Abstract
Unwanted or misfolded proteins are either refolded by chaperones or degraded by the ubiquitin-proteasome system (UPS). When UPS is impaired, misfolded proteins form aggregates, which are transported along microtubules by motor protein dynein towards the juxta-nuclear microtubule organizing center to form aggresome, a single cellular garbage disposal complex. Because aggresome formation results from proteasome failure, aggresome components are degraded through the autophagy/lysosome pathway. Here we report that small molecule isothiocyanates (ITCs) can induce formation of aggresome-like structure (ALS) through covalent modification of cytoplasmic α- and β-tubulin. The formation of ALS is related to neither proteasome inhibition nor oxidative stress. ITC-induced ALS is a proteasome-dependent assembly for emergent removal of misfolded proteins, suggesting that the cell may have a previously unknown strategy in coping with crisis of misfolded proteins.
Keywords: Isothiocyanates, Protein Aggregation, Protein degradation
The cell maintains its biomass and carries out various functions by synthesizing and degrading proteins continuously. The routine degradation of unwanted or abnormal proteins is mainly carried out by ubiquitin-proteasome system (UPS) (1). When the cell senses misfolded proteins, it exercises a triage mechanism for protein quality control (2). At first, a variety of chaperone proteins are activated to retain the original conformations of misfolded proteins. If the repair fails, the cell activates the proteasome-dependent degradation mechanism to remove these misfolded or damaged proteins. However, when the capacity of the proteasome is exceeded or overwhelmed, misfolded proteins form aggregates and these aggregates are transported along the microtubule networks by motor protein dynein to a juxta-nuclear location, microtubule-organizing center (MTOC), to form aggresome/inclusion body (3–6). As demonstrated in cells transfected with misfolded or mislocated proteins, aggresome is a protein complex composed of misfolded proteins, chaperone proteins, proteasome components, mitochondrial components, and ubiquitinated proteins (7–9). Functionally, aggresome, a sign of proteasome failure, sets the stage for high capacity proteolysis by autophagy/lysosome (3–9).
Our recent studies identified that tubulin, a major constituent of microtubule, is a protein target of cancer chemopreventive isothiocyanates (ITCs) for cell growth inhibition (10). Naturally occurring ITCs, including benzyl ITC (BITC), phenethyl ITC (PEITC), and sulforaphane (SFN) (11), are electrophiles that covalently bind to certain cysteine residues in tubulin in vitro and in vivo. The binding induces tubulin conformational changes and microtubule collapse, which contributes to cell cycle G2/M arrest and apoptosis. More recently, we demonstrated that BITC and PEITC, but not SFN, can selectively induce rapid degradation of both α- and β-tubulins in a variety of cancer cells (12). Up to 80% of both α- and β-, but not γ-, tubulins are degraded through ubiquitin-proteasome pathway within 8 h of treatment with ITCs. Interestingly, several lines of evidence obtained support that ITC-induced tubulin degradation is initiated by tubulin aggregation. For example, first, tubulin aggregation occurs prior to tubulin degradation; second, agents that block tubulin aggregation also block tubulin degradation; and third, however, agents that block tubulin degradation have no effects on tubulin aggregation. Although these observations suggest that tubulin degradation is closely related to tubulin aggregation, key questions remain: what is the triggering event leading to tubulin aggregation? What are the components of ITC-induced tubulin aggregates? And what is the relationship between tubulin aggregation and degradation?
Here, we report that ITCs induce tubulin-containing aggregates in cells. The aggregates, insoluble by non-ionic detergents, have aggresome-like structure (ALS), containing some aggresome protein markers such as small molecular chaperone HSP27, proteasome components, mitochondrial components, and ubiquitinated proteins, in addition to ITC-modified tubulins. Our results also show that ITC binding to tubulin, not proteasome inhibition or oxidative stress, is the leading cause of the ALS formation. More importantly, contrary to aggresome by transfection induced misfolded proteins (3–9), ITC-induced ALS has enhanced proteasome activity and rapidly degrades tubulin in a proteasome-dependent manner. The study not only shows for the first time that small molecules may induce aggresome formation through direct modification of intracellular proteins, but also reveals that the cell may have a novel way to cope with misfolded proteins from chemical modifications.
Materials and Methods
Antibodies
Monoclonal anti-α-tubulin (clone B-5-1-2), monoclonal anti-β-tubulin (clone D66), monoclonal anti-ubiquitin (clone 6C1), monoclonal anti-vimentin (clone V9), and monoclonal anti-β-actin (clone AC-15) antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Monoclonal anti-γ-tubulin (clone TU-30) antibody, rabbit polyclonal anti-β-tubulin (H235), and Protein G conjugated agarose beads were purchased from Santa Cruz Biotechnology. Monoclonal anti-HSP90 (clone 9D2), monoclonal anti-HSP70 (clone C92F3A-5), and monoclonal anti-HSP27 (clone G3.1) were purchased from Assay Designs (Ann Arbor, MI). Monoclonal anti-20S proteasome subunit α4 (clone MCP34), anti-19S proteasome ATPase subunit Rpt6 (clone P45–110), and monoclonal anti-Mono- and Poly- ubiquitinated proteins (clone FK2) were purchased from Biomol (Plymouth Meeting, PA). Mouse monoclonal anti-Dynein (74 kDa intermediate chains, clone 74.1) was purchased from Millipore (Billerica, MA).
Lysis Buffers
The lysis buffer used to extract soluble fraction of cell lysate contains: 30 mM phosphate, 1% NP-40, 1 mM EDTA, 0.5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, pH 7.4. Harvested cells were lysed on ice for 20 min before being centrifuged at 13,200 g for 10 min. The supernatant was obtained as the soluble fraction and the pellet as the insoluble. Lysis buffer with 1% non-ionic detergent of either Triton X-100 or Tween 20 was also used to fractionate cell lysate. The results were similar to that of using NP-40. The buffer to extract whole cell lysate and to dissolve the insoluble fraction was SDS lysis buffer consisted of 65 mM Tris-HCl, pH 8.0, 2% SDS, 50 mM DTT, and 150 mM NaCl.
Immunoblotting and Immunofluorescence microscopy
The experiments were performed according to a previously published method (10). Detailed procedures are listed in the Supplemental Materials.
Proteasome activity assay
Purified ALS was diluted in a buffer containing 20 mM Tris-HCl (pH 7.8), 0.5 mM EDTA, 0.1% NP-40, 0.035% SDS before 50 μM of Suc-LLVY-AMC, Boc-LRR-AMC, and Z-LLE-AMC, were added individually. The incubation at 37 °C in dark lasted for 1 h before fluorescence was determined by a Synergy HT 96-well plate reader (BioTeK, Winooski, VT) with an excitation wavelength of 380±20 nm and an emission wavelength of 485±40 nm. Assays were performed in triplicate, and statistical significance was determined with a paired Student’s t test.
Results
ITC treatment induces aggresome-like structure formation
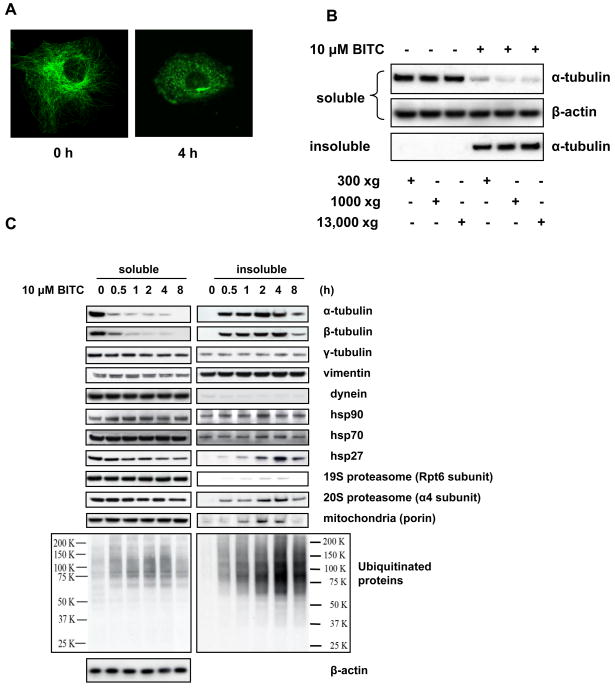
After BITC treatment, tubulin became punctates throughout cytoplasm (Figure 1A) with a brighter dot neighboring the nucleus, suggesting tubulin form aggregates. In fact, the tubulin-containing aggregates remain insoluble in buffers with up to 1% of non-ionic detergents, either NP-40, Triton X-100, or Tween 20. The aggregates can be spun down at only 300 ×g (Figure 1B).
Figure 1. ITCs induce formation of aggresome-like structure in cells.
A, BITC treatment disrupts microtubule network and induces tubulin aggregates. Immunofluorescent staining images of α-tubulin before and after HeLa cells were treated with 5 μM BITC for 4 h. B, ITC-induced tubulin-containing complex can be spun down at low centrifuge speed. HeLa cells were treated with 10 μM BITC for 2 h. The non-ionic detergent soluble and insoluble fractions were separated at various centrifugation speeds and immunoblotted separately for α-tubulin. C, Some aggresome marker proteins are enriched in the ITC-treated tubulin aggregates. HeLa cells were treated with 10 μM BITC for up to 8 h. The soluble and insoluble fractions of the cell lysate was immunoblotted for both α- and β-tubulin and aggresome markers γ-tubulin, vimentin, dynein, HSP90, HSP70, HSP27, proteasome components, mitochondria marker, and ubiquitinated proteins.
The known aggresome components include vimentin, γ-tubulin, dynein, HSP90, HSP70, HSP27, 20S proteasome, mitochondria, and ubiquitinated proteins in addition to the misfolded proteins (5). To study whether ITC-induced complex features these aggresome “signature” proteins, both the soluble and insoluble fractions of ITC-treated cells were analyzed by immunoblotting. The results (Figure 1C) show that within 8 h of ITC treatment, there were no substantial level changes for some aggresome marker proteins such as vimentin, γ-tubulin, dynein, HSP90, HSP70, in both fractions. However, both α- and β-tubulins, HSP27, α4 subunit of 20S proteasome, and ubiquitinated proteins had pronounced enrichment in the aggregates as early as 30 min. Other proteins such as Rpt6 subunit of 19S proteasome and porin (mitochondria component) also had some degree of enrichment. Comparably, in the soluble fraction, other proteins except HSP27 did not show substantial changes to their levels. The ubiquitinated proteins were substantially enriched in the insoluble fraction, while their levels were only slightly increased in the soluble fractions. The enrichment of some aggresome markers in ITC-induced complex indicates that the complex is ALS. The level changes of the protein markers in the insoluble fraction can be used to indicate ALS formation.
Some, but not all, aggresome marker proteins are co-localized with tubulin
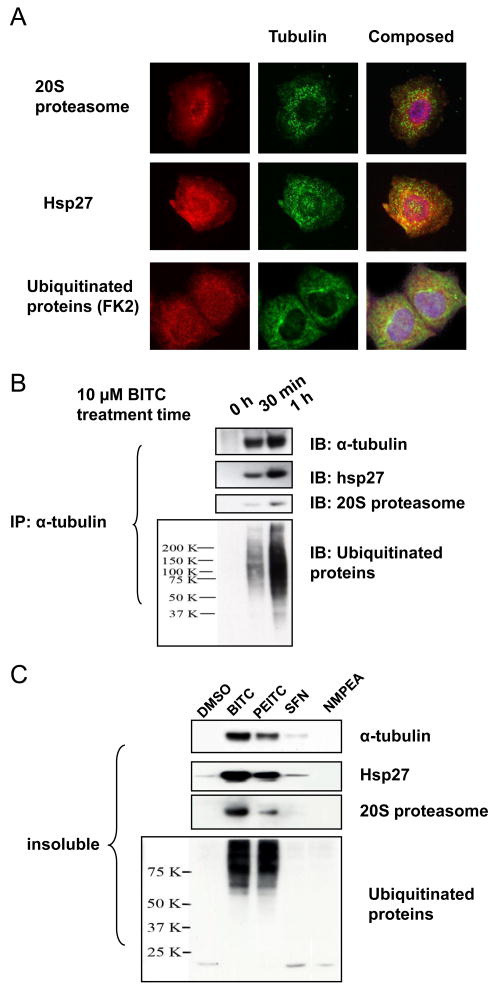
To rule out the possibility that the aggresome markers translocated to nucleus as a result of ITC treatment, we immunofluorescent-stained these three key protein markers, HSP27, 20S proteasome subunit, and ubiquitinated proteins, as well as tubulin, in cells. The results (Figure 2A) show that only a fraction of each marker co-localized with tubulin punctuates, agreeing with the immunoblots results (Figure 1C) that most aggresome marker proteins were still in the soluble fraction of cell lysate. It is clear that none of these aggresome markers were enriched or translocated to the nucleus due to ITC treatment, confirming that BITC induces formation of tubulin-containing ALS in cells.
Figure 2. Aggresome marker proteins binds to tubulin after ITC treatment.
A, ALS marker proteins HSP27, 20S proteasome, and ubiquitinated proteins are co-localized with tubulin aggregates in BITC-treated cells.HeLa cells were treated with 5 μM BITC for 1 h before being fixed and immunofluorescent-stained for ALS markers (in red) and α-tubulin (in green). B, ITC treatment induces rapid binding between tubulin and ALS marker proteins. HeLa cells were treated with 10 μM BITC for up to 1 h. The insoluble fraction was extracted and immunoprecipitated for α-tubulin. The IP products were immunoblotted for ALS markers. C, the potency order of inducing ALS formation is: BITC>PEITC>SFN. HeLa cells were treated with 10 μM BITC for 1 h. The insoluble fraction was extracted and immunoblotted for α-tubulin and ALS markers.
Aggresome marker proteins binds to tubulin after ITC treatment
To study ITC-induced interactions between tubulin and ALS marker proteins, we dissolved the insoluble fraction of ITC-treated cells using high salt (2 M MgCl2) and immunoprecipitated α-tubulin from the solution. All three markers, HSP27, 20S proteasome subunit, and ubiquitinated proteins, were immunodetected in the IP products (Figure 2B), including cells treated with BITC for only 30 min, suggesting that these ALS markers bind to tubulin rapidly after BITC treatment. Neither of three ALS markers was detected by immunoblot in the IP products using IgG, confirming the specificity of the assay (data not shown). As indicated by the time-dependence, the formation of ITC-induced ALS can be characterized by enrichment of α- and β-tubulins, HSP27, 20S proteasome and ubiquitinated proteins in the insoluble fractions.
The potency of ITCs to induce ALS is: BITC>PEITC>SFN
To examine whether other ITC molecules induce ALS, we treated cells with the same concentration (10 μM) of BITC, PEITC, and SFN. The results indicate (Figure 2C) that BITC is the most potent inducer of ALS formation, while SFN is the least potent. N-methyl phenethylamine (NMPEA), a PEITC analog without ITC functional group, was completely inactive. The results suggest that the ITC functional group is essential for the activity and tubulin binding by ITCs may be a triggering event for ALS formation.
Inhibition of proteasome does not induce ALS formation
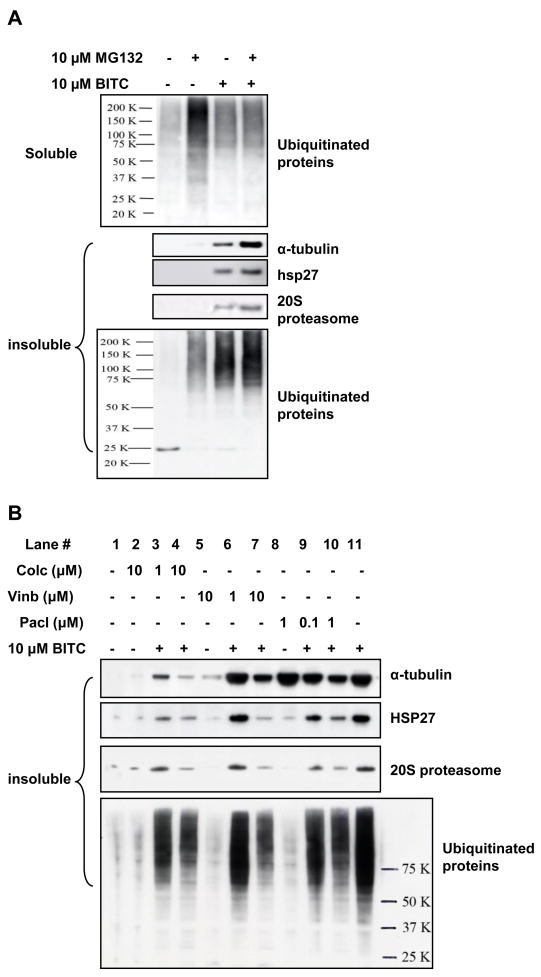
Aggresome is a protein aggregate that forms when cellular UPS is impaired or overwhelmed (3–9). Our results (Figure 3A) show that MG132, as expected, induced significant accumulation of ubiquitinated proteins in the soluble fraction. BITC alone and BITC co-treatment with MG132 also induced ubiquitinated protein build-up in the soluble fraction, but not as much as MG132 treatment alone. In the insoluble fraction, MG132 treatment did not cause enrichment of tubulin, HSP27, and 20S proteasome even though there was a moderate increase of ubiquitinated proteins. In contrast, BITC induced a greater enrichment of ubiquitinated proteins, as well as tubulin, HSP27, and 20S proteasome in the insoluble fraction. The differences in the accumulation of ubiquitinated proteins in both fractions by MG132 and BITC suggest that both may inhibit UPS, however, through two separate mechanisms. The data suggest that the formation of ALS is unique to BITC treatment and, unlike in classical aggresome formation, inhibition of proteasome is not a trigger for ALS formation.
Figure 3.
A, Inhibition of proteasome system did not induce ALS formation. Hela cells were treated with 10 μM proteasome inhibitor MG132, 10 μM BITC individually or combined. Both soluble and insoluble fractions were collected and analyzed by immunoblots. B, ALS formation is likely to be triggered by ITC binding to tubulin. HeLa cells were treated with 1 and 10 μM colchicine, 1 and 10 μM vinblastine, 0.1 and 1 μM taxol for 1 h before treated with 10 μM BITC for another 4 h. The insoluble fraction was detected for ALS marker proteins.
ALS formation is unrelated to oxidative stress
ITCs are known to induce oxidative stress in cells (11,13). To study the relationship between oxidative stress and ALS formation, we treated cells with up to 300 μM H2O2 for 4 h. The results (Supplemental Figure-A) show that exogenous reactive oxygen species (ROS) did not induce enrichment of ALS markers. In our previous studies, ROS generation by 10 μM BITC is much lower than that of 100 μM H2O2 (13). Also, treating cells with cell permeable polyethylene glycol (PEG)-linked catalase before BITC treatment did not alleviate the BITC-induced marker enrichments (Supplemental Figure-A). Additionally, we pretreated cells with catalase specific inhibitor aminotriazole (ATZ). The results (Supplemental Figure-B) show that ATZ pretreatment failed to induce ALS formation. The quenching and inducing of intracellular ROS by PEG-catalase and ATZ, respectively, were previously demonstrated in HeLa cells (10). Taken together, ITC-induced ALS formation is unrelated to the oxidative stress.
Purified tubulin has been shown to form aggregates mediated by disulfide crosslinking in vitro (14). To examine whether formation of ITC-induced tubulin aggregates in cells is mediated through disulfide cross-linking under a possibly oxidative sub-cellular environment, we lysed ITC-treated cells in the presence of up to 20 mM dithiothreitol (DTT, a reducing agent). If protein aggregates through forming disulfides, DTT would reduce the disulfides to free thiols and consequently, less ALS marker proteins would be detected in the insoluble fraction. However, the immunoblots of the insoluble fraction (Supplemental Figure-C) show that levels of tubulin and other ALS markers were not affected by DTT, suggesting that ITC-induced ALS formation is not mediated through cysteine cross-linking. The evidence also seems to confirm that oxidative stress may not involved in ALS formation.
ALS formation is triggered by ITC binding to tubulin
Pretreating cells with colchicine, vinblastine, and taxol, significantly reduces ITC binding affinity to tubulin, prevents tubulin misfolding, and blocks tubulin degradation (12). To further examine the mechanism of ALS formation, we treated cells with these agents before BITC treatment. The results (Figure 4) show that these compounds do not induce ALS formation by themselves (Lane 2, 5, and 8). However, pretreatment of these compounds successfully blocked enrichment of ALS markers, including HSP27, proteasome, and ubiquitinated proteins (Lane 3 and 4, 6 and 7, 9 and 10, respectively compared with Lane 11), suggesting that ALS formation was blocked by tubulin binding agents. Therefore, ITC binding to tubulin may be a triggering event for ALS formation. The blockage of ITC-induced ALS formation by pretreatment of colchicine, vinblastine, and taxol was substantial, but not complete, suggesting that: 1) α- and β-tubulins may be the major target proteins of ITCs probably due to their abundancies in the cell; 2) binding of ITCs to other cellular proteins besides tubulin may also contribute to ALS formation.
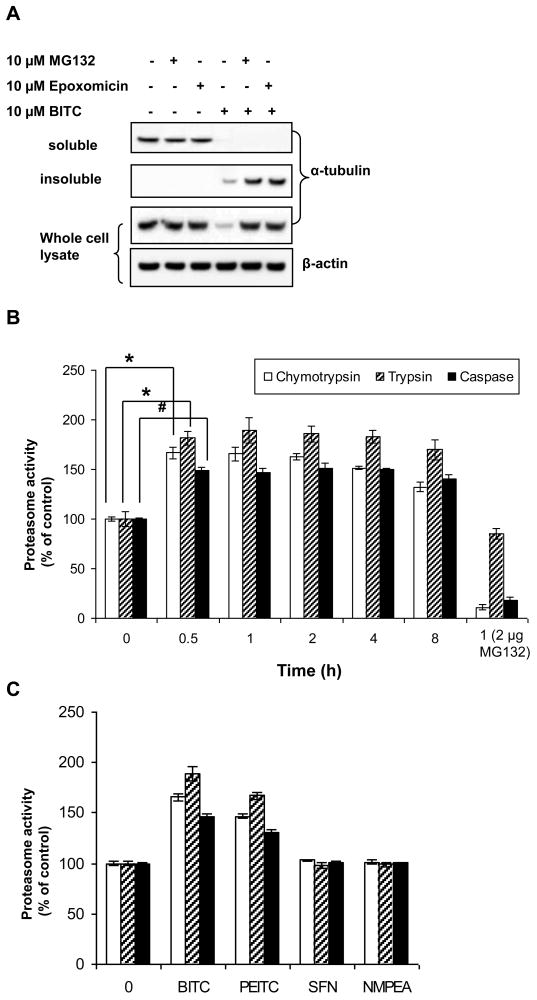
Figure 4. Biological function of ALS is to degrade misfolded proteins.
A, ITC-induced tubulin degradation is proteasome dependent and proteasome inhibitors blocked ITC-induced degradation in the insoluble fraction.HeLa cells were pretreated with 10 μM MG132 or Epoxomicin for 1 h before treated with 10 μM BITC for another 4 h. The levels of α-tubulins in both fractions and the whole cell lysate were determined by immunoblots. B, ALS has enhanced proteasome activities. HeLa cells were treated with 10 μM BITC for up to 8 h. ALS was extracted and its proteasome activities were measured. Open column, trypsin-like activity; shaded column, chymotrypsin-like activity; closed column, caspases-like activity. *, p < 0.005; #, p < 0.002. MG132 (2 μg) was added to the purified ALS of cells treated with 1 h BITC. C, the potency order of enhancing proteasome activities in ALS is: BITC>PEITC>SFN. HeLa cells were treated with 10 μM BITC, PEITC, and SFN for 1 h. ALS was extracted and its proteasome activities were measured. NMPEA treatment served as a negative control.
ALS is high-capacity protein degradation machinery
ITCs were found to selectively induce tubulin degradation in a variety of cancer cells (10). Figure 5A shows that both proteasome inhibitors did not have any effects on tubulin levels in both fractions and the whole cell lysate; however, BITC rapidly induced degradation of α-tubulin, which was blocked by both MG132 and epoxomicin, indicating that proteasome is responsible for the degradation of tubulin (10). The results also show that both inhibitors had no effects on ITC-induced tubulin level decreases in the soluble fraction, suggesting that they did not block tubulin aggregation. However, the complete blockage of tubulin degradation by MG132 and epoxomicin was clear in the insoluble fraction. Since ALS is contained in the insoluble fraction, the results suggest that ALS may be involved in degradation of misfolded tubulin. To confirm this, we purified ALS from the ITC-treated cells and measured its proteasome activities. As expected, all three proteasome activities, including trypsin-like, chymotrypsin-like, and caspase-like, were substantially enhanced as early as 30 min after treatment (Figure 5B). Chymotrypsin-like and caspase-like activities were clearly inhibited by 2 μM MG132, indicating that enhanced proteolytic activity is proteasome-dependent. Figure 5C shows that the potency of enhancing proteasome activities follows the order: BITC>PEITC>SFN; the same that induces ALS formation (Figure 2C), suggesting that the biological function of ALS is to remove misfolded proteins by proteasome.
Discussion
In this study, we found that small molecular ITCs binding to tubulin may trigger formation of protein aggregates in HeLa cells. Similar results were obtained using human non-small lung cancer A549 cells (data not shown), suggesting that the phenomenon may not be cell line-specific. Based on results, we postulate that ITCs covalently modify specific cysteine residues in both α- and β-tubulins and other yet unidentified target proteins, causing their conformation change and misfolding. Rapid generation of a large amount of misfolded proteins may force the cell to switch from the regular-paced ubiquitin-proteasome system to high priority and high-capacity ALS for facile removal of misfolded, potentially toxic proteins (6).
This scheme is supported by characterization of components in the ITC-induced ALS. For example, HSP27 was rapidly enriched in the aggregates. HSP27 belongs to a family of small heat shock proteins (sHsps). sHsps are ATP-independent chaperone proteins that form large oligomeric complex (15). A number of studies have clearly shown that sHsps recognize temperature-induced misfolded proteins and keep them soluble in a defined sHsps-substrate complexes (15). Unlike ATP-dependent chaperones, such as Hsp110, Hsp90, and Hsp70, sHsps are unable to promote substrate refolding (15). However, the formation of large defined complexes with misfolded proteins is a general characteristic of sHsps. Whether HSP27 enriched in ITC-induced ALS acts as a chaperone to accommodate misfolded tubulin requires further investigation.
Proteasome components enriched in the classical aggresome are usually degradation substrates by autophagy/lysosome pathway (3–9). In contrast, the function of 19S and 20S proteasomes enriched in ITC-induced ALS may be to degrade misfolded proteins such as α- and β-tubulins. Their presence in ALS is in agreement with our findings that ITC-induced tubulin degradation is ubiquitin-proteasome dependent. Inclusion of mitochondria in ITC-induced ALS suggests that protein degradation in ALS is not only an energy-consuming process but also a process with a high priority. The higher priority of removing misfolded proteins in ITC-induced ALS is further suggested by the observation that ubiquitinated proteins rapidly accumulated in both the soluble and insoluble-especially the insoluble-fractions. This may reflect a smart strategy adopted by the cell when facing emerging crisis of misfolded proteins: it temporarily gives up degrading its regular substrates, but focuses on the imperative task of removing misfolded proteins. It is important to note that the high capacity protein degradation by ALS still is proteasome-dependent. Interestingly, all three proteasome activities in the soluble fraction (mainly cytoplasmic proteins), chymotryptic-, tryptic-, and caspase- like were moderately inhibited by ITCs in the order of BITC>PEITC>SFN (unpublished results). The inhibition may indicate one of the prices the cell pays when high priority and high capacity tasks are geared from limited resources, such as chaperones, proteasome, and mitochondria. Although we can’t rule out the possibility that cytoplasmic proteasome inhibition is independent of ALS formation, either case supports the notion that ALS is high priority and high capacity protein degradation machinery. At the end of the massive “clean up,” ALS begins to disassemble (8 h in Figure 1C) and the proteasome activities in ALS begin to drop (Figure 5B). Interestingly, ubiquitinated proteins in both fractions become dispersed, suggesting that the temporarily-halted degradation of regular substrates resumes. Additionally, the notion is also supported by the fact that direct inhibition of UPS does not induce the formation of ITC-induced ALS. It is probably because proteasome inhibitors block degradation of regular UPS substrates, but fail to generate misfolded proteins, which are required as a trigger for assembly of ALS. The proteasome-dependence of ITC-induced ALS is also consistent with our previous results that only proteasome inhibitors, but not autophagy, lysosome, caspase, or protease inhibitors, can block ITC-induced tubulin degradation (10). Therefore, our studies suggest that the formation of ALS by ITCs is mechanistically different from classical aggresome, which depends on autophagy/lysosome pathway for protein degradation.
Unlike classical aggresome, ITC-induced ALS formation and its activity are independent of microtubule integrity because microtubule disruption is one of the downstream effects of ITC binding to tubulin. As supporting evidence, γ-tubulin (a MTOC marker) and dynein, two key components which were found to enrich in a classical aggresome, were not detected in the ITC-induced ALS. Tubulin-microtubule equilibrium dynamics is also irrelevant to ITC-induced ALS and its function. It is noted that pretreatment of tubulin binding agents at much lower concentrations, 100 nM for both colchicine and vinblastine and 10 nM taxol, had little effects on ITC-induced ALS formation and tubulin degradation rate, however, effectively induced either microtubule disruption or reinforcement in both HeLa and A549 cells (data not shown). The evidence also rules out ITC-induced microtubule disruption as a possible switch for ALS formation and its activity.
It has been demonstrated that protein aggregations or accumulation of misfolded proteins are toxic and induce G2/M arrest (6). In our previous studies, G2/M phase arrest and apoptosis induction were observed in ITC-treated cells (10,12,13). Besides microtubule disruption, ITC-induced tubulin aggregation and degradation may also contribute to these downstream events. When cells are treated with lower concentration of ITCs (5–10 μM), tubulin is rapidly degraded by ALS. Since tubulin is required to form spindle assembly during mitosis, rapid removal of tubulin results in G2/M arrest. Acute cell death, including both apoptosis and necrosis, was observed when cells are treated with higher concentrations (20–30 μM) of ITCs (13). Under these conditions, tubulin aggregation is aggravated because tubulin degradation is either partially or completely inhibited due to inhibition of cytoplasmic proteasome activity. Misfolded protein aggregates may be at least partially responsible for the acute cell death (6, 16). Accordingly, it is conceivable that ITC-induced ALS formation functions to regulate cytotoxicity caused by misfolded proteins. Although both aggresome and ALS share the common goal of removing misfolded proteins, classical aggresome does it through centralized collection and autophagy/lysosome, while ITC-induced ALS does it locally through activated proteasome. This study provides an insight into a novel mechanism by which the cell deal with emerging crisis of chemically modified and misfolded proteins.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth S. Sztul of Department of Cell Biology at University of Alabama, Dr. David Yang of Department of Chemistry at Georgetown University, Drs. Leslie Wilson and Mary Ann Jordan at University of California Santa Barbara, Dr. Saadi Khochbin at Equipe Epigénétique et Signalisation Cellulaire in France, Dr. Jacques Landry at Centre de recherche en cancérologie l’Hôtel-Dieu de Québec and Université Laval in Canada, and Dr. Changcheng Song of Laboratory of Cancer Prevention at NCI-Frederick, for fruitful discussion and suggestions. This study was supported by NCI grant CA100853.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman S, Wickner S, Maurizi MR. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11(7):815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 3.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143(7):1883–1888. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10(12):524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Mata R, Gao YS, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3(6):388–396. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 6.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 7.Pandey UB, Nie ZP, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447(7146):859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 8.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated Huntingtin. J Biol Chem. 2005;280(48):40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 9.Kalveram B, Schmidtke G, Groettrup M. The ubiquitin-like modifier FAT10 interacts with HDAC6 and localizes to aggresomes under proteasome inhibition. J Cell Sci. 2008;121(Pt 24):4079–88. doi: 10.1242/jcs.035006. [DOI] [PubMed] [Google Scholar]
- 10.Mi L, Gan N, Cheema A, Dakshanamurthy S, Yang DC, Chung FL. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem. 2009;284(25):17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. IARC Handbook on Cancer Prevention 9. Lyon (France): IARC; 2004. Cruciferous vegetables, isothiocyanates and indoles. [Google Scholar]
- 12.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283(32):22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67(13):6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 14.Correia JJ, Lipscomb LD, Lobert S. Nondisulfide crosslinking and chemical cleavage of tubulin subunits: pH and temperature dependence. Archives of biochem Biophys. 1993;300(1):105–114. doi: 10.1006/abbi.1993.1015. [DOI] [PubMed] [Google Scholar]
- 15.Haslbeck M. sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59(10):1649–1657. doi: 10.1007/PL00012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115(6):727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.