Abstract
PDGF and FGF-2 are important regulators of vascular wall assembly. We tested the hypothesis that their embryonic temporal expression facilitates two specific events: 1) the endothelial invasion of the aortic root to form the coronary artery stems and 2) the subsequent growth and development of the arterial tree. Addition of FGF-2 and PDGF-BB proteins to embryonic quail heart explants stimulated a 3 and 7-fold increase, respectively, in tubulogenesis, while neutralizing antibodies to these growth factors attenuated tubulogenesis by 40%. Anti-FGF-2 and anti-PDGF neutralizing antibodies were then introduced in ovo via the vitelline vein at various embryonic (E) days. When injections occurred prior to coronary ostial formation, the embryos usually developed only one coronary artery or lacked coronary arteries. When one or both major coronary arteries formed: 1) their branches had a thinner tunica media, and 2) smooth muscle investment did not progress as far distally as in shams. Other anomalies included smaller diameter coronary artery stems in some hearts. Inhibition of VEGF via injections of aflibercept (VEGF-Trap, a VEGFR-1 and -2 chimera), previously shown to be essential for coronary stem formation, limited development of the coronary arteries even though introduced after formation of coronary ostia (at E9 or EI0). This finding indicates a role for VEGF proteins in the development of the tunica media of coronary arteries. Our data 1) document a role for FGF-2 and PDGF in the temporal regulation of coronary artery stem formation and growth of the coronary arterial tree and 2) reveal that VEGF expression is required for normal artery/arterial formation, even after coronary artery stem formation.
Keywords: arteriogenesis, angiogenesis, VEGF, FGF-2, PDGF, coronary arteries
Most contemporary studies regarding the formation of the coronary vasculature have focused on the formation of the epicardium, epithelial-mesenchymal transformation and factors regulating coronary vascular cell differentiation (see reviews).1, 2 They demonstrated that epicardially-derived cells differentiate into vascular phenotypes, i.e., endothelial, smooth muscle, fibroblasts, and then migrate, proliferate and assemble to form vascular channels. The role of growth factors in the regulation of the events that occur prior to coronary artery formation have also been investigated, i.e. vasculogenesis (migration and assembly of endothelial cells or precursors to form vascular tubes) and angiogenesis (branching and extension of the vascular tubes). We have shown, both in vivo3, 4 and in vitro5, 6 that coronary tubulogenesis is facilitated by VEGF and FGF-2. Moreover, tubulogenesis correlates with an epi-to-endo-cardial VEGF protein gradient.7 Inhibition of VEGFs via aflibercept (VEGF Trap) markedly attenuates tubulogenesis when injected intravascularly in quail eggs on embryonic day 6, which corresponds to the onset of tubulogenesis. A role for FGF signaling in the development of a tubular plexus in mouse embryos has also been recently documented.8 That study showed that FGF triggers hedgehog (HH) activation that is essential for VEGF-A, -B and –C, and angiopoietin-2 expression. The authors noted that the embryonic myocardial vascularization was facilitated by the orchestration of multiple growth factors in response to HH activation.
However, little attention has been paid to the mechanisms regulating formation of the coronary arteries, which occurs subsequent to the formation of an endothelial-lined network, i.e. embryonic (E) day 9 (HH 35) after a capillary-like peritruncal ring penetrates the aorta just above its valves to create the coronary ostia.9–12 Having found that VEGFR-2 and -3 mRNA transcripts are selectively dense at the sites of coronary artery stems during development,6 we inhibited VEGFs in quail embryos by injecting VEGF-Trap prior to the formation of the coronary ostia.9 These experiments revealed that the formation of coronary ostia and stems is dependent on VEGF family members, especially VEGF-B. The data from that study precipitate key questions regarding the roles of other growth factors, their temporal expression and their interactions in both the formation and the growth of the coronary arterial vasculature.
Based on the concept that the coronary vasculature develops in response to temporally and spatially expressed growth factors acting in concert, we focused on two growth factors that are most likely to influence the recruitment and assembly of vascular smooth muscle in the coronary arterial system, namely PDGFs and FGF-2. PDGF-BB plays a key role in endothelial cell proliferation,13 pericyte recruitment and survival14 and the proliferation of mural cells and their precursors.15, 16 A role for PDGF-BB and PDGFR-β in myocardial vasculogenesis/angiogenesis has been suggested because all cell types that contribute to the coronary vasculature express this ligand and receptor in the embryonic avian heart17 and PDGF-BB enhances the production of VEGF in the myocardium.18 FGF-2 is a regulator of both angiogenesis and arteriogenesis (reviewed in Presta et al.),19 as it has been shown to enhance endothelial and smooth muscle cell proliferation.20, 21 We have documented a role for FGF-2 in embryonic myocardial tubulogenesis5 and post-natal arteriogenesis.4
The major goal of the current study was to test the hypothesis that PDGF and FGF-2 play a role in coronary artery formation in the embryo, but that their effects are temporal and specific with regard to 1) formation of the coronary ostia and, 2) the development of the coronary arterial tree. A second goal was to document the temporal effects of PDGF and FGF-2 in coronary tubulogenesis. Finally, we also tested the hypothesis that VEGF plays a role in the development of the tunica media of coronary arteries.
Materials and Methods
Antibodies, Soluble Receptors and Recombinant Proteins
Anti-PDGF and anti-FGF-2 neutralizing antibodies were purchased from R & D Systems (Minneapolis, MN). The anti-PDGF antibody shows a nearly 100% cross-reactivity with rh PDGF-BB, a 10% cross-reactivity with PDGF-AA and a 60% cross-reactivity with PDGF-AB Specificity of the FGF-2 antibody is indicated by data that reveal that it has < 5% cross-reactivity with FGFs 2–6, 9, 10, 15, 17, 19. Aflibercept (VEGF-Trap), which binds ligands for VEGFR-1 (Flt-) and VEGFR-2 (Flk-1), is a VEGFR-1 and VEGFR-2 fusion protein that comprises portions of the human VEGF-1 and VEGF-2 extracellular domains fused to a constant region of human IqG1. Aflibercept was provided by Regeneron Pharmaceutical, Inc. (Tarrytown, NY). Recombinant human PDGF-BB and FGF-2 were purchased from R & D Systems and BD Biosciences, respectively.
Immunohistochemistry and Histology (in vivo studies)
All staining was done on serial cross-sections of the basal halves of the embryonic hearts. Endothelium was visualized by staining with QH1, a specific antibody for quail endothelium (University of Iowa Developmental Studies Hybridoma Bank), using Alexa 488-labeled goat anti-mouse IgG Fab as a chromagen, as previously detailed.9, 22 Cy-3-congugated monoclonal smooth α-actin (clone 1A4) (Sigma St. Louis, MO) was used to visualize arteries and arterioles.23 Immunostaining for FGF-2, PDGF-BB and VEGF proteins was accomplished with rabbit polyclonal primary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and alkaline phosphatase-conjugated goat F(ab)2 anti-rabbit secondary antibodies. The cytokeratin immunolabeling protocol was according to Perez-Pomarez et al. and utilized antibovine cytokeratin (Z622, DAKO Carpinteria CA) and alexa-fluor goat-anti-rabbit IgG 594, Invitogen, Carlsbad, CA).24
Heart Explant Culture Experiments
The apical one-third of embryonic day 6 (E6) quail hearts were placed on collagen gels as previously described.5, 25 Then various doses of PDGF-BB and FGF-2 or anti-PDGF and – FGF-2 neutralizing antibodies were added individually or in combination and the hearts were fixed with paraformaldehyde 48 hours later. Non-treated explants were included for each experiment. Vascular tubes grow out from the explant and are visualized by immunostaining with QH1 antibody. Digitized images of the explants were then quantified, i.e. total length of tubes per perimeter of explant, using Image Plus software (Media Cybernetics).
In Vivo Experiments
Fertilized quail eggs (Coturnix japonica) were incubated (37.8°C & 80% humidity) for 6–10 days. As previously described,9 an incision was made on the inner shell membrane via a window in the shell and neutralizing antibodies to FGF-2 and PDGF (12 or 20μg in 12 μL of saline) were injected into the vitelline vein prior to (E6, E7), during (E8) or after (E9, E10) formation of the coronary ostia and stems, with the aid of a micromanipulator. Shams received 12 μL of saline only. VEGF-Trap as previously described,9 was injected (12μg in 12μL saline) only at embryonic (E) days 9 and 10. Either 2 or 3 days after injection, the hearts were perfused with 4% paraformaldehyde and the basal half of the heart processed and embedded in paraffin. Serial sections, 6 μm in thickness, were then prepared.
Quantifying Tubulogenesis
We incubated deparaffinized tissue sections in QH1 antibody (which labels quail endothelial cells), and then with anti-mouse IgG Alexa Fluor. These slides were digitized using Image Pro Plus software (Media Cybernetics) to determine microvascular volume density in the compact region of the left ventricle, as previously reported.9
Coronary Artery Formation and Growth
Alternate serial sections were stained with either hematoxylin and eosin or smooth muscle α-actin. The approximate apical distance of sections from the aortic root was recorded for all slides and the following data obtained: 1) number of coronary ostia formed, 2) most distal section from aortic root containing arteries or arterioles, 3) extent of tunica media formation (medial thickness), 4) thickness of the compact region of the left ventricle, and 5) ventricular and coronary phenotypes.
Statistical Analyses
A one-way ANOVA with a Dunnett multiple comparison test was used to test for differences between groups. Data are expressed as Mean ± SEM and a ρ ≤ 0.05 was selected to denote statistically significant intergroup differences.
Results
PDGF and FGF-2 Enhance Coronary Tube Formation in Vitro
Incubation of embryonic day (E) 6 heart explants stimulates the formation of a network of vascular tubes within the collagen matrix.5, 9 Addition of FGF-2 protein to the culture medium enhanced tubulogenesis nearly 7-fold in a dose-dependent manner (Figure 1A) consistent with our previously published data.5 PDGF-BB protein also increased tube formation, but only 3-fold. Addition of both growth factors enhanced tube formation, but had an additive effect only at lower doses. Neutralizing antibodies to PDGF and FGF-2 each inhibited tubulogenesis by about 40% (Figure 1B). However, administration of the antibodies in combination did not enhance their inhibitory effect.
Figure 1.
In vitro experiments on endothelial tubulogenesis from embryonic heart explants. A. Addition of various doses of PDGF-BB and FGF-2 proteins individually or in combination enhanced tubulogenesis in a dose-dependent manner. An additive effect of the two growth factors occurred only at lower doses (25 and 50 μg/ml) and did not exceed the maximal affect of FGF-2 (at 100 or 150 μg/ml). B. The effect of neutralizing antibodies on tubulogenesis. Administration of both neutralizing antibodies did not have an additive effect. Means ± SEM are shown. Number of heart explants are indicated within the bars.
Temporal Effect of PDGF and FGF-2 on In Vivo Tubulogenesis
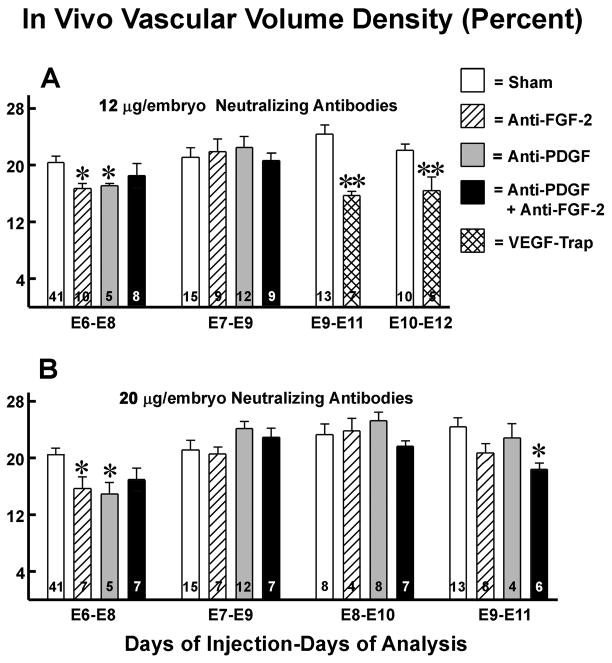
To determine the temporal effects of these growth factors on vasculogenesis/angiogenesis, we injected their neutralizing antibodies at 12μg and 20 μg into the vitteline vein on days E6, 7, 8, 9 and 10. The vascular volumes were then measured two days later. Both anti-PDGF and anti-FGF-2 at both doses significantly attenuated tubulogenesis when injected at E6, which corresponds to the onset of tubulogenesis in the myocardium (Figure 2). Vascular volume densities were 17–23% lower in the embryos injected with the antibodies at the two concentrations, (12 and 20 μg) respectively. Injection of both anti-PDGF and anti-FGF-2 did not have an additive effect. When the embryos were injected at E7, E8 or E9, vascular volume was not affected. However, when both antibodies were injected at E9, vascular volume was 24% lower than the sham group. Injections of both antibodies at earlier time points had no significant effect.
Figure 2.
In vivo inhibition of left ventricular vascular volume density by anti-FGF-2, anti-PDGF, and aflibercept (VEGF-Trap). Quail embryos were injected with these inhibitors (12 or 20 μg/embryo) at various time points. The two embryonic (E) days indicated represent day of inject and day of study, respectively. VEGF-Trap was previously shown to inhibit vascular volume density when injected at E6 or E79. The current data show this effect also occurs after the coronary stems are formed (E9 and later). Data are expressed as Means ± SEM and the number of embryos is indicated within the bars. *, p < 0.05; * *, p < 0.01
Having previously documented the importance of VEGFs in early (E6, E7) tube formation,9 we tested the hypothesis that tubulogenesis continues to be influenced by VEGFs at later time points. Accordingly, VEGF-Trap was injected into embryos after the formation of the coronary artery stems and the establishment of the coronary circulation (E9 and E10). This intervention caused a 37% and 27% reduction in vascular volume density when injected at E9 and E10 respectively. Thus, VEGFs play a role in tubulogenesis after coronary artery stem formation, as well as during the early period of tubulogenesis.
Inhibition of PDGF and FGF-2 Interrupts Formation of Main Coronary Arteries
Injection of anti-FGF-2, anti-PDGF-B or both at E6, 7 and 8 affected, to varying degrees, the formation of coronary ostia, as determined in serial sections of hearts obtained at either E9 or E10 (Figure 3). In shams, both coronary arteries are present by E9. The most dramatic effect was seen in hearts injected with anti-FGF-2 on E6 and studied at E9. Five of six hearts lacked both coronaries. If the injection was delayed by one day (E7), only one of 7 hearts had both coronaries, 4 had one and 2 had none. When the antibodies were injected on E7 or E8 and studied on E10, the effect was more limited, i.e., at least one coronary artery was formed and the majority of hearts had 2 coronary arteries. Antibodies to PDGF also limited the formation of coronary arteries, i.e. most embryos developed only one coronary artery. When the two neutralizing antibodies were given in combination, the results were similar to those from anti-PDGF treatment alone.
Figure 3.
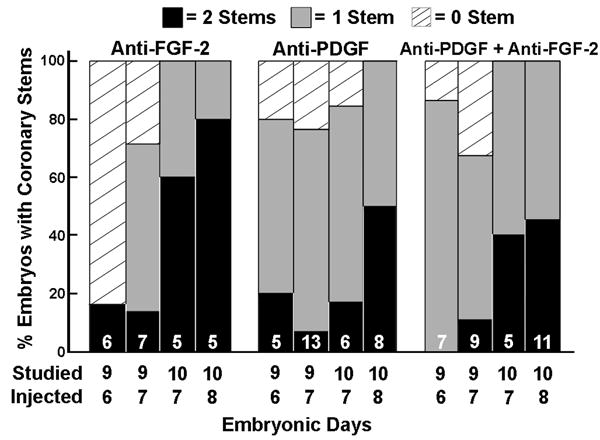
In vivo administration of anti-FGF-2 and anti-PDGF neutralizing antibodies limits coronary artery stem formation, such that neither stem or only one stem is formed. The neutralizing antibodies were injected on embryonic days 6, 7 or 8 and studied 2 or 3 days later. The number of embryos is indicated within the bars.
Artery/Arteriolar Wall Assembly is Attenuated by Temporal Inhibition of FGF-2 and PDGF
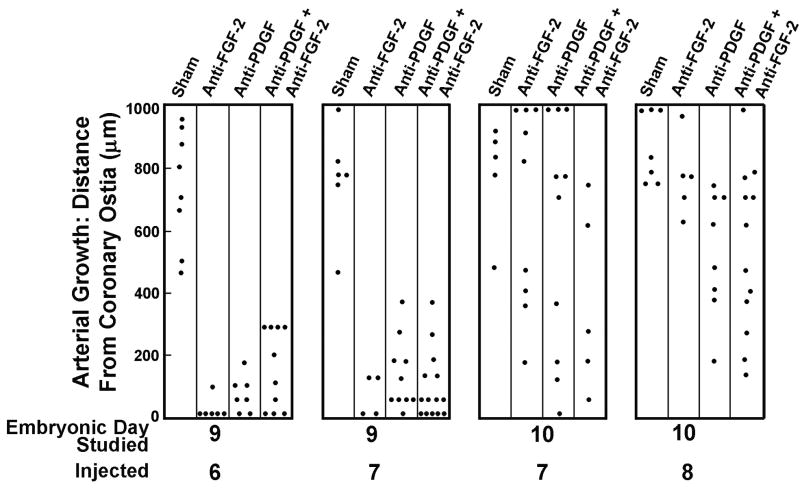
Formation of coronary arteries occurs by the recruitment and assembly of vascular smooth muscle cells (SM) in a base to apex direction; the earliest smooth muscle assembly is at the root of the two main coronary arteries.9 To determine the effect of FGF-2 and PDGF temporal inhibition on the progression of coronary artery/arteriolar formation, we utilized alternate sections stained with smooth muscle α-actin. We then noted the approximate most distal section from the base of the aorta in which an artery or arteriole was found (Figure 4). When neutralizing antibodies to FGF-2 and PDGF are injected at E6, the effect on muscularization of endothelial channels is most affected. This is consistent with the data that reveal most hearts treated with these antibodies either lack coronary artery stems or have only one. Delaying administration of the antibodies tends to lessen the inhibitory effect. Comparison of hearts from E10 embryos reveals two important findings. First, limitations in arterial/arteriolar growth due to anti-FGF-2 were greater when the antibodies were injected at E7 than when injected at E8. Second, the effects on arterial/arteriolar growth for all three antibody treatments were less marked in the hearts of E10 than in E9 quail. This finding suggests that inhibition of FGF-2 or PDGF at E7 does not arrest the muscularization process since E10 hearts tend to have better progression of medial development than E9 hearts.
Figure 4.
Assessment of arterial/arteriolar growth. Data are the furthermost distance from the aortic base where arteries or arterioles were detected based on serial sections and smooth muscle α-actin immunostaining. For the purpose of standardization, the maximum distance included was 1,000 μm.
The inhibitory effects of anti-FGF-2 and anti-PDGF on arterial/arteriolar development are illustrated in Figure 5. The examples provided show that the main effect is a poorly developed tunica media, and most often larger diameters. At least this is true for the largest branches of the main coronary arteries (these are septal arteries).
Figure 5.
Arteriogenesis in sham, anti-FGF-2, anti-PDGF and anti-FGF-2 + anti-PDGF embryos (A–H) studied at E10 and E11 (two days after injection). Development of the tunica media is illustrated via staining (red) of smooth muscle α-actin. E10 hearts (A–D). A sham heart has well-developed tunica media of arteries (about 600 μm distal to the coronary ostia). In contrast, hearts treated with anti-FGF-2 and anti-PDGF individually or in combination show delay in the progression of smooth muscle assembly at comparable distances to coronary ostia. The arteries from these treated embryos have poorly-developed tunica media and usually larger diameters. E11 hearts. Sections from E11 hearts were obtained about 1.2-1.3 mm from the aortic root (EH). Note that unlike the arteries/arterioles in Sham hearts, those from the treatment groups tend to have a thinner media, i.e. fewer layers of smooth muscle and often incomplete muscle investment. Magnification bars = 50 μm.
VEGF Inhibition after Ostial Formation Limits Coronary Artery Assembly
Having previously documented that aflibercept prevents formation of the coronary artery stems,9 we injected this inhibitor at E9 and E10 i.e., after the formation of the coronary stems, to determine the role of VEGF’s in the progression of coronary artery and arteriolar growth. VEGF-Trap limited smooth muscle assembly of arteries and arterioles and the distance of muscularization of these vessels from the aortic root (data not shown). Thus, VEGFs 1) are required for the formation of both the coronary ostia and stems9 and, 2) influence the development of the tunica media of the coronary arteries.
Phenotypes induced by Anti FGF-2 and Anti-PDGF
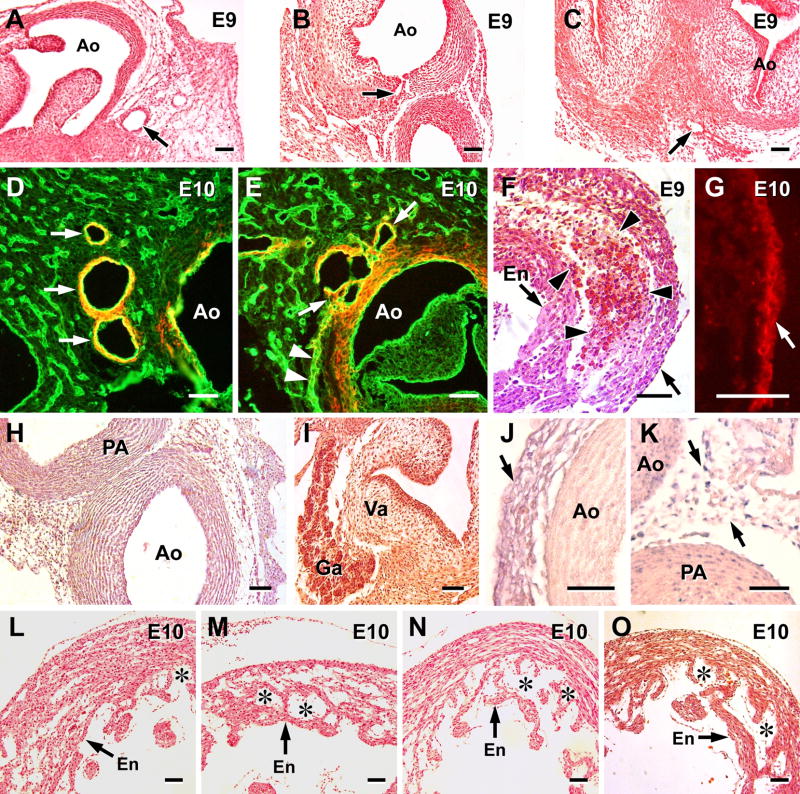
The compact region of the left ventricle is thinner in the hearts treated with the antibodies, compared to shams (Figure 6, L–O). The trabecular spongy component of the ventricular wall also tended to be more dominant, most notably when PDGF and FGF-2 were inhibited prior to E9 (Figure 6). The persistence of a sinusoidal system may be a compensation for an absence of or a limited coronary circulation. Other anomalies observed in some of the hearts of anti-FGF-2 and anti-PDGF treated embryos included 1) narrow coronary ostia; 2) multiple channels emerging from a coronary ostia, and 3) massive accumulations of blood cells either in the subepicardium or in the middle of the ventricular septum (Figure 6). These accumulations were previously documented in embryos treated with VEGF-trap and found to be derivatives of the proepicardium.9
Figure 6.
Coronary and cardiac phenotypes in anti-FGF-2 and anti-PDGF treated embryos. Embryonic day (E) is indicated. Magnification bar = 50 μm. Ao, aorta; PA, pulmonary artery; Va, valve; Ga, ganglion; en, endocardium; *, sinusoidal region of ventricle. A–C: main coronary arteries in E9 embryos (arrows) stained with H and E. In contrast to a normal coronary artery in a sham embryo (A), anomalies that may occur include: a narrow ostium, e.g., in an anti-PDGF embryo (B) and a main narrow coronary artery with little smooth muscle in anti-PDGF + anti-FGF-2 treated embryo (C). Sham (D) and anti-PDGF treated (E) hearts were double-stained for smooth muscle α-actin (orange-yellow) and QH1 (green) to show endothelium. Branching of a main coronary artery occurs downstream and lateral to the aorta in the sham (arrows). One anomaly occasionally encountered in the treated groups is multiple channels emerging from the aorta (arrows) when only one main coronary artery is present. Treatment does not affect the capillary plexus seen at the root of the aorta (arrow heads) Transmural erythrocyte clusters in the interventricular septum of an E9 anti-PDGF-treated embryo (F). Such phenotypes are often noted in all of the treated groups and most often are seen in the subepicardium. Cell types at the base of the aorta (G–K) were not altered by the treatment protocols: 1) epicardial cells remain cytokeratin positive (red cells at arrow) in this anti-FGF-2 treated embryo (G); 2) a rich network of cells surrounds the aorta and pulmonary artery in an anti-FGF-2 embryo (H); 3) ganglion cells (Ga) are seen near the mitral valve (Va) in an anti-PDGF embryo, and 4) the epicardial (arrow) and subepicardial cells at the aortic base in an anti-FGF-2-PDGF treated embryo retain their VEGF expression (J). Many VEGF positive cells (arrows) are seen within the groove between the aorta and pulmonary artery in an anti-PDGF embryo (K). Treatment with anti-FGF-2 (M), anti-PDGF (N) or a combination of the two (O) leads to an exaggerated spongy layer (*) and a thinner compact layer compared to shams (L).
We did not find evidence for cellular alterations at the base of the aorta which might be related to limited ostial formation. Anti-PDGF and –FGF-2 treatments before ostial formation did not appear to limit 1) epicardial cell VEGF or cytokeratin immunoreactivity, and 2) presence of subepicardial cell clusters, parasympathetic ganglia and blood islands at the aorta (Figure 6). The overall growth of embryos treated with the inhibitors was not markedly affected as HH stages were no more than one stage less than shams.
Discussion
Our previous in vivo studies on quail hearts revealed that VEGFR-2 and VEGFR-3 RNA transcripts are in high density at the sites of coronary artery stems6 and that the formation of coronary ostia and stems is dependent on VEGFs, especially VEGF-B.9 The current study has documented three major findings. First, administration of neutralizing antibodies to FGF-2 or PDGF frequently results in coronary anomalies including only one or no coronary artery stem, narrow coronary ostia, and multiple channels in the aorta contributing to a main coronary artery. We also provide both in vivo and in vitro evidence that these growth factors facilitate tubulogenesis during its early period (E6–E8). Second, our data support the conclusion that FGF-2 and PDGF each contribute to smooth muscle cell recruitment and the development of the tunica media. Neutralizing antibodies to these growth factors limit both distance from the ostia that smooth muscle cells are recruited and the thickness of the media. Third, VEGF, previously shown to be essential for coronary ostial formation9, also facilitates smooth muscle recruitment after the coronary artery stems are formed, and continues to be a major regulator of microvessel (capillary) growth.
Vasculogenesis/Angiogenesis
As previously documented, tubulogenesis in quail hearts is first evident at E6.9, 22 Endothelial precursor cells are responsive to growth factors at this stage as shown by our previous studies5, 6 and by the current data. Our in vitro and in vivo data are in accord. The vasculogenic/angiogenic effect of PDGF-BB in heart explants revealed in this study may be due to the ability of PDGF-BB to induce marked increases in VEGF as demonstrated in rabbit hearts18 and retinal pigment cells.26 An earlier in vitro study showed that the PDGFR-β receptor is specific for endothelial tube formation and is responsive to PDGF-BB.13 Our in vitro data demonstrate that PDGF-BB enhances tube formation, but to a lesser degree than FGF-2 and that the effects of the two are additive only at sub-optimal doses. The in vivo experiments support the hypothesis that the effects of FGF-2 and PDGF on tubulogenesis are temporally dependent, as evidenced by the data that indicate their influence wanes over time. However, when both are inhibited after a coronary circulation is established (E9), vascular volume is decreased. Thus, the forces associated with flow may influence the responses of endothelial cells to the two growth factors. In contrast, VEGF-Trap, shown previously to attenuate tubulogenesis when administered at E6 or E79, continued to be effective even when injected at E9 or E10.
Formation of the Coronary Ostia and Stems
The original observation in quail embryos describing the ingrowth of endothelial cells into the aortic wall10 was then detailed in the chick.12 The latter study described multiple penetrating channels with 2 channels becoming dominant to form the coronary ostia and stems at HH32. The process involves fusion of strands of the peritruncal ring of endothelial tubes and a disappearance of the tubes facing the non-coronary sinus.11 As documented in this study and previously,27, 28 failure of ostial formation precludes myocyte recruitment of endothelial channels. Our data support a role for both FGF-2 and PDGF in ostial formation since neutralizing antibodies frequently either limited or prevented coronary artery stem formation. A recent study also reported abnormalities in the number of coronary ostia formed and thinner media of coronary arteries in PDGF-B knockout mouse embryos.29 As seen in Figure 3, the temporal effect was most evident when anti-FGF-2 or anti-PDGF was injected at E6 or E7; 20% or less of these embryos had 2 coronary artery stems. This is not surprising since penetration of the aorta by endothelial cells occurs in quail beginning at E7 or E8.11 The limitations in stem formation seen at E9 when anti-FGF-2 was administered at E7 are similar to those we previously reported.9
Although both PDGF-BB30 and FGF-231 effect VEGF secretion in endothelial cells, the mechanisms underlying their role in coronary ostial formation is still unclear. We did not detect changes in VEGF immuno-reactivity of epicardial/subepicardial cells, nor a loss of the capillary plexus at the aortic root. Furthermore, the presence of parasympathetic ganglia, which are considered essential for the development of coronary arteries32 was also verified in the treated embryos. Thus, the anatomical environment at the aortic root does not appear to underlie failure of ostial formation in PDGF and FGF-2 inhibited embryos.
Formation of the Coronary Arterial Tree
The formation of the two vascular channels (coronary ostia) is immediately followed by mesenchymal cell recruitment to the sites of the forming coronary ostia and their differentiation in situ,33 an event that coincides with the abrupt onset of coronary flow and shear stress. Since shear stress differentially modulates FGF-2 and PDGF-BB in vascular cells,34 the effect of these growth factors may change when flow is initiated and smooth muscle cells give rise to coronary artery stems.11, 22 A recent study in embryonic mice has suggested that the effect of FGF-2 on coronary artery formation is via a Wnt-FGF-2 pathway.35 We previously documented a role for FGF-2 in coronary arteriolar formation and growth during early post-natal development.4 That study also revealed that VEGF-A and FGF-2 together influence the hierarchy of the coronary arterial tree. The current finding that VEGF influences arterial/arteriolar growth, as well as capillary growth, coupled with our previous documentation that VEGF is required for coronary ostial and stem formation, supports the conclusion that this growth factor family plays a key role in events that span a broad period and several events of coronary vascularization.
Conclusions and Limitations of Study
The factors that orchestrate the events required to form the hierarchical coronary arterial tree are numerous and involve complex interactions and signaling events. This study focused on growth factor regulation of ostial and coronary artery formation. Our main goal was to delineate the later stages of the cascade of events leading to the formation of the coronary arterial vasculature. We recognize that other molecules may also play key roles in this process. However, their delineation is beyond the scope of this study.
Acknowledgments
Sources of Funding
This work was supported by NIH grant: 5 R01 HL 075446.
References
- 1.Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- 2.Olivey HE, Compton LA, Barnett JV. Coronary vessel development: the epicardium delivers. Trends Cardiovasc Med. 2004;14:247–251. doi: 10.1016/j.tcm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Tomanek RJ, Haung L, Suvarna PR, O’Brien LC, Ratajska A, Sandra A. Coronary vascularization during development in the rat and its relationship to basic fibroblast growth factor. Cardiovasc Res. 1996;31 Spec No:E116–126. [PubMed] [Google Scholar]
- 4.Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res. 2001;88:1135–1141. doi: 10.1161/hh1101.091191. [DOI] [PubMed] [Google Scholar]
- 5.Tomanek RJ, Zheng W, Peters KG, Lin P, Holifield JS, Suvarna PR. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev Dyn. 2001;221:265–273. doi: 10.1002/dvdy.1137. [DOI] [PubMed] [Google Scholar]
- 6.Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn. 2002;225:233–240. doi: 10.1002/dvdy.10158. [DOI] [PubMed] [Google Scholar]
- 7.Tomanek RJ, Ratajska A, Kitten GT, Yue X, Sandra A. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn. 1999;215:54–61. doi: 10.1002/(SICI)1097-0177(199905)215:1<54::AID-DVDY6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res. 2006;98:947–953. doi: 10.1161/01.RES.0000216974.75994.da. [DOI] [PubMed] [Google Scholar]
- 10.Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, Peault BM, Huysmans HA. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol (Berl) 1989;180:437–441. doi: 10.1007/BF00305118. [DOI] [PubMed] [Google Scholar]
- 11.Ando K, Nakajima Y, Yamagishi T, Yamamoto S, Nakamura H. Development of proximal coronary arteries in quail embryonic heart: multiple capillaries penetrating the aortic sinus fuse to form main coronary trunk. Circ Res. 2004;94:346–352. doi: 10.1161/01.RES.0000112963.79064.09. [DOI] [PubMed] [Google Scholar]
- 12.Waldo KL, Willner W, Kirby ML. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat. 1990;188:109–120. doi: 10.1002/aja.1001880202. [DOI] [PubMed] [Google Scholar]
- 13.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 16.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Van Den Akker NM, Lie-Venema H, Maas S, Eralp I, DeRuiter MC, Poelmann RE, Gittenberger-De Groot AC. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Dev Dyn. 2005;233:1579–1588. doi: 10.1002/dvdy.20476. [DOI] [PubMed] [Google Scholar]
- 18.Affleck DG, Bull DA, Bailey SH, Albanil A, Connors R, Stringham JC, Karwande SV. PDGF(BB) increases myocardial production of VEGF: shift in VEGF mRNA splice variants after direct injection of bFGF, PDGF(BB), and PDGF(AB) J Surg Res. 2002;107:203–209. doi: 10.1006/jsre.2002.6510. [DOI] [PubMed] [Google Scholar]
- 19.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Reidy A, Lindner V. Basic FGF and growth of arterial cells. Ann N Y Acad Sci. 1991;638:290–299. doi: 10.1111/j.1749-6632.1991.tb49039.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- 22.Tomanek RJ, Hansen HK, Dedkov EI. Vascular patterning of the quail coronary system during development. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:989–999. doi: 10.1002/ar.a.20365. [DOI] [PubMed] [Google Scholar]
- 23.Dedkov EI, Christensen LP, Weiss RM, Tomanek RJ. Reduction of heart rate by chronic beta1-adrenoceptor blockade promotes growth of arterioles and preserves coronary perfusion reserve in postinfarcted heart. Am J Physiol Heart Circ Physiol. 2005;288:H2684–2693. doi: 10.1152/ajpheart.01047.2004. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Pomares JM, Macias D, Garcia-Garrido L, Munoz-Chapuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Dev Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 25.Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn. 1999;216:28–36. doi: 10.1002/(SICI)1097-0177(199909)216:1<28::AID-DVDY5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Hollborn M, Bringmann A, Faude F, Wiedemann P, Kohen L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun. 2006;344:912–919. doi: 10.1016/j.bbrc.2006.03.185. [DOI] [PubMed] [Google Scholar]
- 27.Gittenberger-de Groot AC, Vrancken Peeters MP, Bergwerff M, Mentink MM, Poelmann RE. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- 28.Eralp I, Lie-Venema H, DeRuiter MC, van den Akker NM, Bogers AJ, Mentink MM, Poelmann RE, Gittenberger-de Groot AC. Coronary artery and orifice development is associated with proper timing of epicardial outgrowth and correlated fas ligand associated apoptosis patterns. Circ Res. 2005;96:526–534. doi: 10.1161/01.RES.0000158965.34647.4e. [DOI] [PubMed] [Google Scholar]
- 29.Van den Akker NM, Winkel LC, Nisancioglu MH, Maas S, Wisse LJ, Armulik A, Poelmann RE, Lie-Venema H, Betsholtz C, Gittenberger-de Groot AC. PDGF-B signaling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev Dyn. 2008;237:494–503. doi: 10.1002/dvdy.21436. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Huang HJ, Kazlauskas A, Cavenee WK. Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res. 1999;59:1464–1472. [PubMed] [Google Scholar]
- 31.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldo KL, Kumiski DH, Kirby ML. Association of the cardiac neural crest with development of the coronary arteries in the chick embryo. Anat Rec. 1994;239:315–331. doi: 10.1002/ar.1092390310. [DOI] [PubMed] [Google Scholar]
- 33.Ratajska A, Fiejka E. Prenatal development of coronary arteries in the rat: morphologic patterns. Anat Embryol (Berl) 1999;200:533–540. doi: 10.1007/s004290050301. [DOI] [PubMed] [Google Scholar]
- 34.Malek AM, Gibbons GH, Dzau VJ, Izumo S. Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest. 1993;92:2013–2021. doi: 10.1172/JCI116796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]