Abstract
objective
Nonalcoholic fatty liver disease (NAFLD) and its association with insulin resistance are increasingly recognized as major health burdens. The main objectives of this study were to assess the relation between liver lipid content and serum lipids, markers of liver function and inflammation in healthy overweight subjects, and to determine whether caloric restriction (CR) (which improves insulin resistance) reduces liver lipids in association with these same measures.
Methods and Procedures
Forty-six white and black overweight men and women (BMI = 24.7-31.3 kg/m2) were randomized to “control (CO)” = 100% energy requirements; “CR” = 25%; “caloric restriction and increased structured exercise (CR+EX)”= 12.5% CR + 12.5% increase in energy expenditure through exercise; or “low-calorie diet (LCD)” = 15% weight loss by liquid diet followed by weight-maintenance, for 6 months. Liver lipid content was assessed by magnetic resonance spectroscopy (MRS) and computed tomography (CT). Lipid concentrations, markers of liver function (alanine aminotransferase (ALT), alkaline phosphatase (ALK)), and whole-body inflammation (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP)) were measured in fasting blood.
Results
At baseline, increased liver lipid content (by MRS) correlated (P < 0.05) with elevated fasting triglyceride (r = 0.52), ALT (r = 0.42), and hsCRP (r = 0.33) concentrations after adjusting for sex, race, and alcohol consumption. With CR, liver lipid content was significantly lowered by CR, CR+EX, and LCD (detected by MRS only). The reduction in liver lipid content, however, was not significantly correlated with the reduction in triglycerides (r = 0.26; P = 0.11) or with the changes in ALT, high-density lipoprotein (HDL)-cholesterol, or markers of whole-body inflammation.
Discussion
CR may be beneficial for reducing liver lipid and lowering triglycerides in overweight subjects without known NAFLD.
INTRODUCTION
Fatty liver disease that develops in the absence of alcohol abuse is becoming increasingly recognized as a major health burden (1). This disease, termed nonalcoholic fatty liver disease (NAFLD), comprises an histological spectrum that ranges from simple hepatic steatosis (fatty infiltration of liver) to steatohepatitis (fatty liver accompanied by inflammation) and end-stage liver disease (2,3). Recently, it has been suggested that NAFLD is more common than originally expected, and that it may be present even when serum liver enzymes are not elevated (4,5). Most recent estimates suggest that the prevalence is ~20-33% in adults living in the United States and other Western Countries (1,4,5), with a higher prevalence in Hispanics and lower prevalence in blacks than whites (4).
Some degree of hepatic steatosis is present in obesity (6,7) and type 2 diabetes (7,8), and is associated with many features of the metabolic syndrome (9) including central fat accumulation (10), insulin resistance (11,12), hypertriglycemia (10-12), and reduced high-density lipoprotein (HDL)-cholesterol (10,11). Although the pathogenesis of NAFLD is not completely understood, a widely supported theory implicates a key role for insulin resistance leading to hepatic steatosis, and possibly also to steatohepatitis. A “second hit,” or additional oxidative injury may also be required to manifest the inflammatory component of steatohepatitis. Endotoxin-inducible cytokines, particularly tumor necrosis factor-α (TNF-α) which is commonly elevated in obesity, are also hypothesized to play a role (13,14). Treatments aiming at improving insulin sensitivity are commonly recommended (13,15), although only a few studies have documented improvements in hepatic steatosis. One study in obese women found that weight reduction improved insulin sensitivity and reduced hepatic lipid content measured noninvasively by magnetic resonance spectroscopy (MRS) (16), whereas another in obese men and women found reduced hepatic lipids in biopsy samples after a restricted diet and exercise regimen (17).
Although it is known that caloric restriction (CR), weight loss, and exercise training improve insulin sensitivity (18-21), the extent to which these interventions influence liver lipid accumulation has not been adequately explored. The purpose of this study was twofold: (i) to evaluate the relation between liver lipid content (measured by MRS and computed tomography (CT)), and serum lipids, markers of liver function and inflammation in healthy overweight insulin-sensitive subjects (baseline cross-sectional analysis); and (ii) to determine whether CR reduces liver lipid stores in association with serum lipids and with markers of liver function and whole-body inflammation (intervention analysis). A secondary purpose was to compare the use of MRS and CT for analyzing liver lipid content. We hypothesized that fat accumulation in the liver, as measured by both techniques, would be associated with elevated fasting triglyceride concentrations, lower HDL-cholesterol concentrations, and elevated markers of liver function and inflammation, all of which would be improved with CR regardless of the addition of exercise.
METHODS AND PROCEDURES
This evaluation was performed as part of a randomized clinical pilot trial designed to examine the effects of CR on surrogate markers for longevity in nonobese humans referred to as the CALERIE study (Comprehensive Assessment of Long term Effects of Reducing Intake of Energy; Trial registration: ClinicalTrials.gov Identifier: NCT00099151). Details of the study have been published elsewhere (22). In brief, we enrolled 48 overweight men and women (25 ≤ screening BMI < 30 kg/m2) with women between 25 and 45 years of age, men between 25 and 50 years of age with no personal history of type 2 diabetes, cardiovascular disease, high blood pressure (>160/90 mm Hg), liver disease or obesity, no psychiatric or eating disorders, alcoholism or substance abuse, and not taking any medication. Screening for liver disease included history, physical examination, liver function tests, including total bilirubin (normal range = 0.2-1.5 mg/dl), alanine aminotransferase (ALT; normal range = 10-60 IU/l), and alkaline phosphatase (ALK; normal range = 32-130 IU/l), and CT scan. Liver biopsies and serum markers of chronic hepatitis (B and C) were not performed. Screening for alcohol and substance abuse and dependence was by history (no more than three drinks/day was allowed) and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (23) that included evaluation for current substance abuse and dependence. This study was approved by our Institutional Review Board and all participants gave written informed consent before participation.
Intervention
After a 5-week baseline assessment, the 48 participants were randomized into one of four groups (n = 12 for each): 25% CR of baseline energy requirements; 12.5% CR + 12.5% increase in total energy expenditure through structured exercise (CR+EX); low-calorie diet (LCD) until 15% reduction in body weight was achieved followed by maintenance of new lower body weight (i.e., “weight clamped”); and control (CO) with weight-maintenance by a healthy diet (American Heart Association (AHA) Step 1 diet). The LCD group was included as a “weight-clamped control group” so all outcomes of interest (including liver lipid stores) could be compared with a group that would precisely maintain a new energy balance at an intake predicted to be 20-25% below their baseline energy requirements after rapid weight loss (15%). Subjects were stratified according to sex, race, and screening BMI before sequential randomization. Race was self-reported with 17 reporting black, 30 white, and 1 Asian ethnicity. Baseline energy requirements were determined from individual free-living energy expenditure, which was assessed over a 4-week period using doubly labeled water (22). However, for this analysis, the one Asian participant (assigned to CR+EX) was excluded and hence analysis by race could be performed with only black and white subjects. The two subjects who withdrew during the study (one in CO and another LCD) (22) were included in the baseline cross-sectional analysis but not in the intervention analysis.
Diets
For the first 12 weeks after randomization, the diet for all the four groups was provided by the Center’s Metabolic Kitchen (22). During weeks 13-22, participants self-selected their own diet on the basis of their individual calorie target, before returning to the in-feeding protocol for weeks 22-24. All diets (except LCD) were based on the AHA Step 1 recommendations (≤30% fat; ≤10% saturated fat). The very LCD was given in five shakes/day and provided 890 kcal/day (75 g protein, 110 g carbohydrate, 5 g fat: HealthOne, Health and Nutrition Technology, Carmel, CA). An additional 10-g corn oil bolus was added to one shake/day to stimulate cholecystokinin release and prevent gallstones.
Alcohol
Participants were advised to limit alcohol intake during the 5-week baseline assessment and the 6-month intervention to ≤2 standard servings/day (1 serving = 12 ounce beer, 5 ounce wine, or 1.5 ounce of distilled spirits). During the intervention phase, 1 serving of alcohol could be switched for 100 kilocalories of food as long as the participants did not exceed their target energy intake. Participants were asked to keep daily food and beverage (including alcoholic beverages) intake records that were used to assess compliance and assist with behavior modification. These records were later analyzed for alcohol intake (average g/week) over the baseline cross-sectional and intervention phases using Moore’s Extended Nutrient (MENu) Data Base (2000) (Pennington Biomedical Research Foundation, Baton Rouge, LA).
Exercise
CR+EX participants were required to increase their energy expenditure by 12.5% above baseline energy requirements by undergoing structured exercise (i.e., walking, running, or stationary cycling) 5 days/week according to an individualized exercise prescription (22,24). Participants were required to conduct three sessions/week under supervision. A wireless heart rate monitor (Polar S-610; Polar Beat, Port Washington, NY) was used to record exercise duration and average heart rate.
Metabolic tests
Subjects were tested during a 5-day admission to the clinical research center at baseline (month 0) and month 6 (22). Testing included dual-energy X-ray absorptiometry to assess total body composition (QDA 4500A; Hologics, Bedford, MA); MRS to assess liver lipid stores; multislice CT scanning of the abdominal region to assess total, visceral and subcutaneous adipose tissue and liver and spleen tissue densities for measuring liver lipid. A fasting blood sample was drawn for determination of serum lipids and markers of liver function (ALT and ALK) and whole-body inflammation (TNF-α, interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hsCRP)).
Analysis of lipid level in the liver
Intrahepatic lipid (IHL) was determined by proton MRS on a 1.5-T whole-body imaging and spectroscopy system (Picker Edge Eclipse; Picker International, Cleveland, OH) using a PRESS technique. Subjects were positioned on their belly and a single water-suppressed PRESS box (20 × 20 × 20 mm3-voxel, echo time = 40 ms, repetition time = 2 s) was collected using a commercially available 1H body coil in an area of the upper left lobe that was free from heavy vascularization (determined from T2-weighted axial images; echo time = 123 ms, repetition time = 40 ms, echo train length = 32). The peak positions and areas of interest (intrahepatic CH2 (representing IHL) and CH3 of fatty acid chains) were determined by time domain fitting using jMRUI (Java-based Magnetic Resonance User Interface), and referenced to an external oil phantom (peanut oil, one 7.5 × 7.5 × 10 mm3-voxel, repetition time = 2 s, echo time = 35 ms) (25). Duplicate measures of IHL using PRESS acquisitions at 1.5 T are highly correlated (r = 0.99) with a low test-retest coefficient of variation (8.5%) (5).
Liver lipid analysis with CT was performed using a General Electric Light Speed scanner (Milwaukee, WI). Subjects were positioned in the supine position with arms resting above the head. Eight axial images were acquired (120 kVp, 200 mAs, slice thickness = 10 mm, table spacing = 50 mm) following exhale in a single expiration breath hold. Liver and spleen analysis was obtained from the L4/L5 disc space scan. The CT tissue density, in Hounsfield units, was obtained from an average of three distinct 10 × 10 mm2 regions of interest in both the liver and spleen. The liver-to-spleen ratio was calculated as the index of lipid content, i.e., Hounsfield units of spleen was used as an internal normalizer (26).
Serum lipids and markers of liver function and whole-body inflammation
Serum lipids were analyzed according to standardized procedures (27). ALT and ALK were analyzed on a Beckman-Coulter Synchron CX7 (Beckman-Coulter, Brea, CA). TNF-α and IL-6 were analyzed on a Luminex Labmap 100 (Luminex, Austin, TX) using multiplex kits from Linco Research (St. Charles, MO). hsCRP was measured on a DPC 2000 automated immunoassay instrument using chemi-luminescent detection (Diagnostic Products; Siemens Company, St. Louis, MO).
Statistical analysis
Data in text and tables are presented as mean values ± standard deviations. The analysis was carried out using SAS Software Package Version 9.1.3 (SAS, Institute, Raleigh, NC). To assess the association among variables (baseline cross-sectional analysis), Pearson correlation coefficients were calculated at baseline between liver lipid and measurements of adiposity, serum lipids and markers of liver function and whole-body inflammation. In addition, partial correlations were calculated to determine association between the variables of interest adjusting for sex and race as well as for average alcohol consumption measured in g/week. The change from baseline to month 6 (intervention analysis) was assessed on key variables to investigate sex, race, and treatment effects using an analysis of covariance with sex, race, and treatment being the main effects and with all interactions up to the third order included in the model. The baseline variable was included as a covariate in the model. A Tukey adjustment was applied to all pair-wise comparisons to maintain an overall α of 0.05. Serum triglycerides were analyzed using log-transformed data. Alcohol consumption was used as a covariate in analysis of liver lipid. At baseline, simple predictive models with one and two variables were created for liver lipid using sex, race, and alcohol consumption in each model in addition to added variables.
RESULTS
At baseline, one individual (white men assigned to LCD) with a history of social drinking was found to have a severely elevated IHL (IHL = 22% of peanut oil phantom; range of other subjects = 0.3-7.2%) as well as a slightly elevated ALT (ALT = 69 IU/l; normal range ≤60 IU/l). This individual was excluded from all analysis because his IHL was a statistically significant outlier (internally studentized residual = 13.5 (>2.6 = statistical significance)) and confirmed by his low liver-to-spleen ratio (0.726). Thus, analysis for the study included 46 subjects for the baseline cross-sectional analysis (all treatment groups combined) and 44 subjects for the intervention analysis (CR = 12, CR+EX = 11, LCD = 10, CO = 11) owing to necessary exclusions and drop outs.
Cross-sectional analysis
Baseline characteristics
Characteristics of the 46 subjects by sex and race are described in Table 1. The baseline BMI for the group following the 5-week baseline assessment was 27.8 ± 1.7 kg/m2 (24.7-31.3 kg/m2). (Note: some initial BMIs are slightly out of the 25-30 kg/m2 range because of fluctuation during baseline assessment). Significant differences between men and women were found for body mass, percent body fat, central adipose stores, and lipoprotein profile. Markers of liver function were also higher in men than in women while the liver-to-spleen ratio tended to be lower (indicating higher liver lipid stores), particularly in white men (P = 0.01, sex × race effect). IHL also tended to be lower in black than in white participants.
Table 1. Baseline characteristics of subjects by sex and race.
| Men |
Women |
Significance (P) |
|||||
|---|---|---|---|---|---|---|---|
| Black (n = 6) | White (n = 13) | Black (n = 11) | White (n = 16) | Sex | Race | Sex × race | |
| Age (years) | 40 ± 8 (27-47) | 38 ± 6 (28-49) | 38 ± 6 (27-45) | 37 ± 6 (27-45) | |||
| Weight (kg) | 91.1 ± 10.0 (79.3-102.4) | 89.7 ± 8.1 (79.8-104.0) | 72.9 ± 7.6 (61.0-83.1) | 78.0 ± 5.7 (70.4-92.2) | 0.0001 | ||
| BMI (kg/m2) | 28.2 ± 1.8 (25.5-30.5) | 27.9 ± 1.7 (25.1-31.3) | 27.3 ± 1.8 (25.3-30.0) | 27.9 ± 1.7 (24.7-30.5) | |||
| Insulin sensitivity SI (10-4 mU/l/min) | 3.33 ± 1.88 (1.44-5.82) | 3.26 ± 1.57 (0.99-6.78) | 2.12 ± 0.58 (1.3-3.1) | 3.94 ± 1.13 (2.72-5.87) | 0.02 | 0.03 | |
| Body fat (%) | 24.4 ± 2.0 (22.0-27.7) | 24.5 ± 3.8 (16.5-31.0) | 36.2 ± 4.8 (29.1-46.2) | 38.6 ± 3.5 (32.1-42.7) | 0.0001 | ||
| TAT (kg) | 10.3 ± 2.3 (8.4-14.2) | 10.9 ± 2.1 (7.0-14.6) | 10.3 ± 2.8 (7.6-16.5) | 12.2 ± 2.7 (8.1-17.1) | 0.05 | ||
| SAT (kg) | 6.3 ± 1.6 (4.4-9.0) | 6.6 ± 1.5 (4.0-9.1) | 8.6 ± 2.2 (6.5-12.4) | 9.9 ± 2.3 (6.9-14.2) | 0.0001 | ||
| VAT (kg) | 4.0 ± 1.5 (2.4-6.1) | 4.3 ± 1.1 (2.9-6.1) | 1.6 ± 0.9 (0.9-4.1) | 2.2 ± 0.8 (1.2-4.0) | 0.0001 | ||
| IHL (% of oil phantom) | 1.1 ± 0.5 (0.2-1.7) | 2.2 ± 1.9 (0.4-7.2) | 0.9 ± 0.8 (0.3-2.8) | 1.4 ± 1.5 (0.5-6.4) | 0.09 | ||
| IHL (>5%) | 0 | 1 | 0 | 1 | - | - | |
| Liver-to-spleen ratio | 1.4 ± 0.1 (1.2-1.5) | 1.2 ± 0.1 (1.0-1.4) | 1.3 ± 0.1 (1.2-1.5) | 1.3 ± 0.1 (1.2-1.5) | 0.08 | 0.03 | 0.01 |
| Total-cholesterol (mg/dl) | 191 ± 31 (158-231) | 187 ± 31 (142-256) | 156 ± 30 (115-205) | 172 ± 22 (117-210) | 0.006 | ||
| HDL-cholesterol (mg/dl) | 35 ± 9 (22-46) | 36 ± 7 (22-48) | 50 ± 13 (28-77) | 45 ± 9 (33-64) | 0.0002 | ||
| LDL-cholesterol (mg/dl) | 127 ± 37 (68-166.) | 119 ± 27 (81-173) | 88 ± 25 (43-126) | 108 ± 17 (73-145) | 0.003 | ||
| Triglyceride (mg/dl) | 155 ± 114 (72-368) | 157 ± 81 (63-359) | 86 ± 70 (34-260) | 100 ± 44 (28-202) | 0.004 | ||
| ALT (U/l) | 25.9 ± 6.7 (16.0-33.5) | 28.2 ± 9.1 (15.5-42.0) | 12.6 ± 3.8 (7.5-20.0) | 17.3 ± 8.7 (7.0-46.5) | 0.0001 | ||
| ALK (U/l) | 72.8 ± 25.0 (52.0-118.0) | 62.9 ± 10.0 (47.0-75.0) | 47.1 ± 9.5 (32.0-66.0) | 58.7 ± 17.0 (27.0-91.0) | 0.003 | 0.03 | |
| TNF-α (pg/ml) | 3.2 ± 1.5 (1.6-4.7) | 7.7 ± 6.0 (1.6-25.5) | 7.2 ± 6.1 (1.6-18.3) | 8.1 ± 5.7 (1.6-18.2) | |||
| IL-6 (pg/ml) | 170.5 ± 223.3 (1.6-542.8) | 73.3 ± 141.6 (1.6-507.3) | 113.7 ± 147 (1.6-452.5) | 87.8 ± 78.5 (17.1-288.9) | |||
| hsCRP (mg/dl) | 0.37 ± 0.36 (0.08-0.96) | 0.20 ± 0.21 (0.05-0.86) | 0.39 ± 0.33 (0.07-1.21) | 0.30 ± 0.28 (0.04-0.98) | |||
| Average weekly alcohol intake (g) | 7.3 ± 12.3 (0.0-29.6) | 5.3 ± 10.3 (0.0-34.3) | 0.9 ± 2.2 (0.0-7.2) | 3.2 ± 5.5 (0.0-19.8) | |||
Values are means ± s.e.m. (ranges shown in parentheses). Significance between men vs. women and black vs. white shown for P ≤ 0.10.
ALK, alkaline phosphatase; ALT, alanine aminotransferase; Average weekly alcohol intake, average weekly alcohol intake during the 5-week baseline period; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IHL, intrahepatic lipid; IL-6, interleukin-6; LDL, low-density lipoprotein; SAT, subcutaneous abdominal adipose tissue; TAT, total abdominal adipose tissue; TNF-α, tumor necrosis factor-α; VAT, visceral abdominal adipose tissue.
Relations between liver lipid, alcohol intake, and adiposity
IHL was weakly correlated with the liver-to-spleen ratio (r = -0.33, P = 0.02; Figure 1; Note: a lower liver-to-spleen ratio reflects a higher lipid content) but neither IHL nor the liver-to-spleen ratio were significantly correlated with average alcohol intake during the prior 5-week baseline assessment (r = 0.18 and -0.16 respectively, P > 0.05). By simple correlation analysis, IHL was positively correlated with BMI (r = 0.30, P = 0.04), and total abdominal fat (r = 0.42, P = 0.005) and visceral abdominal fat adjusted for total abdominal fat (r = 0.55, P < 0.0001), but not with body weight or other markers of adiposity. The liver-to-spleen ratio was negatively correlated with body mass (r = -0.42, P = 0.004) and visceral abdominal fat adjusted for total abdominal fat (r = -0.37, P = 0.01), and positively correlated with percent body fat (r = 0.31, P = 0.04,). The relation between IHL and percent body fat and total and visceral abdominal tissue was statistically significant after adjusting for sex, race, and alcohol intake (Table 2).
Figure 1.
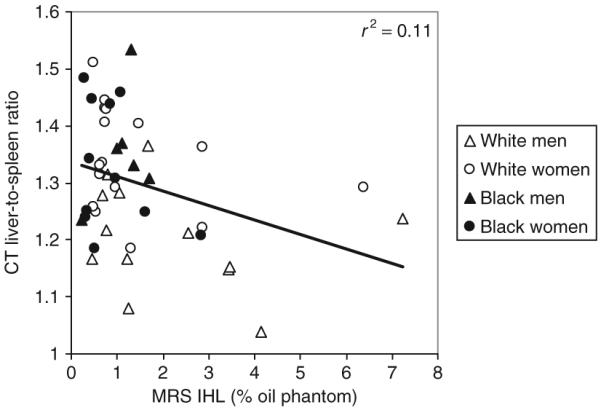
Relation between intrahepatic lipid (IHL) measured by magnetic resonance spectroscopy (MRS) and the ratio of the attenuation (in Hounsfield units (HU)) of liver tissue relative to spleen tissue (liver-to-spleen ratio) (in HU) measured by computed tomography (CT) (P < 0.05). A lower liver-to-spleen ratio reflects a higher lipid content. Data are shown for the group of white men (open triangles), white women (open circles), black men (black triangles), and black women (black circles).
Table 2. Relations between liver lipid content by MRS and selected variables adjusted for sex and race and sex, race, and alcohol intake.
| IHL (% oil phantom) |
Liver-to-spleen ratio |
|||
|---|---|---|---|---|
| Variable | Sex and race | Sex, race, and alcohol intake | Sex and race | Sex, race, and alcohol intake |
| Weight (kg) | 0.077 | 0.11 | -0.33** | -0.37** |
| Body fat (%) | 0.41** | 0.43** | -0.03 | -0.04 |
| TAT (kg) | 0.43** | 0.44** | -0.21 | -0.21 |
| VAT (kg) | 0.56*** | 0.54*** | -0.14 | -0.11 |
| Triglycerides (log mg/dl) | 0.53*** | 0.52*** | -0.15 | -0.13 |
| HDL-cholesterol | -0.23 | -0.21 | -0.07 | -0.10 |
| ALT (U/l) | 0.43** | 0.42** | -0.20 | -0.16 |
| ALK (U/l) | 0.29 | 0.27* | 0.01 | 0.06 |
| hsCRP (mg/dl) | 0.31** | 0.33** | -0.06 | -0.08 |
Values are partial correlation coefficients (rs) between liver lipid stores and the variables of interest adjusted for sex and race as well as for sex, race, and average alcohol consumption measured in g/week during the 5-week baseline assessment. IHL measured by MRS, liver-to-spleen ratio and liver minus spleen by computed tomography.
ALK, alkaline phosphatase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IHL, intrahepatic lipid; MRS, magnetic resonance spectroscopy; TAT, total abdominal adipose tissue; VAT, visceral abdominal adipose tissue.
P 0.05 < 0.10;
P < 0.05;
P < 0.001.
Relations between liver lipid and serum lipids
By simple correlation analysis, IHL (but not the liver-to-spleen ratio) was positively correlated with serum triglyceride (r = 0.57, P < 0.0001) and negatively correlated with HDL-cholesterol concentrations (r = -0.32, P = 0.03). The correlation between IHL and triglyceride concentrations was statistically significant after adjusting for sex, race, and alcohol intake (Table 2).
Relations between liver lipid and markers of liver function and inflammation
By simple correlation analysis, IHL was positively correlated with ALT (r = 0.49, P = 0.0005) and ALK (r = 0.31, P = 0.03) concentrations but not with any markers of whole-body inflammation. The liver-to-spleen ratio was negatively correlated with ALT concentration (r = -0.39, P ≤ 0.01) and positively correlated with IL-6 concentration (r = 0.51, P ≤ 0.001). After adjusting for sex, race, and alcohol intake, IHL was significantly correlated with ALT, ALK, and hsCRP, whereas no significant correlations were found for the liver-to-spleen ratio and markers of liver function and inflammation (Table 2).
Intervention analysis
The characteristics of the 46 subjects assigned to the intervention and CO groups are shown in Table 3. Statistical differences among treatment groups at baseline were not found for any of the variables analyzed. There was a trend (P = 0.07), however, for hsCRP to differ at baseline among the treatment groups.
Table 3. Baseline characteristics of subjects by treatment assignment.
| CR | CR+EX | LCD | CO | |
|---|---|---|---|---|
| Age (years) | 39 ± 5 (30-45) | 36 ± 5 (29-45) | 39 ± 7 (27-49) | 37 ± 7 (27-47) |
| Sex (men/women) | 6/6 | 4/7 | 4/7 | 5/7 |
| Race (black/white) | 5/7 | 5/6 | 4/7 | 3/9 |
| BMI (kg/m2) | 27.8 ± 1.4 (25.7-30.0) | 27.7 ± 1.6 (25.3-29.8) | 27.8 ± 1.9 (24.7-30.5) | 27.8 ± 2.1 (25.1-31.3) |
| Average weekly alcohol intake (g) | 5.5 ± 10.1 (0-29.6) | 2.7 ± 3.9 (0-11.6) | 0.7 ± 1.1 (0-3.16) | 6.0 ± 10.6 (0-34.3) |
Values are means ± s.e.m. (with ranges shown in parentheses).
Average weekly alcohol intake, average weekly alcohol intake during the 5-week baseline period; CO, control; CR, caloric restriction; CR+EX, caloric restriction and increased structured exercise; LCD, low-calorie diet.
Effect of CR
Details on the change in body weight, body composition, and insulin sensitivity have been previously reported (22,24,28). In brief, and as summarized in Table 4, body weight was significantly reduced from baseline (P < 0.001) by 10, 10, and 14% in CR, CR+EX, and LCD groups, respectively at the end of the 6-month intervention. The reduction in body weight was significantly lower in the LCD than the caloric-restricted groups (P < 0.05). Insulin sensitivity and body composition were significantly improved in all caloric-restricted groups (Table 4) but improvements were not statistically different between treatments.
Table 4. Change in body composition, insulin sensitivity and markers of liver function and inflammation with caloric restriction.
| CR |
CR+EX |
LCD |
CO |
|||||
|---|---|---|---|---|---|---|---|---|
| Value | Baseline | %Chg | Baseline | %Chg | Baseline | %Chg | Baseline | %Chg |
| Weighta (kg) (22) | 80.9 ± 114 | -10 ± 3* | 82.3 ± 10.9 | -10 ± 3* | 82.4 ± 11.3 | -14 ± 2* | 81.7 ± 8.9 | 0 ± 4 |
| Insulin sensitivity SI (10-4 mU/l/min)a (24) | 3.3 ± 1.7 | 40 ± 66* | 3.4 ± 1.3 | 79 ± 64* | 3.3 ± 1.7 | 83 ± 106* | 2.8 ± 1.2 | 1 ± 35 |
| Body fata (%) (28) | 30.9 ± 8.3 | -15 ± 9* | 33.1 ± 7.8 | -17 ± 8* | 32.7 ± 8.3 | -21 ± 11* | 32.2 ± 6.6 | -1 ± 6 |
| TATa (kg) (22) | 11.1 ± 2.2 | -27 ± 12* | 11.2 ± 2.5 | -30 ± 9* | 11.0 ± 3.2 | -35 ± 10* | 11.0 ± 2.6 | 0 ± 10 |
| SATa (kg) (22) | 7.8 ± 2.4 | -26 ± 13* | 8.5 ± 2.7 | -30 ± 9* | 8.3 ± 2.7 | -34 ± 9* | 8.1 ± 2.2 | 1 ± 10 |
| VATa (kg) (22) | 3.2 ± 1.8 | -28 ± 12* | 2.6 ± 1.5 | -28 ± 10* | 2.8 ± 1.5 | -36 ± 10* | 3.0 ± 1.3 | -2 ± 13 |
| ALT (U/l) | 23.8 ± 13.0 | -28 ± 19* | 19.6 ± 9.7 | -24 ± 24* | 17.2 ± 5.3 | -2 ± 23 | 20.0 ± 9.1 | -12 ± 11* |
| ALK (U/l) | 65.5 ± 20.2 | -9 ± 6* | 54.7 ± 9.6 | -6 ± 15 | 50.8 ± 13.0 | -10 ± 8* | 63.9 ± 18.1 | -7 ± 14 |
| TNF-α (pg/ml) | 8.3 ± 7.1 | 88 ± 115 | 7.1 ± 6.4 | 62 ± 114 | 5.4 ± 3.4 | 51 ± 83 | 7.8 ± 4.9 | 49 ± 36 |
| IL-6 (pg/ml) | 69.0 ± 64.7 | 71 ± 144* | 134.6 ± 153.7 | 6 ± 31 | 46.1 ± 59.3 | 70 ± 170 | 145.8 ± 197.6 | 10 ± 50 |
| hsCRP (mg/dl) | 0.25 ± 0.26 | 29 ± 184 | 0.15 ± 0.09 | -26 ± 52 | 0.46 ± 0.38 | -50 ± 27* | 0.34 ± 0.28 | -27 ± 28* |
Values are means ± s.d.
ALK, alkaline phosphatase; ALT, alanine aminotransferase; CO, control; CR, caloric restriction; CR+EX, caloric restriction and increased structured exercise; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; LCD, low-calorie diet; SAT, subcutaneous abdominal adipose tissue; TAT, total abdominal adipose tissue; TNF-α, tumor necrosis factor-α; VAT, visceral abdominal adipose tissue.
Data previously published in cited reference.
P < 0.05 vs. baseline.
CR and liver and serum lipids
A significant treatment effect (P < 0.01) was found for IHL levels (Figure 2), which was reduced from baseline in the CR, CR+EX, and LCD treatments (P < 0.01). There was no difference in the response between treatments, but the response in each treatment was significantly different (P < 0.05) from CO. The treatment effect was not influenced by sex or race (P = 0.1 for the sex-by-race-by-treatment interaction). In contrast, the liver-to-spleen ratio (Figure 2) and the density of the liver and spleen (in Hounsfield units) were not significantly altered by CR.
Figure 2.
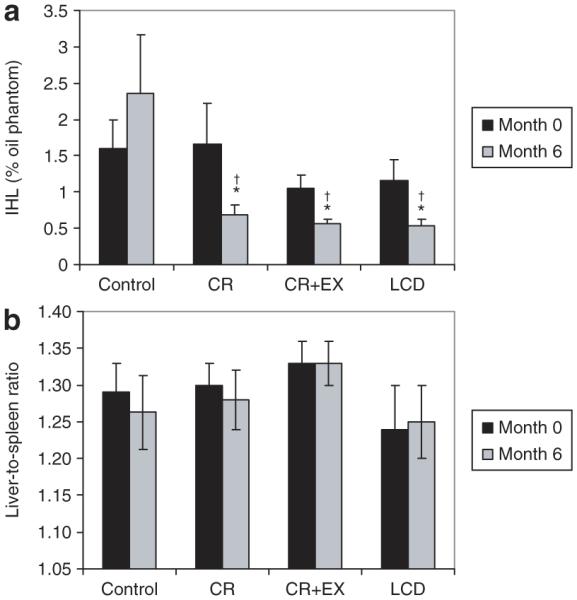
Liver lipid stores at baseline and after 6 months of treatment with caloric restriction (CR), caloric restriction and increased structured exercise (CR+EX), a low-calorie diet (LCD) or control (see text for treatment details) assessed by (a) magnetic resonance spectroscopy and (b) computed tomography. For (a) intrahepatic lipid (IHL), values at 6 months are significantly different than baseline for all treatment groups (CR, CR+EX, and LCD). *Significantly different from control; †significantly different from baseline.
Serum triglyceride concentrations were significantly decreased from baseline in the CR (21 ± 16%), CR+EX (15 ± 18%), and LCD (12 ± 37%) groups and significantly increased from baseline in the CO group (22 ± 31%; P < 0.05). HDL-cholesterol was also increased by 10 ± 11%, 10 ± 9%, and 12 ± 16% in the CR, CR+EX, and LCD groups, respectively, but did not change significantly in the CO group. Details of the changes in the lipoprotein profile are included in a forthcoming article (29).
CR and markers of liver function and inflammation
Baseline concentrations for markers of liver function and inflammation, and the change in these markers with CR are shown in Table 4. ALT was significantly decreased from baseline in the CR, CREX, and CO groups but not in the LCD group, whereas ALK was significantly reduced in the CR and LCD groups. hsCRP was significantly reduced from baseline in the LCD and CO groups and increased slightly (but not significantly) in the CR group. IL-6 tended to increase with treatment (P = 0.08) but was significantly increased only in the CR group. TNF-α tended to increase in all treatment groups as well as the CO group but this increase was not statistically significant.
Changes in liver lipid vs. other parameters
After adjustment for treatment group, the reduction in IHL was positively correlated with the change in visceral adipose tissue (r = 0.33; P = 0.04) and also tended to be correlated with the reduction in serum triglycerides (r = 0.26; P = 0.11). However, IHL was not related to the change in other indices of body adiposity or changes in HDL-cholesterol, ALT, or ALK concentrations. The reduction in IHL was not correlated with the change in hsCRP or TNF-α but was negatively correlated with the change in IL-6 (r = -0.59; P < 0.01). The change in IHL was not significantly correlated with the difference in the liver-to-spleen ratio (r = -0.27, P = 0.10).
DISCUSSION
This study examines liver lipid content measured by both MRS and CT in healthy overweight black and white men and women before and after 6 months of CR with or without exercise. At baseline, we found, as hypothesized, that liver lipid accretion which ranged from 0.3 to 7.2% of peanut oil phantom was correlated with elevated fasting triglyceride and low HDL-cholesterol concentrations, and higher ALT, AST, and hsCRP concentrations. With CR, we found that liver lipid content (measured by MRS but not CT) was significantly reduced but not in association with the improvement in serum lipids and markers of either liver function or whole-body inflammation, as was also hypothesized.
Although a consistent and striking association has been noted between insulin resistance and increased liver lipid stores (11,12,30) and NAFLD (10), it is not clearly established whether insulin resistance is a cause (1) or consequence (31) of liver lipid accumulation. Although some clinical evidence suggests that insulin resistance (or hyperinsulinemia) may promote metabolic abnormality and liver lipid accretion (1,32), a widely accepted hypothesis holds that accumulation of fat in nonadipose tissues (i.e., ectopic fat) such as liver, blood, skeletal muscle, and pancreas interferes with insulin signaling and promotes insulin resistance/secretion (33). Data from animal studies have shown that steatosis and hepatic insulin resistance can be induced by overexpression of liver-specific lipoprotein lipase in mice (34) and by 3 days of high-fat feeding in rats (35). Intracellular accumulation of fatty acid metabolites, including long-chain fatty acyl-CoAs, also reduces insulin activation of IRS 2-associated phosphatidylinositol 3-kinase activity (34) thereby inhibiting insulin signaling (31). Furthermore, accumulation of fatty acids in the liver may damage biological membranes (36) and/or promote liver damage in concert with pro-inflammatory cytokines (13).
In an earlier analysis, we reported that liver lipid accretion was negatively correlated with insulin sensitivity in overweight men and women, and that accretion was greater in those who also had enlarged adipocytes (24). The reduction in liver lipid stores with CR was also found to occur in association with the reduction in body weight but not with the improvement in insulin sensitivity. In this analysis, we extend these findings to show that liver lipid accretion is directly correlated with hsCRP concentration, a marker of whole-body inflammation, after adjusting for sex and race but is not related to concentrations of pro-inflammatory cytokines including TNF-α and IL-6. We also found that liver lipid stores tend to be higher in white than black individuals (particularly white men) despite higher insulin sensitivity in this group (as has been noted in large-scale studies (4)), but these differences were statistically significant only for liver fat assessed by CT (P = 0.03) and not by MRS (P = 0.09). Collectively, our findings from the CALORIE Study suggest that the pathogenesis of NAFLD is complex and may involve an interplay between adipocyte size (24), excess body weight, insulin sensitivity/resistance, and whole-body inflammation that is likely to vary among racial groups independent of alcohol intake.
In agreement with previous studies, we found that liver lipid content and/or hepatic steatosis is associated with many features of the metabolic syndrome (9) including hypertriglycemia (10-12) and reduced HDL-cholesterol (10,11), as well as insulin resistance (11,12,24). Our results, however, also show that CR with or without exercise is an effective lifestyle-modification that simultaneously reduces liver lipid stores and improves the metabolic profile. Quite interestingly, the improvement in liver and serum lipids with a 10% weight loss by CR with or without exercise was similar to that with a 14% rapid weight loss in our weight-clamped control group (24). By comparison, treatment with rosiglitazone (37), and pioglitazone (38) lowers liver lipid content and improves insulin sensitivity, but only pioglitazone treatment impacts serum triglyceride and HDL-cholesterol concentrations (39). Other lifestyle interventions that have reduced body weight via diet (16) or diet plus exercise (17) found only small changes in triglyceride concentrations despite ~39-49% reduction in liver lipid stores (16) or steatosis (17) without changes in HDL-cholesterol concentrations. Differences between these findings and ours may be partially explained by our healthy caloric-restriction diet (i.e., AHA Step 1 diet), which was provided during the first 3 months of the study in the caloric-restricted groups and after weight clamping in the LCD group, and/or by our intense behavioral intervention that educated volunteers on healthy lifestyle modifications. The association between liver lipid accumulation and elevated triglycerides and lowered HDL-cholesterol may indicate that lipid accretion in liver modulates the uptake and utilization of very low-density lipoproteins resulting in elevated triglycerides (12). Normally, insulin acutely suppresses the production of very low-density lipoprotein particles from liver leading to a decrease in serum triglyceride concentrations (40), but this mechanism is defective in insulin-resistant subjects (41). Our results suggest that this disruption may be present as patients develop insulin resistance, and perhaps more importantly that CR may be an effective treatment for reducing both hypertriglyceridemia and liver lipid accumulation.
The employment of both MRS and CT to assess liver lipid content in this study presents some important results for future intervention studies. Although a few studies have validated the use of both techniques against the gold standard of histomorphometrical analysis in liver biopsy samples (42-44) or compared with MRS and CT (42,45), none have compared these two techniques during a lifestyle intervention. Somewhat surprisingly, we found only a modest correlation between IHL assessed by MRS and the liver-to-spleen density ratio (r = -0.33) at baseline, and a weak but not statistically significant correlation (r = -0.27) between the change in IHL and the change in the liver-to-spleen ratio with CR, both lower than those reported by Longo et al. (42) but higher than those reported by Ishii et al. (45). This lack of consistency between the two techniques combined with our data showing no change in the liver-to-spleen ratio despite a change in IHL suggests that MRS may be a more sensitive measurement of liver lipid content than CT, particularly when liver lipid content is low (i.e., in “healthy” overweight subjects but not in obese subjects). In addition, MRS is a more direct method of measuring lipid stores. MRS assesses the hydrogen atoms on the carbon chains of triglyceride molecules stored in tissue, whereas CT is an X-ray imaging technique that distinguishes fat from other tissue based on tissue attenuation characteristics that are a function of tissue density and chemical composition (such that a lower mean attenuation reflects higher lipid content). CT may be influenced by intervention-induced factors (other than lipid concentration) that effect tissue density including hydration and liver glycogen and/or iron content (26). The reliability of the two techniques possibly also differs following weight loss when CT measures may be influenced by differences in patient’s size and shape (26), as noted in this study, and artifacts such as beam hardening (26). In contrast, MRS has not been found to be influenced by technical factors including sampling size or location (5). Our results, nevertheless, should not preclude use of CT for analysis of fatty liver disease but rather suggest that caution be used when employing CT to assess liver lipid accretion in populations with suspected low liver lipid content.
While this study reports some interesting findings that may help gain a better understanding of the pathogenesis and treatment of NAFLD, the study has some limitations. First, our careful screening of participants for good general health likely resulted in recruitment of an overweight sample that was “healthier” than the general population and therefore had a lower prevalence of liver lipid accumulation. Second, our screening to rule out liver disease by history, physical examination, liver function tests, and CT scan was not perfect and did not specifically rule out all causes of liver disease including viral hepatitis. Third, our study size was relatively small and limited our power to detect differences among CR treatment and control groups for improvements in insulin sensitivity (24), hyperinsulinemia and liver lipid accumulation. These limitations, however, were compensated in part by the very tight control of the intervention and control groups and by the number of tests and experimental procedures that allowed a more comprehensive assessment of factors that may contribute to liver lipid accumulation or NAFLD (i.e., elevated hsCRP) or be influenced by its accumulation (e.g., hypertriglyceridemia and hyperinsulinemia) Nevertheless, both mechanistic and larger-scale well-controlled longitudinal studies are needed to elucidate the factors involved in NAFLD pathogenesis and determine the long-term therapeutic effect of CR or other altered lifestyle approaches for preventing and treating NAFLD.
ACKNOWLEDGMENTS
We thank Edward J. Robarge of the Neuroimaging Center for performing the MRS scans. We also thank the remaining members of Pennington CALERIE Research Team including: James DeLany, Lilian de Jonge, Tuong Nguyen, Corby K. Martin, Marlene M. Most, Frank L. Greenway, Emily York-Crow, Steven Anton, Catherine Champagne, Brenda Dahmer, Andy Deutsch, Paula Geiselman, Jennifer Howard, Jana Ihrig, Darlene Marquis, Connie Murla, Sean Owens, Aimee Stewart, and Vanessa Tarver. Our gratitude is extended to the excellent staffs of the Inpatient Clinic, Metabolic Kitchen and Clinical Chemistry Laboratory. Our thanks also go to Health and Nutrition Technology, Carmel, CA for providing us with all the HealthOne formula used in the study. Finally, our profound gratitude goes to all the volunteers who spent so much time in participating in this very demanding research study. This work was supported by grants U01 AG20478 (to E.R.) and K01 DK062018 (to D.E.L.-M.).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Tendler DA, UpToDate Pathogenesis of nonalcoholic fatty liver disease. 2006 < www.uptodate.com>.
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 6.Bizzaro N, Tremolada F, Casarin C, et al. Serum alanine aminotransferase levels among volunteer blood donors: effect of sex, alcohol intake and obesity. Ital J Gastroenterol. 1992;24:237–241. [PubMed] [Google Scholar]
- 7.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 9.Shen YH, Yang WS, Lee TH, et al. Bright liver and alanine aminotransferase are associated with metabolic syndrome in adults. Obes Res. 2005;13:1238–1245. doi: 10.1038/oby.2005.147. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 12.Tiikkainen M, Tamminen M, Häkkinen AM, et al. Liver-fat accumulation and insulin resistance in obese women with previous gestational diabetes. Obes Res. 2002;10:859–867. doi: 10.1038/oby.2002.118. [DOI] [PubMed] [Google Scholar]
- 13.Diehl AM, Poordad F. Nonalcoholic Fatty Liver Disease. Saunders; Philadelphia, PA: 2002. [Google Scholar]
- 14.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 15.Sheth SG, Chopra S, UpToDate Nonalcoholic steatohepatitis. 2006 doi: 10.7326/0003-4819-126-2-199701150-00008. < www.uptodate.com>. [DOI] [PubMed]
- 16.Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 17.Ueno T, Sugawara H, Sujaku K, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 19.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 20.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 21.Franssila-Kallunki A, Rissanen A, Ekstrand A, Ollus A, Groop L. Weight loss by very-low-calorie diets: effects on substrate oxidation, energy expenditure, and insulin sensitivity in obese subjects. Am J Clin Nutr. 1992;56(Suppl 1):S247–S248. doi: 10.1093/ajcn/56.1.247S. [DOI] [PubMed] [Google Scholar]
- 22.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-1, Version 2.0) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 24.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of Calorie Restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson-Meyer DE, Smith SR, Heilbronn LK, et al. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14:73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 27.Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr. 2005;82:957–963. doi: 10.1093/ajcn/82.5.957. quiz 1145-1146. [DOI] [PubMed] [Google Scholar]
- 28.Redman LM, Heilbronn LK, Martin CK, et al. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefevre M, Heilbronn LK, Smith JV, et al. CVD risk factors are improved in overweight individuals by 6-month caloric restriction alone or in combination with increased physical activity. in press. [Google Scholar]
- 30.Banerji MA, Buckley MC, Chaiken RL, et al. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19:846–850. [PubMed] [Google Scholar]
- 31.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanless IR, Bargman JM, Oreopoulos DG, Vas SI. Subcapsular steatonecrosis in response to peritoneal insulin delivery: a clue to the pathogenesis of steatonecrosis in obesity. Mod Pathol. 1989;2:69–74. [PubMed] [Google Scholar]
- 33.Danforth E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 34.Kim JK, Fillmore JJ, Chen Y, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 36.Acosta D, Wenzel DG. Injury produced by free fatty acids to lysosomes and mitochondria in cultured heart muscle and endothelial cells. Atherosclerosis. 1974;20:417–426. doi: 10.1016/0021-9150(74)90023-9. [DOI] [PubMed] [Google Scholar]
- 37.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajaj M, Suraamornkul S, Hardies LJ, Pratipanawatr T, DeFronzo RA. Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int J Obes Relat Metab Disord. 2004;28:783–789. doi: 10.1038/sj.ijo.0802625. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 40.Malmström R, Packard CJ, Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 41.Malmström R, Packard CJ, Caslake M, et al. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes. 1998;47:779–787. doi: 10.2337/diabetes.47.5.779. [DOI] [PubMed] [Google Scholar]
- 42.Longo R, Ricci C, Masutti F, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297–302. [PubMed] [Google Scholar]
- 43.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 44.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by 1H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 45.Ishii M, Yoshioka Y, Ishida W, et al. Liver fat content measured by magnetic resonance spectroscopy at 3.0 tesla independently correlates with plasminogen activator inhibitor-1 and body mass index in type 2 diabetic subjects. Tohoku J Exp Med. 2005;206:23–30. doi: 10.1620/tjem.206.23. [DOI] [PubMed] [Google Scholar]


