activator (tPA) (Castellino and Ploplis, 2005). The conversion of proBDNF to mature BDNF by tPA/plasmin is necessary for hippocampal late-LTP (Pang et al., 2004), suggesting that proBDNF is also secreted in the brain, at least at synapses. Because of the difficulty in detecting proBDNF, ascertaining the amount of BDNF that is secreted as proBDNF in nervous tissues has remained controversial (Matsumoto et al., 2008). However recently, research conducted by the research teams of Barbara Hempstead and Bai Lu has shown that both proBDNF and mature BDNF are secreted from neurons, with proBDNF being the primary form released from dendrites (Nagappan et al., 2009; Yang et al., 2009). Specifically, Nagappan and colleagues reported that both proBDNF and mature BDNF were predominantly secreted by the regulated pathway, and that the high frequency stimulation (HFS) favored the extracellular accumulation of mature BDNF in comparison with the low-frequency (LFS) due to HFS-dependent release of tPA (Nagappan et al., 2009). Importantly, the authors showed that in tPA−/−mice, HFS resulted in increases in extracellular proBDNF and not mature BDNF, indicating that proBDNF is the main form secreted from dendrites and that HFS-induced release of tPA converts these pools to mature BDNF. Taken together, these studies depict a model in which LFS and HFS primarily promote the release of proBDNF in dendrites, with HFS increasing the release of tPA into the extracellular space, thus shifting the extracellular balance from proBDNF to mature BDNF.
Still unknown, however, is the source of proBDNF. The majority of, if not all, proBDNF synthesized in the soma should be processed by furin and convertases when it passes through the trans-Golgi network and is packed into secretory granules (Seidah et al., 1996; Mowla et al., 2001). A recent report showed that BDNF, tPA and plasmin were co-packaged in dense core granules and transported into dendrites (Lochner et al., 2008). This observation further indicates that several proteases are given ample opportunities to convert proBDNF into mature BDNF before release. The co-package data is also incompatible with the observation that both HFS and LFS stimulate proBDNF release whereas only HFS enhances secretion of tPA. The apparent conflicting observations with regard to proBDNF raise the possibility that proBDNF may mainly come from local translation of dendritically localized BDNF mRNA. An and colleagues recently discovered that a large fraction of BDNF in dendrites, especially in distal dendrites, was derived from local translation of dendritic BDNF mRNA (An et al., 2008). Since Golgi outposts, defined by their characteristic mini-Golgi stack ultra-structure and immunoreactivity to the Golgi matrix protein GM130, have only been found as far as dendritic branch points along the dendritic shaft (Gardiol et al., 1999; Horton et al., 2005), dendritically synthesized BDNF is likely present as proBDNF. One mystery to be solved is how dendritically synthesized BDNF is packed into secretory granules without a trans-Golgi network. It is possible that as yet unknown dendritic organelles, whether adapted from and similar to prototypical Golgi networks, or completely different in structure and function from existing organelles, might mediate the activity-regulated secretion of dendritically synthesized BDNF. Electron microscopy did find membranous structures containing some Golgi markers (Pierce et al., 2001) in distal dendrites, suggesting the presence of Golgi-like structures in dendrites. Additional studies are necessary to understand the important question of how dendritically synthesized BDNF is processed, packed into secretory granules, and released.
BDNF-mediated phosphorylation of synaptic proteins
In the previous section we discussed several mechanisms that may restrict the action of BDNF to activated synapses. Evidence accumulated during the past 15 years suggests that BDNF can exert its fast effect on synaptic transmission through posttranslational modifications of synaptic proteins. Application of BDNF to cultured neurons has been shown to potentiate synaptic transmission through either a presynaptic (Lohof et al., 1993) or postsynaptic mechanism (Levine et al., 1995).
Presynaptically, BDNF has been shown to modify synapsin I and RIM1α (Jovanovic et al., 1996; Jovanovic et al., 2000; Simsek-Duran and Lonart, 2008). Synapsin I, a protein found on small synaptic vesicles, aids in the attachment of synaptic vesicles to actin filaments near the presynaptic membrane, thereby restricting their release. Phosphorylation of synapsin I results in vesicle detachment and an increased probability of exocytosis (Greengard et al., 1993). BDNF was shown to induce phosphorylation of synapsin I via the mitogen-activated protein kinase (MAPK) cascade in cortical neurons and PC12 cells (Jovanovic et al., 1996). The inhibition of MAPK with PD98059 caused a significant decrease in BDNF-induced synapsin I phosphorylation, and in neurotransmitter release. Furthermore, the stimulation of neurotransmitter release by BDNF was markedly attenuated in synaptosomes prepared from mice lacking synapsins (Jovanovic et al., 2000). Taken together, these studies suggest that BDNF induces phosphorylation of synapsin I, thus increasing docking of synaptic vesicles and neurotransmitter release. BDNF also acts on the Rab3a signaling pathway to increase efflux of glutamate from CA1 nerve terminals. This was first demonstrated when neurons cultured from Rab3a knockout mice did not display BDNF-enhanced neurotransmitter release (Thakker-Varia et al., 2001; Alder et al., 2005). Rab3a signaling involves the phosphoprotein RIM1α as an effector molecule (Lonart et al., 2003). RIM1α has been shown to be necessary for L-LTP (Huang et al., 2005) and necessary for BDNF-enhanced glutamate release from CA1 synaptoneurosomes (Simsek-Duran and Lonart, 2008). BDNF appears to augment this pathway via ERK2-dependent phosphorylation of RIM1α (Simsek-Duran and Lonart, 2008).
At the postsynaptic site, phosphorylation of N-methyl-d-aspartate (NMDA) receptor subunits has been shown to potentiate NMDA currents in hippocampal neurons (Wang and Salter, 1994), and BDNF appears to at least partially mediate this phosphorylation (Suen et al., 1997). In synaptoneurosome preparations, BDNF application has been demonstrated to rapidly cause phosphorylation of the NMDA receptor subunit 1 (NR1) (Suen et al., 1997). Moreover, BDNF application has also been demonstrated to increase phosphorylation of the NMDA receptor subunit 2B (NR2B) in postsynaptic densities within 5 minutes of application (Lin et al., 1999), and BDNF application to hippocampal slices was shown to increase the amplitude of spontaneous excitatory postsynaptic currents (Levine et al., 1995). These observations have thus demonstrated that BDNF signaling has the ability to stimulate protein modification that functionally alters synapses, independent of protein synthesis.
BDNF also appears to have a role in modifying local proteins that modulate structural plasticity. When paired with theta-burst stimulation, BDNF applied to hippocampal slice culture significantly enhanced the number of phalloidin labeled (F-actin) dendritic spines (Rex et al., 2007). This finding suggests that BDNF may regulate dendritic actin dynamics. In the same set of experiments BDNF application was shown to increase the phosphorylation of p21-activated kinase and cofilin (two dendritically localized actin-regulatory proteins implicated in spine plasticity) in an acute and dose-dependent manner, thus suggesting a mechanism by which BDNF regulates spine dynamics (Rex et al., 2007).
Regulation of local protein synthesis by BDNF
Application of BDNF has been shown to induce long-lasting LTP in hippocampal slices (Kang and Schuman, 1995), which is dependent on local protein synthesis in dendrites (Kang and Schuman, 1996). Local protein synthesis in dendrites is also required for other paradigms of enduring synaptic plasticity (Huber et al., 2000; Miller et al., 2002). These observations suggest that BDNF may modulate synaptic plasticity by regulating dendritic local protein synthesis. A growing number of mRNAs have been localized in neuronal dendrites (Steward and Schuman, 2003). Recent studies indeed found that BDNF stimulates the local synthesis of many different proteins in dendrites via both increasing dendritic mRNA trafficking and facilitating translation of existing dendritic mRNAs.
Moving mRNAs from the soma to dendrites is thought to be accomplished by holding mRNAs translationally silent in RNA granules and transporting them into the dendritic compartment via molecular motors (Kindler et al., 2005; Hirokawa, 2006; Martin and Zukin, 2006). The immediate early gene activity-regulated cytoskeleton-associated protein (Arc) has been shown to be crucial in LTP consolidation at the synapse, and both in vitro and in vivo studies have demonstrated that Arc synthesis is necessary for BDNF to have an effect on LTP at the synapse (Yin et al., 2002; Ying et al., 2002; Messaoudi et al., 2007). Discrete in vivo application of BDNF to the dentate gyrus increased dendritic Arc mRNA, suggesting that BDNF stimulation increases Arc mRNA dendritic trafficking (Ying et al., 2002). However, in this same study overall levels of Arc mRNA were increased, leaving the possibility that BDNF could solely be inducing transcription. Zipcode binding protein 1 (ZBP1), an mRNA binding protein, was shown to be associated with β-actin mRNA and targeted into the dendritic compartment in response to cellular depolarization (Tiruchinapalli et al., 2003). Interestingly, BDNF-induced spine head growth was reduced in cells where ZBP1 was knocked down (Eom et al., 2003). These findings suggest two possibilities. The first possibility is that BDNF stimulates dendritic trafficking of β-actin mRNA via a ZBP1-dependent mechanism. The second possibility is that BDNF induces local translation of β-actin mRNA, but could not do so when local mRNA pools were depleted due to knockdown of ZBP1 and therefore decreased ZBP1-dependent trafficking of β-actin mRNA to dendrites. The strongest support for the possibility that BDNF does stimulate dendritic trafficking of mRNA can be found in a study reporting that BDNF application increased dendritic levels of mRNAs for both TrkB and BDNF (Righi et al., 2000). This study showed that BDNF application to cultured hippocampal neurons in the absence of KCl stimulation was sufficient to increase dendritic levels of both BDNF and TrkB mRNAs in the presence of the transcription inhibitor, actinomycin (Righi et al., 2000). Furthermore, inhibition of BDNF action with scavenger TrkB-IgG, decreased levels of BDNF and TrkB mRNAs in the dendritic compartment after KCl stimulation (Righi et al., 2000). Collectively, these data suggest that BDNF induces an increase in trafficking of mRNA from the soma to dendrites.
A great deal of research has been conducted to address the question of which dendritic mRNAs are translationally induced by BDNF. In cultured neurons, application of BDNF induces the local synthesis of several synaptic proteins in dendrites (Aakalu et al., 2001; Yin et al., 2002; Schratt et al., 2004; Takei et al., 2004; Schratt et al., 2006), including Arc, CaMKIIα, the type 1 inositol 1,4,5-trisphosphate receptor (IP3R1), Homer2, NR1, GluR1, and Lim kinase 1 (Limk1). BDNF-induced local Arc synthesis was completely abolished by inhibition of mammalian target of rapamycin (mTOR), whereas CaMKIIα synthesis was only partially inhibited, suggesting that BDNF induces local translation of CaMKIIα through multiple signaling pathways (Takei et al., 2004). BDNF induction of Arc synthesis appears to be dependent on a signaling synergism between TrkB and NMDA receptors, as demonstrated when BDNF-induced synthesis of Arc in synaptoneurosomes was blocked by the NMDA antagonist MK801 or the tyrosine kinase inhibitor K252a. Finally, BDNF has been shown to stimulate dendritic synthesis of Limk1 via activating an mTOR dependent pathway and relieving translational repression mediated by a brain-specific microRNA, miR-134 (Schratt et al., 2006).
Mechanisms mediating the effect of BDNF on local protein synthesis
It is currently believed that mRNAs are held translationally silent in at least three types of protein-RNA granules found in dendrites, processing bodies (P-bodies), ribonucleoprotein particles (RNPs), and stress granules (SGs), until a signal de-represses the mRNA and allows for translational activation (Kindler et al., 2005; Schuman et al., 2006; Bramham and Wells, 2007; Zeitelhofer et al., 2008). One way in which BDNF induces local translation is by de-repressing RNA granules. It has been shown that BDNF application to cultured hippocampal neurons leads to a 67% reduction in the number of P-bodies in dendrites, suggesting that BDNF is de-repressing dendritic mRNA, thereby enabling the mRNA to be translationally active (Zeitelhofer et al., 2008). RNA granule protein 105 (RNG 105) is a RNA binding protein that associates with RNA granules in hippocampal dendrites and suppresses translation in vivo and in vitro (Shiina et al., 2005). Application of BDNF has been shown to induce the dissociation of RNG105 from RNA granules (Shiina et al., 2005). Importantly, BDNF application to dissociated dendritic cultures was shown to shift the key plasticity mRNAs for CaMKIIα, TrkB, BDNF, and CREB from association with RNA granules to association with translationally-active polyribosomes, ultimately increasing dendritic protein levels (Shiina et al., 2005). Taken together, these findings suggest that BDNF increases dendritic protein synthesis, in part, by dissociating mRNA from repressing RNA granules.
BDNF has also been shown to facilitate local protein synthesis by activating translation initiation factors and modulating elongation factors. The rate-limiting step in cap-dependent translation initiation is the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E), which results in an increased rate of translation (Gingras et al., 2004). Alternatively, phosphorylation of eIF4E binding protein (eIF4E-BP) will also lead to translation initiation via release of eIF4E. In cultured cortical neurons using an 35S-met-labeled protein synthesis assay, BDNF was found to activate protein translation via two pathways, the PI3K-mTOR pathway and the MAPK pathway (Takei et al., 2001). BDNF increased translation initiation via MAPK-induced phosphorylation of eIF4E and via mTOR-induced phosphorylation of eIF4E-BP1 (Takei et al., 2001). These results were extended to demonstrate the local effect of BDNF on translation initiation by utilizing synaptoneurosomes, in which vesicularized pre- and postsynaptic membranes are isolated. In synaptoneurosomes BDNF-mTOR signaling led to phosphorylation of e1F4E–BP and increased translation initiation (Takei et al., 2004). Furthermore, BDNF treatment was shown to result in a rapid and transient activation of eIF4E and the increased expression of CaMKIIα in synaptoneurosome preparations (Kanhema et al., 2006). It also appears that BDNF signaling may increase translation by inducing the local synthesis of ribosomal proteins and translation factors. Transcripts encoding these proteins usually contain a terminal oligopyrimidine tract (TOP) in their 5’UTR, and mTOR activation of ribosomal protein S6 kinase (p70S6K) induces translation of 5’TOP containing mRNAs. BDNF application to synaptoneurosome preparations activated (phosphorylated) p70S6K in an mTOR-dependent manner (Takei et al., 2004), and similarly, BDNF induced phosphorylation of p70S6K in cultured neurons at or near synapses (Schratt et al., 2004).
Fractionation studies have revealed that BDNF can induce translocation of eIF4E to mRNA granule fractions, suggesting that de-repressed mRNA will be released from translational repression for proximal translation (Smart et al., 2003). In the same set of experiments, using immunocytochemistry assays, it was reported that BDNF induced translocation of eIF4E to dendritic spines, thus ensuring that eIF4E is in the right place for local translation initiation (Smart et al., 2003). Using FRET analysis to show translation initiation via a direct interaction of two translation initiation factors, eIF5 and eIF2, Miyata et al. found that the simultaneous application of BDNF and ephrins potentiated local protein synthesis in the dendrites of hippocampal neurons (Miyata et al., 2005). It also appears that BDNF can induce translation initiation by stimulating the dissociation of eIF4E from the repressive mRNA-binding protein CYF1P in the dendritic compartment (Napoli et al., 2008). Taken together, these studies support a model of BDNF as a dynamic facilitator of dendritic translation initiation.
The effect of BDNF on translation elongation appears to be modulatory rather than strictly facilitatory. Elongation factor 2 (eEF2), a GTP binding protein, regulates transfer of peptidyl tRNAs from the A-site to the P-site in ribosomes during elongation. Phosphorylation of eEF2 typically results in release of eEF2 from the ribosome and elongation arrest (Browne and Proud, 2002), however translation of CaMKIIα mRNA has been shown to be increased with phosphorylation of eEF2 (Scheetz et al., 2000). In vivo infusion of BDNF into the rat dentate gyrus increased levels of phosphorylated eEF2, while the infusion of a MAPK inhibitor into the dentate gyrus blocked induction of LTP by BDNF and the increase in phosphorylated eEF2 (Kanhema et al., 2006). In cultured cortical neurons, however, BDNF application increased the rate of elongation (as shown by ribosomal transit time), while decreasing the phosphorylation of eEF2 via an mTOR-dependent pathway (Inamura et al., 2005). These findings might indicate that the role of BDNF and eEF2 in elongation are both site and substrate specific. Further investigation is needed to better elucidate how BDNF, and the phosphorylation status of eEF2, control elongation.
Local synthesis of BDNF and its role in spine morphology and synaptic plasticity
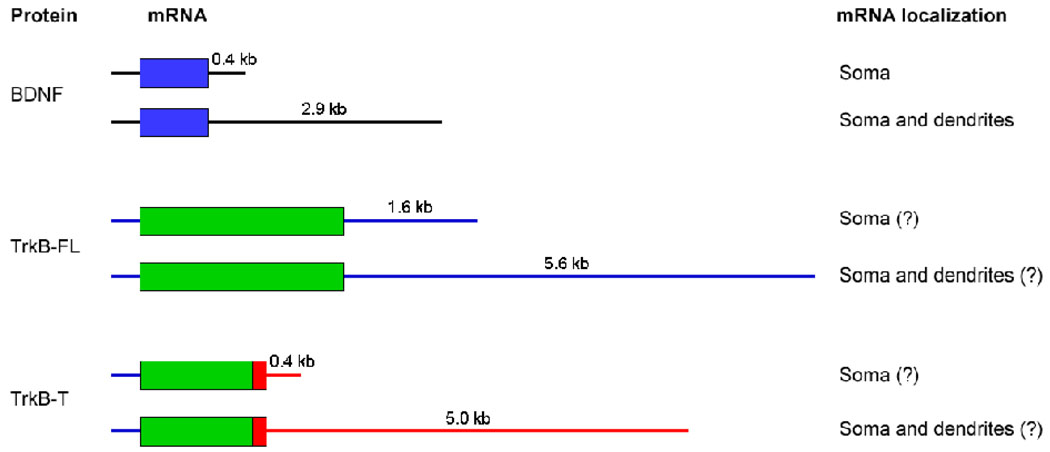
Tongiorgi and colleagues found that BDNF mRNA was transported to dendrites of cultured hippocampal neurons when the neurons were depolarized (Tongiorgi et al., 1997). Their subsequent studies showed that BDNF mRNA was also targeted to dendrites in vivo after epileptogenic stimulation (Simonato et al., 2002; Tongiorgi et al., 2004; Chiaruttini et al., 2008). These observations suggested that translation of dendritically localized BDNF mRNA may play a crucial role in some activity-dependent processes. It has been known for a while that there are two pools of BDNF mRNAs with either a short 3′ UTR or a long 3′ UTR (Timmusk et al., 1993; Ghosh et al., 1994), but it had been a puzzle why cells need two species of transcripts encoding exactly the same protein (Fig. 1A). An et al found that the short 3′ UTR BDNF mRNA was restricted to cell bodies whereas the long 3′ UTR BDNF mRNA was also localized to dendrites. They further demonstrated that the long 3′ UTR was sufficient to target transcripts to dendrites of cultured hippocampal neurons and that truncation of the long 3′ UTR abolished dendritic targeting of BDNF mRNA in vivo (An et al., 2008). These data indicate that the long 3′ UTR controls dendritic targeting of BDNF mRNA and provide an example where mRNAs containing the same coding sequence but distinct 3′ UTRs can have distinct subcellular localization and function.
Figure 1. BDNF and TrkB mRNA structure and subcellular localization.
Transcripts encoding BDNF (A) and TrkB full length (TrkB-FL) and truncated (TrkB-T) (B) undergo alternative polyadenylation generating two mRNA species with identical coding sequences but different length 3’UTRs. The short form of BDNF mRNA is restricted to the soma, however the long form is targeted to the dendrites (An et al., 2008). Although not demonstrated, it is possible that this same sub-cellular localization mechanism is utilized for TrkB mRNAs as well.
The trkB gene encodes two proteins, one full-length TrkB receptor and one truncated TrkB receptor which lacks the tyrosine kinase domain (Klein et al., 1990). TrkB mRNA has been shown to be localized in dendrites in response to activity (Tongiorgi et al., 1997; Simonato et al., 2002), suggesting that TrkB, like BDNF, is also locally synthesized in dendrites. Interestingly, transcripts for both TrkB receptors have either a short 3′ UTR or a long 3′ UTR, and the two sets of 3′ UTRs are completely different (Fig. 1B). Further investigation is necessary to determine whether the long 3′ UTRs target TrkB mRNAs to dendrites and why the trkB gene needs two sets of 3′ UTRs. It was estimated that more than half of all human genes have multiple polyadenylation sites (Zhang et al., 2005). Because these multiple polyadenylation sites could generate transcripts with different lengths of 3′ UTRs that encode the same protein and are expressed in the same cell, an important question for future research is to determine if alternative polyadenylation is a common way that neurons spatially restrict protein expression. Future research is also needed to explore the possible mechanisms that regulate alternative polyadenylation.
In addition to two different lengths of 3′ UTR, each BDNF transcript can have one of several distinct alternatively spliced 5′ UTRs (Liu et al., 2006; Aid et al., 2007). BDNF mRNAs containing different 5′ UTRs were found to be targeted to dendrites of cortical and hippocampal neurons with different efficiencies after epileptogenic stimulation (Pattabiraman et al., 2005; Chiaruttini et al., 2008). Given that the long BDNF 3′ UTR is necessary and sufficient for dendritic targeting of BDNF mRNA (An et al., 2008), it is possible that some of the BDNF 5′ UTRs can modulate the efficiency of BDNF mRNA dendritic targeting mediated by the long 3′ UTR. Alternatively, neuronal activity may increase the usage of the second polyadenylation site for BDNF mRNAs containing the 5′ UTRs that are preferentially targeted to dendrites.
It remains to be determined which sequence elements in the long 3′ UTR and which proteins control trafficking of BDNF mRNA to dendrites. The long BDNF 3′ UTR contains multiple cytoplasmic polyadenylation elements (CPEs) (Du and Richter, 2005) and an AU-rich element (ARE), which is a putative binding site for Hu proteins (HuR, HuB, HuC, and HuD) (Okano and Darnell, 1997). CPEs and their binding protein CPEB1 have been shown to facilitate mRNA transport to dendrites and local protein synthesis in dendrites (Wu et al., 1998; Huang et al., 2003). The interaction of HuR with the ARE sequence is thought to facilitate the relief of microRNA-mediated translational repression of certain mRNAs (Bhattacharyya et al., 2006). While HuR is widely expressed, the other three members of the Hu family are specifically expressed in neuronal tissues (Okano and Darnell, 1997). In the middle of the long BDNF 3′ UTR, there is a G-rich sequence with a potential to form a G quartet structure, a binding motif for fragile X mental retardation protein (FMRP). Recent studies show that FMRP is important for dendritic trafficking of mRNAs (Dictenberg et al., 2008; Estes et al., 2008) and for stimulation-dependent protein translation at synapses (Weiler et al., 2004; Muddashetty et al., 2007). It would be interesting to determine whether these proteins play a role in regulating dendritic trafficking and translation of the long 3′ UTR BDNF mRNA.
By taking advantage of an existing mouse strain in which the long BDNF 3′ UTR is truncated, An and colleagues demonstrated that the long 3′ UTR was necessary for dendritic targeting of BDNF mRNA in vivo (An et al., 2008). Despite the truncation, the Bdnf mouse mutant still produced a normal amount of BDNF mRNA and BDNF protein. Thus, the mouse mutant provides an ideal system to examine the in vivo function of local protein synthesis. Although local protein synthesis is important for long-lasting synaptic plasticity in hippocampal slices (Kang and Schuman, 1996; Huber et al., 2000; Miller et al., 2002), the in vivo physiological function of dendritically localized mRNAs largely remains unknown because it is technically challenging to selectively abolish dendritic local protein synthesis without affecting the total protein level in a neuron. One surprising observation from studying the Bdnf mutant is that levels of BDNF protein are lower in dendrites and higher in somata in hippocampal neurons isolated from the mutant mice (An et al., 2008). This result suggests that granules containing BDNF synthesized in the soma are not transported to dendrites efficiently and that dendritic BDNF is mainly derived from local synthesis. The Bdnf mouse mutant displays an elevated spine density and reduced spine head diameter in distal apical dendrites of CA1 hippocampal neurons at two months of age (An et al., 2008). This observation provides direct evidence tying local protein synthesis to morphology of dendritic spines. The spine phenotype in the Bdnf mouse mutant likely results from deficits in spine pruning, because the spine density measured at the end of spinogenesis is not altered in the mutant hippocampus (An et al., 2008). Future studies using in vivo imaging are necessary to confirm that local BDNF synthesis plays a key role in spine pruning, which is dependent on sensory experience and has been implicated in activity-dependent refinement of synaptic connections (Zuo et al., 2005).
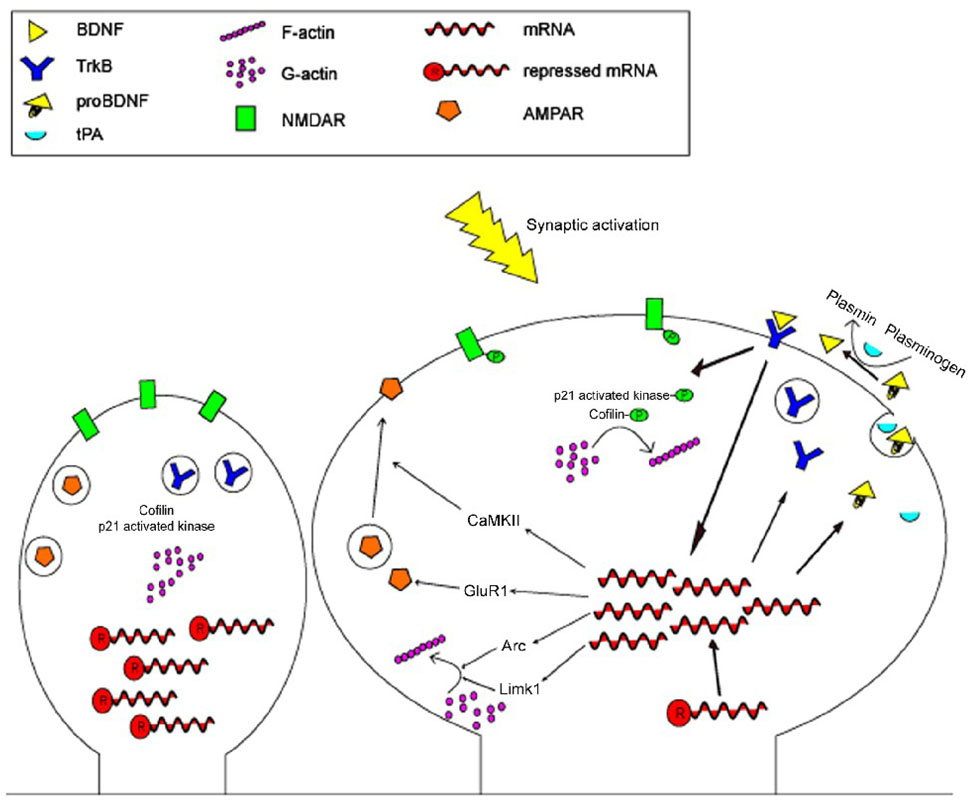
The work done by An et al clearly indicates that dendritically synthesized BDNF regulates spine morphology. We propose a model of activity-dependent spine changes to integrate their findings and findings from other groups (Fig. 2). Dendritically synthesized BDNF is primarily secreted as proBDNF. This is likely due to the fact that the Golgi network is not widely present in distal dendrites, such that dendritic proBDNF packaging and release probably utilizes a novel mechanism. Under LTP-inducing high frequency stimulation, released proBDNF is converted to mature BDNF by the tPA/plasmin system at stimulated synapses due to activity-dependent release of tPA, and subsequently activates TrkB at the same synapses in an autocrine manner. As discussed in the previous sections, TrkB signaling should induce the local synthesis of proteins important for spine growth and plasticity within and/or underneath the stimulated spines. We speculate that these locally synthesized proteins then promote actin dynamics and AMPA receptor trafficking in spines, which leads to growth of the spine head and formation of stable LTP. Under LTD-inducing low frequency stimulation, however, unprocessed pro-BDNF promotes pruning of depressed spines by binding to the sortilin/p75NTR receptor complex with high affinity. This model is based on the following observations. First, both high and low frequency stimulations stimulate release of proBDNF, whereas only high frequency stimulation increases release of tPA (Nagappan et al., 2009). Second, the activation of TrkB-mediated signaling cascades has been shown to induce the local synthesis of several proteins, including Arc, CaMKIIα, Homer2, and Limk1 (Kang and Schuman, 1996; Yin et al., 2002; Schratt et al., 2004). While CaMKIIα is a key regulator of AMPA receptor trafficking at synapses (Derkach et al., 2007), the other three proteins promote actin polymerization required for LTP consolidation (Bramham and Wells, 2007) and the maturation and enlargement of spine heads (Sala et al., 2001; Meng et al., 2002). Third, the gradual phase of spine enlargement induced by synaptic stimulation is dependent on protein synthesis and BDNF action (Tanaka et al., 2008). Finally, the p75NTR receptor is localized to postsynaptic sites in the CA1 area (Woo et al., 2005) and deletion of the gene for the receptor results in increased spine density in apical dendrites of hippocampal neurons (Zagrebelsky et al., 2005). This model predicts that selective inhibition of dendritic BDNF synthesis would reduce the size of spine heads, increase spine number, and impair hippocampal LTP; the phenotypes observed in mice lacking local BDNF synthesis (An et al., 2008).
Figure 2. Mechanisms of BDNF induced synaptic plasticity.
In the non-stimulated state, dendritic spines remain “silent”: mRNAs are repressed in RNA granules, TrkB and AMPA receptors are held intracellularly, and actin dynamics leave the spine head in an immature form. Following synaptic activation, repressed mRNAs are disinhibited, TrkB is inserted into the plasma membrane, pro-BDNF and tPA are packaged either together or separately and released into the synaptic cleft, pro-BDNF is converted into BDNF by plasmin, and BDNF binds to TrkB on the local dendritic membrane. Activation of TrkB by BDNF increases translation of CaMKII, GluR1, Arc, and Limk1, leading to increased the formation and membrane insertion of the AMPA receptor and increased actin polymerization. TrkB signaling also induces phosphorylation of NMDA receptors, synapsin-1, p21 activated kinase, and cofilin, thus increasing receptor activity, vesicle-plasma membrane fusion and neurotransmitter release, and polymerization of actin, respectively.
Concluding remarks
There is substantial evidence that BDNF plays a critical role in synaptic plasticity. Significant progress has been made in our understanding of how BDNF, as a secreted protein, modulates synaptic plasticity that is restricted to activated synapses. Findings from many research groups suggest that activity-dependent local synthesis and release of BDNF and surface expression of TrkB may work in concert to limit the action of BDNF to active synapses. Local BDNF action modulates synaptic transmission quickly by inducing phosphorylation of synaptic proteins or modulates synaptic transmission in a lasting manner by modifying synaptic protein composition via local protein synthesis. One study indicates that dendritic BDNF is mainly derived from local translation of BDNF mRNA in dendrites (An et al., 2008). Since TrkB mRNA has also been found in dendrites (Tongiorgi et al., 1997; Righi et al., 2000), important future research concerning the mechanisms underlying the action of BDNF at synapses will be to elucidate the molecular cascades controlling dendritic trafficking and translation of BDNF and TrkB mRNAs.
Acknowledgements
B.X. is supported by the National Institutes of Health (NS050596 and DK081008), the March of Dimes Foundation, and the American Diabetes Association. We apologize for the fact, owing to space constraints, not all relevant papers could be cited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder J, Thakker-Varia S, Crozier RA, Shaheen A, Plummer MR, Black IB. Early presynaptic and late postsynaptic components contribute independently to brain-derived neurotrophic factor-induced synaptic plasticity. J Neurosci. 2005;25:3080–3085. doi: 10.1523/JNEUROSCI.2970-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Bodian D. A Suggestive Relationship of Nerve Cell Rna with Specific Synaptic Sites. Proc Natl Acad Sci U S A. 1965;53:418–425. doi: 10.1073/pnas.53.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Chiaruttini C, Sonego M, Baj G, Simonato M, Tongiorgi E. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Mol Cell Neurosci. 2008;37:11–19. doi: 10.1016/j.mcn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Davies AM. The role of neurotrophins during successive stages of sensory neuron development. Prog Growth Factor Res. 1994;5:263–289. doi: 10.1016/0955-2235(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150:1423–1434. doi: 10.1083/jcb.150.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Richter JD. Activity-dependent polyadenylation in neurons. Rna. 2005;11:1340–1347. doi: 10.1261/rna.2870505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom T, Antar LN, Singer RH, Bassell GJ. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J Neurosci. 2003;23:10433–10444. doi: 10.1523/JNEUROSCI.23-32-10433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes PS, O'Shea M, Clasen S, Zarnescu DC. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol Cell Neurosci. 2008;39:170–179. doi: 10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Gardiol A, Racca C, Triller A. Dendritic and postsynaptic protein synthetic machinery. J Neurosci. 1999;19:168–179. doi: 10.1523/JNEUROSCI.19-01-00168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–197. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, Sipola I, Larsson K, Akerman KE, Stoilov P, Stamm S, Wong G, Castren E. Regulation of TRKB Surface Expression by Brain-derived Neurotrophic Factor and Truncated TRKB Isoforms. J Biol Chem. 2002;277:43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. Embo J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111:1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci. 2006;26:7139–7142. doi: 10.1523/JNEUROSCI.1821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. Embo J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Huang YS, Carson JH, Barbarese E, Richter JD. Facilitation of dendritic mRNA transport by CPEB. Genes Dev. 2003;17:638–653. doi: 10.1101/gad.1053003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Sudhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Inamura N, Nawa H, Takei N. Enhancement of translation elongation in neurons by brain-derived neurotrophic factor: implications for mammalian target of rapamycin signaling. J Neurochem. 2005;95:1438–1445. doi: 10.1111/j.1471-4159.2005.03466.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Havik B, Ying SW, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control. J Neurochem. 2006;99:1328–1337. doi: 10.1111/j.1471-4159.2006.04158.x. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kindler S, Wang H, Richter D, Tiedge H. RNA transport and local control of translation. Annu Rev Cell Dev Biol. 2005;21:223–245. doi: 10.1146/annurev.cellbio.21.122303.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Koenig E. Synthetic Mechanisms in the Axon. Ii. Rna in Myelin-Free Axons of the Cat. J Neurochem. 1965;12:357–361. doi: 10.1111/j.1471-4159.1965.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczewski N, Porcher C, Ferrand N, Fiorentino H, Pellegrino C, Kolarow R, Lessmann V, Medina I, Gaiarsa JL. Backpropagating action potentials trigger dendritic release of BDNF during spontaneous network activity. J Neurosci. 2008;28:7013–7023. doi: 10.1523/JNEUROSCI.1673-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Len GW, Xu JL, Levine ES, Suen PC, Mount HT, Black IB. Brain-derived neurotrophic factor enhances association of protein tyrosine phosphatase PTP1D with the NMDA receptor subunit NR2B in the cortical postsynaptic density. Brain Res Mol Brain Res. 1999;70:18–25. doi: 10.1016/s0169-328x(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lochner JE, Spangler E, Chavarha M, Jacobs C, McAllister K, Schuttner LC, Scalettar BA. Efficient copackaging and cotransport yields postsynaptic colocalization of neuromodulators associated with synaptic plasticity. Dev Neurobiol. 2008;68:1243–1256. doi: 10.1002/dneu.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. J Neurosci. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Miyata S, Mori Y, Fujiwara T, Ikenaka K, Matsuzaki S, Oono K, Katayama T, Tohyama M. Local protein synthesis by BDNF is potentiated in hippocampal neurons exposed to ephrins. Brain Res Mol Brain Res. 2005;134:333–337. doi: 10.1016/j.molbrainres.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Zaitsev E, Senatorov VV, Jr, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci U S A. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Pru EL, Palacios L, Mendoza RV. Synaptogenetic mechanisms during chick cerebellar cortex development. J Submicrosc Cytol. 1981;13:145–167. [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Tropea D, Chiaruttini C, Tongiorgi E, Cattaneo A, Domenici L. Neuronal activity regulates the developmental expression and subcellular localization of cortical BDNF mRNA isoforms in vivo. Mol Cell Neurosci. 2005;28:556–570. doi: 10.1016/j.mcn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Mayer T, McCarthy JB. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr Biol. 2001;11:351–355. doi: 10.1016/s0960-9822(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M, Tongiorgi E, Cattaneo A. Brain-derived neurotrophic factor (BDNF) induces dendritic targeting of BDNF and tyrosine kinase B mRNAs in hippocampal neurons through a phosphatidylinositol-3 kinase-dependent pathway. J Neurosci. 2000;20:3165–3174. doi: 10.1523/JNEUROSCI.20-09-03165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26:7143–7146. doi: 10.1523/JNEUROSCI.1796-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Shiina N, Shinkura K, Tokunaga M. A novel RNA-binding protein in neuronal RNA granules: regulatory machinery for local translation. J Neurosci. 2005;25:4420–4434. doi: 10.1523/JNEUROSCI.0382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonato M, Bregola G, Armellin M, Del Piccolo P, Rodi D, Zucchini S, Tongiorgi E. Dendritic targeting of mRNAs for plasticity genes in experimental models of temporal lobe epilepsy. Epilepsia. 2002;43 Suppl 5:153–158. doi: 10.1046/j.1528-1157.43.s.5.32.x. [DOI] [PubMed] [Google Scholar]
- Simsek-Duran F, Lonart G. The role of RIM1alpha in BDNF-enhanced glutamate release. Neuropharmacology. 2008;55:27–34. doi: 10.1016/j.neuropharm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Smart FM, Edelman GM, Vanderklish PW. BDNF induces translocation of initiation factor 4E to mRNA granules: evidence for a role of synaptic microfilaments and integrins. Proc Natl Acad Sci U S A. 2003;100:14403–14408. doi: 10.1073/pnas.2436349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Falk PM. Polyribosomes under developing spine synapses: growth specializations of dendrites at sites of synaptogenesis. J Neurosci Res. 1985;13:75–88. doi: 10.1002/jnr.490130106. [DOI] [PubMed] [Google Scholar]
- Steward O, Falk PM. Protein-synthetic machinery at postsynaptic sites during synaptogenesis: a quantitative study of the association between polyribosomes and developing synapses. J Neurosci. 1986;6:412–423. doi: 10.1523/JNEUROSCI.06-02-00412.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Compartmentalized synthesis and degradation of proteins in neurons. Neuron. 2003;40:347–359. doi: 10.1016/s0896-6273(03)00635-4. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci U S A. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–6790. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23:3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Armellin M, Giulianini PG, Bregola G, Zucchini S, Paradiso B, Steward O, Cattaneo A, Simonato M. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. J Neurosci. 2004;24:6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Righi M, Cattaneo A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, Base CK, Greenough WT. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wu L, Wells D, Tay J, Mendis D, Abbott MA, Barnitt A, Quinlan E, Heynen A, Fallon JR, Richter JD. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zeitelhofer M, Karra D, Macchi P, Tolino M, Thomas S, Schwarz M, Kiebler M, Dahm R. Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J Neurosci. 2008;28:7555–7562. doi: 10.1523/JNEUROSCI.0104-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lee JY, Tian B. Biased alternative polyadenylation in human tissues. Genome Biol. 2005;6:R100. doi: 10.1186/gb-2005-6-12-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]