Abstract
Objective
This study assessed longitudinal changes in body composition, fat distribution and energy balance in perimenopausal women. We hypothesized that total fat and abdominal body fat would increase at menopause due to decreased energy expenditure (EE) and declining estrogen, respectively.
Design
Observational, longitudinal study with annual measurements for 4 years.
Subjects
Healthy women (103 Caucasian; 53 African-American), initially premenopausal. During follow-up, lack of menstruation for 1 year and follicle-stimulating hormone >30 mIU ml-1 defined a subject as postmenopausal.
Measurements
Fat and lean mass (dual-energy X-ray absorptiometry), visceral (VAT) and subcutaneous abdominal fat (SAT) (computed tomography), dietary intake (4-day food record), serum sex hormones and physical activity (tri-axial accelerometry). Twenty-four hour EE was measured by whole-room calorimeter in a subset of 34 women at baseline and at year 4.
Results
Body fat and weight increased significantly over time only in those women who became postmenopausal by year 4 (n=51). All women gained SAT over time; however, only those who became postmenopausal had a significant increase in VAT. The postmenopausal group also exhibited a significant decrease in serum estradiol. Physical activity decreased significantly 2 years before menopause and remained low. Dietary energy, protein, carbohydrate and fiber intake were significantly higher 3-4 years before the onset of menopause compared with menopause onset. Twenty-four hour EE and sleeping EE decreased significantly with age; however, the decrease in sleeping EE was 1.5-fold greater in women who became postmenopausal compared with premenopausal controls (-7.9 vs -5.3%). Fat oxidation decreased by 32% in women who became postmenopausal (P<0.05), but did not change in those who remained premenopausal.
Conclusion
Middle-aged women gained SAT with age, whereas menopause per se was associated with an increase in total body fat and VAT. Menopause onset is associated with decreased EE and fat oxidation that can predispose to obesity if lifestyle changes are not made.
Keywords: menopause, intra-abdominal fat, perimenopause, estrogen, physical activity
Introduction
The prevalence of obesity (body mass index >30 kg m-2) is typically higher in females than males globally,1 and is consistently higher in US national surveys.2 While the reasons for this sex difference in obesity are not clear, fluctuations in female sex hormones at menarche, pregnancy and menopause may play a role. Since women in developed countries live approximately one-third of their lives after menopause, and 68% of US women in the 40- to 59-year age group are overweight or obese,2 understanding factors that influence body fat and its distribution in relation to the menopause transition is of critical importance.
Previous research is inconclusive as to whether mid-life body weight gain in women is related to aging per se or hormonal changes at menopause. In a longitudinal study of middle-aged women, Wing et al.3 found there was no difference in weight gain of women who remained premenopausal compared with those who became postmenopausal over follow-up. Similarly, the Study of Women’s Health Across the Nation (SWAN), a survey of ~13 000 multi-ethnic women in the Unite States, reported that body mass index in women who had undergone natural menopause was not significantly different from premenopausal women, although body mass index was higher in women who had experienced surgical menopause.4 A more recent report from SWAN,5 however, indicated that the change in circulating follicle-stimulating hormone (FSH) levels was positively correlated with the change in fat mass over 6 years of follow-up in 543 middle-aged Caucasian and African-American women, and concluded that ovarian aging does play a role in body composition changes at mid-life. Epidemiological studies of postmenopausal hormone replacement therapy (HRT) also suggest that estrogen deficiency is associated with weight gain, whereas exogenous estrogen replacement is associated with weight loss (or less weight gain).6
Data are more consistent in suggesting that estrogens play an important role in regulating body fat distribution in women, although there have been few longitudinal studies of this question. Postmenopausal women who take exogenous estrogens have significantly lower waist-to-hip ratio6 and less visceral adipose tissue (VAT)7 compared with estrogen non-users. Cross-sectional data also suggest that postmenopausal women have higher total and abdominal fat mass8 and lower lean body mass9 than premenopausal women, although it is difficult to fully control for effects of aging in cross-sectional studies.
To our knowledge, there have been no longitudinal studies of the menopause transition using direct measures of both total and intra-abdominal fat and energy expenditure (EE). The purpose of this study was to examine changes in body composition and regional fat distribution in a biethnic cohort of middle-aged women, and to determine the impact of hormonal status, physical activity and diet on these factors. We hypothesized that menopause (that is, estrogen deficiency) would increase both total body fat and VAT due to decreases in EE and circulating estradiol, respectively.
Methods
Subjects
Subjects were recruited by print and radio advertisement and targeted mailings between 1997 and 1998. To be included, subjects had to be healthy, age 43 years or older, have had at least five menstrual periods in the 6 months prior to screening and have serum FSH <30 mIU ml-1. Women were ineligible if they were taking regular medication (including oral contraceptives or other hormones), were not having regular menstrual cycles or had clinically abnormal results on laboratory tests or physical examination. Fifty-five African-American and 103 Caucasian women were enrolled. The Louisiana State University Institutional Review Board approved the protocol and informed consent form and all subjects gave informed consent prior to participating in the study.
Design
The study was observational with all outcomes measured at baseline and annually for 4 years after study enrollment, with the exception of 24-h EE by indirect calorimetry. Because of the high subject burden and cost of 24-h indirect calorimetry, it was only performed at baseline and year 4 in a subset of women who agreed to participate and had become postmenopausal by year 4. Out of 54 women who completed the 24-h indirect calorimetry measures at baseline, 13 women had experienced menopause and had a repeat test at year 4.
Body composition and fat distribution
Height and weight were assessed in overnight fasted subjects wearing a hospital gown. Body composition (fat and lean mass) was determined by dual energy X-ray absorptiometry (Hologic QDR2000; Waltham, MA, USA). Lean mass as reported in this analysis does not include bone mass. Additionally, all subjects had an abdominal computed tomography scan at the level of the interspace between the fourth and fifth lumbar vertebrae (10-mm thick), as previously described,9 for determination of abdominal fat distribution (GE High Speed Advantage, GE Medical Systems, Milwaukee, WI, USA). Deep subcutaneous adipose tissue (SAT) was measured as the area between the clearly demarcated circumferential fascia superficialis and the abdominal muscle wall.10 Superficial SAT was measured as the area between the fascia superficialis and the skin. A single reader performed all image analysis and was blinded to the menopausal status of the subjects. The coefficient of variation for measures of VAT in our laboratory is 10.5% (repeat scans on the same subjects made 1-2 weeks apart).
Free-living EE
To obtain an idea of the volunteers’ habitual level of physical activity, free-living EE was determined by using a triaxial activity monitor (TriTrac R3D; Reining International Ltd., Madison, WI, USA) as previously described.11 Volunteers were instructed to wear the Tritrac on their waist belt for four consecutive days, including one weekend day, from the time they woke up until they went to bed.
Dietary intake and analysis
All subjects completed a 4-day food record following instruction from a dietitian. In most cases, this record was collected during the same 4 days that the subject was wearing the triaxial motion sensor. Intake data were analyzed using Moore’s Extended Nutrient database (MENu) (copyright Pennington Biomedical Research Foundation, 1998).
Metabolic chamber
Details of the metabolic chamber at the Pennington Center have been described previously.12 Briefly, a subset of 34 volunteers was tested at baseline (premenopausal status) and again after 4 years of follow-up (either postmenopausal or premenopausal status). Subjects entered the chamber before breakfast at 0900 hours on each test morning and left the chamber at 0730 hours the next day. They received three meals and two snacks at scheduled times. Treadmill walking at a speed of 2 mph for variable lengths of time was prescribed in order to achieve energy balance in the metabolic chamber. The fixed time period of walking on the treadmill (112±12 min) and energy intake were held constant during both the baseline and follow-up chamber test days. EE and substrate oxidation were calculated from oxygen consumption, CO2 production and urinary nitrogen excretion using the equations of Elia and Livesey.13 Energy intake requirements were determined using the assessment of free-living EE by Tritac for each woman, and the amount of treadmill time necessary to match EE with energy intake was calculated as previously described.12
Laboratory measurements
Total cholesterol, high-density lipoprotein cholesterol and triglyceride levels were measured on the Beckman Synchron CX5 autoanalyzer. The dextran sulphate precipitation method was used for high-density lipoprotein measurement. Low-density lipoprotein level was calculated using the Friedewald equation, assuming triglycerides within normal limits. Glucose was determined using the glucose oxidase method on a Beckman Synchron CX7 instrument (Beckman, Brea, CA, USA). Insulin concentrations were determined using a microparticle enzyme immunoassay on an Abbott IMx analyzer (Abbott Laboratories, Abbott Park, IL, USA). This assay has <1% cross-reactivity with proinsulin. Between-run coefficients of variation for all assays were <2.5% except insulin, which had a coefficient of variation of 6.6%. FSH and estradiol were measured on DPC 2000 using a solid-phase immunometric assay with chemiluminescent detection (Diagnostic Products Corporation, Los Angeles, CA, USA). Insulin sensitivity was estimated using the Quantitative Insulin Sensitivity Check Index (QUIKI) from fasting glucose and insulin as previously described.14
Data analysis
Data were analyzed using SAS version 8.2. Variables that were not normally distributed were log-transformed prior to analysis. The primary endpoint of the study was VAT. Key secondary outcomes included subcutaneous AT, total fat and lean mass, physical activity and energy and macronutrient intake.
A repeated-measure analysis of variance was used (PROC MIXED) to determine main effects of time and menopausal status on outcome variables and a post hoc test with Tukey-Kramer adjustment was used for multiple comparisons. For some analyses, the onset of menopause was marked as ‘0’; dependent variables were set at 100% at that time point and data points from before and after were referenced as a % of that value. In these analyses, ‘TIME 0’ (or menopause onset) refers to the data from the annual visit when a woman reported having at least 12 months without a menstrual period and at which she had an FSH concentration >30 mIU ml-1. Paired comparison tests between years before and after menopause and TIME 0 were adjusted using a Dunnett adjustment with TIME 0 as control point. A P-value less than 0.05 was considered significant.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research and that approval by the Louisiana State University Institutional Review Board for ethics in human research was obtained prior to starting the study.
Results
Characteristics of the subjects at enrollment have been described previously.15 Women were divided post hoc into three groups based on their menopausal status at year 4: (1) women who remained premenopausal throughout the study (PRE, n=34); (2) women who were classified as perimenopausal (that is, irregular cycles and <5 cycles in the 6 months prior to their visit) at year 4 (PERI, n=44) and (3) women who were classified as postmenopausal (that is, reported no menstrual cycles in the past year and FSH>30 mIU ml-1) at year 4 (POST, n=51). A significant main effect of time was observed for body weight (P<0.0001), which increased in all three groups over time but was significantly higher by post hoc analysis in year 4 vs baseline only in the POST group (Table 1). Significant main effects of time were also observed for total abdominal fat and all individual abdominal fat depots, which generally increased over time in all three groups. Of note, the increase in VAT from baseline to year 4 was statistically significant only in the POST group, whereas subcutaneous abdominal fat increased significantly over time in all three groups. There was no main effect of menopause group on any body composition variable, nor did we observe significant group*time interactions (Table 1).
Table 1.
Characteristics of the population by study time point and menopausal status at year 4
|
Pre |
Peri |
Post |
ANOVA P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year4 | Baseline | Year4 | Baseline | Year4 | Time | Meno | Meno*Time | |
| n | 34 | 34 | 44 | 44 | 51 | 51 | |||
| Age | 46.2±0.3 | 50.2±0.3 | 47.6±0.2 | 51.6±0.2 | 48.1±0.3 | 52.1±0.3 | |||
| Weight (kg) | 74.5±3.2 | 76.5±3.3 | 70.4±2.1 | 71.8±2.3 | 70.8±1.8* | 73.1±2.2* | <0.0001 | NS | NS |
| % Body fat | 39.6±1.5 | 39.7±1.6 | 39.6±1.1 | 38.9±1.3 | 40.6±1.1 | 41.2±1.3 | NS | NS | NS |
| Fat mass (kg) | 30.3±2.3 | 31.3±2.5 | 28.2±1.5 | 28.2±1.9 | 29.1±1.5* | 30.7±1.8* | NS | NS | NS |
| Lean mass (kg) | 40.5±1.0 | 41.39±0.9 | 38.6±0.7 | 39.3±0.7 | 38.1±0.6 | 38.6±0.6 | 0.01 | NS | NS |
| Total abdominal fat (cm2) | 404.2±31.7* | 472.1±36.0* | 407.4±25.5 | 465.9±38.1 | 421.4±22.8* | 467.2±27.5* | <0.0001 | NS | NS |
| Visceral fat (cm2) | 88.6±7.5 | 105.0±10.6 | 103.17±10.5 | 109.2±15.0 | 88.0±7.7* | 97.5±6.7* | 0.0002 | NS | NS |
| Subcutaneous fat (cm2) | 318.5±26.2* | 369.2±27.7* | 305.7±18.5* | 356.7±27.3* | 333.4±17.7* | 369.6±22.7* | <0.0001 | NS | NS |
| Deep subcutaneous fat (cm2) | 155.8±12.7* | 193.1±15.9* | 158.5±9.6* | 186.7±13.7* | 174.6±9.3* | 197.7±12.1* | <0.0001 | NS | NS |
| Superficial subcutaneous fat (cm2) | 155.3±14.3* | 185.2±16.1* | 141.4±10.3* | 169.9±15.5* | 152.2±9.5* | 171.8±12.5* | <0.0001 | NS | NS |
| FSH (mIU ml-1) | 8.57±2.6* | 34.2±6.1* | 13.20±1.8* | 50.8±5.6* | 29.3±4.2* | 62.8±5.0* | <0.0001 | <0.0001 | NS |
| Estradiol (pg ml-1) | 110.7±15.4 | 108.1±14.4 | 137.8±16.7 | 104.5±17.3 | 114.1±16.1* | 51.2±8.2* | 0.008 | 0.04 | NS |
Abbreviations: ANOVA, analysis of variance; FSH, follicle-stimulating hormone; NS, not significant. Values are mean±standard deviation.
Significant difference between baseline and year 4 within menopausal status (P<0.05 by post hoc Tukey’s test, adjusted for age).
Significant main effects of menopause group were, however, observed for both FSH and estradiol concentrations. Baseline FSH was lowest in the PRE group and highest in the POST group, whereas the PERI group had intermediate baseline FSH concentrations. All three groups had a significant increase in FSH concentration from baseline to year 4. With regard to estradiol, circulating concentrations remained similar from baseline to year 4 in both PRE and PERI groups, but declined significantly by year 4 in the POST group.
When data were analyzed by race, there were no statistically significant differences in the changes in body composition or hormone levels over time in African-American vs Caucasian women in the three menopausal groups (data not shown).
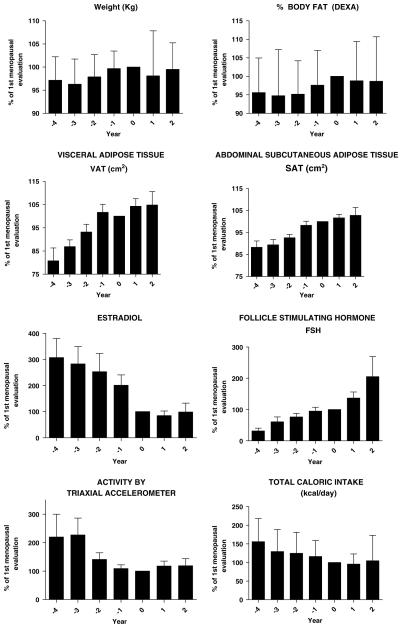
To further determine the impact of menopause on body composition, we analyzed data from only those women who had become menopausal during the course of the study (n=51) relative to the value for each variable at the time of onset of menopause. These data are expressed as a percentage of the value at the time of onset of menopause (‘year 0’) and are shown in Table 2 and Figure 1. There were no significant differences in body weight, fatness or lean mass in relation to menopause onset. VAT was significantly lower in both year -4 and year -3, and increased until the onset of menopause (T=0), at which time there was a plateau in the postmenopausal years +1 and +2. SAT (total, deep and superficial compartments) showed a more linear increase throughout the entire timeline in relation to menopause, and was significantly lower in years -4 through -2 relative to year 0. The increases in abdominal fat from years 4 to 1 - paralleled the decreases in serum estradiol and the increases in serum FSH over time (Figure 1).
Table 2.
Changes in body composition and plasma hormones in perimenopausal women
|
Menopause time |
|||||||
|---|---|---|---|---|---|---|---|
| -4 | -3 | -2 | -1 | 0 | +1 | +2 | |
| n | 17 | 30 | 42 | 48 | 51 | 30 | 20 |
| Weight | 97.2±5.1 | 96.3±5.4* | 97.9±4.8 | 99.7±3.8 | 100 | 98.1±9.8 | 99.5±5.7 |
| % Body fat | 97.9±5.6 | 97.6±8.1 | 96.9±5.9 | 97.8±6.7 | 100 | 98.8±7.1 | 97.9±7.7 |
| Fat mass (kg) | 95.6±9.4 | 94.8±12.5 | 95.2±9.0 | 97.6±9.4 | 100 | 98.8±10.6 | 98.7±12.0 |
| Lean mass (kg) | 99.2±3.8 | 98.6±4.4 | 100.2±4.3 | 101.0±4.4 | 100 | 100.4±3.9 | 101.2±4.6 |
| VAT (cm2) | 80.8±22.2* | 86.9±17.0* | 93.2±20.3 | 101.7±21.5 | 100 | 104.3±18.1 | 104.8±22.4 |
| SAT (cm2) | 88.3±11.6* | 89.1±12.6* | 92.6±9.5* | 98.3±11.8 | 100 | 101.7±8.9 | 102.8±13.9 |
| Deep SAT (cm2) | 88.2±13.9* | 87.7±13.9* | 91.7±12.0* | 99.3±12.1 | 100 | 103.2±9.6 | 105.3±13.8 |
| Superficial SAT (cm2) | 87.7±13.3* | 89.1±12.6* | 93.3±12.1* | 96.6±13.8 | 100 | 100.5±10.5 | 100.1±14.7 |
| FSH (mIU ml-1) | 31.5±38.5 | 60.8±85.8 | 90.9±123.0 | 100.2±88.7 | 100 | 133.9±102.5 | 333.6±619.2* |
| Estradiol (pg ml-1) | 307.0±264.4* | 282.8±289.8* | 245.1±331.5* | 190.7±202.6 | 100 | 82.3±64.0 | 98.5±95.8 |
Abbreviations: FSH, follicle-stimulating hormone; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. Data: Mean±s.d. for each year expressed as the percent of the values at year 0 (menopausal onset).
Significantly different from year 0 with adjusted P<0.05 (Dunnett’s adjusted).
Figure 1.
Longitudinal changes in body composition, sex hormones, EE and energy intake relative to onset of menopause (year 0) in 48 women. Statistical significance is indicated in Tables 2 and 3. EE, energy expenditure.
With regard to EE, we found a large and statistically significant decrease in activity EE in the perimenopausal period as measured over 4 days by accelerometer (Table 3). Overall, in women who transitioned to menopause during the study, activity counts dropped by half from 4 years prior to menopause to menopause onset. Activity EE was similar in years -4 and -3 at about 210% of the menopause-onset level, and had dropped by year -1 and remained at this lower level out through year +2.
Table 3.
Activity, diet and cardiovascular risk at the time of menopause (year 0, standardized to 100%) and at years -4 to +2 relative to menopause onset (shown as percent of year 0)
|
Value at menopause |
Menopause time |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.e.m. | -4 | -3 | -2 | -1 | 0 | +1 | +2 | |
| n | 48 | 16 | 25 | 39 | 40 | 48 | 25 | 17 | |
| Accelerometer activity EE | 388.6 | 45.5 | 219.7±300.4* | 227.1±264.1* | 141.1±134.4 | 108.8±79.1 | 100% | 118.1±82.6 | 118.9±79.3 |
| Intake | |||||||||
| Energy (kcal day-1) | 1455.0 | 70.1 | 156.0±62.1* | 129.6±58.9 | 124.9±56.2 | 116.2±43.0 | 100% | 96.0±26.9 | 104.6±68.4 |
| Protein (kcal day-1) | 246.3 | 10.2 | 156.4±75.7* | 127.3±64.0 | 126.5±65.8 | 117.4±44.6 | 100% | 104.2±32.0 | 93.7±52.5 |
| Fat (kcal day-1) | 485.5 | 25.6 | 133.1±57.1 | 121.8±50.7 | 133.7±75.3 | 127.4±56.7 | 100% | 114.3±56.2 | 144.2±109.4 |
| Carbohydrate (kcal day-1) | 713.2 | 33.8 | 193.61±82.1* | 148.7±84.0* | 130.6±70.3 | 118.3±55.5 | 100% | 91.0±29.1 | 91.6±60.4 |
| MUFA (g day-1) | 20.3 | 1.6 | 136.9±72.1 | 128.5±61.3 | 144.5±104.3 | 137.7±77.0 | 100% | 122.2±72.1 | 158.5±136.4 |
| PUFA (g day-1) | 11.1 | 0.8 | 174.5±109.9* | 139.5±103.0 | 129.8±75.9 | 123.0±57.3 | 100% | 103.6±53.4 | 121.1±78.6 |
| SFA (g day-1) | 18.1 | 1.3 | 117.8±54.9 | 115.4±43.1 | 134.3±81.5 | 126.2±58.9 | 100% | 119.4±59.5 | 150.3±131.4* |
| Calcium (mg day-1) | 687.8 | 45.3 | 123.1±50.7 | 126.2±53.0 | 121.3±62.1 | 125.3±66.0 | 100% | 113.6±52.8 | 99.5±82.1 |
| Cholesterol (mg day-1) | 209.5 | 18.8 | 105.8±68.8 | 110.3±66.9 | 157.5±121.4* | 122.7±91.7 | 100% | 146.3±82.8 | 164.6±125.5* |
| Fiber (g day-1) | 13.4 | 1.2 | 188.7±78.1* | 145.5±73.2* | 139.2±76.0* | 131.8±61.6 | 100% | 97.7±42.7 | 97.1±66.0 |
| Cardiovascular risk factors | |||||||||
| Glucose (IU l-1) | 89.8 | 2.5 | 117.0±12.2 | 107.1±6.8 | 130.0±22.2 | 126.8±20.5 | 100% | 102.5±1.7 | 145.9±45.1 |
| Insulin (mU ml-1) | 9.0 | 0.6 | 88.3±12.2 | 91.5±7.9 | 91.2±5.5 | 115.4±22.0 | 100% | 96.7±7.1 | 96.5±12.4 |
| Cholesterol (mg dl-1) | 209.1 | 5.1 | 94.0±3.0 | 94.9±2.7 | 98.1±2.1 | 100.4±2.0 | 100% | 104.1±2.5 | 109.3±3.0 |
| Triglycerides (mg dl-1) | 121.1 | 11.4 | 82.8±8.6 | 101.1±7.8 | 91.8±5.8 | 108.3±6.2 | 100% | 112.7±7.8 | 96.6±8.7 |
| HDL-cholesterol (mg dl-1) | 60.0 | 2.0 | 84.3±3.1 | 92.1±2.8 | 95.1±2.8 | 94.9±2.2 | 100% | 106.8±2.7 | 118.7±4.9 |
| LDL-cholesterol (mg dl-1) | 121.3 | 4.3 | 97.8±16.6 | 96.4±3.9 | 100.1±3.8 | 101.7±3.0 | 100% | 106.2±3.3 | 115.0±4.1 |
| Insulin sensitivity (QUIKI) | 0.4 | 0.01 | 103.5±3.0 | 102.8±1.7 | 100.8±1.5 | 100.2±1.4 | 100% | 101.7±1.3 | 102.1±2.8 |
Abbreviations: EE, energy expenditure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MUFA, monounsaturated fat; PUFA, polyunsaturated fat; QUIKI, quantitative insulin sensitivity check index; SFA, saturated fat. Data: Mean±s.d. for each year expressed as the percent of the values at year 0 (menopausal onset). Macronutrient intakes are adjusted for total energy intake.
Statistically significant compared to year 0 with adjusted P<0.05 (Dunnett’s adjusted). High values=insulin sensitive; low value=insulin resistant.
Total energy intake tended to decrease across the perimenopausal years and was significantly higher at year -4 compared with menopause onset (Table 3). After adjustment for changes in total energy intake, consumption of protein, polyunsaturated fat and dietary fiber declined over time and were relatively higher in the years preceding onset of menopause than those after menopause. Protein and polyunsaturated fat intake were significantly higher 4 years prior to menopause onset than in the year of onset, whereas fiber intake was significantly higher in years -4, -3 and -2 relative to menopause onset (year 0). In contrast, saturated fatty acid and cholesterol intakes were significantly higher 2 years after menopause onset compared with menopause onset and tended to increase over time.
Changes in 24-h EE and substrate oxidation were examined in more detail in a subset of women studied at baseline and after 4 years of follow-up with a whole-room calorimeter (Table 4). Of this subset, 17 had become postmenopausal by the year 4 follow-up and 17 remained premenopausal at follow-up. Although total daily EE and sleeping EE decreased significantly in both groups of women over time, the decrease was greater in the women who became postmenopausal compared with those who remained premenopausal (-9.3 vs -7% for 24-h EE and -7.9 vs -5.3% for sleeping EE), although this difference was not statistically significant. In addition, despite the fact that planned physical activity in the calorimeter was held constant between both tests, spontaneous physical activity (fidgeting or non-exercise activity thermogenesis, NEAT), declined by 30-40% from baseline to 4 years in both groups (Table 4).
Table 4.
Twenty-four hour EE by whole-room calorimetry at baseline and 4-year follow-up in women who became postmenopausal (n=17) and those who remained premenopausal (n=17)
|
Post-menopausal |
Pre-menopausal |
|||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | % Change | Baseline | Follow-up | % Change | |
| Body weight (kg) | 68.5±11.6 | 70.8±11.3 | +3.4% | 69.7±13.5 | 72.5±14.8** | |
| Fat mass (kg) | 27.1±8.9 | 28.3±8.7 | +4.4% | 28.2±10.6 | 29.4±11.5 | |
| Lean mass (kg) | 37.9±5.7 | 38.6±5.1 | +1.8% | 37.8±4.4 | 39.2±4.1** | |
| 24 h EE (kcal 24 h-1) | 2122.3±200.3 | 1924.9±200.3** | -9.3% | 2118.0±200.5 | 1967.9±200.5** | -7.0 |
| Sleeping EE (kcal 24 h-1) | 1398.0±145.5 | 1287.2±145.5** | -7.9% | 1403.6±141.2 | 1328.8±141.2** | -5.3 |
| Spontaneous physical activity (kcal 24 h-1) | 215.4±85.8 | 150.3±85.8** | -30.2% | 250.3±89.0 | 153.3±89.0** | -38.7 |
| 24-h RQ | 0.86±0.04 | 0.87±0.04 | 1.1% | 0.86±0.03 | 0.85±0.03 | -1.1 |
| Fat oxidation (g 24 h-1) | 79.4±33.5 | 53.6±33.5* | -32.4% | 78.5±30.4 | 70.8±30.4 | -9.8 |
| CHO oxidation (g 24 h-1) | 261.5±69.4 | 246.9±69.4 | -5.5% | 269.6±64.6 | 228.0±64.6 | -15.4 |
| Protein oxidation (g 24 h-1) | 62.2±21.1 | 88.0±21.1** | 41.4% | 56.8±20.2 | 77.2±20.2* | 35.9 |
Abbreviation: EE, energy expenditure.
P<0.05
P<0.01 vs baseline within group. Calorimetry data are adjusted for lean body mass.
We also observed a shift in substrate oxidation, with a significant drop in 24-h fat oxidation (~26 g 24 h-1) at year 4 in women who became postmenopausal, whereas women who remained premenopausal did not have a significant change in fat oxidation (Table 4). Both groups experienced a significant increase in protein oxidation over time, whereas carbohydrate oxidation was not significantly impacted.
Discussion
The present longitudinal study extends findings from previous cross-sectional studies demonstrating that abdominal fat increases over time in women during the perimenopausal years. While increases in SAT occurred in all women regardless of their menopausal status at year 4, significant increases in total body fat and VAT only occurred in those women who became postmenopausal during follow-up. An important finding is that VAT increased prior to the onset of menopause and then remained relatively stable in the first 1-2 years following menopause. Thus, the commonly held notion that weight and abdominal fat gains occur after the onset of menopause must be re-examined. Our results also indicated that 24-h EE and physical activity EE both decline over time in mid-life women; however, the drop in sleeping EE is 1.5-fold greater over time in women who transition to menopause compared with their peers who remain premenopausal. Furthermore, only those women who became postmenopausal during follow-up showed significant decline over time in 24-h fat oxidation, which may predispose them to gain excess body fat.
We observed that VAT increased significantly from 3-4 years prior to menopause compared with menopause onset, whereas circulating estradiol decreased and serum FSH increased significantly during the same time frame. These findings are consistent with the hypothesis that female fat distribution is influenced by sex hormone concentrations and are congruent with the recent epidemiological report by Sowers et al.5 indicating that waist circumference increases with increasing FSH at menopause.
Changes in body fat distribution with declining estrogen level may be due to alterations in adipose tissue metabolism. Several studies have shown that estrogen influences adipose tissue lipoprotein lipase activity and lipolysis. For example, Rebuffe-Scrive et al.16 observed that femoral adipocytes from premenopausal women have higher lipoprotein lipase activity and lower lipolytic responsiveness (promoting fat storage) compared to abdominal adipocytes, while this regional difference was not seen in postmenopausal, estrogen-deficient women. The loss of the relatively higher lipolytic rate in abdominal adipocytes after menopause may predispose estrogen-deficient women to gain fat in this depot. The effects of exogenous estrogens on adipocyte metabolism are somewhat more contradictory. A direct effect of an estradiol transdermal patch in reducing subcutaneous adipose tissue lipoprotein lipase activity in the gluteal fat depot has been reported,17 suggesting that higher levels of circulating estrogen might actually reduce fat storage in this depot, in contrast with the data of Rebuffe-Scrive et al. Estradiol administration had no effect on stimulated lipolysis in gluteal and abdominal fat depots in male-to-female transsexuals18 or in femoral adipocytes from postmenopausal women.19 However, Pedersen et al.20 observed that estradiol administration attenuates lipolytic response in subcutaneous abdominal adipocytes, but not in adipocytes isolated from the intra-abdominal fat depot. This study suggests that higher circulating estrogen levels maintain typical premenopausal fat distribution by causing preferential fat storage in subcutaneous vs intra-abdominal fat depots. After menopause, when estrogen declines, this preferential storage in subcutaneous tissues disappears and, based on the results of Rebuffe-Scrive, there may conversely be preferential storage in abdominal adipose, consistent with the results of the present study.
Our study also showed that free-living EE, as well as 24 h EE and sleeping EE with a whole-room calorimeter, declined over time in mid-life women. In women studied with the calorimeter, it was observed that the decrease in sleeping EE was 1.5-fold greater in those women who became postmenopausal compared with women who remained premenopausal at follow-up. Spontaneous physical activity EE in the whole-room calorimeter also dropped by more than 30% over time in both groups of women. Although we had hypothesized that EE would decrease with menopause, we had expected this might be due to the previously reported decrease in lean body mass at menopause,9 which, somewhat surprisingly, we did not observe in the present study. Nonetheless, the observed reduction in 24-h EE of ~200 kcal day-1 is certainly of a magnitude that could cause significant weight gain and obesity over time. One possible explanation for the observed decline in EE after menopause is the loss of the typical luteal phase increase in EE (~100 kcal day-1) that occurs in women with normal menstrual cycles.21 In our study, the decline in EE appeared to be offset by a reduction in energy intake in our population, thus mean body weight changed minimally by the end of the study. If, however, perimenopausal and postmenopausal women do not voluntarily decrease energy intake, the decline in EE with menopause is enough to result in significant weight gain over time.
In addition to the changes in EE, we also observed changes in 24-h substrate oxidation, with a significant decline in fat oxidation that appeared to be due to menopause per se as this change was not observed in women who remained premenopausal at follow-up. Both groups of women experienced significant increase in protein oxidation over time. A limitation of these results was the small number of subjects available for follow-up measures of 24-h EE; nonetheless, our findings are suggestive that estrogen deficiency influences metabolism in a manner that would favor overall energy and fat storage.
The observed decline in free-living activity EE (Tritrac) coinciding with the decline in serum estradiol and the onset of menopause is consistent with the literature in rodents. Female estrogen-receptor-knockout mice have been shown to exhibit decreased spontaneous physical activity relative to wild-type females, an effect independent of estrogenic effects on energy intake and only seen in estrogen-receptor-α knockouts and not estrogen-receptor-β knockouts.22 Although a reduction in activity EE would primarily impact overall body weight and fatness, previous studies have identified physical inactivity as a major determinant of abdominal fat deposition as well.23 Thus, it is possible that the observed changes in abdominal fat distribution over time were due in part to the decrease in physical activity as well as the change in circulating sex hormones, although our study did not directly test this hypothesis.
In addition to the changes in EE, we also observed changes in diet composition in relation to the onset of menopause. There is a large body of literature on the role of sex steroids in regulating macronutrient preference in both humans and other mammals (reviewed in Geiselman and Smith24). Previously published data suggest that estrogen deficiency in female rodents results in hyperphagia with a macronutrient-specific increase in dietary fat intake.25 In the present study, although we did not observe significant longitudinal change in total fat intake across the perimenopausal years, we did observe that saturated fat and cholesterol intake increased significantly in the years post-menopause. Importantly, we also observed a decline in relation to menopause onset in several nutrients that are important in satiety and body weight regulation, including protein and fiber. We previously reported baseline data from this study population indicating ethnic differences in protein and fiber intake that relate to body composition, and demonstrating that fiber is the strongest independent dietary predictor of body fatness in the cross-sectional analysis.26 Thus, the observed changes in dietary composition in the present study could indicate a risk for positive energy balance and weight gain over time.
Although increased VAT and decreased physical activity are well-known risk factors for metabolic and cardiovascular disease, the observed changes these variables in our population were not accompanied by changes in health risk factors such as blood lipids, glucose or insulin. Possible reasons for this finding include the relatively small sample size, which might preclude the detection of small changes, or that longer follow-up is needed before changes in fat distribution and EE to result in measurable changes in risk factors. It is also possible that other abdominal fat-associated risk factors not measured in the present study, such as low-density lipoprotein particle size and C-reactive protein,27 did change in our population.
The question of the effects of menopause on insulin sensitivity is an important and controversial. An extensive and conflicting literature exists on the effects of exogenous estrogens and progestins on insulin sensitivity in postmenopausal women, with beneficial, neutral or adverse effects observed with oral HRT.28-30 Transdermal 17β-estradiol, on the other hand, appears to have a neutral or positive effect.30-32 A recent meta-analysis of 107 clinical trials found that HRT resulted in 13% improvement in insulin sensitivity as measured by homeostasis model assessment (HOMA), and reduced the risk of new-onset diabetes in postmenopausal women.33 In addition to these studies of HRT, several previous studies have reported that estrogen deficiency at natural menopause has no effect on insulin resistance,34,35 which is consistent with our findings in the present study. One possible reason for the variability in findings related to female sex hormones and insulin sensitivity include the fact that visceral adiposity may mediate the impact of HRT on insulin sensitivity.7
A limitation of our study was that clinic visits occurred annually and thus, we typically did not have a study visit that was timed precisely 1 year post cessation of menses (that is, a ‘true’ year 0 or menopause onset). Given the standard definition of menopause of 12 months without a menstrual cycle, theoretically an individual’s year 0 visit could have occurred close to 2 years after cessation of menses rather than the 1 year assumed in our analysis. Regardless of these menopause-onset timing issues of plus or minus 9-12 months, our finding that visceral fat level changes occur prior to or at the onset of menopause rather than after menopause is still valid. An additional limitation is that the duration of follow-up post-menopause was relatively short (2 years) and the sample size was smaller during the postmenopausal period and the early premenopausal period (year -4). Thus, interpretation of changes in body composition and energy balance post-menopause should be made with caution. We are currently continuing follow-up of this cohort for an additional 3 years, which should provide considerably longer postmenopausal longitudinal data.
In summary, the present study suggests that menopausal transition years place women at high risk for abdominal fat gain. The increases in VAT are greatest up to the onset of menopause, at which point there is little further increase at least for the first several years after menopause. The reduction in both basal EE and physical activity EE at menopause, along with observed reductions in dietary protein and fiber intakes, may significantly increase risk postmenopausal women’s risk for weight gain. These data support the importance of focusing on the early perimenopausal years, with the goal of increasing physical activity and encouraging healthy dietary choices to prevent weight and visceral fat gain in menopausal transition.
Acknowledgements
We thank the study participants and their families for their support during this study. We also thank the staff at the Pennington Biomedical Research Center clinic and the inpatient unit for assistance with all aspects of the study, Mr Tuong Nguyen, BSE, for expert maintenance of the metabolic chambers and the case managers who helped retain so many participants in the study. This study was funded by the National Institutes of Health (DK 2 R01 DK050736).
Footnotes
All authors do not have conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this work.
References
- 1.WHO . Report of a WHO Consultation. World Health Organization; Geneva: 2000. Obesity: preventing and managing the global epidemic. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 4.Matthews KA, Abrams B, Crawford S, Miles T, Neer R, Powell LH, et al. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord. 2001;25:863–873. doi: 10.1038/sj.ijo.0801618. [DOI] [PubMed] [Google Scholar]
- 5.Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SW, Waclawiw MA, James MK, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. J Clin Endocrinol Metab. 1997;82:1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 7.Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res. 2002;10:424–431. doi: 10.1038/oby.2002.59. [DOI] [PubMed] [Google Scholar]
- 8.Kanaley JA, Sames C, Swisher L, Swick LL, Ploutz-Snyder CM, Steppan KS, et al. Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age and menopausal status. Metabolism. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 9.Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y. Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas. 2002;42:301–306. doi: 10.1016/s0378-5122(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 10.Smith SR, Lovejoy JC, Greenway F, Ryan D, Dejonge L, De La Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 11.Westerterp KR. Physical activity assessment with accelerometers. Int J Obes Relat Metab Disord. 1999;23(Suppl 3):S45–S49. doi: 10.1038/sj.ijo.0800883. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 13.Elia S, Livesey G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. In: Simopoulus AP, editor. Metabolic Control of Eating, Energy Expenditure and the Bioenergetics of Obesity World Review on Nutrition and Diet. Karger; Basel: 1992. pp. 68–131. [DOI] [PubMed] [Google Scholar]
- 14.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 15.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: the Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 16.Rebuffe-Scrive M, Eldh J, Hafstrom LO, Bjorntorp P. Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metabolism. 1986;35:792–797. doi: 10.1016/0026-0495(86)90217-9. [DOI] [PubMed] [Google Scholar]
- 17.Price TM, O’Brien SN, Welter BH, George R, Anandjiwala J, Kilgore M. Estrogen regulation of adipose tissue lipoprotein lipase—possible mechanism of body fat distribution. Am J Obstet Gynecol. 1998;178:101–107. doi: 10.1016/s0002-9378(98)70634-9. [DOI] [PubMed] [Google Scholar]
- 18.Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. 1999;48:1371–1377. doi: 10.1016/s0026-0495(99)90146-4. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg UB, Crona N, Silfverstolpe G, Bjorntorp P, Rebuffe-Scrive M. Regional adipose tissue metabolism in postmenopausal women after treatment with exogenous sex steroids. Horm Metab Res. 1990;22:345–351. doi: 10.1055/s-2007-1004917. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Gallagher D, Poehlman ET, Wolper C, Nonas K, Nelson D, et al. Menopausal changes in body composition and energy expenditure. Exp Gerontol. 1994;29:377–389. doi: 10.1016/0531-5565(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S, Chan J, Gustafsson J-A, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 23.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 24.Geiselman PJ, Smith SR. Estrogen’s role in the regulation of appetite and body fat. In: Kohlstadt I, editor. Scientific Evidence for Musculoskeletal, Bariatric, and Sports Nutrition. CRC Press, an imprint of Taylor and Francis Group, LLC; Boca Raton, London, and New York: 2006. pp. 231–251. [Google Scholar]
- 25.Geiselman PJ, Martin JR, Vanderweele DA, Novin D. Dietary self-selection in cycling and neonatally ovariectomized rats. Appetite. 1981;2:87–101. doi: 10.1016/s0195-6663(81)80002-5. [DOI] [PubMed] [Google Scholar]
- 26.Lovejoy JC, Champagne CM, Smith SR, de Jonge L, Xie H. Ethnic differences in dietary intakes, physical activity, and energy expenditure in middle-aged, premenopausal women: the Healthy Transitions Study. Am J Clin Nutr. 2001;74:90–95. doi: 10.1093/ajcn/74.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Despres JP. The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients’ risk. Obes Res. 1998;6(Suppl 1):8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 28.Ryan AS, Nicklas BJ, Berman DM. Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care. 2002;25:127–133. doi: 10.2337/diacare.25.1.127. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox JG, Hwang J, Hodis HN, Sevanian A, Stanczyk FZ, Lobo RA. Cardioprotective effects of individual conjugate equine estrogens through their possible modulation of insulin resistance and oxidation of low-density lipoprotein. Fertil Steril. 1997;67:57–62. doi: 10.1016/s0015-0282(97)81856-0. [DOI] [PubMed] [Google Scholar]
- 30.Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC. Effects of oral and transdermal 17β-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion and elimination in postmenopausal women. Metabolism. 2000;49:742–747. doi: 10.1053/meta.2000.6238. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan AJ, Ho KKY. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1783–1788. doi: 10.1210/jcem.80.6.7775623. [DOI] [PubMed] [Google Scholar]
- 32.Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low doses of transdermal 17β-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocinol Metab. 1992;74:1396–1400. doi: 10.1210/jcem.74.6.1317387. [DOI] [PubMed] [Google Scholar]
- 33.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 34.Wing RR, Matthews KA, Kuller LH, Smith D, Becker D, Plantinga PL, et al. Environmental and familial contributions to insulin levels and change in insulin levels in middle-aged women. JAMA. 1992;268:1890–1895. [PubMed] [Google Scholar]
- 35.Toth MJ, Sites CK, Eltabbakh GH, Poehlman ET. Effect of menopausal status on insulin stimulated glucose disposal. Diabetes Care. 2000;23:801–806. doi: 10.2337/diacare.23.6.801. [DOI] [PubMed] [Google Scholar]