Abstract
Individuals with asthma have twice the risk of developing mood and anxiety disorders as individuals without asthma and these psychological factors are associated with worse outcomes and greater need for medical intervention. Similarly, asthma symptom onset and exacerbation often occur during times of increased psychological stress. Remission from depression, on the other hand, is associated with improvement in asthma symptoms and decreased usage of asthma medication. Yet research aimed at understanding the biological underpinnings of asthma has focused almost exclusively on the periphery. An extensive literature documents the relationship between emotion and asthma, but little work has explored the function of affective neural circuitry in asthma symptom expression. Therefore, the following review integrates neuroimaging research related to factors that may impact symptom expression in asthma, such as individual differences in sensitivity to visceral signals, the influence of expectation and emotion on symptom perception, and changes related to disease chronicity, such as conditioning and plasticity. The synthesis of these literatures suggests that the insular and anterior cingulate cortices, in addition to other brain regions previously implicated in the regulation of emotion, may be both responsive to asthma-related bodily changes and important in influencing the appearance and persistence of symptom expression in asthma.
Introduction
The interrelatedness of immune system activity and emotion is deeply rooted and highly conserved in evolutionary development. At its foundation, the role of emotion is to attach salience to sensory information, both internal and external, and to motivate physiological and behavioral responses to that information that best promote survival. In this way, the activities of the immune system are just as integral to emotion as hunger or the presence of predators. An effective defense against pathogens is metabolically expensive and requires reallocation of energy from other physiological processes. Indeed, some suggest that immune defense is such a high priority to survival that it trumps most other sensory information in driving behavioral and physiological changes (Dantzer & Kelley, 2007; Miller, 1964). It is not only important for the brain to receive information pertaining to immune system activity, but the ability for the brain to regulate this activity is also critical in order, for example, to mobilize immune cells for the possibility of injury when danger is sensed. The adaptiveness of this association is obvious when considering that for most forms of life, emotions such as anger and fear represent an increased likelihood for injury and pathogenic infection and pathogenic infection poses a serious risk to survival.
In recent decades, empirical evidence has amassed which demonstrates that products of the immune system can cause psychological and behavioral changes that minimize energy expenditure needed for the demands of immune mobilization, such as depressed mood (Capuron et al., 2004; Wright et al., 2005), lethargy (Swiergiel & Dunn, 2007), and decreased social interaction and appetite (Dantzer et al., 2008). In turn, perceived stress and negative emotion can provoke increases in circulating levels of inflammatory markers (Kiecolt-Glaser et al., 2005; Pace et al., 2009). While these physiological relationships are advantageous for most organisms, humans today typically face situations that evoke stress or negative emotion that do not benefit from prophylactic inflammation and mobilization of energy stores. This mismatch may contribute to the rapidly increasing prevalence of chronic inflammatory disease and obesity. Likewise, ongoing inflammation may signal continuous internal threat and lead to chronic activation of stress- and emotion-related neural circuitry, potentially contributing to the overrepresentation of mood and anxiety disorders in populations of individuals with chronic inflammatory diseases. Despite well-established links between inflammation and psychological factors, little is known about the involvement of emotional neural circuitry in the response to and the regulation of peripheral inflammation during inflammatory disease, or about the mechanisms through which this reciprocal modulation is accomplished in humans. Therefore, the following review examines the relevant neuroimaging literature from the perspective of one chronic inflammatory disease infamous for its responsiveness to stress and emotion — asthma.
Asthma is a chronic inflammatory disease, characterized by hypersensitivity of the airways, which leads to bronchoconstriction, inflammation and, ultimately, airway obstruction. Following exposure to an allergen or irritant, mediators released from activated mast cells in the lungs act on bronchial smooth muscle and vessels to cause bronchoconstriction, or a narrowing of the airways (Bloemen et al., 2007). Bronchoconstriction causes the sensation of dyspnea, often described as breathlessness or shortness of breath, which is associated with the immediate response to allergen exposure. The immediate response typically resolves and lung function returns to normal within an hour. However, in a subset of individuals with asthma, a second decline in lung function begins 4 to 8 hrs after allergen exposure and can persist for days. This late response is due, in large part, to the increased recruitment of eosinophils from the blood into the lung tissue, where they cause vasodilation, mucus secretion, and tissue damage (Calhoun et al., 1991). A sustained increase in the production of eosinophils in bone marrow following allergen exposure has been observed in asthmatics that show a late response (Dorman et al., 2004; Wood et al., 1998), perhaps leading to the greater numbers that infiltrate the lungs.
The vulnerability of asthmatics to stress- and emotion-related exacerbations has been acknowledged for decades (e.g. Clarkson, 1937). Published scientific reports discussing psychological contributions to asthma symptom expression date back to the late 19th century. One noteworthy observation involved the elicitation of an asthmatic episode in response to an artificial rose in a patient with a pollen allergy (MacKenzie, 1961). More recent investigations have confirmed these early clinical reports (for review, see Lehrer et al., 1993). For example, Leigh and colleagues (2003) found that 6 of 17 asthmatic participants had a clinically relevant decline in lung function, and 14 of 17 experienced dyspnea in response to a sham bronchoconstrictor, whereas 0 of the 17 showed a decline (or an improvement) or experienced dyspnea in response to a sham bronchodilator. Ritz et al. (2000) manipulated emotional arousal, through presentation of medical and emotionally evocative film clips, and observed an increase in respiratory resistance. Moreover, Liu and colleagues (2002) demonstrated that undergraduate asthmatic subjects had greater airway inflammation and a larger decrement in lung function in response to allergen challenge during final examination week—a period of significantly heightened stress—compared to an identical challenge during a relatively stress-free period. Thus, cognitive and emotional factors clearly have the capacity to modulate, if not evoke, objectively measured asthma-related symptoms.
The mind-body influences in asthma have significant public health and socioeconomic implications. A recent article in the Journal of the American Medical Association reported that individuals with asthma have a two-fold higher risk of having one or more anxiety or depressive disorders (Kuehn, 2008). To compound the weight of this finding, depression and anxiety are associated with poorer asthma control, leading to increased frequency of symptoms, as well as more physician and emergency room visits (Strine et al., 2008). The fact that these psychological factors can influence asthma symptoms underscores the critical role of the brain since it is through the brain, and only through the brain, that such influences can be processed and exert downstream influences on peripheral biological systems of import to asthma. Yet, research directed toward understanding the pathophysiology of asthma has ignored the role of the brain almost entirely. Preciously few investigations have examined the mutual influence of activity in emotional neural circuitry and the appearance, persistence, and resolution of asthma symptoms. Therefore, this review integrates functional neuroimaging research related to factors that may impact symptom expression in asthma.
The synthesis of this research suggests that the insula and anterior cingulate cortex (ACC) are major components of a circuitry through which emotional and cognitive processes and peripheral inflammation are mutually influential. Thus, we first discuss the evidence that these brain structures are involved in the physiological changes that occur during an asthma exacerbation. Next we discuss the potential nature of asthma-related changes in insula and ACC activity, based on anatomical connectivity. We then review the evidence that these regions are involved in sensing and responding to physiological changes, depending on the cognitive and emotional context, through a discussion of neuroimaging data related to sensitivity to visceral signals, influence of expectation and emotion on the perception of visceral signals, and the plasticity and conditioning that lead to hypersensitivity when inflammation is chronic. Finally, we make recommendations for future research that address some of the limitations of the work described above in understanding the role of emotional neural circuitry in asthma.
Is insula and ACC activity altered during an asthma exacerbation?
In one of the only efforts to examine the neural response in humans to the development of airway inflammation following allergen exposure, we and our colleagues designed a study that would allow us to isolate inflammation as the afferent signal that modulated brain function (Rosenkranz et al., 2005). Asthmatic participants who were previously screened and determined to demonstrate a late phase response were challenged with both methacholine – a smooth muscle constrictor – and inhaled subject-specific allergen on separate days. Methacholine challenge was included as a control for the mechanical aspects of the asthmatic response. During subsequent brain imaging sessions, participants viewed asthma-related, general negative and valence-neutral words in the context of a Stroop task. Participants were required to press 1 of 4 buttons to indicate the color of the printed word. The collection of functional magnetic resonance imaging (fMRI) data occurred 4h post-challenge, timed to coincide with the development of the late response. Changes in blood oxygen-level dependent (BOLD) signal in response to asthma words were compared to that of negative and neutral words in order to assess the reactivity of emotional neural circuitry to disease-specific emotional information. We contrasted this differential response to asthma-related words during allergen challenge with that of methacholine challenge, which allowed us to determine the responsivity of this circuitry specifically during the development of inflammation. We found that activation in the left mid-insular cortex and perigenual ACC strongly predicted the magnitude of the subsequent fall in lung function and infiltration of eosinophils into lung tissue that is associated with the late asthma response (Figs. 1 and 2).
Figure 1. Anterior cingulate cortex activity predicts peripheral measures of inflammatory potential.
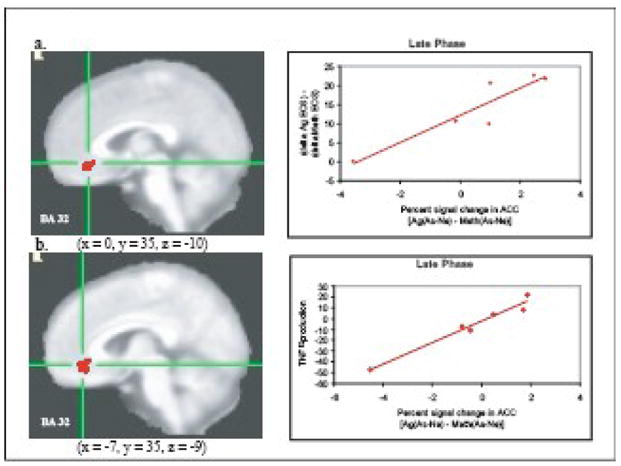
Percent signal change in the ACC in response to asthma compared to neutral words [As-Ne] and (a) percentage of eosinophils during late phase antigen relative to methacholine challenge [Ag-Meth] (r = .92, P < .001) and (b) peripheral blood leukocyte production of TNF-α (% of production relative to no dexamethasone) during late phase antigen relative to methacholine challenge [Ag-Meth] (r = .98, P < .001). Note: clusters in (a) and (b) were identified independently and are not identical, though they largely overlap. From Rosenkranz, M.A. et al. (2005). Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proceedings of the National Academy of Science of the United States of America, 102, p. 13322.
Figure 2. Insula activity predicts peripheral measures of inflammatory potential and lung function.
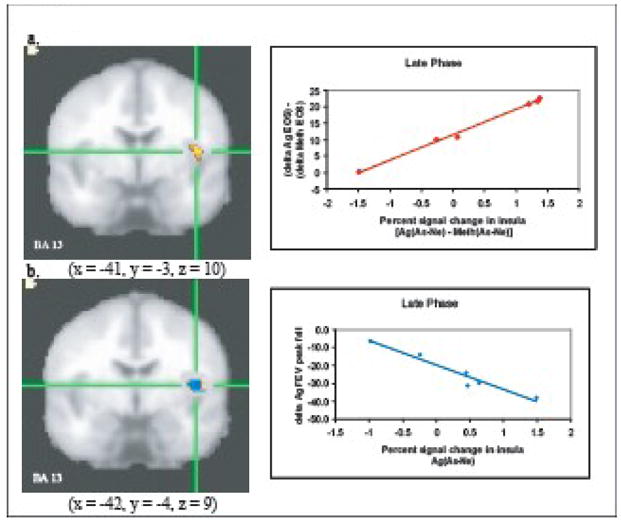
Percent signal change in the left insula in response to asthma compared to neutral words [As-Ne] and (a) percentage of eosinophils during late phase antigen relative to methacholine challenge [Ag-Meth] (r = .99, P < .001) and (b) peak fall FEV1 (L.) during late phase antigen [Ag] challenge (r = −.97, P < .001). Note: clusters in (a) and (b) were identified independently and are not identical, though they largely overlap. For analyses with FEV1 only values for the Ag challenge were used because one participant was missing data from the Meth challenge. From Rosenkranz, M.A. et al. (2005). Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proceedings of the National Academy of Science of the United States of America, 102, p. 13323.
We replicated this finding in a second study with a larger sample size (Rosenkranz et al., in prep), where we again showed that activity in the mid-insula (right-sided this time) predicted lung function decline and the infiltration of eosinophils into the airway. Moreover, in this study, asthmatics that have a late response (significant inflammation) were compared with asthmatics that have only an immediate response (minimal inflammation) and non-asthmatic controls. Those with a late response showed significantly more activation in the right anterior insula in response to asthma words, compared to negative and neutral words, during allergen compared to methacholine challenge, than asthmatics with only an immediate response.
In the two studies described above, it is likely that the observed modulations in brain activity reflect a combination of afferent and efferent processes. Given that lung function had not yet significantly declined at the time that fMRI data were collected, and that the differential neural response predicted the future influx of sputum eosinophils, it would seem that the neural response patterns reflect efferent activity that contributes to the development of these symptoms. However, the differential responses were specific to the allergen challenge, suggesting that initial changes in afferent signaling from the lungs were necessary. Thus, we will turn to anatomy to provide some insight into the possible roles of these two brain regions.
What does anatomy tell us about the role of the insula and ACC in asthma?
The anatomical projections to and from the insular and anterior cingulate cortices implicate these structures in monitoring changes in physiological status, integrating this information with external sensory, cognitive, and emotional information and directing the appropriate behavioral and peripheral physiological (i.e. homeostatic) responses1. Indeed, the insula and ACC have previously been referred to as components of the limbic sensory and limbic motor cortices, respectively (Craig, 2002; Craig, 2009; Öngür & Price, 2000). In primates, the insula receives sensory input from all tissues in the body ascending in small diameter afferent fibers (A∂ and C) that synapse in lamina I of the dorsal horn or the solitary nucleus (NTS). The lamina I neurons ascend in the lateral spinothalamic tract, which projects, together with the NTS, to other brainstem nuclei (e.g. parabrachial nucleus) and then on to the basal ventral medial nucleus of the thalamus, or directly to the posterior aspect of the ventral medial thalamic nucleus. These thalamic nuclei provide a topographically organized map of ongoing physiological status to the posterior insula (Craig, 2002; Craig, 2003a; Craig, 2003b). This information is then re-represented in the middle insula, and again in the anterior insula (e.g. Craig, 2009). In addition to the thalamic inputs, the posterior insula receives input from all regions of the cingulate cortex, while it provides input only to the posterior portion. The posterior insula also has reciprocal connections with the secondary somatosensory cortex (Mesulam & Mufson, 1982a, Mesulam & Mufson, 1982b). The middle insula has reciprocal connections with the both posterior and anterior insula, primary and secondary sensory and motor cortices, and amygdala. In addition, the middle insula receives input from the supragenual region of the ACC and sends projections to frontal cortical sites such as the lateral orbitofrontal cortex and the mid to posterior cingulate gyrus (Bagaev & Aleksandrov, 2006; Mesulam & Mufson, 1982a, Mesulam & Mufson, 1982b, Mufson et al., 1981). Importantly, the middle insula also has efferent projections to subcortical and brain stem regions that are important in maintaining homeostasis such as the NTS, bed nucleus of the stria terminalis, parabrachial nucleus, and lateral hypothalamus (Bagaev & Aleksandrov, 2006). The anterior insula has reciprocal connections with the middle insula and is noteworthy for its strong connections with frontal cortical sites, such as the peri- and subgenual ACC, orbitofrontal cortex and frontal operculum (Mesulam & Mufson, 1982a; Mesulam & Mufson, 1982b).
The differences in input and output of the insular sub-regions is reflected in their response characteristics. Activity in the posterior insula varies closely with the strength of a sensory stimulus, reflecting its direct connections with afferent signals from the thalamus, as well as its interconnectedness with the somatosensory cortex. Activity in the anterior insula varies with the subjective perception of the stimulus (Craig et al., 2000; Olausson et al., 2005), perhaps reflecting a greater degree of top-down processing or integration with information from the prefrontal cortex and ACC. This differential activation was clearly illustrated by Craig and colleagues (2000) in a study where they applied cold stimulation of increasing intensity to the palm of the hand and measured the associated changes in neural activity using positron emission tomography (PET). They found that activity in the posterior aspect of the dorsal insula was most strongly correlated with the intensity of the cold stimulus. On the other hand, activity in the anterior insula was most strongly correlated with participants’ experience of the stimulus intensity. A similar relationship has been observed with breathlessness – a stimulus more relevant to asthma – where activity in the anterior insula predicted the perceived discomfort associated with dyspnea (Peiffer et al., 2001). Further, lesions to the anterior insula tend to disrupt the unpleasantness associated with the experience of dyspnea, but not the intensity (Schön et al., 2008). In contrast, disruption of function in the posterior insula diminishes the intensity of a thermal stimulus (Greenspan & Winfield, 1992). It is unclear precisely what activity in the mid-insula reflects. Activity in this region has been observed when experiencing a sense of personal agency or awareness of movement of one’s own body (Farrer & Frith, 2002; Tsakiris et al., 2007) and damage to this area can result in a loss of awareness of hemiplegia (Karnath et al., 2005). Thus, it has been proposed that this region of the insula contains a representation of the feelings associated with one’s current movements (Craig, 2009). However, its connections with the subcortical structures and autonomic control centers suggest that the mid-insula may also be involved in modulating descending homeostatic responses. Indeed, stimulation of this region causes various autonomic-driven effects, including changes in heart rate, blood pressure, respiration and gastric motility (Augustine, 1996; Bagaev & Aleksandrov, 2006; Yasui, et al. 1991)2. Thus activity in this region may more generally reflect, perhaps in tandem with the ACC (see below), descending responses to changes in the perceived status of the body.
The ACC is also a target of ongoing homeostatic status updates from all tissues in the body and, like the insula, it receives internal sensory input through the lamina I spinothalamic tract from small-diameter afferent fibers. Input to the ACC from the spinothalamic tract and NTS, however, arrives via the medial dorsal thalamic nucleus, rather than the ventral medial nucleus (Craig, 2002). The connectivity of areas within the ACC with other cortical regions, as well as with subcortical and brain stem structures has led to various sub-classifications that all seem to suggest two or three relatively distinct functional regions. One region lies along the rostral curve of the genu of the corpus callosum extending below to slightly above the genu. This region has strong connections with several areas of the prefrontal cortex, especially the orbitofrontal cortex. In addition, this infragenual region of the ACC shows significant connectivity with the anterior insula, as well as many areas of the subcortex and brain stem including the amygdala, periaqueductal grey, hypothalamus, hippocampus, striatum, and NTS. (Arikuni et al., 1994; Devinsky et al., 1995; Morecraft & Van Hoesen, 1998; Öngür & Price, 2000; Vogt & Pandya; 1987). A second domain of the ACC lies just posterior to the previously described region, dorsal to the corpus callosum and posterior to the genu. This region receives input from the more rostral and ventral aspect of the ACC, as well as the orbitofrontal cortex. What distinguishes this region from its rostral neighbor, however, is its strong connections with the premotor cortex (Arikuni et al., 1994; Morecraft & Van Hoesen, 1998). Moreover, the more caudal regions of the ACC (mid-cingulate) show an even greater association with motor control in their connections to the primary motor cortex, supplementary motor area (SMA) and spinal cord (Devinsky et al., 1995). As an illustration, activation in the more dorsal and posterior area of the ACC and the SMA predicted the urge to cough in response to inhalation of capsaicin (Mazzone et al., 2007). Thus there seems to be a continuum of motor control in the ACC from the viscera in the rostral and ventral aspects to the skeletal in the more dorsal and caudal areas. Due to this pattern of connectivity, it has been proposed that the ACC plays an important role in coordinating the collection of responses that restore homeostasis in the face of a challenge (Öngür & Price, 2000). The infralimbic portion receives inputs from sensory regions in the prefrontal cortex and insula and in turn may drive changes in descending visceromotor activity, as well as relaying the sensory information to the more caudal regions. The caudal regions may be involved in guiding behavior, such as avoidance, based on changes in the physiological status of the body. Similarly, it has been proposed that the ACC may act to bring about the peripheral physiological changes to meet the demands of concomitant challenging mental processes (both cognitive and emotional), and in this way serves to integrate higher level cortical processes with physiological activity in the body (Critchley et al., 2003; Luu & Posner, 2003).
Insula and ACC activity in relation to sensitivity to visceral stimulation
In a series of recent publications (Craig, 2002; Craig, 2003; Craig, 2004; Craig, 2009) the theory has been developed that the basis for emotional awareness and an ongoing sense of current wellbeing emerges from an integration of external sensory, cognitive, and emotional information with ascending information pertaining to the physiological state of the body in the insula. The ongoing adjustments to behavior and physiology that restore or maintain our wellbeing are proposed to be based in the ACC. In relation to our data in asthmatic individuals, this hypothesis would suggest that, in the context of an allergen exposure, the insula response to asthma-relevant cognitive information is indicative of one’s sensitivity to a physiological perturbation — in other words, how threatening the situation is — and therefore will predict the degree of the subsequent protective response (e.g. inflammation) to follow (engendered in rostral ACC activity). Individual differences in sensitivity to visceral stimuli is important in considering the relations between emotion and asthma symptom expression. This is not a new idea. Several prominent theories of emotion are based on physiological changes in the body, such as the James-Lang theory of emotion (James, 1884; Lange, 1885) and Damasio’s somatic marker hypothesis (1999). As such, compelling evidence exists that suggests that those who are more sensitive to internal sensory stimuli experience more intense emotion (e.g. Critchley et al., 2004; Pollatos et al., 2007a; Pollatos et al., 2007b; Pallatos et al., 2007c; Weins, 2000). For example, Wiens et al. (2000) presented emotional film clips to participants previously classified as “good” or “poor” at detecting their own heartbeat. Participants rated their emotional experience of the film clips on valence and intensity dimensions: those who were good heartbeat detectors reported significantly more intense emotions than those who were poor detectors. In a similar study, Pollatos et al. (2007a) demonstrated that good heartbeat detectors had a stronger increase in heart rate in response to viewing emotionally-laden images and rated those images as significantly more arousing than poor heartbeat detectors. In another experiment by the same group, heartbeat detection ability was positively correlated with trait anxiety and neuroticism (Pollatos et al., 2007b). Moreover, fMRI data were collected during the heartbeat detection task. Regions activated significantly more by the heartbeat detection task (counting heartbeats) than by the control task (counting barely audible tones) included the anterior and mid-insula (bi-lateral), inferior/middle frontal gyrus (bi-lateral), medial frontal gyrus/dorsal ACC (left), inferior parietal lobule (right), and thalamus (right). In addition, greater activation in the anterior insula, inferior parietal lobule, and the medial frontal/cingulate gyrus during heartbeat detection, relative to tone detection, was positively associated with heartbeat detection ability, where greater activation in the right anterior insula showed the strongest association. Self-reported trait anxiety and neuroticism, on the other hand, were associated only with greater activation in the medial frontal/cingulate gyrus.
Critchley and colleagues (2004) conducted a similar study where participants judged the synchronicity of an auditory tone with their heartbeat during collection of fMRI data. This interoceptive task was compared to a task where participants judged whether the pitch of string of auditory tones were all the same. Relative to the control condition, accuracy in heartbeat detection was a strong predictor of variance in self-reported anxiety and a less strong (trend-level) predictor of self-reported depression and trait negative affect. Interestingly, heartbeat detection accuracy did not correlate with self-reported visceral awareness, which suggests that increased sensitivity does not always indicate greater awareness. Neural circuitry engaged more by an internal compared to an external focus of attention included the lateral somatomotor (bilateral), anterior insular (bilateral), inferior frontal (bilateral), dorsal anterior cingulate (right), and supplementary motor cortices (left). Within these regions, activity in the right anterior insula/frontal operculum, specifically during heartbeat detection trials, showed the strongest relationship with heartbeat detection accuracy (interoceptive awareness) and self-reported anxiety. Importantly, grey matter volume in the right anterior insula/frontal operculum, right orbitofrontal cortex, and medial cerebellum predicted accuracy of heartbeat detection. Moreover, grey matter volume in the right anterior insula/frontal operculum predicted self-reported visceral awareness. Combined, the results of these studies suggest that individuals who are more sensitive to internal stimulation show a stronger physiological response to emotional information, experience more anxiety, neuroticism, depression and negative affect and that these qualities are reflected in greater activity particularly in the anterior and mid-insula, but also in the ACC and related prefrontal cortical sites, when attention is directed inward. These observations lead to the very testable hypothesis that increased sensitivity to internal cues following allergen exposure in asthmatics may lead to an amplified protective response and may differentiate those who typically experience a late phase response from those who do not. Though we do not have information on the interoceptive ability of those individuals in our sample, we did show that the anterior insula of those asthmatics that went on to have a late response responded more strongly to disease-relevant stimuli in the context of an asthma exacerbation (Rosenkranz, in prep). These data regarding interoception also provide a theoretical framework in which to consider the increased prevalence of mood and anxiety disorders in asthma sufferers: the ongoing, low level inflammation that is often present in asthmatic lungs may chronically heighten the activity of the insula and ACC in asthmatic individuals who are especially sensitive to interoceptive stimuli, causing more frequent and prolonged experiences of anxiety and negative affect.
Do emotion and expectation modulate the perception of and response to visceral signals through activity in the insula and ACC?
To extend this logic, one can easily imagine a scenario in which a pre-existing stressor or expectation could also cause a heightened insula/ACC response to what would otherwise be benign fluctuations in afferent signals from the body, leading to efferent adjustments that are out of line with the actual physiological state. This phenomenon has been investigated through the manipulation of cognitive and affective input. For example, von Leupoldt and colleagues (2008) coupled dyspnea (the sensation of breathlessness or air hunger) induction in healthy individuals to the presentation of positive, negative, and neutral visual images. This manipulation was based on previous research that showed that presentation of negative emotional images during dyspnea results in an increase in self-reported dyspnea unpleasantness, while presentation of positive images led to a decrease in unpleasantness (von Leupoldt et al., 2006). In a subsequent fMRI study, von Leupoldt et al. (2008) compared the difference in BOLD signal between negative picture presentation during dyspnea and at baseline to that of the difference in BOLD signal during positive picture viewing with dyspnea and at baseline (i.e. Neg(dys – base) - Pos(dys – base)). This comparison revealed activation in a small region of the right anterior insula and the right sublenticular extended amygdala. In a similar study, Phillips and colleagues (2003) paired non-painful esophageal stimulation with the presentation of fearful and neutral faces, i.e. information that allows us to make predictions about the salience of sensory information. Activation in the anterior insula and dorsal ACC regions was greater when stimulation was paired with fearful, compared to neutral faces in this design. Moreover, self-reported discomfort associated with the esophageal stimulation, anxiety, and activation in these brain regions were positively associated with the intensity of the fearful expression. Grey et al. (2007) did the inverse of this experiment and showed that neutral facial expressions were perceived to have significantly more emotional intensity when presented with the false feedback of elevated heart rate, and further, activation of the right anterior insula strongly predicted the self-reported intensity ratings.
The manipulation of one’s expectations concerning a sensory stimulus is another useful strategy for gaining insight into the mechanisms through which cognition influences our experience of sensation. Nitschke et al. (2006) showed that an aversive taste paired with an accurate or misleading expectation produced differential mid- and posterior insula activity, such that the expectation of a less aversive taste produced less insula activation than the expectation of a more aversive taste, in response to the identical stimuli. In addition, insula activity predicted the self-reported aversiveness of the taste. Likewise, Rainville and colleagues (1997) reported increases in mid-insula and dorsal ACC activation in response to the hypnotic suggestion of the increasing intensity of a constant pain stimulus. These observations hint at the possible role of the mid-insula and dorsal ACC in modulating the sensitivity of spinal cord and brain stem neurons to ascending stimulation, based on input from prefrontal cortical sites. The apparent cognitive modulation of posterior insula activity reported by Nitschke et al. (2006) may belie its reciprocal connections with more rostral insula regions and contradicts the assertion that activity in this region varies closely with stimulus magnitude, rather than subjective experience; unless descending modulation of spinal cord and brain stem neurons is indeed increasing the gain. In this case, the posterior insula would be reflecting its ascending thalamic input with high fidelity.
Disease-related changes in insula and ACC responsivity to visceral stimulation
Though informative, all of the previous studies employed healthy individuals and thus, were not able to address the conditioning, plasticity, and compensatory changes that often accompany a chronic disease. To date, the relative interoceptive ability of asthmatics has not been examined and it is unknown whether asthmatics, or a subset of asthmatics, experience more intense emotion than individuals without asthma. However, as discussed earlier, asthma is associated with increased risk for anxiety and depression (Kuehn, 2008). Moreover, asthma is characterized as a hypersensitivity of the airways, including the sensory nerves that innervate the airway (Joos et al., 1989). The sensitization of ascending pathways, combined with plasticity that occurs in tissues where inflammation is chronic, likely leads to an amplified noxious signal to the brain. In addition to sensitization at the level of the sensory nerve, sensitization can also occur in the spinal cord and brainstem (Chen et al., 2001; Hermann et al., 2005; Joad et al., 2004), and perhaps the cortex as well. Though this hypothesis has not been addressed in an asthmatic sample, it has been examined in other chronic inflammatory conditions, such as irritable bowel syndrome (IBS). IBS is not as unrelated to asthma as it may seem. In fact, a significant number of those diagnosed with IBS show airway hyperresponsiveness to methacholine challenge (White et al., 1991).
Several studies have shown that individuals with IBS are hypersensitive to visceral stimulation. Both PET and fMRI have been used to investigate changes in neural activity in response to painful and non-painful rectal pressure in individuals with IBS and healthy controls. Individuals with IBS tend to report feeling pain at lower pressures (Yuan et al., 2003) and to rate painful pressure as significantly more intense (Mertz et al., 2000; Verne et al., 2003), painful, unpleasant (Yuan et al., 2003), and extensive (Mertz et al., 2000) than controls. In addition, individuals with IBS reported significantly more fear and anxiety associated with rectal stimulation, and these measures accounted for a significant portion of the variance in pain ratings (Verne et al., 2003). Examination of changes in painful pressure-evoked neural activation that differentiated IBS and control groups revealed increased activity in the ACC (subregion unspecified: Mertz et al., 2000; left perigenual: Verne et al., 2003), insula (subregion unspecified: Mertz et al., 2000; bi-lateral anterior: Verne et al., 2003; subregion unspecified: Yuan et al., 2003), prefrontal cortex (left dorsolateral: Verne et al., 2003; subregion unspecified: Yuan et al., 2003) somatosensory cortex (right: Verne et al., 2003), and thalamus (right ventroposteriolateral and dorsomedial nuclei: Verne et al., 2003; subregion unspecified: Yuan et al., 2003) in those with IBS. These findings likely reflect either hyperresponsiveness of the insula, ACC, and portions of the prefrontal cortex to disease-relevant stimulation; or the response of these regions to an amplified afferent signal. Further, this activity may underlie a potentiation of the ACC and/or mid-insula efferent response that stimulates gastrointestinal motility.
In addition to rectal distention, Verne and colleagues (2003) compared neural responses evoked by painful cutaneous heat in IBS patients to that in healthy controls. Again, IBS patients rated pain from the cutaneous stimulation as more intense and unpleasant than those in the control group, though the groups did not differ in fear or anxiety related to cutaneous stimulation. In response to painful cutaneous stimulation, individuals with IBS showed greater activation than controls in similar regions to that observed in response to painful rectal stimulation, including the left mid-insula, right somatosensory cortex, and the right dorsal ACC. However, the dorsal, rather than perigenual ACC and the mid, rather than anterior insula appear to be involved in responding to cutaneous pain in this study. These subtle differences may relate to the greater emotional salience attributed to disease-relevant stimuli. Indeed, in a similar study by the same group, blood pressure response was inversely associated with visceral pain in IBS patients, whereas cutaneous pain showed a slight positive relationship with blood pressure (Gupta et al., 2002), suggesting that the homeostatic response, and by extension, the degree of threat differed between these two stimuli. It is also noteworthy that a greater extent of prefrontal cortex was activated in response to rectal stimuli, suggesting that affective and/or regulatory processes may play a larger role in processing stimuli that are disease-related. Importantly, these data indicate that hypersensitivity is more generalized and not limited to the diseased compartment in this population and likely reflects increased signaling that originates above the level of the sensory neurons that innervate the gut.
In order to more directly investigate the contributions of cognitive input on visceral stimulation and the consequent neural changes, Naliboff and colleagues used PET to identify brain regions selectively activated during expected rectal distention compared with actual rectal distention in IBS patients (Mayer et al., 2005; Naliboff et al., 2001; Naliboff et al., 2006). Anticipation trials followed actual distention trials and participants were told that they would receive a “more intense stimulus” during these trials. During anticipation, IBS patients reported significantly higher intensity and unpleasantness ratings of sensation than the control group (Mayer et al., 2005). In all three studies, increased activation in the perigenual ACC was observed in response to actual rectal distention relative to controls (Mayer et al., 2005; Naliboff et al., 2001) or to baseline (Naliboff et al., 2006). Additionally, in two of three studies (Mayer et al., 2005; Naliboff et al., 2006) the dorsal medial prefrontal cortex (bilateral and right, respectively) showed increased activation in this comparison. During anticipation of aversive rectal distention, the perigenual ACC again showed increased activation in all three studies, whereas the subgenual ACC and dorsal medial prefrontal cortex showed increased activation in two of three studies, suggesting that these regions may be involved in the facilitation of pain in individuals with IBS. Interestingly, Naliboff et al. (2001) also observed increased activation in the control group, relative to the IBS group, in medial orbitofrontal cortex (BA 11) and the frontopolar area (BA 10), extending into the perigenual ACC and medial frontal cortex (BA 24/32) during anticipation of painful stimulation. The authors suggest that this differential activation may reflect mechanisms that allow the control group to more effectively regulate or cope with the aversive visceral stimulus. A study published by Berman et al. (2008) supports this hypothesis. In this study, BOLD signal decreased in the right posterior insula and bilateral dorsal brainstem during anticipation of painful rectal distention in the control group, whereas this deactivation in IBS group was significantly attenuated. Further, the lack of decrease in dorsal brainstem activation predicted self-reported negative affect, such that the smaller the decrease in activation, the greater the negative affect. Importantly, the magnitude of the anticipatory attenuation of dorsal brainstem activity predicted greater right orbitofrontal cortex (OFC) and bilateral supragenual ACC activation during actual distention. Thus, downregulation of these regions in anticipation of pain, as well as activation of the OFC and supragenual ACC during pain, may reflect an adaptive coping strategy which individuals with IBS fail to adequately engage leading to visceral hypersensitivity. Nonetheless, roughly this same region of ACC is activated when interoceptive stimuli are paired with negative information (e.g. fearful faces, or the hypnotic suggestion of increasing stimulus intensity), suggesting that it is active when modulation (either up or down) of a sensory stimulus occurs. The absence of anterior and mid-insula activity in these anticipation studies is noteworthy and suggests that perhaps these regions may not be directly involved in modulating brain stem and spinal cord sensitivity.
Summary and Conclusions
The anterior insula and ACC appear to be critical components of the circuitry that links the development of peripheral symptoms with emotion and cognition in asthma. The insular and cingulate cortices, and to a lesser extent adjacent prefrontal regions, were overwhelmingly the most commonly reported foci of activation related to sensing and responding to physiological perturbations in the body in both healthy and ill populations. Overall, there were 13 distinct regions described, across 22 studies, which showed increased activation during an experimental manipulation related to peripheral physiological disturbances. Across these 22 studies, there were 18 reports of insula/frontal operculum activation and 15 reports of ACC activation. In addition, there were 11 reports of foci in other frontal cortical regions, predominantly the ventral and medial PFC. No other brain region appeared more than 2 times.
Based on the empirical evidence reviewed here and the posited functions of the insula and ACC, we propose that it is the interaction of cognitive and emotional context with afferent homeostatic signaling that is integrated in the anterior insula, and the subsequent efferent consequences driven by the ACC, that underlie the reciprocal relationship between emotion and symptom expression in asthma and other chronic inflammatory diseases. According to this model, the presence of asthma symptoms (e.g. inflammation and dyspnea) would activate all regions of the insula resulting in a conscious perception of breathing difficulty and a sense of the deterioration of wellbeing. To the degree to which one is sensitive to these physiological changes, they may also cause hypervigilance and misattribution of threat to external stimuli, anxiety, and increased negative affect. The presence of asthma symptoms would also activate the ACC, leading to protective responses manifested in autonomic and endocrine changes that spur continuing development of symptoms. ACC activation may also enhance the salience of asthma-related physiological and cognitive cues and, through projections to the anterior insula, lead to heightened sensitivity to interoceptive information. Alternatively, perceived stress, negative emotion, or cognitive cues like expectation may activate this same cascade via projections from the amygdala and prefrontal cortex, leading to increased anterior insula activation and a deterioration in the sense of wellbeing, increased ACC activation and a defensive efferent homeostatic response that may contribute to the development or progression of asthma symptoms.
In our asthma samples, we found that increases in mid-insula and perigenual ACC activation in response to asthma-relevant stimuli were related to objective measurements of symptom development, rather than perceived intensity of symptoms, during an asthma exacerbation. Given its projections to brain stem and spinal cord neurons that have the capacity to influence the development of an inflammatory response, its not surprising that this relationship was observed with the mid-insula rather than the anterior insula. On the other hand, it was the anterior insula that showed a heightened response to asthma-related words, in a straight group comparison when asthmatics that experience a late phase response were compared to asthmatics that do not and to controls. This difference in location may reflect the influence of cognitive processes more than efferent regulatory processes. In addition, it is noteworthy that activity in the perigenual ACC predicted the development of asthma symptoms, but did not show a group difference in response to asthma words. Again, this may reflect its greater relationship to the output, rather than the input. Thus it is likely that in our asthmatic sample, the observed changes in anterior insula and ACC activation reflect both afferent and efferent processes constantly working in tandem to assess and respond to the perceived physiological condition.
Limitations and future directions
Due to the lack of prospective studies, the literature reviewed here cannot speak to the cause of the relationship between increased rates of anxiety and depression in individuals with asthma. In other words, we don’t know whether inflammation or other asthma-related peripheral pathological processes cause central changes that can lead to anxiety and depression; or whether central changes the underlie psychopathology predispose one to develop inappropriate peripheral inflammatory responses. Given the bi-directionality of influence between inflammatory immune signaling and central nervous system function, it would seem that both could be true. Thus, longitudinal studies that could access the onset and progression of symptoms, though logistically difficult, would be valuable in this regard.
Another major limitation of the current literature to providing a more complete understanding of the role of the insula and ACC in asthma symptom expression is the relatively poor temporal resolution of the neuroimaging techniques that are currently available. Because of the discrepancy between the speed of neuronal activity and changes in BOLD signal, we cannot determine for certain whether the observed neural changes reflect afferent or efferent signals or both. Relatedly, given the current limits of spatial resolution and the common use of spatial smoothing, it can be difficult to attribute activity to a precise region within these larger structures.
A more precise account of the afferent and efferent contributions to the neural changes observed during the development of asthma symptoms could be accomplished with direct manipulation of either the afferent and efferent signaling pathways. For example, a bronchoconstrictor could be administered under the guise of an environmental allergen (nocebo challenge) in individuals who typically experience a late asthmatic response to manipulate the efferent path. This would mimic the experience of an early phase response, but should not cause an immune cascade leading to the late response. Thus any changes in neural activity and asthma-related peripheral physiology occurring during the time frame when a late phase would develop could be attributed to expectation. Alternatively, pharmacological manipulation could be paired with allergen administration to isolate inflammation as the afferent signal, while eliminating the development of asthma symptoms. For example, administration of a corticosteroid will selectively block the late phase response, while the early phase remains intact. On the other hand, administration of a β2-agonist after resolution of the early response will block the lung function decline associated with the late response, but not the development of inflammation. Therefore, the afferent signal associated with the development of inflammation in the late phase response will be intact. In either condition, the participant will not experience late phase symptoms and will be explicitly told to expect no symptoms.
The identification of the anterior insula and ACC as important contributors to the neural circuitry underlying the relationship between emotion and asthma symptom expression has important implications for treatment. Thus far, the development of interventions aimed at preventing and treating asthma symptoms has exclusively targeted the periphery. However, the collective results of the studies reviewed here suggest that interventions aimed at reducing anterior insula and ACC reactivity, alone or in concert with peripheral-acting pharmacotherapy may offer promise. Indeed, the few reports that have evaluated drugs targeting the central nervous system in the treatment of asthma have yielded positive results. Lechin et al. (1998) report that the antidepressant tianeptine was very effective in treating asthma in a double-blind and randomized, placebo-controlled study. In a more recent investigation, Brown et al. (2005) report a decline in corticosteroid use in depressed asthmatics treated with the antidepressant citalopram (an SSRI) compared to a group treated with placebo, despite similar improvements in self-reported functional impairment and quality of life related to asthma symptoms in the two groups. Unfortunately, this study did not report the effect of citalopram on any objective measures of lung function. Similarly, antidepressant medication known to impact ACC activity has shown efficacy in the treatment of IBS (Bahar et al., 2008; Morgan et al., 2005). Morgan et al. (2005) showed that amitriptyline reduced the hyperresponsivity of the ACC to rectal distention in individuals with IBS, in addition to reducing subjective symptoms and objective disease measures.
In the future, studies should be designed to directly test the effect of antidepressant and anti-anxiety medication on the increased ACC and insula activity evoked by asthma symptom expression, and on the subsequent progression of symptoms. In addition, the efficacy of behavioral interventions that target emotion regulation and stress reactivity in reducing asthma symptom expression should be explored. Mindfulness-based stress reduction training, for example, has been used to buffer the effects of psychosocial stress on the development of inflammation in the skin (Rosenkranz et al., unpublished data). A future study could use this same strategy in asthmatics. Deterioration in lung function and increases in airway inflammation in response to psychological stress have already been demonstrated in asthma (Liu et al., 2002). Lung function and airway inflammation in response to an experimental psychological stressor alone, or accompanied by allergen exposure, could be assessed before and after mindfulness training. Neuroimaging could be employed in this context to quantify neural activity associated with stress exposure and how it relates to the training-related changes in symptom expression. The goal of the proposed research would be to determine if stress-induced symptom exacerbations could be buffered by mindfulness training, via changes in neural responsivity, as it was in the skin of healthy individuals. This type of translational research, which investigates the physiological basis for the application of behavioral interventions is essential to bringing new and efficacious therapeutic options to individuals with symptoms that are unresolved by the currently available pharmaceutical treatments or those unable or unwilling to tolerate their adverse effects.
Acknowledgments
The authors would like to thank Alexander J. Shackman and Brendon Nacewicz for their feedback. Support was provided by NIH grants P50-MH084051 and P01-AT004952 to RJD.
Footnotes
Note that the list of connections that follows is not meant to be exhaustive.
Note that this evidence comes from mid-insula anatomy in the rat (Bagaev & Aleksandrov, 2006). Substantial evidence suggests that important differences exist in regional insula function in sub-primates compared to primates, and so the autonomic motor control of the mid-insula reflected in this study may be represented in a more posterior insula region in humans. Nonetheless, stimulation and epileptic activity in the human insula does confirm visceromotor function in the human insula, though the precise location is not described (Augustine, 1996).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikuni T, Sako H, Murata A. Ipsilateral connections of the anterior cingulate cortex with frontal and medial temporal cortices in the macaque monkey. Neurosci Res. 1994;21:19–39. doi: 10.1016/0168-0102(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152:685–689. doi: 10.1016/j.jpeds.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Begaev V, Aleksandrov V. Visceral-related area in the rat insular cortex. Auton Neurosci-Basic. 2006;125:16–21. doi: 10.1016/j.autneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Berman SM, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen K, et al. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Brown ES, et al. A randomized trial of Citalopram versus placebo in outpatients with asthma and major depressive disorder: a proof of concept study. Biol Psychiat. 2005;58:865–870. doi: 10.1016/j.biopsych.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Calhoun WJ, Sedgwick J, Busse WW. The role of eosinophils in the pathophysiology of asthma. Ann NY Acad Sci. 1991;629:62–72. doi: 10.1111/j.1749-6632.1991.tb37961.x. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, et al. Extended allergen exposure in asthmatic monkeys induces neuroplasticity in nucleus tractus solitarius. J Allergy Clin Immunol. 2001;108:557–562. doi: 10.1067/mai.2001.118132. [DOI] [PubMed] [Google Scholar]
- Clarkson K. The nervous factor in juvenile asthma. Br Med J. 1937:845–850. doi: 10.1136/bmj.2.4008.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003a;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003b;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Brandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dorman SC, et al. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;1:565–72. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Grey MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. Plos One. 2007;2:1–9. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Winfield JA. Reversible pain and tactile deficits associated with a cerebral tumor compressing the posterior insula and parietal operculum. Pain. 1992;50:29–39. doi: 10.1016/0304-3959(92)90109-O. [DOI] [PubMed] [Google Scholar]
- Gupta V, Sheffield D, Verne GN. Evidence for autonomic dysregulation in the irritable bowel syndrome. Dig Dis Sci. 2002;47:1716–1722. doi: 10.1023/a:1016424007454. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Holmes GM, Rogers RC. TNF(alpha) modulation of visceral and spinal sensory processing. Curr Pharm Design. 2005;11:1391–1409. doi: 10.2174/1381612053507828. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Joad JP, et al. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- Joos GF. The role of sensory neuropeptides in the pathogenesis of bronchial asthma. Clin Exp Allergy. 1989;19:9–13. [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiat. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kuehn B. Asthma linked to psychiatric disorders. J Amer Med Assoc. 2008;299:158–160. doi: 10.1001/jama.2007.54-a. [DOI] [PubMed] [Google Scholar]
- Lange CG. The mechanism of the emotions. In: Rand B, editor. The classical psychologists. Boston: Houghton Mifflin; 1885. pp. 672–684. [Google Scholar]
- Lechin F, et al. Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: A double-blind, cross-over placebo-controlled study. Clin Pharmacol Ther. 1998;64:223–232. doi: 10.1016/S0009-9236(98)90156-4. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Isenberg S, Hochron SM. Asthma and emotion: a review. J Asthma. 1993;30:5–21. doi: 10.3109/02770909309066375. [DOI] [PubMed] [Google Scholar]
- Leigh R, MacQueen G, Tougas G, Hargreave FE, Bienenstock J. Change in forced expiratory volume in 1 second after sham bronchoconstrictor in suggestible but not suggestion-resistant asthmatic subjects: a pilot study. Psychosom Med. 2003;65:791–795. doi: 10.1097/01.psy.0000079454.48714.1b. [DOI] [PubMed] [Google Scholar]
- Liu LY, et al. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Luu P, Posner MI. Anterior cingulate cortex regulation of sympathetic activity. Brain. 2003;126:2119–2120. doi: 10.1093/brain/awg257. [DOI] [PubMed] [Google Scholar]
- MacKenzie M. Rose cold. Historical document. Ann Allergy. 1961;19:298–304. [PubMed] [Google Scholar]
- Mayer EA, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- Mertz H, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterol. 2000;118:82–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- Mesulam EJ, Mufson M-M. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982a;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam EJ, Mufson M-M. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982b;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Miller NE. Some psychophysiological studies of the motivation and of the behavioral effects of illness. Bull British Psychol Soc. 1964;17:1–20. [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M-M, Mesulam EJ, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome. Gastroenterol. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat Neurosci. 2006;9:435–442. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- Olausson H, et al. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neurosci Lett. 2005;389:1–5. doi: 10.1016/j.neulet.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pace TWW, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer C, Poline J-B, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. 2001;163:951–957. doi: 10.1164/ajrccm.163.4.2005057. [DOI] [PubMed] [Google Scholar]
- Phillips ML, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Herbert BM, Matthias E, Schandry R. Heart rate response after emotional picture presentation is modulated by interoceptive awareness. Int J Psychophysiol. 2007a;63:117–124. doi: 10.1016/j.ijpsycho.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007b;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Traut-Mattausch E, Schroeder H, Schandry R. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. J Anxiety Disord. 2007c;21:931–943. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Ritz T, Steptoe A, DeWilde S, Costa M. Emotions and stress increase respiratory resistance in asthma. Psychosom Med. 2000;62:401–412. doi: 10.1097/00006842-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, et al. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Nat Acad Sci USA. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön D, et al. Reduced perception of dyspnea and pain after right insular cortex lesions. Am J Respir Crit Care Med. 2008;178:1173–1179. doi: 10.1164/rccm.200805-731OC. [DOI] [PubMed] [Google Scholar]
- Strine TW, Mokdad AH, Balluz LS, Berry JT, Gonzalez O. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J Asthma. 2008;45:123–33. doi: 10.1080/02770900701840238. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Verne GN, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177:1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A, Mertz C, Kegat S, Burmester S, Dahme B. The impact of emotions on the sensory and affective dimension of perceived dyspnea. Psychophysiology. 2006;43:382–386. doi: 10.1111/j.1469-8986.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- White AM, Stevens WH, Upton AR, O’Byrne PM, Collins SM. Airway responsiveness to inhaled methacholine in patients with irritable bowel syndrome. Gastroenterol. 1991;100:68–74. doi: 10.1016/0016-5085(91)90584-8. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa ES, Katkin ES. Heartbeat detection and the experience of emotions. Cogn Emotion. 2000;14:417–427. [Google Scholar]
- Wood LJ, et al. Changes in bone marrow inflammatory cell progenitors after inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1998;157:99–105. doi: 10.1164/ajrccm.157.1.9704125. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005a;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cochetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- Yuan Y-Z, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]


