Abstract
Objective
BisGMA, a widely used component in dentin adhesive has very good mechanical properties after curing, but is relatively hydrophobic and thus, does not adequately infiltrate the water wet demineralized dentin collagen. Developing techniques that would lead to optimum infiltration of the hydrophobic component into the demineralized dentin matrix is very important. The purpose of this study was to evaluate interfacial morphological and chemical characteristics of the resultant adhesive-dentin interface when the ethanol wet bonding technique is used with hydrophobic adhesives.
Materials and methods
The occlusal one-third of the crown was removed from six unerupted human third molars; a uniform smear layer was created with 600 grit SiC. The dentin surface was etched with 35% phosphoric acid for 15 seconds before applying BisGMA/HEMA model adhesive using either water wet or ethanol wet bonding technique. Five-micro-thick sections of adhesive/dentin interface specimens were cut and stained with Goldner’s trichrome for light microscopy. Companion slabs were analyzed with SEM and micro-Raman spectroscopy.
Results
The presence of ethanol in the demineralized dentin increased adhesive collagen encapsulation as indicated by trichrome staining. The SEM results confirmed that the ethanol wet bonding improved the quality of the interface. Micro-Raman spectral analysis of the dentin/adhesive interface indicated there was a gradual decrease in penetration of BisGMA component for specimens using water wet bonding, while relatively homogeneous distribution of the hydrophobic BisGMA component was noted in the interface with ethanol wet bonding.
Significance
Wet bonding with ethanol instead of water permits better BisGMA infiltration improving the quality of interface. We speculate that the higher infiltration of hydrophobic BisGMA and better collagen encapsulation observed from the specimens using ethanol wet bonding would lead to more durable bonds because of improved resistance to hydrolytic attack.
Keywords: Dentin, ethanol wet bonding, BisGMA, interface, Raman
1. Introduction
Dental adhesives are widely used in modern dentistry to provide retention of composite restorations and reduce associated marginal micro-leakage. The most important components of dental adhesives are monomers, such as 2,2-bis[4(2-hydroxy-3-methacryloyloxy-propyloxy)-pheny] propane (BisGMA) [1]with cross-linking capability during polymerization [2]. BisGMA monomer provides better mechanical strength than monomethacrylates such as hydroxyethylmethacrylate (HEMA) because it forms cross-linked polymer compared to the linear polymer of HEMA upon curing [2]. However, BisGMA is a hydrophobic monomer with limited water solubility and as a result, exhibits phase separation in the presence of water [1, 3, 4].
In the water wet bonding technique, after acid etching, the continued presence of water in the demineralized dentin is critical to prevent the collapse of the dentin matrix. This is related to the fact that the Hoy’s hydrogen bonding force of water is higher than that of the exposed collagen fibrils and as such, the presence of water prevents the formation of collagen interpeptide hydrogen bonds that lead to dentin matrix collapse [2]. The space that is occupied by the water surrounding the non-collapsed collagen fibers can then be infiltrated by the resin monomers to form the “hybrid layer”. This layer is composed of 50% collagen matrix and 50% resin by volume if the resin completely replaces the space previously occupied by water [2, 5-7].
However, due to the presence of water in the current wet bonding techniques [8], there is the potential for phase separation of the hydrophobic BisGMA monomer which has limited water solubility. To avoid this problem, hydrophobic BisGMA monomer is blended with hydrophilic HEMA monomer [4]. However, is has been reported that the current generation of dentin adhesives are too hydrophilic [9] and absorb increased amounts of moisture following polymerization [10]. The consequence of this increased moisture sorption under clinical conditions is accelerated degradation and decreased mechanical properties that potentially affect the long-term survival of the interface and associated restoration [3, 11]. To address this issue, it is important to find a technique which will optimize the penetration of hydrophobic monomers like BisGMA into the wet demineralized dentin.
A new technique has been developed called “ethanol wet-bonding” [12] in which ethanol instead of water is used to support the demineralized dentin collagen matrix. Theoretically, with this technique, the acid etched demineralized collagen matrix is less hydrophilic, and should also prevent phase separation [5, 6] of the hydrophobic adhesive monomer. Moreover, because most hydrophobic monomers are also soluble in ethanol, it is possible to use an adhesive with a higher ratio of hydrophobic to hydrophilic monomers. Using more hydrophobic adhesives will create a less hydrophilic hybrid layer that, in theory, should absorb less water and be more durable [6]. Dentin bonding using the ethanol wet technique produced the highest microtensile bond strengths when compared to dentin bonding under moist with water or dry conditions [10]. However, little information on the interfacial chemistry and structure of ethanol wet bonding is available. The purpose of this study was to compare the morphological and chemical differences using two different bonding techniques, water wet and ethanol wet bonding, with a BisGMA/HEMA model adhesive. The null hypotheses tested were that as compared to water wet bonding techniques, the adhesive/dentin (a/d) bond formed using the ethanol wet bonding technique would show enhanced collagen encapsulation by resin and better hydrophobic monomer penetration into the interface.
2. Materials and Methods
2.1 Specimen preparation
Six extracted unerupted human third molars stored in 0.96% w/v phosphate buffered saline (PBS) containing 0.002% sodium azide at 4°C were used. These teeth were collected after the patients’ informed consent was obtained under a protocol approved by the University of Missouri-Kansas City Adult Health Sciences Institutional Review Board. The roots of the teeth were removed 2-3 mm below the cemento-enamel junction by means of a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL, USA). This created a flat base to attach the teeth to an aluminum block by means of a cyanoacrylate adhesive (Zapit, Dental Vetures of America, Inc, CA, USA). Then the occlusal one-third of the crowns of the teeth including occlusal enamel were removed with the same water-cooled low-speed diamond saw. To make sure there was no residual enamel, a light microscope was used to view the exposed dentin surface. A smear layer was created by abrading the exposed dentin surface with 600-grit sandpaper (Buehler, Lake Bluff, IL) under water until the surface was seemed to be smooth under light microscopy.
A model adhesive, with a composition similar to Single Bond (3M ESPE Dental Products, St. Paul, MN, USA) was used for this study [13]. The monomer mixture consisted of 2.25 g hydroxyethylmethacrylate (HEMA, Acros Organics, NJ, USA) and 2.75 g 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]-propane (Bis-GMA, Polysciences, Washington, PA, USA) with a mass ratio of 45/55. The solvent was 100% ethanol (Fisher, Fair Lawn, NJ, USA) with the ratio of solvent to monomer mixture at 40/60 totaling 1 g. The resultant solution contained 600 mg of the monomer mixture and 400 mg of 100% ethanol. Three-component visible light photoinitiators (Aldrich, Milwaukee, WI, USA) were also used: camphorquinone (CQ, 0.5 wt%), 2-(dimethylamino) ethyl methacrylate (DMAEMA, 0.5 wt%), and diphenyliodonium hexafluorophosphate (DPIHP 1.0 wt%). The concentration of the photoinitiators was calculated with respect to the total amount of monomer.
Before applying the adhesives using the water wet technique, the prepared dentin surface was acid etched for 15 s with 35% phosphoric acid. After acid etching, the surface was rinsed with distilled water for 10 s. Excess water was removed from the surface with absorbing paper (Kimwipes, Kimberly-Clark Global Sales,Inc, GA, USA) and the dentin remained moist. Two layers of adhesive were applied with gentle air drying for 10 s after each layer application followed by light polymerization for 20 s (550 mW/cm2, Spectrum® 800; Densply, Milford, DE, USA).
Applying the adhesive using the ethanol wet bonding technique was similar to the water wet technique; however, after acid etching and rinsing the demineralized dentin, the surface was treated with 2 drops (Transfer Pipets, Fisher Scientific, PA, USA) of 100% ethanol followed by a 10 s waiting period. This allowed the ethanol and water to mix together and therefore, in theory, remove water from the interfibrillar space in the demineralized dentin. The surface was then gently air dried for 5 s before applying another 2 drops of 100% ethanol and waiting another 10 s. Excess ethanol was removed from the surface with the same absorbent paper while keeping the dentin moist, before applying 2 coats of adhesive followed by light polymerization using the same protocol as with the water wet bonding technique. All adhesive-dentin specimens were stored for a minimum of 24 h in H2O at room temperature before being sectioned into slabs. The treated dentin surface was cut perpendicular and parallel to the bonded surface in ~1.8 mm increments by means of the water-cooled low-speed diamond saw. The final dimensions of the slabs were ~10 × 2 × 1.8 mm (3 slabs / tooth).
2.2 Differential staining technique
The rectangular, ~10 × 1.8 × 2 mm3, adhesive/dentin slabs (1 slab per tooth) were mounted on a PMMA support and 5-μm thick sections were cut from the slab using a tungsten carbide knife mounted on a Polycut S “sledge” microtome (Leica, Germany). Differential staining was accomplished with Goldner’s trichrome [5, 14] and the sections were examined and photographed at 100 X magnification with a Nikon E800 light microscope. To determine the width of the dentin demineralization and exposed collagen layer, photomicrographs whose exact magnification was established with a stage micrometer were used.
2.3 Scanning electron microscopy
Following sectioning for trichrome staining, the remaining a/d interface slabs were used for field emission scanning electron microscopy observations. In order to observe the presence of the interface and resin tags, specimens were observed last with field emission scanning electron microscopy. In order to determine the quality of the interface, specimens were prepared for SEM examination by treatment for 30 s of 5N HCL, washed with water, followed by soaking in 5 % NaOCl for 30 min. After water rinsing, the specimens were then dehydrated using increasing concentrations of ethanol starting with 50 %, 70 %, and 85 % for 15 min each, followed with 95 %, 100 %, and 100 % ethanol for 30 min each. After drying overnight, the prepared specimens were mounted on aluminum stubs and sputter coated with ~20 nm of gold-palladium. Specimens were examined at a variety of magnifications and tilt angles in a Philips XL#) ESEM-FEG (Philips, Eindhoven, Netherlands) at 5 kV.
2.4 Micro-Raman spectroscopy
Adjacent sections were analyzed using micro-Raman spectrometer (Jasco NRS-2000) consisted of an argon ion laser beam (514.5 nm) focused through an X100 Olympus Plan Neofluor water-immersion objective to a 1-μm beam diameter. Raman back-scattered light was collected through the objective and resolved with a monochromator. The spectra were recorded with a software-controlled CCD detector. The laser power was approximately 8 mW; an imaging system and high-resolution monitor were incorporated to enable visual identification of the position at which the Raman spectrum was obtained. Spectra were Raman-shift-frequency-calibrated using the known line of silicon.
The adhesive/dentin interface specimens were mounted and covered with distilled water in preparation for micro Raman spectroscopy analysis. Mapping spectra were acquired a minimum of 3 different positions corresponding to 1-μm intervals across the adhesive/dentin interface using the computer controlled x-y-z stage with a minimum step width of 50 nm. Spectra were obtained at a resolution of ~6 cm-1 over the spectral region of 875 cm-1 to 1785 cm-1 and with an integration time of 120 s. Two consecutive scans were obtained from each site.
3. Results
3.1 Staining light microscopy
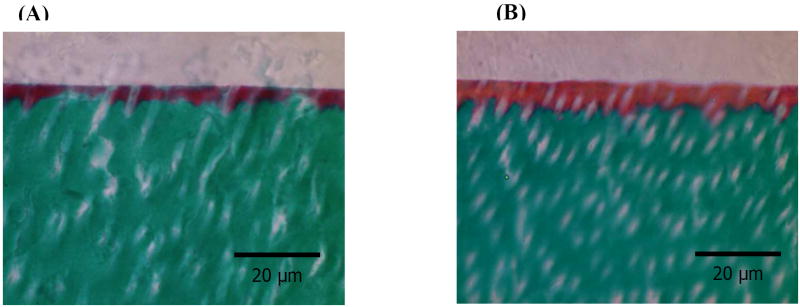
Representative light micrographs of Goldner’s trichrome stained sections for water and ethanol wet bonding groups are shown in Figure 1. In the trichrome differential staining, mineralized dentin collagen usually stains green, unprotected, demineralized dentin collagen/protein stains red, and pure adhesive will either be unstained or a faint beige color [15, 16]. If the collagen is totally encapsulated, the hybrid layer would be beige or unstained [5]. Representative micrographs of stained dentin interface for water wet bonding showed a red interface color. The width of this interface was ~ 3.1 um. Representative micrographs of stained dentin interface for ethanol wet bonding showed an orange interfacial color. The difference in color represents the extent of exposed collagen in the interface. The orange color indicates that the collagen was more encapsulated with adhesive. This width of this hybrid layer was ~ 5.7 um. This interfacial zone for the specimens using the ethanol wet bonding technique was much wider than that of the specimens using water wet bonding.
Figure 1.
Representative Goldner’s trichrome stains for water wet bonding (A) and ethanol wet bonding (B). Green color represents dentin, beige color represents adhesive, red color represents exposed collagen fibrils, and orange color represents partially encapsulated collagen fibrils.
3.2 Scanning electron microscopy
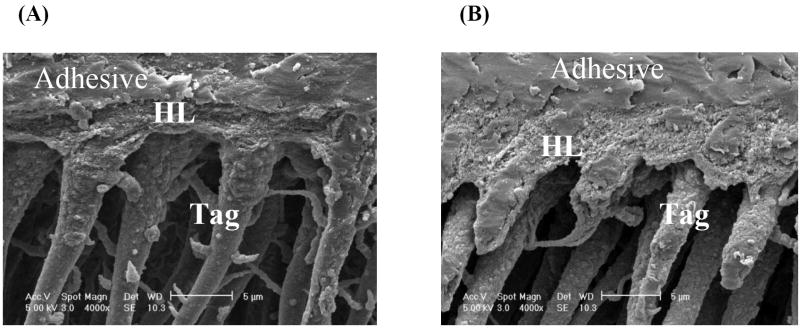
Representative SEM micrographs of the unpolished adhesive/dentin interface specimens with water wet and ethanol wet bonding are shown in Figure 2. Both interface specimens were treated with 5 N HCl (30 s) followed by 5% NaOCl (30 min) (acid-bleach treatment). For the specimens using water wet bonding technique, the acid-resistant layer were observed, however, the layer was porous and uneven indicating the quality of this layer was low from the acid/bleach treatment. The thickness of this resin-infiltrated layer was ~ 2.6 um. For the specimens that use ethanol wet bonding, the well defined, resin-infiltrated layer thickness was ~ 4.0 um, which was wider than that of water wet bonding specimens. It was also noted that all specimens contained long resin tags indicating high infiltration into tubules.
Figure 2.
Representative SEM micrographs of acid-bleach treated dentin interface with water wet bonding (A), ethanol wet bonding (B). HL stands for hybrid layer.
3.3 Raman microscopy
The Raman spectra of the model adhesive resin and dentin are shown in Figure. 3. The major peaks associated with the dentin collagen appear at 1242 cm-1 (amide III), 1273 cm-1 (amide III), 1453 cm-1 (CH2), and 1667 cm-1 (amide I) [17, 18]. The spectral features associated with the mineral of dentin occur at 961 cm-1 (phosphate) and 1072 cm-1 (carbonate) [17, 18]. The peaks associated with the adhesive occur at 1720 cm-1 (carbonyl), 1609 cm-1 (phenyl C=C), 1453 cm-1 (CH2 def), 1113 cm-1 (C-O-C). These peaks are associated with the model adhesive methacrylate monomers. In particular, the peaks at 1609 and 1113 cm-1 are associated with the BisGMA monomer. The Raman intensities at 1453 and 1113 cm-1, which are associated with all monomers in adhesive and BisGMA monomer in model adhesive respectively, were used to monitor the adhesive concentration as a function of position across the interface.
Figure 3.
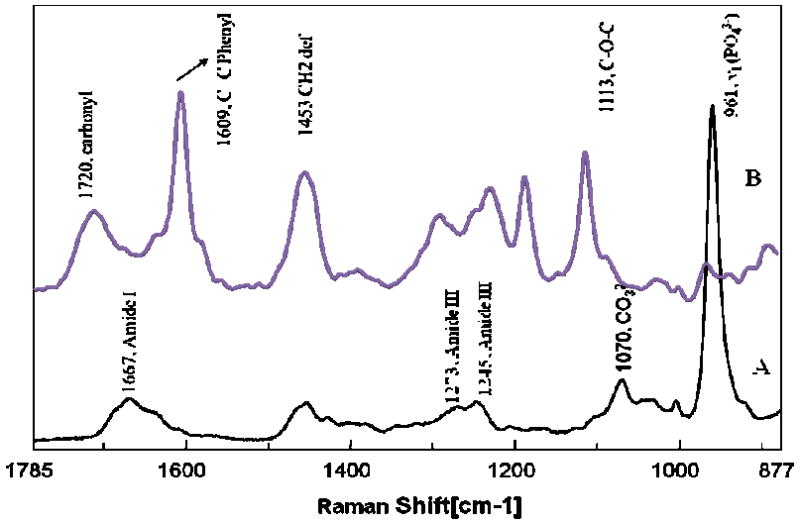
Raman spectra of dentin (A) and pure model adhesive resin (B).
Figure 4 shows the Raman microscopic images of model adhesive/dentin interfaces using water (Figs. 4A, B) and ethanol (Figs. 4C, D) wet bonding techniques. The images were collected from a 20×14 μm2 area. The relative intensity of the spectral features associated with adhesive resins, i.e. 1113 (C-O-C) and the mineral (P-O, 961 cm-1) are represented by the differences in color. Red represents the highest relative intensity while black represents the lowest. Images of these two spectral parameters (1113 and 961 cm-1) provide information about the spatial distribution of the BisGMA monomer and mineral (apatite) across the adhesive/dentin interface. Investigation of all of the images indicates that the interface is not a simple, uniform layer. Such survey maps of the molecular structure provided a reliable and powerful means of identifying the distributions of adhesive monomers and dentin demineralization. As shown in Fig. 4A, C, BisGMA monomer penetrated into dentin tubules and spread into intertubular region through open tubules. The extent of BisGMA monomer penetration was higher in the intertubular regions close to tubules as compared to the middle regions between the tubules. The images of mineral revealed a demineralized layer and tubules (Fig. 4B, D). The diffusion of BisGMA monomer differed between water and ethanol wet bonding. BisGMA monomer penetrated more into the demineralized dentin, especially, the intertubular area in ethanol wet bonding specimens (Fig. 4C).
Figure 4.
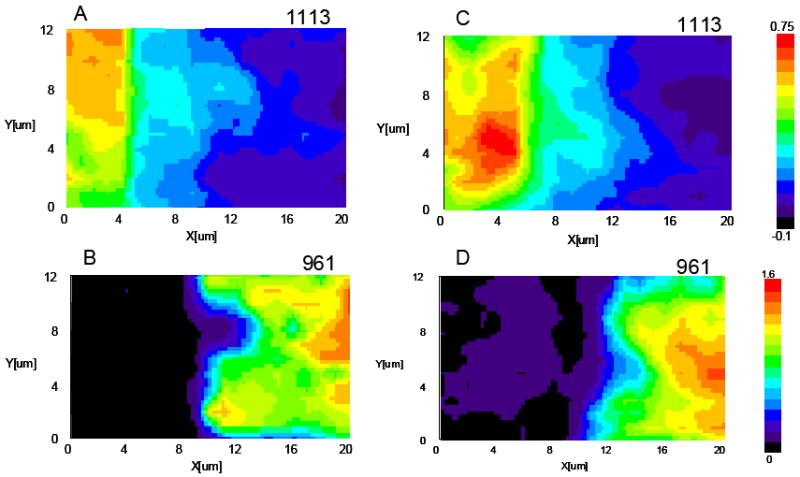
Micro-Raman images of adhesive/dentin interfaces. Water wet bonding (A, B); ethanol wet bonding (C, D). 1113 cm-1 is associated with BisGMA, 961 cm-1 is associated with mineral phosphate.
Since the micro-Raman images contained hundreds of very high quality spectra at a resolution of 1 μm, the difference in relative composition and other chemical information between water and ethanol wet bonding can be determined across the length and breadth of the adhesive/dentin interface. As an example, a series of micro-Raman mapping spectra across the interfaces prepared using water and ethanol wet bonding are represented in Figure 5A and B. In Figure 5A, the first six spectra were acquired from pure adhesive. Peaks associated with the adhesive and collagen components of dentin were noted in the seventh spectrum. The strong peak of P-O group (961 cm-1) in the twelfth spectrum suggested the bottom of the demineralized dentin layer. Similarly, dentin was demineralized to a similar depth for the all specimens using both wet bonding techniques of roughly 5-6 μm. In Figure 5B, the first four spectra were from pure adhesive, the fifth to tenth spectra were from the interface, and the eleventh spectrum was from mineralized dentin. Major spectral changes have been marked with arrows (Fig. 5). The relative intensities of the Raman peaks attributed to the adhesive (1113, 1609, 1720 cm-1) were higher in the interface that use ethanol wet bonding when comparing to water wet bonding interface spectra.
Figure 5.
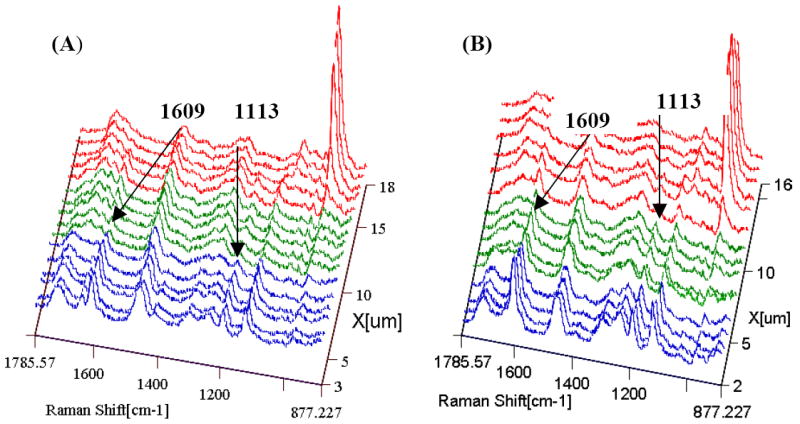
Micro-Raman mapping spectra of adhesive/dentin interfaces. Blue color represents pure adhesive; green color represents the interface; red color represents the dentin. Water wet bonding (A); ethanol wet bonding (B). Peaks associated with BisGMA are 1609 cm-1 and 1113 cm-1.
To quantitatively determine differences in adhesive infiltration into demineralized dentin at different depths, the ratios of the relative integrated intensities of peaks associated with adhesive and collagen were calculated. The CH2 in HEMA and BisGMA (1453 cm-1) and C-O-C in BisGMA monomer (1113 cm-1) were used to monitor the adhesive monomers concentration and the amide I peak (1667 cm-1) was selected for collagen. Figure 6 represents the BisGMA monomer and the adhesive monomers penetration as a function of depth in the intertubular region of dentin between water and ethanol wet bonding. As compared to water wet bonding, the ratios of 1453/1667 for adhesive monomers/collagen as a function of spatial position were always higher in the interface using ethanol wet bonding (Fig. 6B). The ratios of 1113/1667 (BisGMA/collagen) showed a gradual decrease in the concentration of BisGMA monomer across the adhesive/dentin interface using water wet bonding, while homogeneous distribution of the hydrophobic BisGMA component was noted in the interface when ethanol wet bonding was used (Fig. 6A).
Figure 6.
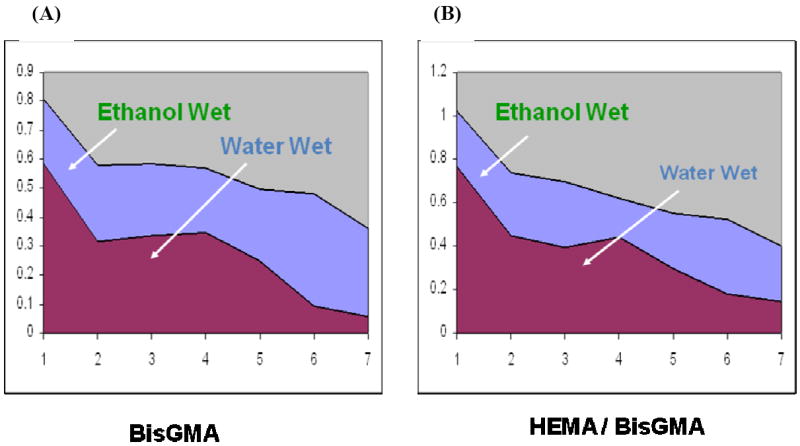
Raman intensity ratios of 1113/1667 (A), 1453/1667 (B) as a function of spatial position across the adhesive/dentin interface.
4. Discussion
There are ongoing problems associated with the dentin adhesives such as poor infiltration of wet dentin with hydrophobic monomers yielding a less than optimum hybrid layer; however, when more hydrophilic adhesive monomers are used, the resultant hybrid layer tends to be more prone to hydrolytic degradation. To potentially address these issues, in this study, ethanol wet bonding was examined to test the hypotheses that hydrophobic monomers, like BisGMA, can better encapsulate collagen fibrils and better infiltrate into demineralized dentin saturated with ethanol rather than with water. The results of this study support both hypotheses.
SEM observations of the resin-dentin interface of specimens exposed by the acid-bleach method indicated that the quality of the hybrid layer was low with the specimens using water wet bonding (Fig. 2). Consistent with the SEM results, staining light microscopic studies suggested that the extent of exposed collagen in the interfaces was different between these two bonding techniques. Light microscopy revealed a dark red layer in the micrograph of the interface specimens using water wet bonding. The color of this layer was pale orange for specimens using ethanol wet bonding (Fig. 1). The difference in color of the a/d interfacial zone is dependent on the availability of exposed collagen for reaction with the Goldner’s trichrome stains. As previously reported, unprotected, exposed collagen stained dark red, partially encased collagen by resin stained light color (i.e. orange) [14, 16]. If the collagen was totally encapsulated by adhesive resins and it would not be available for staining [5]. Thus, adhesive penetration, or more specifically, collagen encapsulation by resins varied when using the two different bonding techniques. Ethanol-saturated demineralized dentin resulted in better resin-collagen interactions/encapsulation. This may be due to ethanol having a lower vapor pressure than water, and also lowering the vapor pressure of water when mixed together; therefore removing/evaporating more water-ethanol from around the collagen fibrils for the adhesive monomers to bond to. However, the fact which the interface still stained orange also indicated that collagen fibrils at the interface were not totally encased by resin even in the ethanol saturated demineralized dentin. Micro-voids might exist in the a/d interface using ethanol wet bonding, which was probably attributed to incomplete ethanol evaporation [5].
Besides the difference in color, the thickness (~3.1 um) in the interface of specimens that use water wet bonding was also much lower than that (~ 5.7 um) of specimens using ethanol wet bonding. This was also confirmed by SEM results, although the thicknesses measured by SEM were lower than those by staining light microscopy. This is mainly due to the damaging characteristics of the SEM preparation technique, which removes underlying dentin and measures acid-bleach resistance. The higher thickness in the ethanol wet bonding interface might be associated with the following reasons. For example, ethanol used to wet the collagen matrix may create some interpeptide hydrogen bonds, which will stiffen the matrix to minimize its shrinkage/collapse [10]. This situation would be advantageous since it would likely lead to better infiltration of the monomers into the collagen matrix which was confirmed by the micro-Raman results.
The micro-Raman mapping/imaging technique is a powerful means for obtaining chemical images of the adhesive/dentin interface. The diffusion and/or distribution of adhesive monomers into the interface and the degree of dentin demineralization have been directly observed using micro-Raman imaging (Fig. 4). Adhesive monomers readily penetrated into dentinal tubules, which also served as avenues for infiltration of suitable monomers into the intertubular regions. However, there was a distinct difference in the resin monomer penetration between the regions close to tubules and the regions away from tubules in the intertubular dentin. For example, the extent of BisGMA monomer penetration was higher in the intertubular regions close to tubules as compared to the middle regions between the tubules. In addition, although it is only qualitatively shown in the images, the ethanol wet bonding increased the penetration of BisGMA in the intertubular regions as compared to water wet bonding. The results were further confirmed by more quantitative analyses (Fig. 6)
The diffusion of resin monomers differed substantially in the model adhesive when compared ethanol wet bonding to water wet bonding technique. The adhesive penetration dramatically increased in the ethanol-saturated demineralized dentin (Fig. 6). Micro-Raman results indicated that there was a gradual decrease in penetration of BisGMA component for specimens using water wet bonding, while relatively homogeneous distribution of the hydrophobic BisGMA component was noted in the interface when ethanol wet bonding was used (Fig. 6). The dramatic increase in BisGMA penetration may be due to the following reasons. BisGMA is soluble in ethanol, not water. The ethanol-solvated model adhesive could easily penetrate into the ethanol-saturated demineralized dentin, instead of undergoing phase-separation as occurs in water-saturated dentin [4, 19]. In addition, it was previously reported that a more than 80% volume increase in the interfibrillar area occurs due to the shrinkage of the collagen fibrils [12]. It was speculated that ethanol might collapse the highly hydrated glycosaminoglycans in interfibrillar spaces [20]. Maintaining interfibrillar spaces would promote better penetration of monomers.
In summary, ethanol wet bonding technique allows for hydrophobic monomers in adhesives to penetrate more/deeper into dentin, encapsulate collagen fibers better, and be more resistant to acid-bleach treatment when compared to water wet bonding technique. As mentioned earlier, having ethanol in the demineralized dentin plays a major role on BisGMA infiltration, resulting in more protection of the collagen by the adhesive resin. No matter what technique is used, water must be displaced from around the collagen fibrils in order for the adhesive monomers to bond. We speculate that the higher infiltration of hydrophobic BisGMA and better collagen encapsulation observed from the specimens using ethanol wet bonding would lead to more durable bonds because of improved resistance to hydrolytic attack. However, the above results were obtained in the laboratory under relatively optimum conditions. Future laboratory studies of ethanol wet bonding should include modifications to more closely simulate clinical conditions such as using teeth with restoration preparations rather than flattened molars and adding simulated pulpal pressure throughout the etching/adhesive procedures. Another area of interest that will be important to this technique includes studying the effect of ethanol on odontoblasts. Finally, as with all research, the laboratory results must be validated with future clinical trials that include the ethanol wet bonding technique.
Acknowledgments
This study was supported by the UMKC SOD Rinehart Foundation and U.S. Public Health Service (USPHS) Research Grant DE 015281 (YW) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Maryland. The authors would like to acknowledge Dr. David H Pashley from Medical College of Georgia for scientific suggestions, and also to acknowledge the SEM technical support of Dr. Vladimer Dusevich from UMKC School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 3.Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B Appl Biomater. 2009;88:339–48. doi: 10.1002/jbm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res. 2003;82:141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 6.Becker TD, Agee KA, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Infiltration/evaporation-induced shrinkage of demineralized dentin by solvated model adhesives. J Biomed Mater Res B Appl Biomater. 2007;80:156–65. doi: 10.1002/jbm.b.30580. [DOI] [PubMed] [Google Scholar]
- 7.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 8.Kanca J., 3rd Improving bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:35–43. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 9.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726–31. [PubMed] [Google Scholar]
- 10.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–21. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res. 2008;87:829–33. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, Tay KC. Bonding BisGMA to dentin--a proof of concept for hydrophobic dentin bonding. J Dent Res. 2007;86:1034–9. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res Part A. 2007;80A:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Spencer P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner’s trichrome. Am J Dent. 2005;18:66–72. [PubMed] [Google Scholar]
- 15.Wang Y, Spencer P. Effect of acid etching time and technique on interfacial characteristics of the adhesive-dentin bond using differential staining. Eur J Oral Sci. 2004;112:293–299. doi: 10.1111/j.1600-0722.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 16.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79:1458–1463. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Spencer P, Walker MP. Chemical profile of adhesive/caries-affected dentin interfaces using Raman microspectroscopy. J Biomed Mater Res Part A. 2007;81A:279–286. doi: 10.1002/jbm.a.30981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Spencer P. Exploring the nature of acid-resistant hybrid layer with wet bonding. Oper Dent. 2004;29:650–655. [PubMed] [Google Scholar]
- 20.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192(Pt 3):391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]