Abstract
Purpose
The emergence of antiviral resistance can negate the benefits of antiviral therapy in patients with chronic hepatitis B (CHB). This study aimed to assess how physicians in Asia manage suspected antiviral resistance.
Methods
Randomly selected CHB-treating physicians in Mainland China, South Korea, Taiwan, and Thailand underwent a face-to-face interview. A standardized questionnaire was used to assess how physicians identify, monitor, and manage suspected resistance and its associated medical costs.
Results
We interviewed 575 physicians from January to May 2008. Most physicians preferred a “prevention-of-antiviral resistance” strategy over a “rescue-once-resistance-develops” strategy. Physicians had encountered lamivudine resistance most frequently (96–100% of respondents), followed by the resistance to adefovir (18–58%) and entecavir (3–7%). While physicians in South Korea and Taiwan have access to resistance testing, physicians in Mainland China and Thailand have limited access to resistance testing but rely on HBV DNA and alanine aminotransferase (ALT) tests to identify resistance. Once resistance is suspected, 60% of the physicians in Mainland China, South Korea, and Thailand monitored these patients quarterly and the remaining 40% opted for monthly follow-up. In comparison, 70% of the Taiwanese physicians monitored these patients monthly. The average total direct medical costs, excluding antiviral costs, to manage a patient during the first year after suspected resistance is identified ranged from USD $319 to USD $709.
Conclusions
Limited access to HBV resistance tests causes physicians in Asia to manage suspected resistance by various HBV DNA assays and ALT tests. This raises concerns that resistance may not be detected early enough to be rescued efficiently.
Keywords: Chronic hepatitis B, Nucleoside/nucleotide analogs, HBV DNA, ALT, Antiviral resistance, Asia
Introduction
Chronic hepatitis B (CHB) remains a global health problem, with more than 350 million people showing evidence of chronic infection. The disease prevalence is particularly high in Asia where 8–10% of the general population is chronically infected. Many of these individuals are at risk of developing complications such as cirrhosis, hepatocellular carcinoma, and end-stage liver disease [1–3]. Overall, hepatitis B virus (HBV) remains the most common cause of death from liver disease, with an annual estimate of 1 million deaths worldwide.
The ultimate goal of CHB treatment is to prevent or reduce long-term complications and prolong survival by permanently suppressing HBV DNA replication to the lowest possible level. To achieve this goal, the long-term use of antivirals is frequently indicated [4]; however, the development of antiviral-resistant mutations is a concern with the prolonged use of oral nucleoside/nucleotide analogs (NA). It has been shown that the initial clinical benefit of NA therapy is lost among patients who develop NA resistance [5]. The emergence of NA-resistant mutations may lead subsequently to a loss of histologic improvement and, in some cases, severe exacerbations of liver diseases [6, 7].
Information is lacking regarding how physicians in Asia manage CHB patients, particularly in identifying, monitoring, and managing antiviral resistance. This is important not only because HBV infection is highly prevalent in the region but also because a large number of patients receive various antiviral treatments. Through a face-to-face survey, this current study examined how practicing physicians in Mainland China, South Korea, Taiwan, and Thailand manage suspected antiviral resistance in CHB. In addition, the study estimated the direct medical costs (excluding the costs of antivirals) of managing these patients in the first year after suspected resistance is identified.
Materials and methods
Five hundred seventy-five CHB-treating physicians were randomly selected from Mainland China, South Korea, Taiwan, and Thailand. A standardized questionnaire was used to conduct the face-to-face interview with these physicians from January 2008 through May 2008.
Survey questionnaire
The questionnaire was developed by 14 physicians from Mainland China (n = 4), Taiwan (n = 5), Thailand (n = 3), and South Korea (n = 2) who participated as coinvestigators in this project with the medical affairs department of Bristol-Myers Squibb Company Asia Pacific Regional Office. The questionnaire contained three components: (1) basic information about the participating physicians, such as specialty and type of practice; (2) CHB management practices, such as frequency of monitoring patients while on NA treatment and laboratory tests ordered for monitoring resistance; and (3) estimate of direct medical costs (excluding antiviral costs) associated with managing resistance during the first year following the identification of suspected resistance. The questionnaire was originally developed in the English language. Translation into Chinese, Korean, and Thai languages was done by Synovate Healthcare, an international company that specializes in market research. The translated questionnaire was cross-referenced to the original English language version and checked for accuracy by Bristol-Myers Squibb medical affairs staff in Mainland China, South Korea, and Thailand, respectively. The interviews were conducted in local languages of the four geographic entities.
Randomized selection of physicians
A total of 4852 CHB-treating physicians specializing in hepatology, gastroenterology, and infectious diseases were selected from medical specialist registers for the initial randomization. Physicians from this list were randomly selected if they met the following criteria:
Treating a minimum number of CHB patients per month: 50 for Mainland China, 30 for South Korea and Taiwan, and 15 for Thailand. Random codes for this first pass were generated separately for each country. Phone calls were made to these randomly selected CHB-treating physicians to schedule face-to-face appointments. Subsequent random codes were generated and calls were made to fill in the shortfall if any physicians from the first pass refused to be interviewed for any reason. The primary reason for having the requisite numbers of patients per month was to ensure that the interviewed physicians were experienced in managing CHB patients with NAs and antiviral resistance.
Willing to participate in the face-to-face interview. A face-to-face method was chosen rather than a mail survey to improve response rate and data quality.
A total of 575 physicians were interviewed, with 300 physicians coming from Mainland China, 100 each from South Korea and Taiwan, and 75 from Thailand. These physicians represented 5, 25, 18, and 50% of the estimated total CHB-treating physicians in the four Asian geographies, respectively. From January 2008 through May 2008, face-to-face interviews were conducted by trained interviewers from Synovate Healthcare. Each interview lasted approximately 30 min.
Estimate of direct medical costs
Direct medical costs, excluding costs of antivirals, were estimated for managing patients in the first year immediately after resistance was suspected. The costs reported herein are incremental to the cost inherent to managing a patient in these settings for whom resistance is not suspected. The number of physician visits, laboratory tests, and procedures performed to manage/detect resistance are included for both outpatient and inpatient care. Medical utilization information was collected from the interviews and subsequently translated into direct costs. Information on costs of medical services, which differs by geographic entity, was collected from the following sources: (1) average per-unit costs based on the payment schedule of three medical centers was calculated for Mainland China; (2) the National Health Insurance Corporation and Health Insurance Review Agency information was employed for South Korea; (3) data from the Bureau of National Health Insurance and the payment schedule of one medical center were used for Taiwan; and (4) for Thailand, data from the General Department Procurement were used; unit cost information from a university hospital was used if such information was not found from the General Department Procurement.
Data collection and analysis
All raw data collected from the interviews was double entered to ensure quality control. Data analysis was conducted by the statistics department of Synovate Health using the SPSS® (SPSS, Inc., Version 15.0).
Results
The interviewed physicians
For the four geographic entities, hepatologists comprised a majority (43%) of the 575 physicians interviewed, followed by an almost equal number of gastroenterologists (27%) and infectious disease (28%) specialists (Table 1). Chronic hepatitis B was managed by different specialties in different countries. For example, gastroenterologists predominated in Thailand and Taiwan compared with hepatologists, who formed the major group in Korea. In Mainland China, CHB was most often managed by infectious disease specialists (52%).
Table 1.
Characteristics of the physicians interviewed (N = 575)
| Mainland China (n = 300) | South Korea (n = 100) | Taiwan (n = 100) | Thailand (n = 75) | |
|---|---|---|---|---|
| Specialty, % | ||||
| Hepatology | 39 | 85 | 41 | 7 |
| Gastroenterology | 7 | 5 | 59 | 93 |
| Infectious disease | 52 | 5 | – | – |
| Others | 2 | 5 | – | – |
| Monthly visits | ||||
| Number of CHB cases seen/month per physician | 227 | 219 | 175 | 38 |
| Number of NA-naive CHB patients among cases seen/month (%) | 34.8 | 11.9 | 72.0 | 44.7 |
| Number of NA-naive patients who started NA among the cases seen/month (%) | 31.6 | 42.3 | 9.5 | 41.2 |
CHB chronic hepatitis B, NA, nucleoside/nucleotide analog
The estimated number of patients seen monthly was much higher than the minimum number specified in the inclusion criteria (Table 1). For Taiwan, it was noted that the percentage of NA-naive patients seen is the highest (72.0%) but the percentage of these patients who eventually receive NA treatment is the lowest (9.5%), compared with other geographic entities included in this survey (Table 1).
Clinical experience of encountering resistance and attitudes toward resistance management strategies
Almost all interviewed physicians had encountered CHB patients with resistance to antivirals. The majority of these patients had lamivudine resistance (Table 2), followed by the number of physicians who had ever encountered patients with adefovir and entecavir resistance.
Table 2.
Experience of encountered resistance and preference of resistance management strategies (N = 575)
| Mainland China (n = 300) | South Korea (n = 100) | Taiwan (n = 100) | Thailand (n = 75) | |
|---|---|---|---|---|
| Experience with resistance in practice | ||||
| Ever encountered CHB patients with NA resistance | 100 | 98 | 96 | 95 |
| Ever encountered resistance when NA-naive patients initiated on LVD | 96 | 100 | 99 | 99 |
| Ever encountered resistance when NA-naive patients initiated on ADV | 32 | 58 | 18 | 28 |
| Ever encountered resistance when NA-naive patients initiated on ETV | 6 | 3 | 4 | 7 |
| Preference of resistance management strategies | ||||
| Prevention | 77 | 68 | 42 | 69 |
| Prediction | 15 | 15 | 13 | 4 |
| Rescue | 6 | 14 | 29 | 20 |
| Not important | 1 | 3 | 16 | 6 |
Telbivudine and clevudine were included in the survey; information is not available for all four geographic entities due to the market-approval status of these two products during the study period. As such, information for the two products is not shown. Values given are in percentages
NA nucleoside/nucleotide analog, LVD lamivudine, ADV adefovir dipivoxil, ETV entecavir
The majority of the interviewed physicians in all geographic entities indicated that resistance prevention at the point of initiating CHB treatment is the preferred strategy in their practices (Table 2). Twenty percent to 29% of physicians in Thailand and Taiwan considered using the rescue approach once resistance develops as an acceptable strategy, whereas 15% of the physicians in Mainland China and South Korea would utilize on-treatment monitoring to predict resistance in managing the patients. Of note, 16% of interviewed physicians in Taiwan did not consider antiviral resistance to be a major issue in their practice.
Availability of laboratory tests for the identification of suspected resistance
Tests used to monitor possible emergence of resistance include liver chemistries, HBV DNA, and resistance-specific tests such as gene sequencing and line-probe assay. With the exception of the line-probe assay, Tables 3 and 4 suggest that almost all other tests were available and performed by the interviewed physicians to either identify or confirm suspected resistance. However, the availability of specific resistance tests varied significantly among the Asian practices. Almost all treating physicians in South Korea (99%) and Taiwan (85%) had access to direct confirmatory antiviral resistance tests compared with a lower percentage in Thailand (56%) and Mainland China (49%).
Table 3.
Utilization of laboratory tests/procedures to monitor suspected resistance (N = 575)
| Percentage of physicians using tests/procedures (median-interval between tests/procedure) | Mainland China (n = 300) | South Korea (n = 100) | Taiwan (n = 100) | Thailand (n = 75) |
|---|---|---|---|---|
| Alanine aminotransferase (month) | 98 (1.4) | 100 (2.6) | 100 (1.5) | 97 (2.3) |
| HBV DNA (month) | 99 (2.8) | 92 (2.9) | 93 (5.7) | 97 (5.5) |
| Serology (month) | 89 (3.0) | 86 (3.0) | 90 (3.2) | 64 (5.8) |
| Resistance-specific testsa | 49 | 99 | 85 | 56 |
Values given are in percentages
aResistance-specific tests, such as PCR sequencing and INNO-LiPA and restriction fragment mass polymorphism
Table 4.
Tests used to indirectly confirm suspected resistance when resistance-specific tests are unavailable/not accessible (N = 201)
| Mainland China (n = 152) | South Koreaa | Taiwan (n = 16) | Thailand (n = 33) | |
|---|---|---|---|---|
| HBV DNA | 52 | – | 56 | 88 |
| ALT | 9 | – | 25 | 12 |
| Hepatic flare | 3 | – | 13 | – |
| HBV DNA and ALT | 14 | – | 6 | – |
| HBV DNA and hepatic flare | 22 | – | – | – |
Based on physicians who indicated they had no access to any resistance tests. Values given are in percentages
ALT alanine aminotransferase
aAll interviewed physicians, except 1 in South Korea, indicated that they had access to resistance-specific tests
Table 3 shows that the three conventional laboratory tests (alanine aminotransferase [ALT], aspartate aminotransferase, and HBV DNA) for CHB patients were used similarly across all interviewed physicians to identify resistance. However, variations were found in the frequency of HBV DNA testing. The frequency with which HBV DNA tests are used was similar in Mainland China and South Korea (e.g., almost every 3 months), whereas the same tests were conducted less frequently in Thailand (every 5.5 months) and Taiwan (every 5.7 months).
As shown in Table 4, HBV DNA test was the most commonly used surrogate marker of resistance emergence when confirmatory resistance tests were not available, whereas ALT testing remained an alternative. In Mainland China, 22% of interviewed physicians identified suspected resistance cases with a combination of HBV DNA and ALT tests when they have limited access to resistance-specific tests. Information from this survey indicated that the percentage of physicians who would use locally made HBV DNA test assays varied from 7% (South Korea) to 47% (Mainland China). In addition, there are marked variations in the sensitivities of these locally made assays and their dynamic ranges (e.g., lower limit of quantification of 15 IU/ml [Thailand] to 7500 IU/ml [Mainland China]) (Table 5).
Table 5.
Timing of ordering resistance-specific tests to identify/confirm antiviral resistance (N = 373)
| Mainland China (n = 148) | South Korea (n = 99) | Taiwan (n = 84) | Thailand (n = 42) | |
|---|---|---|---|---|
| Immediately when a resistance is suspected | 45 | 58 | 24 | 69 |
| After virologic breakthrough | 47 | 37 | 45 | 29 |
| After both virologic and biochemical breakthrough | 8 | 5 | 31 | 2 |
Based on physicians who indicated that they do have access to resistance tests. Values given are in percentages
Clinical experience of managing antiviral resistance in practice
While a majority of the interviewed physicians in South Korea (58%) and Thailand (69%) ordered confirmatory resistance tests immediately when antiviral resistance was suspected, a smaller proportion waited for virologic breakthrough (37 and 29%, respectively) to occur before doing so. The reverse was found in Taiwan, with 24% of physicians ordering a confirmatory resistance test immediately whereas a higher proportion (45%) would wait for virologic breakthrough before ordering a confirmatory resistance test. In addition, 31% of the Taiwan physicians would wait to order confirmatory tests until both virologic and biochemical breakthroughs had occurred. In Mainland China, almost equal proportions of physicians would order confirmatory tests immediately (45%) or after a virologic breakthrough (47%).
While follow-up visits every 3–6 months are recommended when nucleoside-naive patients are initiated on therapy [4, 8–10], information shown in Table 6 suggests that a good proportion of the physicians increased the frequency of follow-up visits to “once a month for patients with suspected resistance.”
Table 6.
Frequency of follow-up visits when resistance is suspected (N = 570)
| Mainland China (n = 299) | South Korea (n = 100) | Taiwan (n = 100) | Thailand (n = 71) | |
|---|---|---|---|---|
| Once every month | 36 | 46 | 69 | 41 |
| Once every 3 months | 61 | 53 | 29 | 55 |
| Once every 6 months | 3 | 1 | 2 | 4 |
Values given are in percentages
The data in Table 7 describe how the surveyed physicians modified treatment of confirmed cases of antiviral resistance. In patients with lamivudine resistance, add-on adefovir was commonly used across all geographic entities. The strategies were more varied when managing patients with adefovir or entecavir resistance. The data also suggest that physicians were more experienced in managing lamivudine resistance than either adefovir resistance or entecavir resistance. However, the great majority of interviewed physicians had not encountered entecavir resistance and did not have experience to manage it.
Table 7.
Treatment management for suspected antiviral resistance (N = 566)
| Mainland China (n = 295) | South Korea (n = 100) | Taiwan (n = 96) | Thailand (n = 75) | |
|---|---|---|---|---|
| On LVD | ||||
| Switch to ADV | 17 | 38 | 22 | 4 |
| Switch to ETV | 2 | 7 | 7 | 7 |
| Add on ADV | 73 | 51 | 65 | 77 |
| Add on ETV | 2 | – | 1 | 5 |
| On ADV | ||||
| Switch to ETV | 27 | 24 | 40 | 18 |
| Switch to interferon | 10 | 2 | 13 | 8 |
| Switch to LVD | 8 | 5 | 8 | 2 |
| Add on LVD | 33 | 19 | 20 | 20 |
| Add on ETV | 15 | – | 15 | 21 |
| Not sure what to do | – | 44 | – | 15 |
| On ETV | ||||
| Switch to ADV | 17 | 8 | 31 | 14 |
| Switch to LVD | 7 | 3 | 6 | 8 |
| Switch to interferon | 18 | – | 28 | 9 |
| Add on ADV | 45 | – | 18 | 31 |
| Add on LVD | 4 | 1 | 4 | 5 |
| Not sure what to do | 2 | 87 | – | 22 |
Values given are in percentages
LVD lamivudine, ADV adefovir, ETV entecavir
Direct medical costs of managing antiviral resistance
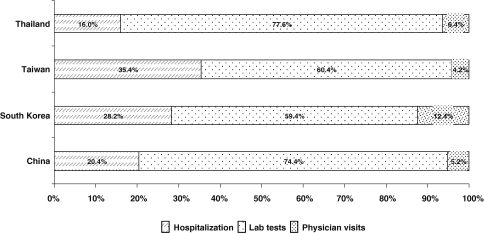
The direct medical costs of managing antiviral resistance during the first year after suspected resistance is identified were estimated for outpatient and inpatient care separately. These estimated costs did not include the cost of drugs (e.g., continuing the same antiviral, switching to or adding another antiviral agent). The estimated costs are incremental to the medical costs for managing CHB patients without antiviral resistance. The estimated total costs of consultation, laboratory tests, and hospitalization, if required, associated with managing suspected resistance per patient were US $580, US $709, US $572, and US $319 for Mainland China, South Korea, Taiwan, and Thailand, respectively. Distributions of the cost components are shown in Fig. 1, with the bulk of these costs arising from laboratory tests and related procedures.
Fig. 1.
Direct medical costs of resistance management by categories
Discussion
To our best of knowledge, this is the first study of its kind in the four geographic entities, or in Asia, at the time the study was conducted. This study consisted of two key features: first, all interviewed physicians were randomly selected, ensuring that the interviewed group represented the treating physicians in the four geographic entities; second, the study was conducted through a face-to-face interview with a large number of physicians. However, this study was based on a survey of a predesigned questionnaire by physicians’ recall but not actual patient-level information. Therefore, a key limitation of this study is the inability to differentiate between what these interviewed physicians “believe they do” or would “like to do” under ideal circumstances versus what they “actually do” in clinical practice. For example, a majority of the physicians expressed the desire to prevent antiviral resistance beginning with their choice of initial treatment; yet, lamivudine is the antiviral most often prescribed in these four Asian geographies despite its high resistance rate and the likelihood of developing cross-resistance with other antivirals [6, 11, 12]. Additional research using patient level information would enable a deeper understanding of the challenge that antiviral resistance presents in the management of CHB. The findings of this study area are confined to two areas pertaining to the management of antiviral resistance in CHB patients in these four Asian geographies: (1) the ability to detect antiviral resistance early enough and (2) the economic burden of managing antiviral resistance.
From this survey, resistance-specific testing was widely available only in Korea (Table 3). For physicians in the other three geographies, serum HBV DNA was often used to confirm the emergence of antiviral resistance (Table 4). A sensitive HBV DNA test therefore becomes crucial in making decisions to initiate treatment, in monitoring the patient’s response to treatment, and in detecting the emergence of antiviral resistance [6, 13]. This study found a heterogeneous dynamic range of the available HBV DNA testing methods, with the lower limit of quantification (LLOQ) of HBV DNA assays ranging from less than 6 IU/ml to less than 7500 IU/ml (or from <36 copies/ml to <4.5 × 105 copies/ml) in these four geographical areas. This wide range influenced the interpretation of laboratory results when comparing different assays. Furthermore, a relatively insensitive assay (i.e., with higher LLOQ) may fail to detect the virologic breakthrough in the lower range of HBV DNA levels, for example, a rise of HBV DNA from 3000 to 10000 copies/ml.
Of particular note, ALT testing, a cheaper and more readily available test than the HBV DNA test, was also reported as one of the primary means to identify suspected resistance in these Asian entities (except South Korea). Because biochemical breakthrough occurs even later than virologic breakthrough [4], reliance upon ALT elevation may lead to further delay in antiviral resistance rescue.
In addition to the clinical implications associated with antiviral resistance, the increase in clinical visits and laboratory tests impacts productivity and imparts additional financial burden to both patients and payers. This is particularly true in Asia where the prevalence of HBV infection and disease burden is especially high [14–18]. Before this survey, information was lacking about the direct medical costs of resistance management in Asia. By estimating the costs related to managing antiviral resistance in the first year after identification, this study showed that even without considering the costs of additional antivirals, the incremental direct medical costs to manage resistance are substantial. These findings are consistent with the study conducted by Tafesse et al. [19], who showed that lamivudine resistance in CHB patients was associated with a substantial increase in healthcare utilization. This information is important to payers in national reimbursement systems and healthcare insurance programs. It is also meaningful to CHB patients in the four geographic entities, particularly for patients paying out of pocket for the entire management of the disease in Asia. Therefore, the cost of resistance management should be considered when the total financial impact of antiviral therapy is evaluated.
To conclude, the development of antiviral resistance during CHB treatment is common in clinical practice in Asia. Although most of the interviewed physicians from Mainland China, South Korea, Taiwan, and Thailand favor a resistance-prevention strategy, medical practices in Asia vary by region and each region has its own set of challenges, ranging from the variability and frequency of resistance monitoring, limited access to specific resistance testing, and various scopes of reimbursement policies. These barriers may lead to a delay in the early identification of antiviral resistance and in the optimal timing for the initiation of rescue therapy. Managing suspected antiviral resistance can also incur significant additional costs. When initiating antiviral treatment, information on the direct medical costs of managing resistance needs to be considered by both treating physicians and CHB patients in addition to the clinical benefits and costs of each antiviral agent.
Acknowledgments
The authors thank Doctors Anna Lok, Bruce Kreter, Helena Brett-Smith, Suzy Ren, Iris Bian, Hyejin Hwang, Wonjoo Jung, Jennifer Lin, Alice Chen, Eric Lin, Atithep Mooreangratana, Ricardo Tamez, Simon Jones, Brenda Lau, Amy Chai, and Julie Newman for their review of the questionnaire and assistance in the project and the manuscript. In addition, the authors express their gratitude to the 575 physicians who participated in this survey. The fund for the study was provided by Bristol-Myers Squibb Company.
Footnotes
Authors contributed equally to the conception and preparation of the manuscript and are listed alphabetically by their last names.
Contributor Information
Siwaporn Chainuvati, Email: siswf@mahidol.ac.th.
Jun Cheng, Email: jun.cheng.ditan@gmail.com.
Jin Lin Hou, Email: jlhousmu@yahoo.com.cn.
Chao Wei Hsu, Email: hsu2406@adm.cgmh.org.tw.
Ji Dong Jia, Email: jia_jd@ccmu.edu.cn.
Piyawat Komolmit, Email: pkomolmit@yahoo.com.uk.
So Young Kwon, Email: sykwonmd@kuh.ac.kr.
Chang Hong Lee, Email: chlee@kuh.ac.kr.
Hong Li, Phone: +65-6500-9163, FAX: +65-6345-0932, Email: hong.li@bms.com.
Ying Li, Email: lisa.li@bms.com.
Chun Jen Liu, Email: cjliu@ntu.edu.tw.
Boon Leong Neo, Email: bl.neo@bms.com.
Cheng Yuan Peng, Email: cypeng@mail.cmuh.org.tw.
Tawesak Tanwandee, Email: sittw@mahidol.ac.th.
Suchat Wongcharatrawee, Email: suchat.wongcharatrawee@bms.com.
Jaw Ching Wu, Email: jcwu@vghtpe.gov.tw.
Ming Lung Yu, Email: fishya@ms14.hinet.net.
Xin Xin Zhang, Email: xin-xin-zhang@163.com.
References
- 1.Chen G, Lin W, Shen FM, IIoeje UH, London T, Evans AA. Chronic hepatitis B virus infection and mortality from non-liver causes: results from the Haimen City cohort study. Int J Epidemiol. 2005;34:132–137. doi: 10.1093/ije/dyh339. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Iloeje UH, Yang HY, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;30:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS, McMahon BJ. AASLD practice guidelines on chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, Cirrhosis Asian Lamivudine Multicenter Study Group et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 6.Keeffe EB, Dieterich DT, Han SB, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Leung N. Recent data on treatment of chronic hepatitis B with nucleos(t)ide analogues. Hepatol Int. 2008;2:163–178. doi: 10.1007/s12072-008-9061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asia-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Hepatitis B Virus Drug Resistance Working Group et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Lampertico P, Vigano M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414–1419. doi: 10.1002/hep.20939. [DOI] [PubMed] [Google Scholar]
- 12.Locarnini S. Primary resistance, multidrug resistance, and cross-resistance pathways in HBV as a consequence of treatment failure. Hepatol Int. 2008;2:147–151. doi: 10.1007/s12072-008-9048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlotsky JM, Dusheis G, Hatzakis A, Lau D, Lau G, Liang TJ, et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology. 2008;134:405–415. doi: 10.1053/j.gastro.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li SC, Ong SC, Lim SG, Yeoh KG, Kwong KS, Lee V, et al. A cost comparison of management of chronic hepatitis B and its associated complications in Hong Kong and Singapore. J Clin Gastroenterol. 2004;38(Suppl 3):S136–S143. doi: 10.1097/00004836-200411003-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CR, Kuo CW. Cost of chronic hepatitis B virus infection in Taiwan. J Clin Gastroenterol. 2004;38(Suppl 3):S148–S152. doi: 10.1097/00004836-200411003-00006. [DOI] [PubMed] [Google Scholar]
- 16.Guan ZQ, Dong ZH, Wang QH, Cao DX, Fang YY, Liu HT, et al. Cost of chronic hepatitis B infection in Mainland China. J Clin Gastroenterol. 2004;38(Suppl 3):S175–S178. doi: 10.1097/00004836-200411003-00010. [DOI] [PubMed] [Google Scholar]
- 17.Yang BM, Kim CH, Kim JY. Cost of chronic hepatitis B infection in South Korea. J Clin Gastroenterol. 2004;38(Suppl 3):S153–S157. doi: 10.1097/00004836-200411003-00007. [DOI] [PubMed] [Google Scholar]
- 18.Lesmana LA, Leung NWY, Mahachai V, Phiet PH, Suh DJ, Yao GB, et al. Hepatitis B: overview of the burden of disease in the Asia-Pacific region Liver Int 2006263–10. 10.1111/j.1478-3231.2006.01370.x17051681 [DOI] [Google Scholar]
- 19.Tafesse E, Claxton A, Granger A, Sanders J, Apelian D, Atillasoy E, et al. Estimates of health care costs for lamivudine-refractory chronic hepatitis B (CHB) patients. In Proceedings of the ISPOR 10th annual international meeting; 15–18 May 2005; Washington, DC