Abstract
A catalyst system based on a new biarylmonophosphine ligand (BrettPhos) that shows excellent reactivity for C–N cross-coupling reactions is reported. This catalyst system enables the use of aryl mesylates as a coupling partner in C–N bond-forming reactions. Additionally, the use of BrettPhos permits the highly selective monoarylation of an array of primary aliphatic amines and anilines at low catalyst loadings and with fast reaction times, including the first monoarylation of methylamine. Lastly, oxidative addition complexes of BrettPhos are included, which provide insight into the origin of reactivity for this system.
Palladium-catalyzed C–N cross-coupling reactions are an important technology both in industry and academia.1 Despite considerable advances in the field,2 notable limitations remain for which improved methods will have an immediate impact on the chemistry community. Herein, we report a catalyst comprised of a new biaryldialkylphosphine ligand that shows excellent reactivity and stability in C–N cross-coupling reactions and overcomes many restrictions that previous catalyst systems have possessed. This improved ligand enables the aminations of aryl mesylates as well as, for the first time, the highly selective monoarylation of primary amines using low catalyst loadings of a monophosphine-based catalyst.
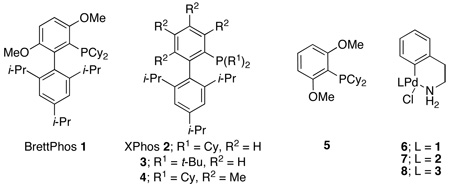
Due to their high stability, good atom economy, and low cost, aryl mesylates represent an important substrate class for C–N cross-coupling reactions.3,4,5 Recently, we demonstrated that substitution of the phosphine-containing arene in biarylmonophosphine ligands can have profound effects of the observed reactivity in catalytic reactions.6 In continued efforts to explore this effect, we have prepared a new ligand with methoxy substitution on the phosphine-containing arene (BrettPhos, 1)7 and have found it to be effective in the amination of aryl mesylates.
Initial studies focused on the coupling of 4-t-butylphenyl methanesulfonate and aniline. Whereas catalyst systems based on the combination of Pd2(dba)3 and 1 failed to produce product (Table 1, entry 1), precatalyst8 6 provided a 98% yield in 3 h (Table 1, entry 2). Similarly, utilization of water-mediated catalyst activation with 1 and Pd(OAc)2 gave the desired product in 99% yield (Entry 3).9 In contrast, the use of ligand 2 (XPhos), which has been shown to be efficient in couplings of other aryl sulfonates,3c but lacks the methoxy groups, provided only trace amounts of product when used either as precatalyst 7 or with the water-mediated activation protocol (Table 1, entries 4 and 5).
Table 1.
Screen of Ligands and Pd Sources for the Coupling of Aniline and 4-t-Butylphenyl Methanesulfonatea
 | |||
|---|---|---|---|
| Entry | Ligand | Pd Source | Yieldc |
| 1 | 1 | Pd2(dba)3 | 0% |
| 2 | 1 | 6 | 98% |
| 3b | 1 | Pd(OAc)2 | 99% |
| 4 | 2 | 7 | 4% |
| 5b | 2 | Pd(OAc)2 | 2% |
| 6 | 3 | 8 | 0% |
| 7b | 4 | Pd(OAc)2 | 0% |
| 8b | 5 | Pd(OAc)2 | 0% |
ArOMs (1mmol), amine (1.2 mmol), Pd (1 mol%), ligand (2 mol%), K2CO3 (1.4 mmol), t-BuOH (12 mL/mmol), 110 °C, 3 h.
Using water activation protocol (ref. 9c).
GC Yields.
Because these results clearly implicate the importance of substitution in the upper arene in 1, we also examined the use of the tetra-methyl substituted ligand 4, a congener of a ligand which has been shown to be effective in amidation reactions.6 Unlike reactions employing 1, reactions employing 4 failed to provide even detectable amounts of the desired product (Table 1, entry 7). These results demonstrate that the nature of the arene substituent is critical to the performance of 1. Further, in order to show that the activity of 1 does not only arise from the ortho methoxy substituent dimethoxy ligand 5, was synthesized. As with 4, the use of 5 as the ligand failed to provide detectable product (Table 1, entry 8). These results, taken together, reveal a cooperative effect between the methoxy substituents and the biaryl motiff and demonstrate that both are required for the observed reactivity in catalytic reactions employing ligand 1.
Having defined an efficient catalytic system, the scope of aryl mesylate coupling reactions was next explored. Highlighted below (Table 2), a number of electron-deficient anilines, which are less reactive in coupling reactions than electron-rich or -neutral anilines,10 were successfully reacted with both electron-rich and electron-deficient aryl mesylates in excellent yields. Ortho substituents on both the aniline and aryl mesylate and several functional groups were well tolerated (Table 2).11
Table 2.
Formation of Diarylamines Using Aryl Mesylates
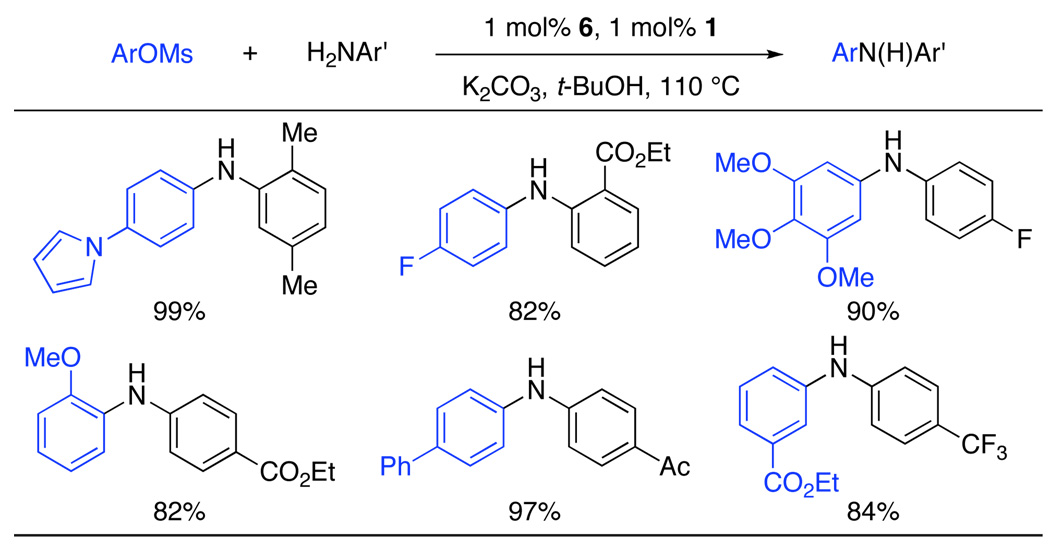 |
ArOMs (1 mmol), amine (1.2 mmol), 1 (1 mol%), 6 (1 mol%), K2CO3, (1.4 mmol), t-BuOH (12 mL/ mmol), 110 °C, 16 h.
Encouraged by the level of reactivity that catalysts based on 1 displayed in cross-coupling reactions involving aryl mesylates, we decided to examine the monoarylation of primary amines. Although this transformation has long been proficient with aryl bromides,12 recent progress has extended the method to aryl chlorides.2b,13 However, despite this success, challenges still remain, including the monoarylation of methylamine, which has yet to be described. Because it is the smallest aliphatic primary amine and therefore most likely to undergo diarylation, methylamine is a particularly challenging coupling partner to monoarylate. Using 6, methylamine was successfully coupled with 4-chloroanisole with a selectivity of >97:3 for monoarylation over diarylation (Table 3, entry 1). The analogous reaction using 7 did not give any product at room temperature. By increasing the temperature to 80 °C, the reaction proceeded but favored diarylation, reversing the selectivity to 20:80 (Table 3, entry 3). Using the more bulky ligand 3,14 the selectivity increased to 82:18 (Table 3, entry 4) but was still not nearly as selective as 1. The use of ligand 1 successfully inhibits reactions involving disubstituted amines and allows for the highly selective monarylation of methylamine at room temperature (Table 4).
Table 3.
Screen of Ligands for the Arylation of Methylaminea
| Entry | Precat | Yieldb | Temp | MeN(H)Ar : MeNAr2c |
|---|---|---|---|---|
| 1 | 6 | 92% | rt | >97:3 |
| 2 | 7 | 0% | rt | -- |
| 3 | 7 | 11% | 80 °C | 20:80 |
| 4 | 8 | 70% | rt | 82:18 |
ArCl (1 mmol), 2M MeNH2 in THF (2.0 mmol), Pd [6, 7, or 8] (1 mol %),15 NaOt-Bu (1.2 mmol).
GC yields.
Selectivities determined by GC.
Table 4.
Mono-arylation of Methylaminea
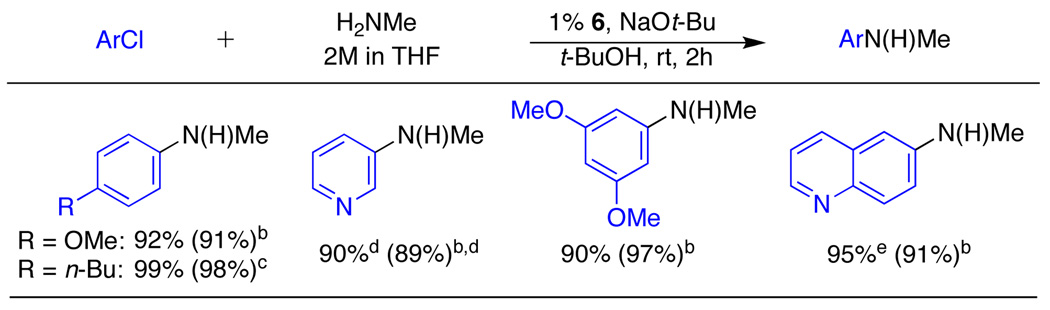 |
ArCl (1 mmol), 2M MeNH2 in THF (2.0 mmol), 6 (1 mol %), NaOt-Bu (1.2 mmol).
0.3 mol % 6.
0.1 mol % 6.
35:1 mixture of monoarylation to diarylation.
17 h reaction time.
Selectivities determined by GC.
We then turned our attention to the selective monoarylation of other primary aliphatic amines, which have been difficult to achieve using biarylphosphine ligands. With a catalyst system using 1 as ligand, we were able to successfully couple several primary aliphatic amines and aryl chlorides in excellent yields at 0.05 mol% catalyst loading in 1 h (Table 5). It is also noteworthy that less than 1% of the diarylation product was observed in all cases. Common perception has been that chelating bisphosphine ligands are required for these couplings in order to suppress diarylation. However, these results not only show that biarylmonophosphines can efficiently support cross-coupling reactions involving primary aliphatic amines, but in some cases they are more efficient than bisphosphine systems. For example, the coupling of octylamine and 4-chloroanisole with a bisphosphine based catalyst system formerly required 0.1 mol% Pd and a reaction time of 48 h.13 With a catalyst system based on 1, the reaction of hexylamine and 4-chloroanisole was complete after 1 h using only 0.05 mol% Pd.
Table 5.
Coupling Reactions at Low Catalyst Loadings and Short Reaction Times
 |
ArCl (1 mmol), amine (1.4 mmol), 1 (0.05 mol%), 6 (0.05 mol%), NaOt-Bu (1.2 mmol).
ArCl (1 mmol), amine (1.2 mmol), 1 (0.01 mol%), 6 (0.01 mol%), NaOt-Bu (1.2 mmol).
We recently reported a catalyst system using ligand 2, in which anilines were successfully coupled with aryl chlorides at catalyst loadings as low as 0.05 mol %.9c By switching to ligand 1 we were able to lower the catalyst loadings to 0.01 mol % while keeping the reaction times at 1 h (Table 5). This is the lowest palladium loading that has been reported in C–N bond-forming reactions of primary anilines with aryl chlorides.2a These results demonstrate clearly the exceptional activity of 1 in these reactions in comparison to previously reported catalyst systems.
The results described above suggested that we should see high levels of chemoselectivity for the arylation of a primary amine over a secondary amine. Using 1, we were able to couple the primary amino group of 9 in the presence of a secondary anilino group with >40:1 selectivity (Table 6). This result is complimentary to a previous report by our group in which anilines reacted in preference to aliphatic amines.16 Further in the intramolecular competition between a primary and cyclic secondary amine in 10, and between a primary and secondary aniline in 11, the primary amino group was preferentially N-arylated and proceeded in excellent yields with selectivities of >20:1 (Table 6).17
Table 6.
Selectivity of Primary Amines Over Secondary Aminesa
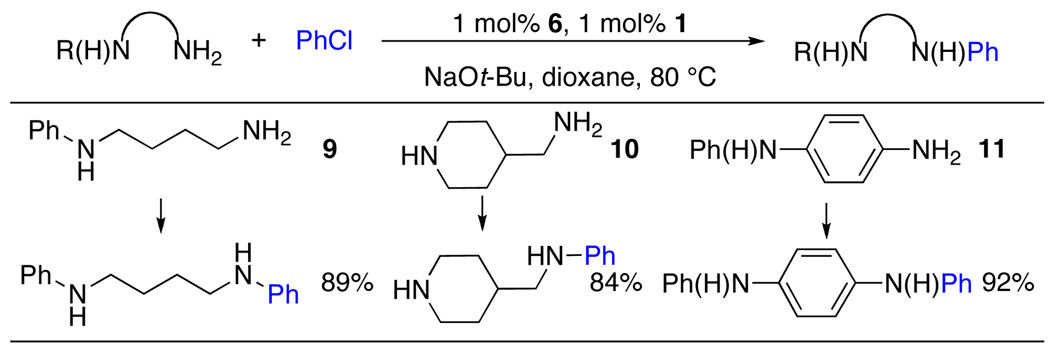 |
ArCl (1 mmol), amine (1.2 mmol), NaOt-Bu (2.0 mmol), 2 – 15 h.
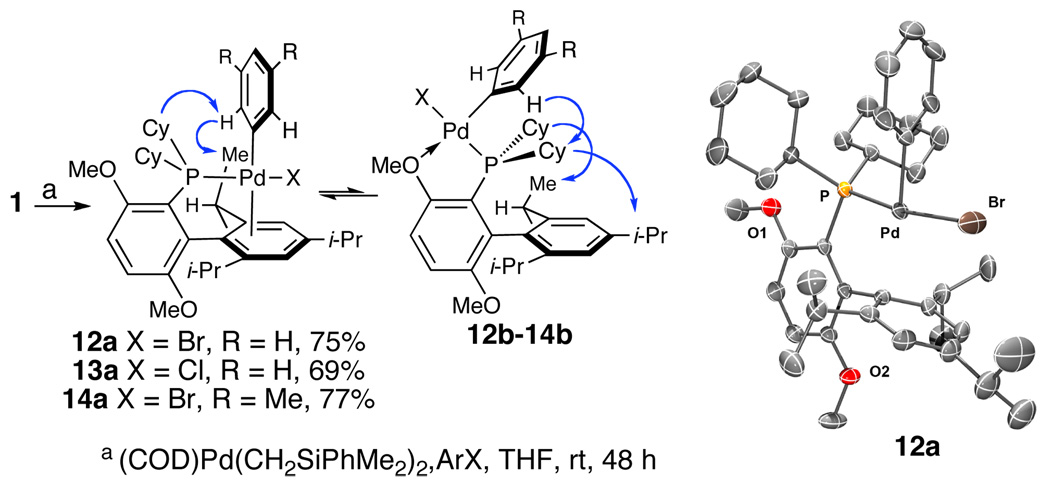
In an attempt to understand the unique reactivity of 1 compared to other ligands, we have prepared a series of 1·Pd(II)ArX (X = Cl or Br, Scheme 1) complexes by combining (COD)Pd(CH2SiPhMe2)2,18 1 and ArX. These complexes exist in solution as a mixture of two well-defined conformational isomers. Examination of the reaction mixture by in situ 31P NMR revealed two products in an approximate 2:1 ratio. Addition of pentane to the solution induces precipitation of the Pd(II) complex, which crystallizes as a single conformer. Freshly prepared solutions of the isolated complexes display only signals from the major conformer (1H and 31P NMR). However, rapid isomerization is observed, with the minor isomer becoming detectable within 5 minutes at room temperature.19
Scheme 1.
Synthesis and NOESY NMR cross-peaks of 12, 13, and 14; crystal structure of 12a.
Subjecting either 2 or 4 to the conditions shown in Scheme 1 provided dramatically different results from those obtained using 1. The in situ 31P NMR spectrum from the reaction of 2 is complex and shows broad resonances that we have yet to deconvolute. The reaction with 4 results in the formation of Pd black. These differences, to the extent that they reflect the behavior of the resulting Pd(II) complexes, may be related to the observed differences in reactivity observed with these ligands.
X-ray crystal analysis of 12 revealed that the complex exists as a monomer in solid state, and, although some disorder about the Br atom was observed, the resulting structure clearly demonstrates that the Pd center is bound over the tri-isopropylphenyl ring (Scheme 1). In solution, the conformations of the major and minor rotamers are analogous in 12, 13, and 14 (1H NMR).20 For all three complexes, both conformers display 1H NMR resonances consistent with D2 symmetry. NOESY NMR analysis of an equilibrated sample of 14 (2:1 mixture) allowed the assignment of the solution state conformation of both rotomers. The conformation of the major rotamer 14a is the same as that observed in the solid state. In the minor isomer 14b, the P–CAr bond is rotated by 180°, and the palladium atom is chelated by the phosphine atom and the proximal methoxy group. Important cross-peaks used in these assignments are summarized in Scheme 1. 12, 13 and 14 are active precatalysts in C–N bond-forming reactions.
Two notable points arise from the structural data. First, the NMR data demonstrate that the Pd(II) aryl halide complexes of 1 likely remain monomeric in solution and are not in equilibrium with the non-D2 symmetric dimeric form.15 Second, the observed monomeric equilibrium demonstrates that the proximal methoxy does not prevent rotation about the P–CAr bond. Theoretical studies have shown that this rotation may play an important role in catalytic systems with other biarylphosphines.9 The implications of these findings are not yet fully understood, and further studies to clarify the role of the methoxy groups in 1 are ongoing.
In summary, a new ligand with unprecedented reactivity for C–N cross-coupling reactions has been developed. Use of this ligand has allowed Pd-catalyzed amination reactions of aryl mesylates to take place in high yield. Arylations of methylamine were also performed for the first time with exceptional selectivities for monoarylation. Primary aliphatic amines and anilines were coupled with aryl chlorides at low catalyst loadings and with fast reaction times, demonstrating the exceptional reactivity and stability of the catalyst derived from 1. Finally, isolation of oxidative addition complexes of 1 has led to insight into the importance of the methoxy substitutent proximal to the phosphine for the reactivity of this catalyst system. Further studies into the origin of the reactivity of 1 are currently underway in our laboratories.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (NIH) for financial support of this project (GM-58160). We thank Merck and BASF (Pd compounds) for additional support. M. R. B. thanks the NIH for a postdoctoral fellowship (GM-F32-75685). We thank Dr. David S. Surry for helpful discussions and Dr. Kelvin Billingsley for the preparation of 4. The Varian NMR instrument used was supported by the NSF (CHE 9808061 and DBI 9729592).
Footnotes
Supporting Information Available: Procedural, spectral, and crystallographic data. This material is available free of charge via http://pubs.acs.org.
REFERENCES
- 1.a) Schlummer B, Scholz U. Adv. Synth. Catal. 2004;346:1599. [Google Scholar]; b) Jiang L, Buchwald SL. In: Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. de Meijere A, Diederich F, editors. Weinheim: Wiley-VCH; 2004. [Google Scholar]; c) Hartwig JF. Synlett. 2006:1283. [Google Scholar]
- 2.a) Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP. J. Am. Chem. Soc. 2006;128:4101. doi: 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]; b) Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Angew. Chem. Int. Ed. 2005;44:1371. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]; c) Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M. Chem. Eur. J. 2004;10:2983. doi: 10.1002/chem.200306026. [DOI] [PubMed] [Google Scholar]
- 3.Amination of aryl tosylates, benzenesulfonates, and nonaflates are known. See:Anderson KW, Mendez-Perez M, Priego J, Buchwald SL. J. Org. Chem. 2003;68:9563. doi: 10.1021/jo034962a.Roy AH, Hartwig JF. J. Am. Chem. Soc. 2003;125:8704. doi: 10.1021/ja035835z.Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w.
- 4.A paper disclosing the amination of aryl mesylates was released after this work was completed;So CM, Zhou Z, Lau C, Kwong F. Angew. Chem. Int. Ed. 2008;47:6402. doi: 10.1002/anie.200802157.
- 5.a) Percec V, Golding GM, Smidrkal J, Weichold O. J. Org. Chem. 2004;69:3447. doi: 10.1021/jo049940i. [DOI] [PubMed] [Google Scholar]; b) Munday RH, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2008;130:2754. doi: 10.1021/ja711449e. [DOI] [PubMed] [Google Scholar]
- 6.Ikawa T, Barder TE, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2007;129:13001. doi: 10.1021/ja0717414. [DOI] [PubMed] [Google Scholar]
- 7.Details of the synthesis of 1 are in the supporting information.
- 8.Biscoe MR, Fors BP, Buchwald SL. J. Am. Chem. Soc. 2008;130:6686. doi: 10.1021/ja801137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Ozawa F, Kubo A, Hayashi T. Chem. Lett. 1992:2177. [Google Scholar]; b) Amatore C, Carre E, Jutand A, M’Barki MA. Organometallics. 1995;14:1818. [Google Scholar]; c) Fors BP, Krattiger P, Strieter E, Buchwald SL. Org. Lett. 2008;10:3505. doi: 10.1021/ol801285g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwig JF. Inorg. Chem. 2007;46:1936. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]
- 11.a) An extra equivalent of ligand was added to reactions at temperatures above 80 °C to help stability. No attempt has been made to minimize the quantity of additional ligand. b)Attempts to use primary aliphatic amines or amides in cross couplings reactions with mesylates have been unsuccessful due to sulfonyl transfer side reactions.
- 12.Wolfe JP, Buchwald SL. J. Org. Chem. 2000;65:1144. doi: 10.1021/jo9916986. [DOI] [PubMed] [Google Scholar]
- 13.Shen Q, Ogata T, Hartwig JF. J. Am. Chem. Soc. 2008;130:6586. doi: 10.1021/ja077074w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KW, Tundel RE, Ikawa T, Altman RA, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:6523. doi: 10.1002/anie.200601612. [DOI] [PubMed] [Google Scholar]
- 15.For these reactions conducted at room temperature, an extra equivalent of 1 was not required in order to create the most stable catalytic system.
- 16.Biscoe MR, Barder TE, Buchwald SL. Angew. Chem. Int. Ed. 2007;46:7232. doi: 10.1002/anie.200702122. [DOI] [PubMed] [Google Scholar]
- 17.Cabello-Sanchez N, Jean L, Maddaluno J, Lasne M, Rouden J. J. Org. Chem. 2007;72:2030. doi: 10.1021/jo062301i. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Young GB. J. Organomet. Chem. 1999;577:257. [Google Scholar]
- 19.Concentrations measured versus an internal standard.
- 20.In particular, the methoxy and isopropyl resonances are diagnostic in this assessment, see Supporting Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.