Summary
Introduction
Alcohol and nicotine both alter learning, locomotion, and anxiety, yet no study has directly examined the interactive effects of these drugs across these behaviors within subjects. Such a comparison would determine if the drugs produce independent effects on each behavior. The plus-maze discriminative avoidance task (PMDAT) allows within-subject measurement of these behaviors.
Methods
For training, each mouse explored the elevated plus-maze for five minutes and each time a mouse entered the aversive enclosed arm, a light and white noise were turned on. For testing, each mouse was returned to the center of the maze and, for three minutes, the time in each arm or in the center area was recorded. No cues were turned on during testing. The effects of ethanol (0.6–2.6 g/kg 15 minutes before training) and nicotine (0.045–0.18 mg/kg 5 minutes before training), alone or in combination, on behavior were examined.
Results
Ethanol dose-dependently decreased anxiety, increased locomotion, and decreased learning but different doses altered each behavior. Nicotine dose-dependently increased anxiety and locomotion and decreased learning but different doses altered each behavior. Nicotine (0.09 mg/kg) reversed ethanol-associated changes in learning (1.0 and 1.4 g/kg), locomotion (1.4 g/kg), and anxiety (1.4 g/kg).
Conclusions
The effects of nicotine or ethanol on learning occurred at different doses than those that altered anxiety or locomotion, suggesting that the drug effects on learning are independent of the effects on anxiety and locomotion. With combined administration, nicotine reduced ethanol-associated deficits in learning and changes in anxiety and locomotion.
Keywords: Addiction, Memory, Motor Behavior, Alcohol, Acetylcholine
Introduction
Alcohol and nicotine may have opposing effects on learning and memory that could contribute to the high frequency of co-use of these drugs (Dawson, 2000). Ethanol impairs the acquisition of learning, and this has been demonstrated in many paradigms, including contextual and cued fear conditioning, the radial arm maze, and passive avoidance (Bammer and Chesher, 1982; Gibson, 1985; Gould, 2003; Higgins et al., 1992; Weitemier and Ryabinin, 2003). In contrast, nicotine can enhance the acquisition of learning and other cognitive processes such as attention, as demonstrated in the radial arm maze, passive avoidance learning, and contextual fear conditioning (Gould and Wehner, 1999; Hefco et al., 2004; Rezvani and Levin, 2003). Nicotine can also reverse ethanol-induced learning deficits. For instance, acute nicotine ameliorated ethanol-induced deficits in contextual and cued fear conditioning, radial arm maze, and passive avoidance(Gould and Lommock, 2003; Gulick and Gould, 2008; Rezayof et al., 2008; Tracy et al., 1999). However, it is unclear whether the effects of nicotine on these ethanol-induced deficits in fear conditioning are due to direct effects on learning or to effects such as changes in anxiety and locomotor activity that can modify the acquisition or expression of learned responses.
Both nicotine and alcohol alter anxiety (Abreu-Villaca et al., 2008; Biala and Budzynska, 2006; Durcan and Lister, 1988) and locomotion (Durcan and Lister, 1988; Elliott et al., 2004). Because the dependent measure of learning in the studies that examined the interactive effects of nicotine and ethanol on fear conditioning was freezing, drugs that can alter locomotion could either mask drug effects on learning in fear conditioning or could produce changes in fear conditioning that could be misinterpreted as changes in learning. Similarly, fear conditioning by its nature can be affected by changes in anxiety (Lissek et al., 2008). Thus, ethanol and/or nicotine effects on anxiety could also confound interpretation of drug-related changes in fear conditioning. Therefore, to facilitate understanding the interactive effects of alcohol and nicotine on learning, it must be established if drug-induced changes in locomotion and/or anxiety contribute to purported drug effects on learning. If the effects of nicotine and/or alcohol on learning, locomotion, and anxiety are independent, the doses of each drug that produce these effects may also differ.
Previous work found that within subjects, the ethanol disruption of learning in the plus-maze discriminative avoidance task (PMDAT) was independent of the effects of ethanol on anxiety or locomotion (Kameda et al., 2007); however, it remains unknown if the same holds true for nicotine alone and co-administration of nicotine and ethanol. Developed by Silva and colleagues (1997), the PMDAT uses an elevated plus-maze consisting of two opposing, open arms and two opposing, enclosed arms to examine changes in anxiety, locomotion, and learning within subjects. In the present series of experiments, we used the PMDAT to investigate the dose response effects of ethanol and nicotine, administered alone and together, on learning, anxiety, and locomotion. We hypothesized that ethanol and nicotine would dose-dependently alter learning, anxiety and locomotion, but that the effects on each behavior would emerge at different drug doses. In addition, it was hypothesized that nicotine would reverse deficits in learning induced by a dose of ethanol that did not alter anxiety or locomotion.
Methods
Subjects
One hundred and eighty-six male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were tested at 8–12 weeks of age (20–30 g). Mice were housed in groups of 4 mice per cage and had ad libitum access to food and water. A 12-hr light–dark cycle (lights on at 7:00 am) was maintained, with all testing done between 9:00 am and 5:00 pm. Procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate salt was procured from Sigma (St. Louis, MO). Ethanol was procured from Fisher Scientific (Pittsburgh, PA). Ethanol and nicotine were prepared in physiological saline (pH = 6.1) and administered via intraperitoneal injection (i.p.). Ethanol (0.0, 0.6, 1.0, 1.4, 1.8, 2.6 g/kg; pH = 6.2) was administered 15 minutes before training and nicotine (0.0, 0.045, 0.09 or 0.18 mg/kg, freebase weight; pH = 4.8) was administered 5 minutes before training (methods based on Gulick and Gould, 2008; Kameda et al., 2007). Injection volume for nicotine was 0.01 ml/g body weight, and for ethanol it was 20% vol/vol in saline.
Apparatus
The modified elevated plus-maze consisted of a wood base and grey Plexiglas floors and walls with no top. The base of the maze was 36 inches off the ground, with two, grey opposing enclosed (walled) arms (12 × 3 × 6 inches, L × W × H, 5 lx) and two opposing open arms (12 × 3 inches, L × W, 10 lx). A 75-watt lamp (600 lx) was placed directly over one enclosed arm, and a speaker connected to a noise generator (85 dB) was placed directly below the same arm.
Procedure
For all experiments, groups consisted of 6–9 animals per condition. Data for mice that jumped off of the maze or never entered the aversive arm during training were excluded from the analysis. For training, each mouse was placed in the center of the apparatus and, for a period of five minutes, the animal freely explored the maze. This length of time is shorter than has been used previously (Kameda et al., 2007), but was necessary as longer training durations produced a ceiling effect on learning that could have obscured any enhancement of learning. The time spent in each arm or in the center area was recorded by a researcher who was blind to the drug treatment. Total number of entries into each arm was also recorded. Each time that the mouse entered the aversive enclosed arm, the 75-watt light and the 85 dB white noise were turned on by the researcher. Both cues were turned off when the mouse exited the aversive enclosed arm.
For testing 24 hours later, each mouse was returned to the center of the apparatus and, for a period of three minutes, was again free to explore the maze. Time in each arm and arm entries were again recorded. No cues were turned on during the testing session. The maze was cleaned with 70% ethanol before each training and testing session. This procedure was based on Silva and Frussa-Filho (2000).
Scoring
Entry into an arm was counted when all four paws crossed into that arm. Time in the center arm was not counted toward any measure. Total number of entries into all arms was used as an index of locomotion. Percent time in the aversive enclosed arm vs. percent time in the non-aversive enclosed arm (time in arm/time in all arms) was used as an index of learning (increased time in the aversive enclosed arm and decreased time in the non-aversive enclosed arm = decreased learning), and percent time in the open arms (time in open arms/time in all arms) was used as an index of anxiety (increased time in the open arms = decreased anxiety).
If mice learn to avoid the aversive enclosed arm, then time in the aversive enclosed arm should be lower at testing in trained mice compared to untrained mice. To establish if learning occurred, we put a group of mice (n = 7) through the training and testing sessions without presenting any stimulus at training. If the arm to be associated with aversive stimuli is aversive itself, then we would expect animals to spend less time in it during testing even when stimuli were not presented at training. Paired t-tests revealed a significant difference at training, t(6) = 3.98, p<0.05 (percent time in the non-aversive enclosed arm: 45.6±2.3 [mean ± standard error]; percent time in the aversive enclosed arm: 31.5±2.4), but not at testing, t(6) = 0.519, p>0.05 (percent time in the non-aversive enclosed arm: 53.9±11.8; percent time in the aversive enclosed arm: 42.1±11.2). Thus, untrained animals spent a similar amount of time in the (to be) aversive and non-aversive arms at testing, suggesting that avoidance of the aversive arm in trained animals reflects a learned association between the aversive stimuli and the arm in which they were presented. The disparity in time spent in the aversive versus the non-aversive arm at training may be due to slight differences between the arms. For example, the presence of the lamp above the aversive arm may have resulted in a slight effect at training but such differences were not sufficient to produce a learned aversion for the arm since no difference was seen at testing.
Statistical Analyses
Differences between groups were analyzed by one-way or two-way Analysis of Variance (ANOVA). Levene’s test for homogeneity of variance was used to determine appropriate post-hoc tests, and either Tukey’s (homogeneous variability) or Duncan’s (non-homogeneous variability) post-hoc tests were run at the level of p<0.05. Statistics were calculated with SPSS (Version 14; SPSS, Chicago, IL).
Effects of Ethanol and Nicotine in the PMDAT
We first examined the dose-dependent effects of ethanol (0.6–2.6 g/kg) administered at training on plus-maze discriminative avoidance. To determine whether the effects of ethanol on learning are due to state-dependent effects, we then compared the effects of ethanol (1.0 g/kg) administered before training or before training and testing on plus-maze discriminative avoidance. If memory for the plus-maze is state–dependent, then animals that receive the same treatment during both training and testing (i.e. saline on both days or drug on both days) should perform better than animals that receive different treatments during training and testing (e.g. saline at training and drug at testing). In a second experiment, we examined the dose-dependent effects of nicotine (0.045–0.18 mg/kg) administered at training on the same task. It has previously been demonstrated that systemic nicotine only enhances learning when administered before training and testing in the fear conditioning paradigm (Gould and Higgins, 2003); to determine whether state-dependent effects underlie the impairment of learning by the 0.18 mg/kg dose of nicotine, we administered this dose before training or before training and testing. Since previous studies have shown that nicotine can reverse ethanol-induced learning deficits even when nicotine alone does not enhance performance (Gulick and Gould, 2008), we tested the interactive effects of ethanol and nicotine in the PMDAT. We chose three ethanol doses (1.0, 1.4, 2.6 g/kg) that produced diverse effects on anxiety, learning, and locomotion in the initial dose response experiment, and the 0.09 mg/kg dose of nicotine based on the dose-response experiment and previous work in our laboratory (Gould, 2003; Gould and Lommock, 2003).
Results
Ethanol Dose-Response in the PMDAT
Percent time in the aversive enclosed arm
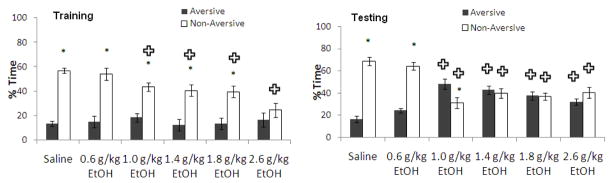
Ethanol administered at training dose-dependently altered learning in the PMDAT. At training, two-way ANOVA revealed significant effects of drug treatment, F(5,37) = 10.03, p<0.001 and of arm, F(1,41) = 61.23, p<0.001, as well as a significant interaction, F(5,37) = 9.07, p<0.001. Tukey’s post-hoc tests revealed that all groups except the group administered 2.6 g/kg ethanol spent more time in the non-aversive enclosed arm than the aversive enclosed arm (p<0.05); there was no difference in time in the aversive vs. non-aversive enclosed arm for the group administered 2.6 g/kg ethanol. In addition, the groups administered 1.0–2.6 g/kg ethanol spent less time in the non-aversive enclosed arm than the saline-treated group (p<0.05) but there were no differences in time in the aversive enclosed arm. At testing, two-way ANOVA revealed no significant effect of drug treatment, but a significant effect of arm, F(1,41) = 15.63, p<0.001, as well as a significant interaction, F(5,37) = 55.68, p<0.001. Tukey’s post-hoc tests revealed that the groups administered saline or 0.6 g/kg ethanol were not significantly different and both groups spent significantly more time in the non-aversive enclosed arm than the aversive enclosed arm (p<0.05). The groups administered 1.0–2.6 g/kg ethanol spent more time in the aversive enclosed arm and less time in the non-aversive enclosed arm than saline controls (p<0.05), and the groups administered 1.4–2.6 g/kg ethanol showed no arm preference at testing while the 1.0 g/kg showed a preference for the aversive enclosed arm (Figure 1). This suggests that a wide range of ethanol doses administered at training decreased learning assessed 24 hours later.
Figure 1.
Effects of ethanol (0.6–2.6 g/kg; i.p.) administered at training on learning [percent time in the aversive and non-aversive enclosed arms]. (n=6–8; Mean ± SEM; * = significant difference between aversive and non-aversive enclosed arms at p<0.05; + = significant difference from controls at p<0.05).
A separate set of mice was administered 1.0 g/kg ethanol before training or before both training and testing to determine whether the effect of ethanol on learning was state-dependent. At training, two-way ANOVA revealed a significant effect of arm, F(1,17) = 81.19, p<0.001, but no significant effect of drug treatment, and no interaction (Table 1). Tukey’s post-hoc test revealed that all three groups spent significantly more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05). Two-way ANOVA revealed no effect of drug but a significant effect of arm at testing, F(1,17) = 49.21, p<0.001, and a significant interaction F(2,16) = 24.79, p<0.001 (Table 1). Tukey’s post-hoc tests revealed that the group administered saline and the group administered ethanol on both days spent more time in the non-aversive than the aversive enclosed arm (p<0.05) and that both groups administered ethanol spent significantly less time in the non-aversive enclosed arm and more time in the aversive enclosed arm than saline controls (p<0.05); the group administered ethanol at both training and testing spent less time in the aversive arm than the group administered ethanol at training only, but this difference did not reach statistical significance (p=0.1). Thus, the effects of ethanol on learning are not due to state-dependent changes.
Table 1.
Changes in locomotion [number of arm entries] during training in the PMDAT with training-day administration of saline (i.p.), ethanol (0.6–2.6 g/kg; i.p.), or nicotine (0.045–0.18 mg/kg; i.p.)
| Training | Testing | |||
|---|---|---|---|---|
| Aversive Arm | Non-Avers. Arm | Aversive Arm | Non-Avers. Arm | |
| Saline | 14.4±1.7 | 56.6±5.5 | 19.4±4.3 | 67.3±3.9 |
| EtOH at Training | 14.8±2.3 | 47.5±4.4 | 38.2±3.5* | 45.7±4.9* |
| EtOH at Both | 13.3±1.9 | 45.9±5.5 | 29.2±3.3* | 45.1±4.1* |
n = 6–9; Mean ± SEM;
= significantly different from controls at p<0.05
Percent time in the open arms
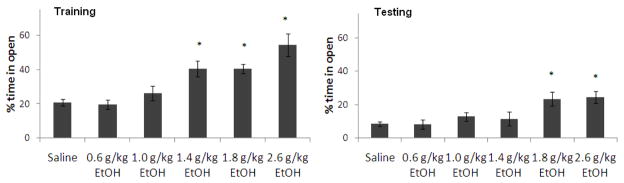
In addition to the effects of ethanol on learning, the effects of ethanol administered at training on anxiety were also examined. During training, there was a significant effect of ethanol dose on percent time in the open arms, F(5,37) = 11.02, p<0.01. Duncan’s post-hoc tests revealed that the groups administered 1.4–2.6 g/kg ethanol spent a significantly higher percentage of time in the open arms compared to saline controls (p<0.05). There was also a significant effect of ethanol dose on percent time in the open arms during testing, F(5,37) = 5.04, p<0.01. Tukey’s post-hoc tests revealed that the groups administered 1.8 and 2.6 g/kg ethanol at training spent a higher percentage of time in the open arms compared to saline controls at testing (p<0.05), while the groups administered 0.6–1.4 g/kg ethanol were not significantly different from controls (Figure 2). As expected, higher doses of ethanol decreased anxiety on training day; interestingly, the two highest doses of ethanol also decreased anxiety on testing day, although ethanol administration occurred only before training.
Figure 2.
Effects of ethanol (0.6–2.6 g/kg; i.p.) administered at training on anxiety [percent time in the open arms]. (n=6–8; Mean ± SEM; * = significantly different from controls at p<0.05).
Total entries into all arms
Ethanol administration at training altered locomotor activity. During training, there was a significant effect of ethanol dose on mean total entries, F(5,37) = 4.86, p<0.01. Duncan’s post-hoc test revealed that the group administered 1.4 g/kg ethanol had significantly more arm entries than saline controls (p<0.05) while all other groups were similar to saline controls (Table 2). There was also a testing day effect of ethanol on mean total entries, F(5,37) = 4.13, p<0.01. Duncan’s test revealed that the groups administered 1.8 and 2.6 g/kg ethanol at training had significantly more entries than saline controls during testing (p<0.05) but all other groups were similar to controls (Table 3). Thus, a moderate dose of ethanol increased locomotion on training day, and higher doses of ethanol administered at training only were associated with increased locomotion on testing day.
Table 2.
Changes in locomotion [number of arm entries] during testing in the PMDAT with training-day administration of saline (i.p.), ethanol (0.6–2.6 g/kg; i.p.), or nicotine (0.045–0.18 mg/kg; i.p.).
| Training | Number of Entries |
|---|---|
| Saline | 23 ± 0.94 |
| 0.6 g/kg EtOH | 25 ± 2.40 |
| 1.0 g/kg EtOH | 27.87 ± 2.24 |
| 1.4 g/kg EtOH | 35.57 ± 2.10* |
| 1.8 g/kg EtOH | 27.83 ± 4.43 |
| 2.6 g/kg EtOH | 21.86 ± 2.01 |
| Saline | 19.33 ± 1.62 |
| 0.045 mg/kg Nicotine | 21.67 ± 1.85 |
| 0.09 mg/kg Nicotine | 20.50 ± 2.28 |
| 0.18 mg/kg Nicotine | 19.33 ± 1.71 |
| Saline | 24.00 ± 2.01 |
| 0.09 Nicotine | 21.14 ± 2.02 |
| 1.0 g/kg EtOH | 25.8 ± 2.81 |
| Ethanol + Nicotine | 25.00 ± 2.42 |
| Saline | 21.42 ± 1.31 |
| 0.09 Nicotine | 21.43 ± 1.54 |
| 1.4 g/kg EtOH | 28.33 ± 1.43* |
| Ethanol + Nicotine | 22.00 ± 3.26 |
| Saline | 20.33 ± 1.51 |
| 0.09 Nicotine | 19.33 ± 1.99 |
| 2.6 g/kg EtOH | 22.5 ± 1.87 |
| Ethanol + Nicotine | 16.00 ± 1.43 |
n=6–9; Mean ± SEM;
= significantly different from controls at p<0.05
Table 3.
Effects of ethanol (1.0 g/kg; i.p.) administered at training only vs. at training and testing on learning [percent time in the aversive and non-aversive enclosed arms].
| Testing | Number of Entries |
|---|---|
| Saline | 11.57 ± 0.32 |
| 0.6 g/kg EtOH | 10.86 ± 1.64 |
| 1.0 g/kg EtOH | 14.50 ± 1.21 |
| 1.4 g/kg EtOH | 13.71 ± 2.01 |
| 1.8 g/kg EtOH | 16.67 ± 0.83* |
| 2.6 g/kg EtOH | 17.14 ± 1.26* |
| Saline | 4.83 ± 1.03 |
| 0.045 mg/kg Nicotine | 12.50 ± 2.17* |
| 0.09 mg/kg Nicotine | 12.0 ± 1.81* |
| 0.18 mg/kg Nicotine | 11.67 ± 2.46* |
| Saline | 10.86 ± 2.56 |
| 0.09 Nicotine | 10.43 ± 1.29 |
| 1.0 g/kg EtOH | 12.20 ± 2.06 |
| Ethanol + Nicotine | 9.86 ± 1.91 |
| Saline | 7.00 ± 1.01 |
| 0.09 Nicotine | 12.29 ± 1.79 |
| 1.4 g/kg EtOH | 13.50 ± 1.83* |
| Ethanol + Nicotine | 9.17 ± 1.75 |
| Saline | 7.33 ± 2.46 |
| 0.09 Nicotine | 13.17 ± 1.80 |
| 2.6 g/kg EtOH | 15.67 ± 2.24* |
| Ethanol + Nicotine | 12.71 ± 2.04 |
n = 6–7; Mean ± SEM;
= significantly different from controls at p<0.05
Nicotine Dose-Response in the PMDAT
Percent time in the aversive enclosed arm
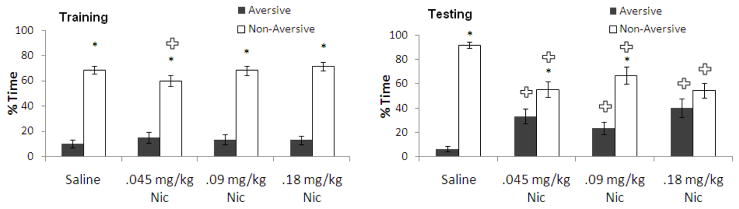
The dose-dependent effects of nicotine administration (0.045–0.18 mg/kg) at training on behavior in the PMDAT were examined. For training, two-way ANOVA revealed a significant effect of arm, F(1,22) = 93.96, p<0.001, but no significant effect of drug treatment and no significant interaction. Tukey’s post-hoc tests revealed that all groups spent more time in the non-aversive than the aversive enclosed arm (p<0.05). For testing, two-way ANOVA revealed no effect of drug treatment but a significant effect of arm, F(3,20) = 57.56, p<0.001 and a significant interaction, F(3,20) = 25.24, p<0.001. Tukey’s post-hoc tests revealed that all groups except the 0.18 mg/kg group spent more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05); there was no difference between time in either enclosed arm for the 0.18 mg/kg nicotine group. In addition, post-hoc tests demonstrated that all three nicotine groups spent significantly less time in the non-aversive enclosed arm and more time in the aversive enclosed arm than saline controls (p<0.05) (Figure 3). Interestingly, all doses of nicotine decreased learning, but only the 0.18 mg/kg dose of nicotine on training day fully disrupted preference for the non-aversive enclosed arm.
Figure 3.
Effects of nicotine (0.045–0.09 mg/kg; i.p.) administered at training on learning [percent time in the aversive and non-aversive enclosed arms]. (n=7–8; Mean ± SEM; * = significant difference between aversive and non-aversive enclosed arms at p<0.05; + = significant difference from controls at p<0.05).
In order to determine whether nicotine disruption of learning in the PMDAT was due to state-dependent effects, the highest dose of nicotine (0.18 mg/kg) or saline was administered before training or before training and testing in separate groups of mice. For training, two-way ANOVA revealed a significant effect of arm, F(1,16) = 85.48, p<0.01, but no effect of drug treatment and no interaction (Table 4). Tukey’s post-hoc tests revealed that all groups spent more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05). For testing, two-way ANOVA revealed no effect of drug treatment but a significant effect of arm, F(1,16) = 87.67, p<0.001, and a significant interaction, F(3,14) = 4.44, p<0.01 (Table 4). Tukey’s post-hoc tests revealed that all three groups spent significantly more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05). Thus, all three groups showed learning; however, the group administered nicotine at training only spent more time in the aversive enclosed arm than both saline controls and the group administered nicotine at both training and testing(p<0.05). Nicotine did not impair memory in the PMDAT when administered before both training and testing, suggesting that a state-dependent memory deficit occurred when nicotine was administered before training only.
Table 4.
Effects of nicotine (0.18 mg/kg; i.p.) administered at training only vs. at training and testing on learning [percent time in the aversive and non-aversive enclosed arms].
| Training | Testing | |||
|---|---|---|---|---|
| Aversive Arm | Non-Avers. Arm | Aversive Arm | Non-Avers. Arm | |
| Saline | 7.94±2.3 | 60.6±8.0 | 17.2±1.0 | 70.6±3.0 |
| Nic at Training | 17.1±3.5 | 57.8±4.4 | 31.8±3.3* | 62.0±4.7* |
| Nic at Both | 17.9±1.7 | 67.2±3.6 | 18.9±1.7 | 67.2±3.6 |
n = 6–7; Mean ± SEM;
= significantly different from controls at p<0.05).
Percent time in the open arms
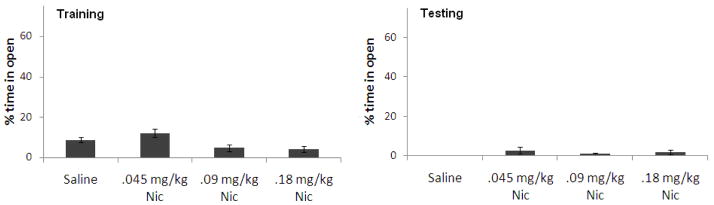
There was a significant effect of nicotine treatment on percent time in the open arms at training, F(3,20) = 6.11, p<0.05. Tukey’s post-hoc tests revealed that the group administered 0.045 mg/kg nicotine spent significantly more time in the open arms than the other nicotine groups (p<0.05), but there was no significant difference from controls. There was no effect of nicotine treatment on percent time in the open arms at testing (Figure 4).
Figure 4.
Effects of nicotine (0.045–0.09 mg/kg; i.p.) administered at training on anxiety [percent time in the open arms]. (n=7–8; Mean ± SEM).
Total entries into all arms
There was no significant effect of nicotine treatment on mean total entries at training (Table 2), but there was a significant effect of nicotine treatment on mean total entries at testing, F(3,20) = 5.41, p<0.05. Tukey’s post-hoc tests revealed that all three nicotine-treated groups had more arm entries than saline controls at testing (p<0.05) (Table 3). Thus, nicotine administration on training day was associated with increased locomotion at testing. Since there was no drug on board when the changes in locomotion were observed, it is likely that the changes in locomotion were due to drug effects on training day. It may be that nicotine alters attention to environmental stimuli on training day, and this altered experience in the maze produces changes in behavior on testing day. However, the locomotor activity in the saline controls was lower than in other experiments, suggesting that the differences may be due to changes in the control group rather than the experimental groups.
Interactions of 1.0 g/kg Ethanol and 0.09 mg/kg Nicotine in the PMDAT
Percent time in the aversive enclosed arm
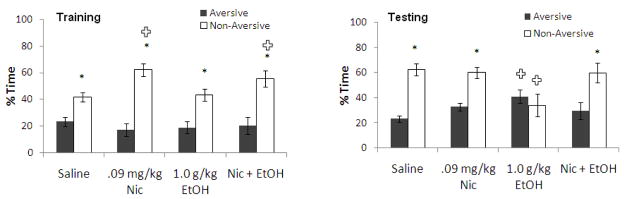
We next examined whether the 0.09 mg/kg dose of nicotine would reverse the learning deficits associated with a dose of ethanol (1.0 g/kg) that did not alter anxiety or locomotion. Both drugs were administered at training only. For training, two-way ANOVA revealed no significant effect of drug treatment but a significant effect of arm, F(1,23) = 65.36, p<0.001, and a significant interaction, F(3,21) = 7.46, p<0.01. Tukey’s post-hoc tests revealed that all groups spent more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05) and that both groups treated with nicotine spent significantly more time in the non-aversive enclosed arm than saline controls (p<0.05). For testing, two-way ANOVA revealed no significant effect of drug treatment but a significant effect of arm, F(1,23) = 39.38, p<0.001, and a significant interaction, F(3,21) = 22.05, p<0.001. All groups except the ethanol-alone group spent significantly more time in the non-aversive enclosed arm than the aversive enclosed arm (p<0.05); the ethanol-alone group showed no preference for either arm. Furthermore, the group administered ethanol alone spent more time in the aversive enclosed arm and less time in the non-aversive enclosed arm than saline controls (p<0.05), but there were no other group differences (Figure 5). Thus, ethanol decreased learning, and nicotine reversed this deficit.
Figure 5.
Effects of 1.0 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on learning [percent time in the aversive and non-aversive enclosed arms]. (n=6–7; Mean ± SEM; * = significant difference between aversive and non-aversive enclosed arms at p<0.05; + = significant difference from controls at p<0.05).
Percent time in the open arms
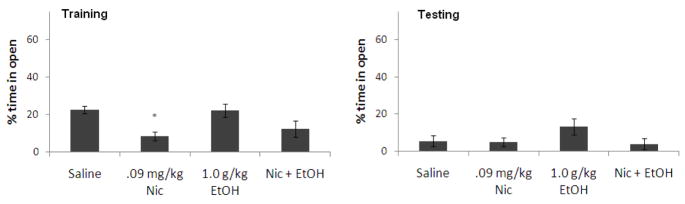
There was no significant effect of ethanol on percent time in the open arms during training. There was a significant effect of nicotine on percent time in the open arms during training, F(1,23) = 18.4, p<0.05, but no interactive effect. Tukey’s post-hoc test revealed that the group administered nicotine alone spent significantly less time in the open arms at training than saline controls (p<0.05) but there were no other group differences. There was no significant effect of ethanol or nicotine on percent time in the open arms during testing, nor was there a significant interaction (Figure 6).
Figure 6.
Effects of 1.0 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on anxiety [percent time in the open arms]. (n=6–7; Mean ± SEM; * = significantly different from controls at p<0.05).
Total entries into all arms
There was no significant effect of ethanol or nicotine on mean total entries at training, nor was there a significant interactive effect (Table 2). Similarly, there was no significant effect of ethanol or nicotine on mean total entries at testing, nor was there a significant interactive effect (Table 3).
Interactions of 1.4 g/kg Ethanol and 0.09 mg/kg Nicotine in the PMDAT
Percent time in the aversive enclosed arm
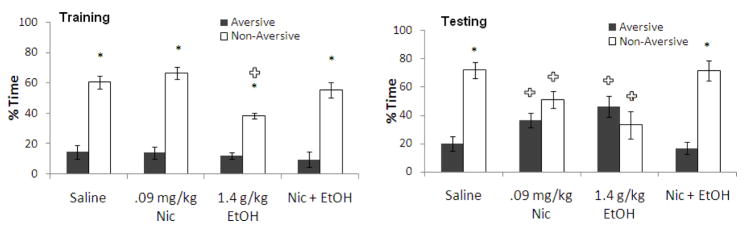
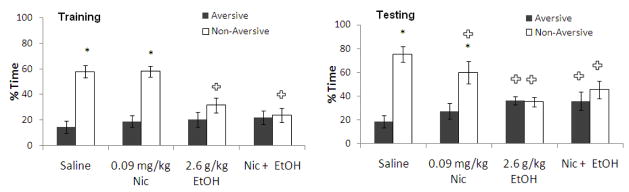
The interactive effects of training day administration of 0.09 mg/kg nicotine and 1.4 g/kg ethanol, a dose that also decreased anxiety and increased locomotion, on learning were assessed. For training, two-way ANOVA revealed a significant effect of drug treatment, F(3,22) = 5.63, p<0.05, as well as an effect of arm, F(1,24) = 79.76, p<0.001, and a significant interaction, F(3,22) = 4.05, p<0.001. Tukey’s post-hoc tests revealed that all groups spent significantly more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05). In addition, the group administered ethanol alone spent less time in the non-aversive enclosed arm than saline controls (p<0.05). For testing, two-way ANOVA revealed no significant effect of drug treatment but a significant effect of arm, F(1,24) = 33.17, p<0.001, and a significant interaction, F(3,22) = 35.36, p<0.001. Tukey’s post-hoc tests revealed that the control group and the group administered ethanol with nicotine spent significantly more time in the non-aversive enclosed arm than the aversive enclosed arm (p<0.05), while time in the aversive and non-aversive enclosed arms for the group administered nicotine alone and the group administered ethanol alone were not significantly different. The groups administered ethanol alone and nicotine alone also spent significantly more time in the aversive enclosed arm and less time in the non-aversive enclosed arm than saline controls (p<0.05) (Figure 7). Thus, ethanol and nicotine each impair learning when administered alone, but co-administration of ethanol and nicotine at training reversed the learning deficit at testing associated with both anxiolytic (1.4 g/kg) and non-anxiolytic (1.0 g/kg) doses of ethanol.
Figure 7.
Effects of 1.4 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on learning [percent time in the aversive and non-aversive enclosed arms]. (n=6–8; Mean ± SEM; * = significant difference between aversive and non-aversive enclosed arms at p<0.05; + = significant difference from controls at p<0.05).
Percent time in the open arms
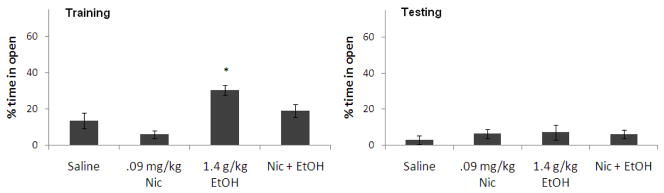
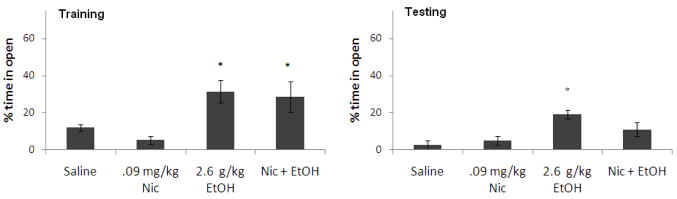
There was a significant effect of the 1.4 g/kg dose of ethanol on percent time in the open arms during training, F(1,25) = 14.76, p<0.05. There was also a significant effect of nicotine on percent time in the open arms during training, F(1,25) = 7.67, p<0.05, but no interactive effect. Tukey’s post-hoc tests revealed that the group administered ethanol alone spent significantly more time in the open arms than saline controls at training (p<0.05), but there were no other group differences. There was no significant effect of ethanol or nicotine on percent time in the open arms during testing, nor was there a significant interaction (Figure 8). Thus, ethanol decreased anxiety at training and nicotine lessened this effect, but there were no effects on anxiety at testing.
Figure 8.
Effects of 1.4 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on anxiety [percent time in the open arms]. (n=6–8; Mean ± SEM; * = significantly different from controls at p<0.05).
Total entries into all arms
There was a significant effect of ethanol on mean total entries at training, F(1,25) = 5.14, p<0.05. There was no significant effect of nicotine on mean total entries at training, but there was an interactive effect of ethanol and nicotine, F(1,25) = 4.73, p<0.05. Tukey’s post-hoc tests revealed that the group administered ethanol alone made significantly more entries than saline controls at training (p<0.05), but the group administered ethanol and nicotine was not significantly different from controls (Table 2). There was no significant effect of ethanol on mean total entries at testing nor was there a significant effect of nicotine on mean total entries at testing but there was an interactive effect of ethanol and nicotine, F(1,25) = 9.91, p<0.05. Tukey’s post-hoc tests revealed that the group administered ethanol alone at training made significantly more entries than saline controls at testing (p<0.05), but the group administered ethanol and nicotine at training was not significantly different from controls (Table 3). Thus, 1.4 g/kg ethanol increased locomotion, and this effect was reversed by nicotine co-administration.
Interactions of 2.6 g/kg Ethanol and 0.09 mg/kg Nicotine in the PMDAT
Percent time in the aversive enclosed arm
Finally, we examined the interactive effects of training day administration of 0.09 mg/kg nicotine with a dose of ethanol (2.6 g/kg) that also decreased anxiety but did not alter locomotion at training. For training, two-way ANOVA revealed a significant effect of drug treatment, F(3,21) = 12.86, p<0.001, a significant effect of arm, F(1,23) = 40.08, p<0.001, and a significant interaction, F(3,21) = 23.43, p<0.001. Tukey’s post-hoc tests revealed that the groups administered saline or only nicotine spent significantly more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05) and did not show a preference for either arm. The groups administered ethanol spent significantly less time in the non-aversive enclosed arm than saline controls (p<0.05). For testing, two-way ANOVA revealed no effect of drug treatment, but a significant effect of arm, F(1,23) = 22.80, p<0.001, and a significant interaction, F(3,21) = 43.43, p<0.001. Tukey’s post-hoc tests revealed that the groups administered saline or nicotine only spent significantly more time in the non-aversive enclosed arm than in the aversive enclosed arm (p<0.05). In addition, all experimental groups spent significantly less time in the non-aversive enclosed arm than saline controls (p<0.05); the groups administered ethanol also spent significantly more time in the aversive enclosed arm than saline controls (p<0.05) and showed no arm preference (Figure 9). Thus, nicotine administration at training was not able to reverse learning deficits due to a higher dose of ethanol.
Figure 9.
Effects of 2.6 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on learning [percent time in the aversive and non-aversive enclosed arms]. (n=7–9; Mean ± SEM; * = significant difference between aversive and non-aversive enclosed arms at p<0.05; + = significant difference from controls at p<0.05).
Percent time in the open arms
There was a significant effect of ethanol on percent time in the open arms during training, F(1,24) = 13.74, p<0.05, but no significant effect of nicotine on percent time in the open arms during training, nor an interactive effect. Duncan’s post-hoc tests revealed that the groups administered ethanol alone and ethanol with nicotine at training spent significantly more time in the open arms at training than saline controls (p<0.05). There was a significant effect of ethanol administered at training, F(1,24) = 17.97, p<0.05, but not nicotine, on time in the open arms during testing; there was no significant interaction. Tukey’s post-hoc tests revealed that the group administered ethanol alone at training spent significantly more time in the open arms than saline controls at testing (p<0.05) (Figure 10). Thus, ethanol decreased anxiety at training, and nicotine failed to reverse this effect, although nicotine administered at training blocked anxiolysis assessed at testing in ethanol-treated animals.
Figure 10.
Effects of 2.6 g/kg ethanol and 0.09 mg/kg nicotine administered i.p. at training on anxiety [percent time in the open arms]. (n=7–9; Mean ± SEM; * = significantly different from controls at p<0.05).
Total entries into all arms
There was no significant effect of ethanol or nicotine on mean total entries at training but there was an interactive effect of ethanol and nicotine on mean total entries at training, F(1,24) = 5.02, p<0.05. Tukey’s post-hoc tests revealed that the group administered ethanol alone made significantly more entries than the group administered ethanol and nicotine at training (p<0.05), but there were no significant differences from controls (Table 2). There was a significant effect of ethanol administered at training on mean total entries at testing, F(1,24) = 4.24, p<0.05, but there was no significant effect of nicotine administered at training on mean total entries at testing, and no interactive effect. Tukey’s post-hoc tests revealed that the ethanol-alone group made more entries than controls at testing (p<0.05) (Table 3). Thus, neither drug significantly altered locomotor behavior compared to controls in the PMDAT at training, although nicotine did attenuate the ethanol-induced increase in locomotion at testing.
Discussion
The current study extends prior work demonstrating that nicotine ameliorates ethanol-induced deficits in fear conditioning (Gould and Lommock, 2003; Gulick and Gould, 2008; Gulick and Gould, 2009) by demonstrating that nicotine also ameliorates ethanol deficits in learning the PMDAT. The PMDAT differs from fear conditioning on several measures. First, the PMDAT is an operant task in which mice learn to avoid an area of the maze, whereas fear conditioning is a traditional classical conditioning paradigm. In addition, the PMDAT does not use shocks to train the mice. Thus, the interactive effects of ethanol and nicotine on learning generalize across different types of learning. Interestingly, in the present study saline-treated mice showed a consistant decrease in locomotion between training day and testing day. This decrease may reflect habituation. Adminstration of higher doses of ethanol at training blocked the development of this habituation and nicotine prevented this ethanol-associated change in behavior. These interactive effects on locomotion at testing occurred even though nicotine and ethanol were administered at training, suggesting that ethanol and nicotine interact at training to produce long-term changes in behavior. Nicotine has also been shown to ameliorate ethanol-induced deficits in other cognitive processes. For example, ethanol-associated deficits in reference and working memory measured in the 8-arm radial maze were prevented by nicotine pretreatment (Tracy et al., 1999). In addition, testing day administration of nicotine decreased ethanol-induced impairments in memory retrieval for passive avoidance (Rezayof et al., 2008). Thus, ethanol produced deficits in both learning the PMDAT and in habituation, but at different doses; and nicotine reversed both deficits. These findings, along with prior research, indicate that nicotine can counter maladaptive effects of alcohol on learning and cognitive processes.
The ability of nicotine to reverse deficits in learning the PMDAT did not depend on changes in anxiety or locomotion. Specifically, the dose of nicotine used in the co-administration experiments did not produce any consistant effects on locomotor activity or anxiety but did reverse learning deficits induced by 1.0 and 1.4 g/kg ethanol. In addition, the 1.0 g/kg dose of ethanol altered learning but did not alter locomotor activity or anxiety, further suggesting that the ability of nicotine to ameliorate learning deficits associated with this dose of ethanol is not due to effects on anxiety or locomotion.
This is also the first study to examine the effects of nicotine on learning, anxiety and locomotion within the same subjects and the same task. Although previous research has demonstrated that nicotine enhances learning in other tasks (Gould and Wehner, 1999; Hahn et al., 2002; Rezvani and Levin, 2003), nicotine did not enhance learning the PMDAT. In fact, the highest dose of nicotine produced a state-dependent learning deficit that was absent when nicotine was administered before testing as well as training, whereas the ethanol-induced learning deficit was attenuated but still significant when ethanol was administered on both days. Thus, the impairment of learning by nicotine may be due to state-dependent changes, but this was not the case for ethanol. Furthermore, the lack of enhancement of learning in the PMDAT by nicotine may be due to the specificity of nicotine enhancement of learning. We have previously shown that the doses of nicotine used here enhance hippocampus-dependent learning but not simple tone-shock associative learning that is hippocampus-independent (Gould et al., 2004; Gould and Higgins, 2003). Thus, learning in the PMDAT may not be critically dependent on the hippocampus; however, this needs further investigation.
Nicotine administration did not alter locomotor activity in the PMDAT on training day but did produce a slight, though inconsistent, anxiogenic effect. Previous studies examining the effects of nicotine on anxiety have likewise met with variable results (Biala and Budzynska, 2006; Costall et al., 1989). Using similar dose ranges to that of the current study, Costall and colleagues (1989) found that acute nicotine decreased anxiety in a light-dark box, while Biala and Budzynska (2006) found that acute nicotine increased anxiety in the elevated plus-maze. Although factors such as mouse strain (Costall and colleagues used BKW mice and Biala and Budzynska used Swiss mice), and methodological differences may contribute to the differential effects of nicotine across studies, nicotine may also have variable effects on anxiety depending on the task being examined.
Nicotine did reduce the anxiolytic effects of 1.4 g/kg ethanol assessed at training but did not alter the anxiolytic effects of the highest dose of ethanol. Previous work found that nicotine reduced the anxiolytic effects of ethanol in adolescent mice tested in the elevated plus-maze but one month after nicotine and ethanol exposure, these mice showed an anxiogenic response (Abreu-Villaca et al., 2008). Another study found that ethanol and nicotine worked synergistically to decrease anxiety measured with the mirrored chamber task but this effect was dependent on genotype as mice bred for short sleep times after alcohol administration showed this effect and mice bred for long sleep times did not (Cao et al., 1993). Thus, alcohol and nicotine clearly interact to alter anxiety but the effects may depend on genetics and perhaps on how anxiety is assessed.
The current results replicate previous work demonstrating that ethanol disruption of learning in the PMDAT was independent of the effects of ethanol on anxiety or locomotion (Kameda et al., 2007) and show that this finding extends across different strains of mice, as we used C57BL/6 mice and Kameda and colleagues used Swiss mice. The finding that a wide range of ethanol doses impairs learning is consistent with previous research (Bammer and Chesher, 1982; Gibson, 1985; Gould, 2003; Higgins et al., 1992; Kameda et al., 2007; Weitemier and Ryabinin, 2003); however, in the PMDAT study by Kameda and colleagues (2007), learning was altered by lower doses of ethanol than those used in the current study. This disparity may be due to strain or methodological differences; Kameda and colleagues used a 10-minute training session, whereas the current study used a 5-minute session to reduce ceiling effects that could obscure any potential enhancement of learning. Nonetheless, ethanol clearly disrupts learning.
Ethanol also dose-dependently decreased anxiety in the current study, which is in agreement with previous literature on the anxiolytic effects of ethanol (Blanchard et al., 1993; Durcan and Lister, 1988; Gallate et al., 2003; Kameda et al., 2007). The anxiolytic effects of ethanol in the current study were weaker than the effect observed by Kameda and colleagues (2007); again, this may be due to genetic or methodological differences. We also found that a moderate dose of ethanol stimulated locomotion. This result is similar to a previous report that in the PMDAT moderate doses of ethanol stimulate, while higher doses depress, motor activity (Kameda et al., 2007). Overall, ethanol disrupted learning (1.0–2.6 g/kg ethanol), decreased anxiety (1.4–2.6 g/kg ethanol), and increased locomotor activity (1.4 g/kg ethanol), but these effects occurred at different doses.
In conclusion, nicotine ameliorates ethanol induced deficits across a wide range of cognitive processes. This effect of nicotine could contribute to the high incidence of co-use and abuse of alcohol and tobacco products as individuals attempt to reduce cognitive impairments associated with alcohol use. However, recent work suggests that tolerance for this effect of nicotine occurs with chronic administration and cessation of chronic nicotine administration itself can produce cognitive deficits (Gulick and Gould, 2008). The ability of acute nicotine to reverse learning deficits appears to be independent of changes in anxiety and locomotion. Finally, the effects of nicotine and alcohol administered alone on learning, anxiety, and locomotion were also independent.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant AA015515 (T.J.G.). All research complies with current US laws for the care and use of laboratory animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaca Y, Nunes F, do EQGF, Manhaes AC, Filgueiras CC. Combined exposure to nicotine and ethanol in adolescent mice differentially affects anxiety levels during exposure, short-term, and long-term withdrawal. Neuropsychopharmacology. 2008;33:599–610. doi: 10.1038/sj.npp.1301429. [DOI] [PubMed] [Google Scholar]
- Bammer G, Chesher GB. An analysis of some effects of ethanol on performance in a passive avoidance task. Psychopharmacology (Berl) 1982;77:66–73. doi: 10.1007/BF00436101. [DOI] [PubMed] [Google Scholar]
- Biala G, Budzynska B. Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels. Life Sci. 2006;79:81–88. doi: 10.1016/j.lfs.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Magee L, Veniegas R, Blanchard DC. Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:171–182. doi: 10.1016/0278-5846(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Cao W, Burkholder T, Wilkins L, Collins AC. A genetic comparison of behavioral actions of ethanol and nicotine in the mirrored chamber. Pharmacol Biochem Behav. 1993;45:803–809. doi: 10.1016/0091-3057(93)90124-c. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Lister RG. Time course of ethanol’s effects on locomotor activity, exploration and anxiety in mice. Psychopharmacology (Berl) 1988;96:67–72. doi: 10.1007/BF02431535. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Morley KC, Ambermoon P, McGregor IS. The consequences of beer consumption in rats: acute anxiolytic and ataxic effects and withdrawal-induced anxiety. Psychopharmacology (Berl) 2003;166:51–60. doi: 10.1007/s00213-002-1291-z. [DOI] [PubMed] [Google Scholar]
- Gibson WE. Effects of alcohol on radial maze performance in rats. Physiol Behav. 1985;35:1003–1005. doi: 10.1016/0031-9384(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008;196:483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits via different effects at nicotinic receptors. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.45. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hefco V, Yamada K, Hefco A, Hritcu L, Tiron A, Nabeshima T. The interaction between the cholinergic and dopaminergic system in learning and memory process in rats. Rom J Physiol. 2004;41:21–30. [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Hughes JR, Bickel WK, Lynn M, Capeless MA. Effects of cocaine and alcohol, alone and in combination, on human learning and performance. J Exp Anal Behav. 1992;58:87–105. doi: 10.1901/jeab.1992.58-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda SR, Frussa-Filho R, Carvalho RC, Takatsu-Coleman AL, Ricardo VP, Patti CL, Calzavara MB, Lopez GB, Araujo NP, Abilio VC, Ribeiro Rde A, D’Almeida V, Silva RH. Dissociation of the effects of ethanol on memory, anxiety, and motor behavior in mice tested in the plus-maze discriminative avoidance task. Psychopharmacology (Berl) 2007;192:39–48. doi: 10.1007/s00213-006-0684-9. [DOI] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Alijanpour S, Zarrindast MR, Rassouli Y. Ethanol state-dependent memory: involvement of dorsal hippocampal muscarinic and nicotinic receptors. Neurobiol Learn Mem. 2008;89:441–447. doi: 10.1016/j.nlm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav. 2003;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Silva RH, Bellot RG, Vital MA, Frussa-Filho R. Effects of long-term ganglioside GM1 administration on a new discriminative avoidance test in normal adult mice. Psychopharmacology (Berl) 1997;129:322–328. [PubMed] [Google Scholar]
- Silva RH, Frussa-Filho R. The plus-maze discriminative avoidance task: a new model to study memory-anxiety interactions. Effects of chlordiazepoxide and caffeine. J Neurosci Methods. 2000;102:117–125. doi: 10.1016/s0165-0270(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Tracy HA, Jr, Wayner MJ, Armstrong DL. Nicotine blocks ethanol and diazepam impairment of air righting and ethanol impairment of maze performance. Alcohol. 1999;18:123–130. doi: 10.1016/s0741-8329(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]